Abstract
Pyruvate kinase type M2, which is expressed in multiple tumor cell types and plays a key role in aerobic glycolysis, also has nonglycolytic functions and can regulate transcription and cell proliferation. The results of this study show that epidermal growth factor receptor activation induces pyruvate kinase type M2 nuclear translocation. To further determine the relationship between pyruvate kinase type M2 and epidermal growth factor receptor, we analyzed pathological data from mammary glands and performed epidermal growth factor receptor/human epidermal growth factor receptor 2 knockdown to reveal that pyruvate kinase type M2 is associated with epidermal growth factor receptor and human epidermal growth factor receptor 2. Lapatinib is a small molecule epidermal growth factor receptor tyrosine kinase inhibitor that can inhibit epidermal growth factor receptor and human epidermal growth factor receptor 2, though its effect on pyruvate kinase type M2 remains elusive. Accordingly, we performed Western blotting and reverse transcription polymerase chain reaction and analyzed pathological data from mammary glands, with results suggesting that lapatinib inhibits pyruvate kinase type M2 expression. We further found that the antitumor drug lapatinib inhibits breast cancer cell proliferation by influencing pyruvate kinase type M2 expression, as based on Cell Counting Kit-8 analyses and pyruvate kinase type M2 overexpression experiments. Signal transducer and activator of transcription 3, which is a transcription factor-associated cell proliferation and the only transcription factor that interacts with pyruvate kinase type M2, we performed pyruvate kinase type M2 knockdown experiments in Human breast cancer cells MDA-MB-231 and Human breast cancer cells SK-BR-3 cell lines and examined the effect on levels of Signal transducer and activator of transcription 3 and phosphorylated Signal transducer and activator of transcription 3. The results indicate that pyruvate kinase type M2 regulates Signal transducer and activator of transcription 3 and phospho-Stat3 (Tyr705) expression. Together with previous reports, our findings show that lapatinib inhibits breast cancer cell proliferation by influencing pyruvate kinase type M2 expression, which results in a reduction in both Signal transducer and activator of transcription 3 and phosphorylated Signal transducer and activator of transcription 3.
Keywords: pyruvate kinase, isoenzyme, lapatinib, cell proliferation, breast cancer
Introduction
Breast cancer is one of the most common malignant tumors in females. Occurrence of this cancer is related to apoptosis imbalance, aberrant cell proliferation, and signal transduction abnormalities as well as many other factors.
The metabolism of tumor cells is different from that of normal cells, with tumor cells requiring sufficient nutrition, nucleic acids, and biomolecules to synthetize substances required for proliferation. Tumor cells metabolize large quantities of glucose to produce lactic acid, and when there is a large accumulation under high-oxygen conditions, a phenomenon known as the Warburg effect, or aerobic glycolysis, occurs.1 Pyruvate kinase (PK) is an important rate-limiting enzyme in glycometabolism, with 4 known isoenzyme forms: L type, R type, M1 type, and M2 type. Pyruvate kinase type M2 (PKM2), which is mainly expressed in proliferating cells, such as embryonic tissue, adult stem cells, and tumor cells, is associated with nucleic acid synthesis.2-6 There are 3 forms of PKM2: a nonactive monomer, a slightly active dimer, and a highly active tetramer.7 In tumor cells, PKM2 exists mainly in the dimeric form, and dimeric PKM2 is known as tumor M2-PK. In addition, PKM2 is allosterically regulated by fructose 1,6-bisphosphate.4,8,9 A number of studies have shown that malignant transformation is accompanied by changes in the isoenzyme spectrum, with PK activity and isozyme spectrum alterations being universal to the processes of tumorigenesis and development.10-13
Epidermal growth factor receptor (EGFR), which belongs to the tyrosine kinase receptor family, plays a key role in cell growth, proliferation, and differentiation. Epidermal growth factor receptor is activated by its growth factor ligands and can homodimerize or heterodimerize.14 Human epidermal growth factor receptor 2 (HER2) is an orphan receptor that forms heterodimers with 3 other receptors belonging to the V-Erb-B2 Avian Erythroblastic Leukemia Viral Oncogene Homolog (ErbB) family.15 Once activated, the intracellular tyrosine kinase domain autophosphorylates tyrosine residues, which eventually leads to cell division, migration, engraftment, and differentiation via cellular signaling. In breast cancer, the overexpression of EGFR and HER2 is associated with poor prognosis, low rates of survival and disease-free survival, high transfer rates, and resistance to chemotherapy and hormone treatment. Some studies have shown that EGFR activation regulates PKM2 expression16,17 and that there is a relationship between HER2 and PKM2.18
Molecular-targeted therapy is a novel category of biological treatment that is performed after surgery, radiotherapy, and chemotherapy and is currently the subject of many research endeavors in the field of breast cancer. Some patients with breast cancer exhibit EGFR and HER2 overexpression and have serious disease and poor prognosis. Lapatinib is an EGFR tyrosine kinase inhibitor that blocks the functions of both EGFR and HER2. Clinical trials have shown that it is well tolerated and can be used alone or with other drugs for breast cancer treatment.19-25 Although it is known to target EGFR and HER2, the effect of lapatinib on PKM2 remains unclear; in addition, further research is required to determine whether the inhibition of tumor cell proliferation by lapatinib is associated with PKM2. In this report, we show that suppression of PKM2 expression via lapatinib-mediated inhibition of EGFR and HER2 results in reduced Stat3 and pStat3 expression, which leads to lower levels of gene transcription and impedes tumor cell proliferation.
Methods and Materials
Patients
The study was approved by Tianjin Medical University’s Institutional Human Assurance Committee and was performed in accordance with the Declaration of Helsinki. Eighty-two primary breast cancer tissues and adjacent normal breast tissues were obtained from patients who were diagnosed according to the modified Scarff system at Tianjin Medical University Cancer Institute & Hospital from 2013 to 2014. All participants were female with a mean age of 45 (31-54) years. The tissues were obtained from the Tissue Bank Facility of Tianjin Medical University Cancer Institute & Hospital. The tissue collection procedure was approved by the institutional review board. All participants were informed that their tissue samples were being collected in a tissue bank, and they provided written informed consent.
Western Blotting
The primary antibodies used included a rabbit monoclonal anti-EGF receptor antibody (1:1000, CST, Danvers, Massachusetts, #4267), rabbit monoclonal anti-HER2/ErbB2 antibody (1:1000, CST, #4290), rabbit polyclonal anti-HER3/ErbB3 antibody (1:500, Santa Cruz Biotechnology, Dallas, Texas, sc-285), rabbit monoclonal anti-PKM2 antibody (1:1000, CST, 4053), rabbit monoclonal anti-PKM2 (Tyr(P)-105) antibody (1:500, CST, #3827), rabbit monoclonal anti-Stat3 antibody (1:500, CST, #4904), and rabbit monoclonal anti-Stat3 (Tyr(P)-705) antibody (1:500, CST, #9145).
Statistical Analysis
Data are expressed as the means (SD). One-way analysis of variance with Bonferroni correction for multiple comparisons and Student t test and continuous correction for the χ2 test were employed to analyze significant differences (SPSS 19.0 Inc, Chicago, Illinois). A value of P < .05 was considered to be significant.
Results
Pyruvate Kinase Type M2 Expression Is Upregulated and Positively Correlated With EGFR and HER2 Expression in Breast Cancer Tissues
Previous studies demonstrated that PKM2 is expressed in multiple types of tumor cells.2-6 To determine the level of PKM2 expression in breast cancer, we analyzed pathological data by performing immunohistochemistry analysis of 82 primary breast cancer tissues and adjacent normal tissues from patients diagnosed according to the modified Scarff system at Tianjin Medical University Cancer Institute & Hospital from 2013 to 2014. The results showed that the expression of PKM2 in invasive ductal carcinomas (88.24%) was significantly increased compared with that in adjacent normal tissues (15.85%) and in ductal carcinoma in situ (71.43%) compared with that in adjacent normal tissues (15.85%). For different breast cancer Classification of Malignant Tumours (TNM) stages, PKM2 expression (T1: 77.50%; T2: 94.12%; T3: 87.50%) was significantly increased compared to that in adjacent normal tissues (15.85%). Pyruvate kinase type M2 expression was also significantly increased in breast cancer with (90.00%) and without (82.69%) lymph node metastasis compared to that in adjacent normal tissues (15.85%; Table 1). Immunohistochemical staining and Western blotting showed PKM2 to be highly expressed in breast cancer tissues (Figure 1). These results indicate that PKM2 expression is increased in breast cancer tissues compared to adjacent normal tissues.
Table 1.
Expression of PKM2 in Breast Tissues.
| Parameters | N | PKM2 Expression | |
|---|---|---|---|
| Negative, n (%) | Positive, n (%) | ||
| Breast tissues | |||
| Normal tissues | 82 | 69 (84.15) | 13 (15.85) |
| Tumor type | |||
| Invasive ductal carcinoma | 68 | 8 (11.76) | 60 (88.24)a |
| Ductal carcinoma in situ | 14 | 4 (28.57) | 10 (71.43)a |
| TNM stage | |||
| T1 | 40 | 9 (22.50) | 31 (77.50)a |
| T2 | 34 | 2 (5.88) | 32 (94.12)a |
| T3 | 8 | 1 (12.50) | 7 (87.50)a |
| Lymph node metastases | |||
| N0 | 52 | 9 (17.31) | 43 (82.69)a |
| N1-3 | 30 | 3 (10.00) | 27 (90.00)a |
Abbreviation: PKM2, pyruvate kinase type M2; TNM, TNM Classification of Malignant Tumours.
aCompared to normal breast tissues, P < .05.
Figure 1.
Pyruvate kinase type M2 is highly expressed in breast cancer tissues. A, Immunohistochemical staining with an anti-PKM2 antibody was performed on breast cancer tissues and adjacent normal tissues. (a), (c), and (e) Positive staining of PKM2 in tumor tissues (at ×400). (b), (d), and (f) Negative results for PKM2 in normal tissues (at ×400). (g) Negative control, with the primary antibody against PKM2 omitted and replaced with preimmune serum (at ×400). B, Western blot of breast cancer tissues and adjacent normal tissues was performed with an anti-PKM2 antibody. β-Actin was used as a loading control. PKM2 denotes pyruvate kinase type M2.
Pathological data for mammary glands from the above-mentioned 82 patients with breast cancer showed that PKM2 expression was increased in HER2-positive (96.43%) compared to HER2-negative (79.63%) breast cancer tissues (Table 2). Pathological data for mammary glands also showed that in invasive ductal carcinoma, PKM2 expression in EGFR-positive tissues (96.30%) was increased compared to that in EGFR-negative tissues (80.00%; Table 2). Therefore, the results of these analyses indicate a positive relationship between PKM2 and EGFR expression in breast cancer tissues.
Table 2.
Correlation Between EGFR/HER2 and PKM2.
| n | PKM2 Expression | χ2 | P | ||
|---|---|---|---|---|---|
| Negative, n (%) | Positive, n (%) | ||||
| Breast cancer | |||||
| HER-2-negative | 54 | 11 (20.37) | 43 (79.63) | 2.93 | <.05 |
| HER-2-positive | 28 | 1 (3.57) | 27 (96.43) | ||
| EGFR-negative | 55 | 11 (20.00) | 44 (80.00) | 2.66 | <.05 |
| EGFR-positive | 27 | 1 (3.70) | 26 (96.30) | ||
Abbreviations: EGFR, epidermal growth factor receptor ; HER2, human epidermal growth factor receptor 2; PKM2, pyruvate kinase type M2.
To further determine the relationship between PKM2 and EGFR, we performed EGFR knockdown. Knocking down EGFR expression in the breast cancer cell line MDA-MB-231 (Figure 2A) resulted in a significant reduction in PKM2 expression compared to that in wild-type and negative control cells (Figure 2B).
Figure 2.
Expression of EGFR, HER2, HER3 and PKM2 in breast cancer cell lines. Inhibition of PKM2 expression by siEGFR in MDA-MB-231 cells, inhibition of PKM2 expression by siHER2 in SK-BR-3 cells, and determination of the working concentrations of lapatinib and EGF. A, Epidermal growth factor receptor was expressed in MDA-MB-231 cells, whereas HER2 and HER3 were not. HER2 and HER3 were expressed in SK-BR-3 cells, whereas EGFR was not. Human epidermal growth factor receptor 3 was expressed in MCF-7 cells, whereas EGFR and HER2 were not. Three cell lines expressed PKM2. The experiments were repeated 3 times. B, Epidermal growth factor receptor knockdown analyses were performed in MDA-MB-231 cells. Cells were transfected with 20 μM siEGFR for 48 hours and lysed for Western blotting. We successfully achieved EGFR gene silencing with Stealth RNAi siRNA oligoribonucleotide duplexes of EGFR. Knocking down EGFR resulted in a reduction in PKM2 expression compared to control groups. WT represents the wild-type control group. Scr represents the negative control group. The experiments were repeated 3 times. C, Human epidermal growth factor receptor 2 knockdown analyses were performed in SK-BR-3 cells. Cells were transfected with 20 μM siHER2 for 48 hours and lysed for Western blotting. We successfully achieved HER2 gene silencing with Stealth RNAi siRNA oligoribonucleotide duplexes of HER2. Knocking down HER2 resulted in a reduction in PKM2 expression compared to control groups. WT represents the wild-type control group. Scr represents the negative control group. The experiments were repeated 3 times. D-F, The working concentration of lapatinib was 1.0 μM. Expression of PKM2 was significantly reduced with 1.0 μM lapatinib treatment in MDA-MB-231 (D) and SK-BR-3 (E) cells compared with lower concentrations of 0 μM and 0.5 μM; expression was not notably changed compared with 2.0 μM lapatinib treatment. In MCF-7 cells, the effect of Lapatinib on the expression of PKM2 was not obvious (F). All cells were treated for 48 hours. The experiments were repeated 3 times. G, The working concentration of EGF was 10 ng/mL. Expression of PKM2 was significantly increased with 10 ng/mL EGF treatment of MDA-MB-231 cells compared to lower concentrations of 0 and 5 ng/mL, and there was no marked change compared to higher concentrations of 20, 50, and 100 ng/mL. All cells were treated for 48 hours. The experiments were repeated 3 times. H, The working concentration of EGF was 50 ng/mL. Expression of PKM2 was significantly increased with 50 ng/mL EGF treatment of SK-BR-3 compared to lower concentrations of 0, 5, 10, and 20 ng/mL, and there was no obvious change compared to higher concentration of 100 ng/mL. All cells were treated for 48 hours. The experiments were repeated 3 times. I, In MCF-7 cells, there was a trend of increasing the protein levels of PKM2 in MCF-7 cells induced with EGF. All cells were treated for 48 hours. The experiments were repeated 3 times. EGFR indicates epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; HER3, human epidermal growth factor receptor 3; PKM2, pyruvate kinase type M2; siRNA, small inhibitory RNA; siEGFR, small inhibitory EGFR; siHER2, small inhibitory HER2; MDA-MB-231, Human breast cancer cells MDA-MB-231; MCF-7, Michigan Cancer Foundation-7; SK-BR-3, Human breast cancer cells SK-BR-3.
We also performed HER2 knockdown in the breast cancer cell line SK-BR-3 (Figure 2A), which resulted in a significant reduction in PKM2 expression compared to wild-type and negative control cells (Figure 2C). These results show that PKM2 expression is upregulated and positively correlated with EGFR and HER2 expression in breast cancer tissues.
Lapatinib Inhibits PKM2 Expression in Breast Cancer Cell Lines
The small EGFR tyrosine kinase inhibitor lapatinib can block the functions of EGFR and HER2. We previously showed that PKM2 is associated with EGFR and HER2, and we next examined whether lapatinib influences PKM2 expression in breast cancer cells. First, Western blot analyses of breast cancer cell lines MDA-MB-231, SK-BR-3, and MCF-7 were performed. As shown in Figure 2A, MDA-MB-231 cells expressed EGFR and expression of HER2 and HER3 was not detected in these cells. In contrast, we found that SK-BR-3 cells expressed HER2 and HER3, but not EGFR. MCF-7 cells expressed HER3, but not EGFR and HER2. Three cell lines expressed PKM2. Although some studies have reported that SK-BR-3 cells expresses EGFR,26,27 we did not observe expression of EGFR in these cells, possibly because the level was below the limit of detection.
The ligand EGF activates EGFR to regulate cell proliferation,28,29 and some studies have reported that EGF stimulates SK-BR-3 cells and regulates proliferation.30 To determine the working concentrations of lapatinib and EGF, we established a concentration gradient using lapatinib at 0, 0.5, 1.0, and 2.0 μM and EGF at 0, 5, 10, 20, 50, and 100 ng/mL. MDA-MB-231, SK-BR-3, and MCF-7 cells were seeded into 60-mm dishes; after attachment, the cells were starved overnight to synchronize them in the same proliferative cycle. Lapatinib and EGF were then added at different concentrations, and the cells were cultured for 48 hours. Because lapatinib’s half-life is 24 hours, we changed the culture medium of the cells treated with lapatinib and added the inhibitor after 24 hours of culture. After culturing for 48 hours, the cells were lysed to obtain protein extracts for Western blotting to detect PKM2 expression. The results showed that PKM2 expression was significantly reduced in both MDA-MB-231 and SK-BR-3 cell lines treated with 1.0 μM lapatinib compared with lower concentrations. Furthermore, expression was not markedly changed compared to that with 2.0 μM lapatinib (Figure 2D and E). In MCF-7 cells, the effect of lapatinib on the expression of PKM2 was not obvious (Figure 2F). These results suggested that the inhibition of EGFR and HER2 by lapatinib in MDA-MB-231 and SK-BR-3 cells, respectively, induces a downregulation of PKM2. In addition, expression of PKM2 was significantly increased in MDA-MB-231 cells treated with 10 ng/mL EGF compared to lower concentrations, and there was no obvious change compared to higher concentrations (Figure 2G). In SK-BR-3 cells, expression of PKM2 was significantly increased with 50 ng/mL EGF compared with lower concentrations; furthermore, there was no marked change compared to 100 ng/mL EGF (Figure 2H). In MCF-7 cells, there was a trend of increasing the protein levels of PKM2 in MCF-7 cells induced with EGF (Figure 2I). These results suggest that 50 ng/mL EGF is an appropriate working concentration for treating MDA-MB-231 and SK-BR-3 cell lines in subsequent experiments. Additionally, MCF-7 cells expressed PKM2 and they did not express EGFR and HER2. We will do an additional study to further elucidate the mechanisms of PKM2 regulation may exits, whether HER3 was also involved in the regulation of PKM2.
To examine the influence of lapatinib on PKM2, we performed Western blotting and reverse transcription polymerase chain reaction (RT-PCR). As shown in Figure 3, PKM2 expression in MDA-MB-231 cells stimulated by EGF was increased compared with controls treated with dimethyl sulfoxide (DMSO) alone. Furthermore, expression of PKM2 after EGF stimulation for 48 hours was increased more than at 24 hours. Pyruvate kinase type M2 (Tyr(P)-105) expression in MDA-MB-231 cells stimulated by EGF was increased compared with the DMSO group (Figure 3A left and C), and PKM2 and PKM2 (Tyr(P)-105) expression in MDA-MB-231 cells stimulated by EGF and treated with lapatinib showed no obvious changes compared with controls (Figure 3A right and C). In both cell lines, expression of PKM2 in the DMSO treatment group was increased more than that in the lapatinib treatment group with the same duration of EGF stimulation. At 48 hours, PKM2 and PKM2 (Tyr(P)-105) expression in SK-BR-3 cells stimulated by EGF was increased compared with the DMSO group. Pyruvate kinase type M2 (Tyr(P)-105) expression was more enhanced by EGF stimulation for 48 hours than 24 hours (Figure 3B left and D). Moreover, PKM2 and PKM2 (Tyr(P)-105) expression in SK-BR-3 cells stimulated by EGF showed no notable difference compared with the lapatinib treatment group (Figure 3B right and D). At the same duration of EGF stimulation, expression of PKM2 was increased in SK-BR-3 cells treated with DMSO compared to those treated with lapatinib. The results indicate that messenger RNA (mRNA) levels of PKM2 are not decreasing with lapatinib treatment alone, which indicates that lapatinib is not downmodulating the expression of PKM2 at the transcriptional level and lapatinib modulates PKM2 by regulating its phosphorylation.
Figure 3.
Lapatinib inhibits PKM2 expression in breast cancer cell lines. A, Lapatinib inhibits PKM2 and PKM2 (Tyr (P)-105) expression in MDA-MB-231 cells. In DMSO treatment groups, PKM2 expression was increased after stimulation with EGF compared to controls, and PKM2 expression after EGF stimulation for 48 hours was more increased than after 24 hours. PKM2 (Tyr(P)-105) expression was increased after stimulation with EGF compared to controls (left). In lapatinib treatment groups, PKM2 and PKM2 (Tyr(P)-105) expression was not notably changed after stimulation with EGF compared to controls (right). The PKM2 expression in DMSO treatment groups increased more than in lapatinib treatment groups with EGF stimulation for the same time period. The experiments were repeated 3 times. B, Lapatinib inhibits PKM2 and PKM2 (Tyr (P)-105) expression in SK-BR-3 cells. In DMSO treatment groups, PKM2 expression was increased after stimulation with EGF for 48 hours compared to controls. The PKM2 (Tyr(P)-105) expression was increased after stimulation with EGF compared to controls. The PKM2 (Tyr(P)-105) expression with EGF stimulation for 48 hours was increased more than after 24 hours (left). In lapatinib treatment groups, PKM2 and PKM2 (Tyr(P)-105) expression were not obviously changed after stimulation with EGF compared to controls (right). PKM2 expression in DMSO treatment groups was increased more than in lapatinib treatment groups with EGF stimulation for the same time period. The experiments were repeated 3 times. C, Lapatinib inhibits mRNA levels of PKM2 in MDA-MB-231. In DMSO treatment groups, PKM2 expression was significantly increased after stimulation with EGF compared to controls (P < .05). In lapatinib treatment groups, PKM2 expression was not markedly changed after stimulation with EGF compared to controls. The PKM2 expression in DMSO treatment groups was increased more than in lapatinib treatment groups with EGF stimulation for the same time period (P < .05). The experiments were performed in triplicate. Data are presented as means (SD). D, Lapatinib inhibited mRNA levels of PKM2 in SK-BR-3 cells. In DMSO treatment groups, PKM2 expression was significantly increased after stimulation with EGF compared to controls (P < .05). In lapatinib treatment groups, PKM2 expression was not obviously changed after stimulation with EGF compared to controls. PKM2 expression in DMSO treatment groups was increased more than in lapatinib treatment groups with EGF stimulation for the same time period (P < .05). The experiments were performed in triplicate. Data are presented as means (SD). DMSO denotes dimethyl sulfoxide; EGF, epidermal growth factor; mRNA, messenger RNA; PKM2, pyruvate kinase type M2; SD, standard deviation; MDA-MB-231, Human breast cancer cells MDA-MB-231; SK-BR-3, Human breast cancer cells SK-BR-3.
Plasma PKM2 Levels Are Significantly Reduced in Patients With Breast Cancer Treated With Lapatinib
To further support the finding that lapatinib inhibits PKM2 expression, we performed in vivo experiments by analyzing pathological data from 120 patients with HER2 (+ + +) tissues according to immunohistochemistry or HER2 gene amplification according to fluorescence in situ hybridization (FISH). The 120 patients included 60 patients treated with adjuvant lapatinib chemotherapy as the experimental group and 60 treated with chemotherapy as the control group. All patients underwent 4 phases of treatment: pretherapy, treatment for 1 cycle, treatment for 3 cycles, and treatment for 6 cycles, and the level of plasma PKM2 was detected by enzyme-linked immunosorbent assay during different treatment phases. The results showed significantly reduced plasma PKM2 levels during the treatment periods of 3 cycles and 6 cycles compared with pretherapy in the same group. In addition, plasma PKM2 levels in the lapatinib treatment group were markedly reduced compared to the control group during the treatment periods of 3 cycles and 6 cycles (Table 3). Data represent the means (standard deviation, SD) of 3 independent experiments. The results indicate that lapatinib can reduce plasma PKM2 levels in patients with breast cancer.
Table 3.
Plasma PKM2 Levels in Patients With Breast Cancer Undergoing Lapatinib Treatment.a,b
| Groups | Treatment Period | n | (SD), U/mL |
|---|---|---|---|
| Lapatinib treatment group | Pretherapy | 60 | 22.05 (7.71) |
| Treatment for 1 cycle | 60 | 20.87 (5.65) | |
| Treatment for 3 cycles | 60 | 15.58 (4.34)c,d | |
| Treatment for 6 cycles | 60 | 11.39 (3.89)c,d | |
| Control group | Pretherapy | 60 | 21.98 (7.45) |
| Treatment for 1 cycle | 60 | 20.75 (5.43) | |
| Treatment for 3 cycles | 60 | 17.88 (5.26)c | |
| Treatment for 6 cycles | 60 | 16.31 (4.22)c |
Abbreviations: PKM2, pyruvate kinase type M2; HER2, human epidermal growth factor receptor 2; SD, standard deviation.
aOne hundred twenty patients exhibited HER2 (+++) based on immunohistochemistry or HER2 gene amplification of the HER2 gene according to fluorescence in situ hybridization (FISH).
bData represent the means (SD) of 3 independent experiments.
cCompared to pretherapy: P < .05.
dCompared to the same period in the control group: P < .05.
Lapatinib Inhibits Breast Cancer Cell Proliferation by Influencing PKM2 Expression
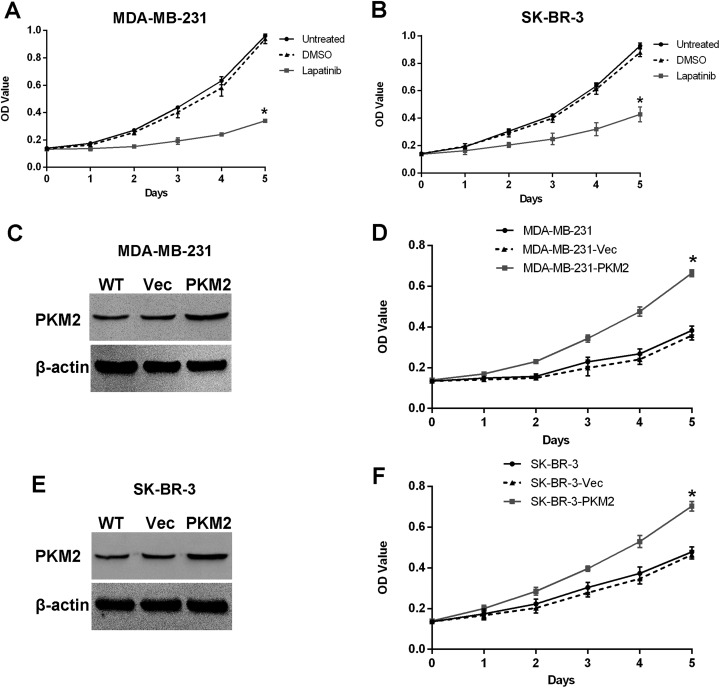
Some researchers have shown that PKM2 regulates gene transcription and cell proliferation.6,31-33 As an anticancer drug, lapatinib inhibits tumor cell proliferation, and we previously demonstrated that lapatinib inhibits PKM2 expression. Thus, we next examined whether lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. Cell Counting Kit-8 (CCK-8) analyses showed that lapatinib-treated cells proliferated at a significantly slower rate than control cells after culturing for 1 day, with no obvious changes between DMSO and untreated groups (Figure 4A and B). To assess whether lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression, we established MDA-MB-231 and SK-BR-3 cell stably overexpressing PKM2, as shown in Figure 4C and E. Cell proliferation tests showed that after culturing for 1 day under the effect of lapatinib, PKM2-overexpressing cells proliferated at a significantly faster rate than those in negative control groups, and there was no obvious change between negative control and wild-type groups, as shown in Figure 4D and F. Data represent the means (SD) of 6 independent experiments. We hypothesized that lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression.
Figure 4.
Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. A, Lapatinib inhibits MDA-MB-231 cell proliferation. MDA-MB-231 cells were treated with lapatinib and DMSO. The results showed that cells proliferated at a significantly slower rate in the lapatinib treatment group compared with DMSO treatment and untreated groups (P < .05). There were no obvious changes in the DMSO treatment group compared to the untreated group in terms of cell proliferation. Both DMSO treatment groups and untreated groups served as control groups. Data represent the means (SD) of 6 independent experiments. B, Lapatinib inhibits SK-BR-3 cell proliferation. SK-BR-3 cells were treated with lapatinib and DMSO. The results showed that cells proliferated at a significantly slower rate in the lapatinib treatment group compared to DMSO treatment and untreated groups (P < .05). There were no obvious changes in cell proliferation in the DMSO treatment group relative to the untreated group. Dimethyl sulfoxide treatment groups and untreated groups served as control groups. Data represent the means (SD) of 6 independent experiments. C and E, The pcDNA3.1-PKM2 recombinant plasmid was successfully transfected into MDA-MB-231 and SK-BR-3 cell lines. WT represents the wild-type group. Vec represents the negative control group. The experiments were repeated 3 times. D and F, Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. The PKM2 sequences were transfected into MDA-MB-231 and SK-BR-3 cell lines under treatment with lapatinib for overexpression. The CCK-8 results show that cells proliferated at a significantly faster rate in transfected groups compared to control groups (P < .05), and there was no obvious change between negative control groups and wild-type groups. WT represents the wild-type group. Vec represents the negative control group. Data represent the means (SD) of 6 independent experiments. CCK-8 denotes Cell Counting Kit-8; DMSO, dimethyl sulfoxide; PKM2, pyruvate kinase type M2; SD, standard deviation; MDA-MB-231, Human breast cancer cells MDA-MB-231; SK-BR-3, Human breast cancer cells SK-BR-3.
Lapatinib-Mediated Inhibition of Breast Cancer Cell Proliferation Reduces Stat3 and Phosphorylated Stat3 Levels
The results presented above demonstrate that lapatinib inhibits cell proliferation by influencing PKM2 expression. Stat3 is correlated with cell proliferation34-37 and the only transcriptional factor that interacts with PKM2.33 We thus examined whether inhibition of PKM2 by lapatinib reduces levels of Stat3 and phosphorylated Stat3. Pyruvate kinase type M2 knockdown analyses after the transfection of PKM2 small inhibitory RNA (siRNA) into MDA-MB-231 and SK-BR-3 cells revealed a reduction in Stat3 and Stat3 (Tyr(P)-705) expression compared to control groups in both MDA-MB-231 and SK-BR-3 cell lines (Figure 5A and B). The results indicate that PKM2 regulates Stat3 and Stat3 (Tyr(P)-705) expression in breast cancer cell lines.
Figure 5.
Inhibition of PKM2 by lapatinib reduces levels of Stat3 and phosphorylated Stat3. A, Knockdown of PKM2 results in reduced Stat3 and Stat3 (Tyr(P)-705) expression in MDA-MB-231 cells. Cells were transfected with 20 μM siPKM2 for 48 hours and lysed for Western blotting. Knockdown of PKM2 resulted in decreased Stat3 and Stat3 (Tyr(P)-705) expression compared to WT and negative control groups. The experiments were repeated 3 times. B, Knockdown of PKM2 results in reduced Stat3 and Stat3 (Tyr(P)-705) expression in SK-BR-3 cells. Cells were transfected with 20 μM siPKM2 for 48 hours and lysed for Western blotting. Knockdown of PKM2 resulted in decreased Stat3 and Stat3 (Tyr(P)-705) expression compared to WT and negative control groups. The experiments were repeated 3 times. C and D, Inhibition of PKM2 by lapatinib reduces levels of Stat3 and phosphorylated Stat3. MDA-MB-231 and SK-BR-3 cell lines were treated with lapatinib. The results showed that PKM2 expression was significantly reduced in lapatinib treatment groups compared to control groups; Stat3 and Stat3 (Tyr(P)-705) expression was also significantly reduced after treatment with lapatinib compared to control groups in both cell lines. The experiments were repeated 3 times. PKM2 denotes pyruvate kinase type M2; Stat3, Signal transducer and activator of transcription 3; Stat3(Tyr(P)-705), phospho-Stat3 (Tyr705); MDA-MB-231, Human breast cancer cells MDA-MB-231; SK-BR-3, Human breast cancer cells SK-BR-3; WT, Wild type.
We further evaluated whether PKM2 regulates Stat3 and Stat3 (Tyr(P)-705) expression under the effect of lapatinib. As shown in Figure 5C and D, PKM2 expression was significantly reduced in lapatinib-treatment groups compared to control groups, and Stat3 and Stat3 (Tyr(P)-705) expression was also significantly reduced after treatment with lapatinib compared to control groups in both cell lines.
The results indicate that inhibition of PKM2 by lapatinib reduces levels of Stat3 and phosphorylated Stat3. As activated Stat3 can induce cell proliferation,34-37 all these results indicate that lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression, which results in reduced levels of Stat3 and phosphorylated Stat3 (Figure 6).
Figure 6.
Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression, which reduces levels of Stat3 and phosphorylated Stat3. Downregulation of PKM2 expression by lapatinib-mediated suppression of EGFR and HER2 reduces Stat3 and pStat3 expression, which leads to a lower level of gene transcription and inhibition of tumor cell proliferation. EGFR denotes epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PKM2, pyruvate kinase type M2; Stat3, Signal transducer and activator of transcription 3; pStat3, Phosphorylated Stat3.
Discussion
Tumor cell metabolism differs from that of normal cells. With a sufficient oxygen supply, tumor cells carry out glycolysis to produce energy, and aerobic glycolysis in tumor cells represents a special pattern of glycometabolism that provides the material basis for biosynthesis.38 Pyruvate kinase is the key enzyme of glycometabolism, of which there are 4 known isoforms: Pyruvate kinase type L, Pyruvate kinase type R, Pyruvate kinase type M1, and Pyruvate kinase type M2. Pyruvate kinase type M2 plays a crucial role in aerobic glycolysis in tumor cells39 and exists in 3 forms: a nonactive monomer, a low-activity dimer, and a high-activity tetramer. Pyruvate kinase type M2, which mainly exists in the dimeric form in tumor cells,40 increases the rate of ATP generation in aerobic glycolysis compared to oxidative phosphorylation.41 Additionally, dimeric PKM2 induces a larger accumulation of intermediate products of glycometabolism, thus providing a material basis for the synthesis of nucleic acids and other biological macromolecules.42 Some recent studies have reported a nonglycolytic function of PKM2. Indeed, PKM2 is expressed in multiple tumor cells43 and can regulate gene transcription and cell proliferation.6,31-33
Epidermal growth factor receptor belongs to the tyrosine kinase receptor family and plays a key role in cell growth, proliferation, and differentiation. Epidermal growth factor receptor is activated by growth factor ligands and forms homodimers or heterodimers.14 As an orphan receptor, HER2 can form heterodimers with 3 other receptors belonging to the ErbB family.15 Upon activation, the intracellular tyrosine kinase domain autophosphorylates tyrosine residues, eventually leading to cell division, migration, engraftment, and differentiation through various signaling pathways.
Epidermal growth factor receptor activation induces nuclear translocation of PKM2, which regulates cyclin D1 expression.16 To further determine the relationship between PKM2 and EGFR, we analyzed pathological data from mammary glands and EGFR knockdown experiments. The results demonstrated that PKM2 is associated with EGFR. Previous studies have shown that activation of EGFR regulates the expression of PKM2.17 In addition, analyses of pathological data from mammary glands and HER2 knockdown experiments revealed that PKM2 is associated with HER2. Human epidermal growth factor receptor 2 plasma levels have previously been reported to be associated with tumor type M2 PK.18
Lapatinib is a small molecule tyrosine kinase inhibitor that can block EGFR and HER2. Clinical trials show that this drug is well tolerated and can be used alone or with other drugs for breast cancer treatment.19-25 Although it is a known EGFR and HER2 inhibitor, lapatinib’s effect on PKM2 is still unclear. In this study, we demonstrate that lapatinib inhibits PKM2 expression, as based on in vitro Western blotting and RT-PCR data in MDA-MB-231 and SK-BR-3 cells as well as analysis of pathological data for mammary glands from 120 patients. These patients exhibited HER2 (+++) according to immunohistochemistry analysis or amplification of the HER2 gene according to FISH. To further examine whether lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression, we performed CCK-8 analyses. MDA-MB-231 and SK-BR-3 breast cancer cell lines stably overexpressing PKM2 were established, and after treatment with lapatinib, overexpressing cells proliferated significantly faster than control groups. These results suggested that the inhibition of EGFR and HER2 by lapatinib in MDA-MB-231 and SK-BR-3 cells, respectively, induces a downregulation of PKM2. Quantitative PCR results indicated that mRNA levels of PKM2 are not decreasing with lapatinib treatment alone, which indicates that lapatinib is not downmodulating the expression of PKM2 at the transcriptional level, and lapatinib modulates PKM2 by regulating its phosphorylation. All the results indicated that lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression.
Stat3 is a transcription factor associated with cell proliferation34-37; to date, it is the only transcription factor known to interact with PKM2.33 We next explored the involvement of Stat3 and phosphorylated Stat3. Pyruvate kinase type M2 knockdown via transfection of PKM2 siRNA into MDA-MB-231 and SK-BR-3 cells showed that knocking down PKM2 led to reduction in Stat3 and Stat3 (Tyr(P)-705) expression compared to control groups. The results indicate that PKM2 regulates Stat3 and Stat3 (Tyr(P)-705) expression. We further examined whether PKM2 modulates Stat3 and Stat3 (Tyr(P)-705) expression under the effects of lapatinib and found that PKM2 expression was significantly reduced in lapatinib-treated cells compared to control cells, and Stat3 and Stat3 (Tyr(P)-705) expression was also significantly reduced after treatment with lapatinib compared to control groups in both cell lines. The results show that inhibition of PKM2 by lapatinib reduces Stat3 and phosphorylated Stat3 levels.
Together with previous studies, we found that lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression and reduces Stat3 and phosphorylated Stat3 levels.
Acknowledgments
The authors thank Dr R. Niu for providing the laboratory and Dr F. Zhang for providing technological guidance.
Abbreviations
- CCK-8
Cell Counting Kit-8
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- FISH
fluorescence in situ hybridization
- HER2
human epidermal growth factor receptor 2
- mRNA
messenger RNA
- PK
pyruvate kinase
- PKM2
pyruvate kinase type M2
- RT-PCT
reverse transcription polymerase chain reaction
- siRNA
small inhibitory RNA
- SD
standard deviation.
Authors’ Note: Mingxiu Guan and Yingna Tong contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Tianjin Municipal Health Bureau Project Foundation (2011KZ77) to Y. Zhou, the National Natural Science Foundation of China (81201653) to Y. Zhou, the National Natural Science Foundation of China (31501091) to J. Shao, and the National Natural Science Foundation of China (81502519) to D. Dong.
References
- 1. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi:10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi:10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 3. Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969–980. doi:10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4. Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15(4):300–308. doi:10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5. Bluemlein K, Grüning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2(5):393–400. doi:10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (Pkm2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40(5):1043–1054. doi:10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7. Morgan HP, O’Reilly FJ, Wear MA, et al. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci U S A. 2013;110(15):5881–5886. doi:10.1073/pnas.1217157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun Q, Chen X, Ma J, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108(10):4129–4134. doi:10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44(27):9417–9429. doi:10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 10. Hathurusinghe HR, Goonetilleke KS, Siriwardena AK. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann Surg Oncol. 2007;14(10):2714–2720. doi:10.1245/s10434-007-9481-x. [DOI] [PubMed] [Google Scholar]
- 11. Weinberger R, Appel B, Stein A, Metz Y, Neheman A, Barak M. The pyruvate kinase isoenzyme M2 (Tu M2-PK) as a tumour marker for renal cell carcinoma. Eur J Cancer Care. 2007;16(4):333–337. doi:10.1111/j.1365-2354.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 12. Oremek G, Kukshaĭte R, Sapoutzis N, Ziolkovski P. The significance of TU M2-PK tumor marker for lung cancer diagnostics [in Russian]. Klin Med (Mosk). 2007;85(7):56–58. [PubMed] [Google Scholar]
- 13. Kumar Y, Gurusamy K, Pamecha V, Davidson BR. Tumor M2-pyruvate kinase as tumor marker in exocrine pancreatic cancer a meta-analysis. Pancreas. 2007;35(2):114–119. doi:10.1097/mpa.0b013e3180537237 [DOI] [PubMed] [Google Scholar]
- 14. Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26(5):1443–1451. doi:10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 15. Olayioye MA. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3(6):385–389. doi:10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118–122. doi:10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang W, Zheng Y, Xia Y, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–1304. doi:10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lüftner D, Mazurek S, Henschke P, et al. Plasma levels of HER-2/neu, tumor type M2 pyruvate kinase and its tyrosine-phosphorylated metabolite in advanced breast cancer. Anticancer Res. 2003;23(2A):991–997. [PubMed] [Google Scholar]
- 19. Ito Y, Tokudome N, Sugihara T, Takahashi S, Hatake K. Does lapatinib, a small-molecule tyrosine kinase inhibitor, constitute a breakthrough in the treatment of breast cancer? Breast Cancer. 2007;14(2):156–162. doi:10.2325/jbcs.971. [DOI] [PubMed] [Google Scholar]
- 20. Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6(2):667–674. doi:10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 21. Konecny GE, Venkatesan N, Yang G, et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer. 2008;98(6):1076–1084. doi:10.1038/sj.bjc.6604278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vazquez-Martin A, Oliveras-Ferraros C, Colomer R, Brunet J, Menendez JA. Low-scale phosphoproteome analyses identify the mTOR effector p70 S6 kinase 1 as a specific biomarker of the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) in human breast carcinoma cells. Ann Oncol. 2008;19(6):1097–1109. doi:10.1093/annonc/mdm589. [DOI] [PubMed] [Google Scholar]
- 23. Gilmer TM, Cable L, Alligood K, et al. Impact of common epidermal growth factor receptor and HER2 variants on receptor activity and inhibition by lapatinib. Cancer Res. 2008;68(2):571–579. doi:10.1158/0008-5472.CAN-07-2404. [DOI] [PubMed] [Google Scholar]
- 24. Trowe T, Boukouvala S, Calkins K, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14(8):2465–2475. doi:10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 25. Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36(4):695–701. doi:10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 26. Diermeier S, Horváth G, Knuechel-Clarke R, Hofstaedter F, Szöllosi J, Brockhoff G. Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res. 2005;304(2):604–619. doi:10.1016/j.yexcr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27. Manfroid I, Van de Weerdt C, Baudhuin A, Martial JA, Muller M. EGF stimulates Pit-1 independent transcription of the human prolactin pituitary promoter in human breast cancer SK-BR-3 cells through its proximal AP-1 response element. Mol Cell Endocrinol. 2005;229(1-2):127–139. doi:10.1016/j.mce.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28. Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20(10):408–412. doi:10.1016/S0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 29. Kozlova N, Samoylenko A, Drobot L, Kietzmann T. Urokinase is a negative modulator of Egf-dependent proliferation and motility in the two breast cancer cell lines MCF-7 and MDA-MB-231. Mol Carcinog. 2016;55(2):170–181. doi:10.1002/mc.22267. [DOI] [PubMed] [Google Scholar]
- 30. Diermeier-Daucher S, Breindl S, Buchholz S, Ortmann O, Brockhoff G. Modular anti-EGFR and anti-Her2 targeting of SK-BR-3 and BT474 breast cancer cell lines in the presence of ErbB receptor-specific growth factors. Cytometry Part A. 2011;79(9):684–693. doi:10.1002/cyto.a.21107. [DOI] [PubMed] [Google Scholar]
- 31. Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–744. doi:10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoshino A, Hirst JA, Fujii H. Regulation of cell proliferation by interleukin-3-induced nuclear translocation of pyruvate kinase. J Biol Chem. 2007;282(24):17706–17711. doi:10.1074/jbc.M700094200. [DOI] [PubMed] [Google Scholar]
- 33. Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi:10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang W, Dong Z, Wang F, Peng H, Liu JY, Zhang JT. A small molecule compound targeting STAT3 DNA-binding domain inhibits cancer cell proliferation, migration, and invasion. ACS Chem Biol. 2014;9(5):1188–1196. doi:10.1021/cb500071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nichane M, Ren X, Bellefroid EJ. Self-regulation of Stat3 activity coordinates cell-cycle progression and neural crest specification. EMBO J. 2010;29(1):55–67. doi:10.1038/emboj.2009.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puram SV, Yeung CM, Jahani-Asl A, et al. STAT3-iNOS signaling mediates EGFRvIII-induced glial proliferation and transformation. J Neurosci. 2012;32(23):7806–7818. doi:10.1523/JNEUROSCI.3243-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ding BB, Yu JJ, Yu RY, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111(3):1515–1523. doi:10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007;(4):99–124. doi:10.1007/2789_2008_091. [DOI] [PubMed] [Google Scholar]
- 39. Dang CV. PKM2 tyrosine phosphorylation and glutamine metabolism signal a different view of the Warburg effect. Sci Signal. 2009;2(97):pe75 doi:10.1126/scisignal.297pe75. [DOI] [PubMed] [Google Scholar]
- 40. Hitosugi T, Kang S, Vander Heiden MG, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2(97):ra73 doi:10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi:10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 42. Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209(2):211–215. doi:10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi:10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]