Abstract
Apolipoprotein L1 (ApoL1) genetic variants are strongly associated with kidney diseases. We investigated the role of ApoL1 variants in monocyte differentiation and eicosanoid production in macrophages, as activated tissue macrophages in kidney might contribute to kidney injury. In human monocyte THP-1 cells, transient overexpression of ApoL1 (G0, G1, G2) by transfection resulted in a 5- to 11-fold increase in CD14 and CD68 gene expression, similar to that seen with phorbol-12-myristate acetate treatment. All ApoL1 variants caused monocytes to differentiate into atypical M1 macrophages with marked increase in M1 markers CD80, TNF, IL1B, and IL6 and modest increase in the M2 marker CD163 compared with control cells. ApoL1-G1 transfection induced additional CD206 and TGFB1 expression, and ApoL1-G2 transfection induced additional CD204 and TGFB1 expression. Gene expression of prostaglandin E2 (PGE2) synthase and thromboxane synthase and both gene and protein expression of cyclooxygenase-2 (COX-2) were increased by ApoL1-G1 and -G2 variants compared with -G0 transfection. Higher levels of PGE2 and thromboxane B2, a stable metabolite of thromboxane A2, and transforming growth factor (TGF)-β1 were released into the supernatant of cultured THP-1 cells transfected with ApoL1-G1 and -G2, but not -G0. The increase in PGE2, thromboxane B2, and TGF-β1 was inhibited by COX-2-specific inhibitor CAY10404 but not by COX-1-specific inhibitor SC-560. These results demonstrate a novel role of ApoL1 variants in the regulation of monocyte differentiation and eicosanoid metabolism, which could modify the immune response and promote inflammatory signaling within the local targeted organs and tissues including the kidney.
Keywords: apolipoprotein L1, cyclooxygenase-2, macrophage polarization, TGF-β1
INTRODUCTION
Apolipoprotein L1 (ApoL1) is an innate immune protein that is a component of trypanosome lytic factor (TLF), a subfraction of high density lipoprotein (7, 12, 26, 55). Liver-produced ApoL1 is secreted into plasma (45), where it associates with TLF complexes and can be endocytosed by trypanosomes (13). Intracellular ApoL1 is thought to form anionic pores in the lysosomal membrane of the parasite, leading to parasite killing (26, 55). The ApoL1 variants G1 (rs73885319; p.S342G and rs60910145; p.I384M), and G2 (rs71785313; p.N388del:Y389del) protect against trypanosomal infection at the cost of increased risk of chronic kidney diseases (CKD), including focal segmental glomerulosclerosis (FSGS) and arterionephrosclerosis in African Americans (12, 13, 18). The data associating ApoL1 renal risk variants with CKD has been complemented by demonstration that transgenic mice that express the ApoL1 risk variants in podocytes develop FSGS (4).
FSGS is characterized by macrophage infiltration into glomeruli and the interstitium (8). Similarly, the Buffalo Mna rat model of FSGS also manifests increased interstitial macrophages (24). In general, CKD is characterized by chronic inflammation and activation of the innate immune system (1). Macrophages function in both innate and adaptive immune responses. Activated macrophages are differentiated from circulating monocytes upon entering tissues (47). Differentiated macrophages classically have two states. These can be summarized as follows: M1 macrophages mediate host defense and promote inflammation, and M2 macrophages suppress immune activities and resolve inflammation (29, 31).
Eicosanoid signaling contributes to both innate and adaptive immunity (9, 33). Eicosanoid products play major roles in proinflammatory responses (39). Prostaglandins are produced abundantly during acute inflammation, which contribute to proinflammatory eicosanoid response to infection (33). Cyclooxygenases (COX) catalyze the conversion of arachidonic acid to prostaglandins such as prostaglandine E2 (PGE2) and thromboxane A2 (TXA2) (21). COX-1 is constitutively expressed in almost all cell types (28). COX-2 is highly inducible by proinflammatory cytokines in a variety of cell types, including monocytes (36). Both COX-1 and COX-2 contribute to the synthesis of TXA2 and PGE2 in macrophages (35).
ApoL1 gene expression is upregulated by proinflammatory cytokines and, as noted above, suppresses trypansomal infection (26, 38). Furthermore, ApoL1 reduces HIV-1 replication in interferon (IFN)γ-differentiated M1 macrophages (26, 51). Macrophage activation is a hallmark of trypanosomal infection (38). However, the role of ApoL1 and its risk variants in eicosanoid signaling and subsequent inflammatory responses in macrophages are unknown. In this study, we investigated the roles of ApoL1 variants on the activation and differentiation of macrophages and on macrophage prostaglandin production. Our results suggest a novel mechanism by which ApoL1 risk variants may promote renal injury.
METHODS
Cell culture.
THP-1 cells (American Type Culture Collection, Rockville, MD), a monocyte cell line derived from a patient with acute monocytic leukemia, were cultured in suspension in RPMI 1640 (4.5 g/liter glucose) supplemented with 10% heat-inactivated fetal bovine serum. Cells were exposed to phorbol myristate acetate (PMA, 320 nM) for 6 h with subsequent treatment for 18 h of IFNγ (20 ng/ml) and lipopolysaccharides (LPS, 0.1 µg/ml) for differentiation into M1-polarized macrophages or interleukin-4 (IL-4) and IL-13 (both 20 ng/ml) for differentiation into M2-polarized macrophages. THP-1 cells treated only with PMA for 24 h were designated M0 macrophages (53).
Transient transfection and cell viability assay.
Transfection was performed using Lipofectamine 2000 reagent following the manufacturer’s instructions (Invitrogen, Gaithersburg, MD). ApoL1-expressing constructs (1.5 µg) of p-Sport-ApoL1 G0 (designated G0), p-Sport-ApoL1 G1 (designated G1), p-Sport-ApoL1 G2 (designated G2), and the empty vector pCMV-Sport (designated EV) were transfected into 50,000 THP-1 cells/well in six-well plates. A second construct (pEGFP-N1) was used for monitoring the transfection efficiency. The cells were subsequently exposed to vehicle, cytokines, or cyclooxygenase inhibitors and were harvested to determine mRNA and protein expression levels.
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously (25). The formazan product was dissolved in DMSO, and its optical density was measured spectrophotometrically at 570 nm in a microplate reader.
Quantification of mRNA.
Cellular total RNA was extracted using NucleoSpin RNA Kit (Macherey-Nagel, Bethlehem, PA). Isolated mRNA was reverse transcribed to cDNA with GoScript reverse transcriptase (Promega, Madison, WI). One microliter of cDNA was used for real-time polymerase chain reaction (PCR) (SYBR Green qPCR Supermix UDG Kit, Invitrogen) under the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Real-time PCR reactions were carried out in a total volume of 10 µl, using specific primers for eicosanoid synthetic enzymes and ribosomal S26 (RPS26). Primer sequences used in this study are described in Table 1. Real-time PCR reactions were performed in triplicate using the SYBR Green PCR Master Mix in a 7500 Real-time PCR System (Applied Biosystems). The gene expression relative to RPS26 was analyzed using the comparative ΔCT method as previously described (25).
Table 1.
Primer sets used for real-time PCR amplificatio
| Genes | Primer Names | Primer Sequences (5′-3′) |
|---|---|---|
| CD14 | CD14-F | GTCATCAGGACACTGCCAGG |
| CD14-R | GTGAACCCTGATCACCTCCC | |
| CD68 | CD68-F | GGCTACTGGCAGAGAGCAC |
| CD68-R | CTGTGAGTGGCAGTTGAGGG | |
| CD80 | CD80-F | TTGCCCCAAGATGCAGAGAG |
| CD80-R | GCTACTTCTGTGCCCACCAT | |
| CD163 | CD163-F | GAGACAGCGGCTTGCAGTTT |
| CD163-R | CAGAATGGCCTCCTTTTCCATTCC | |
| CD204 | CD204-F | CGAAAGTTCGACTGGTCGGT |
| CD204-R | TGTCCCCCATTGCCGAATTT | |
| CD206 | CD206-F | TGTTTTGCGTCTTAGTTCCGC |
| CD206-R | CCGTTTTTGATGGCACTCCC | |
| COX-1 | COX-1-F | GGGACTCCTTTTGGTCAGGC |
| COX-1-R | ATGGGTGTGGGGCAATCTTT | |
| COX-2 | COX-2-F | GATGATTGCCCGACTCCCTT |
| COX-2-R | TGAAAAGGCGCAGTTTACGC | |
| IL-1β | IL-1β-F | CAACAGGCTGCTCTGGGATT |
| IL-1β-R | CATGGCCACAACAACTGACG | |
| IL-6 | IL-6-F | AATAACCACCCCTGACCCAAC |
| IL-6-R | TCTGAGGTGCCCATGCTACA | |
| PGDS | PGD2S-F | CTCTTCACCAGAGCCTAGCA |
| PGD2S-R | AGGCGCATTATACGTGAGCA | |
| PGES | PGE2S-F | ATCCTCTCCCTGGAAATCTCG |
| PGE2S-R | CGTACTTGCCCACCTCTCTT | |
| PGIS | PGI2S-F | TGTGCTTGATAGCGTGCTGA |
| PGI2S-R | TCTTCAGCCGTTTCCCATCC | |
| TBXAS1 | TBXAS1-F | GTGGCTCGCCCATCTCACA |
| TBXAS1-R | GTCTCCTCCCTCAGGCACAT | |
| TNF | TNF-F: | CACCACTTCGAAACCTGGGA |
| TNF-R: | AGGAAGGCCTAAGGTCCACT | |
| TGF-β1 | TGF-β1-F | ACCTGCCACAGATCCCCTAT |
| TGF-β1-R | CTCCCGGCAAAAGGTAGGAG | |
| RPS26 | RPS26-F | TTCCGCCATCCGGCTAAATA |
| RPS26-R | TGGCACGACCATTGTTCCTT |
F, forward; R, reverse; CD, cluster of differentiation; COX, cyclooxygenase; IL, interleukin; PG, prostaglandin; TBX, thromboxane; TNF, tumor necrosis factor; TGF, transforming growth factor; RPS26: ribosomal protein S26.
ELISA assay.
Prostanoids including PGE2 and thromboxane B2 (TBX2; Cayman Chemical, Ann Arbor, MI) and transforming growth factor-β1 (TGF-β1, R & D Systems) levels were determined using ELISA kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. Briefly, following 24-h transfection, cells were cultured in serum-free culture medium (Invitrogen) treated with SC-560 (Cayman Chemical) or CAY10404 (Cayman Chemical) or vehicle for 12 h. Subsequently, cell suspension was centrifuged, supernatant was collected, and absorbance was read at 420 and 450 nm, respectively.
Western blot analysis.
ApoL1, COX-1, and COX-2 protein expression in transiently transfected THP-1 cells was characterized by Western blot analysis. Cell pellets were harvested and protein concentration was determined using a BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Cell lysate proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Bedford, MA). The membranes were then blocked with 5% nonfat milk and then incubated overnight at 4°C with anti-ApoL1 rabbit (Sigma, St. Louis, MO) and anti-COX-1/2 (Abcam, Cambridge, MA) antibodies. After incubation with proper secondary antibodies, the protein bands were detected by LI-COR near-infrared fluorescence technology (Lincoln, NE).
Statistical analysis.
Data are presented as means ± SD. All experiments were performed using a minimum of triplicate wells and were repeated biologically at least two times. Differences between two groups were assessed by Student’s t-test, and differences among three or more groups were assessed by one-way factorial ANOVA with Bonferroni’s multiple comparison test. P values <0.05 were considered statistically significant (Prism5; GraphPad, La Jolla, CA).
RESULTS
Overexpression of ApoL1 proteins in transiently transfected THP-1 cells.
To study potential roles in immune activities, we transiently overexpressed ApoL1 wild-type (G0) and renal risk variants G1 and G2 into monocytic THP-1 cells. All the ApoL1 variants were successfully overexpressed in THP-1 cells for at least 36 h (Fig. 1A). Of note, the protein expression levels of ApoL1-G0, -G1, and -G2 were very similar, indicating that the COOH-terminal domain variants of ApoL1-G1 and -G2 had no effect on ApoL1 expression. Consistent with previous observation (26), we also observed cytotoxicity in our system with overexpression of ApoL1-G0, -G1, and -G2 (Fig. 1B).
Fig. 1.
Apolipoprotein L1 (ApoL1) overexpression in transiently transfected THP-1 cells. A: ApoL1 protein expression was observed in THP-1 cells transiently transfected with ApoL1-G0, ApoL1-G1, and ApoL1-G2 and compared with empty vector (EV) or mock transfection, as indicated (see methods for definition of constructs). Cell lysates were subjected to SDS-PAGE and immunoblotted with anti-ApoL1 antibody (top) or anti-actin antibody (bottom) for loading control. Immunoblotting shown is a representative of 2 independent experiments. The density of ApoL1-G0, ApoL1-G1, and ApoL1-G2 over β-actin was quantified by densitometry and normalized to EV; n = 6. Expression levels of each of the ApoL1 variants were similar; ns, not significanct (P > 0.05). B: THP-1 cells (20,000/well) transfected with EV for 12 h and with ApoL1-G0, -G1, and -G2 for the indicated time were incubated for 12 h in complete culture medium containing 0.4 mg/ml MTT in 5% CO2 at 37°C. Formazan production was measured spectrophotometrically at 570 nm in a microplate reader. Cell viability was normalized with EV transfection after 12 h; n = 3. P < 0.05 vs. EV.
ApoL1 induced monocyte differentiation into atypical M1 macrophage state.
Macrophage polarization is considered a sign of immune response initiation. Therefore, we investigated whether overexpression of ApoL1 differentiates monocytes into activated macrophages. PMA alone activated monocytes as assessed by the increase in gene expression of CD14 (5.27 ± 1.5-fold relative to vehicle) and CD68 (5.87 ± 0.9-fold relative to vehicle; Fig. 2A). Additional treatment with IFNγ and LPS in the presence of PMA induced monocyte differentiation into M1 macrophages (Fig. 2B) and with IL-4 and IL-13 into M2 macrophages (Fig. 2C), assessed by the gene expression profile of M1 and M2 markers. These data indicate normal macrophage polarization in vitro.
Fig. 2.
Characterization of macrophage subtypes differentiated from THP-1 cells. THP-1 cells were treated with phorbal myristol acetate (PMA; A), IFNγ and LPS (B), and IL-4 and IL-13 (C). mRNA expression was quantified by qRT-PCR. Values of relative mRNA levels of each marker were expressed as fold increase compared with vehicle treatment. PMA was used to generate the M0 state, whose macrophage markers are CD14 and CD68; IFNγ and LPS were used to induce M1 state, the putative markers are CD80, TNF, IL1B, and IL6; and IL-4 and IL-13 were used to induce the M2 state, whose macrophage markers are CD163, CD204, CD206, and TGFB1. Veh, vehicle; n = 8–9. †P < 0.05 vs. vehicle, §P < 0.05 vs. M0.
Overexpression of each of the ApoL1 variants induced a 5.0–10.8-fold increase in gene expression of CD14 and CD68 (Fig. 3), levels similar to those induced by PMA. Furthermore, the gene expression of selective M1 markers (CD80, TNF, IL1B, IL6) was substantially increased throughout; expression of some M2 marker genes also increased significantly, although to a much lesser extent than the M1 markers: CD163 (4.95 ± 1.3-fold, P < 0.05 vs. EV) in ApoL1-G0; CD163 (3.07 ± 0.69-fold, P < 0.05 vs. EV), CD206 (4.35 ± 0.21-fold, P < 0.05 vs. EV), and TGF-β1 (4.83 ± 0.07-fold, P < 0.05 vs. EV) in ApoL1-G1 cells, and CD204 (4.29 ± 1.92-fold, P < 0.05 vs. EV) and TGFB1 (6.85 ± 0.87-fold, P < 0.05 vs. EV) in ApoL1-G2-overexpressing THP-1 cells (Fig. 3). We designated this ApoL1-induced polarization as atypical M1 state, since it differed from the classical M1 state described (29–31, 47). Of note, the extent of gene expression of M1 markers was different among the wild-type ApoL1-G0 and risk variants ApoL1-G1 and -G2. TNF gene expression in ApoL1-G2 cells showed a greater increase compared with that of ApoL1-G0- and -G1-overexpressing cells, and IL6 gene expression in ApoL1-G1 and -G2 cells showed greater increase compared with that of ApoL1-G0-overexpressing cells as well (Fig. 3).
Fig. 3.
Profile of cell surface markers induced by ApoL1 overexpression. Cultured THP-1 cells were transfected with EV or ApoL1-G0 (A), ApoL1-G1 (B), or ApoL1-G2 (C) variants as indicated. The average value of empty vector was used to normalize the relative mRNA expression for each marker. For all ApoL1 variants, mRNA expression of M1 markers was markedly increased and there was modest increase in gene expression of some M2 markers, indicating atypical M1 macrophage differentiation. As shown, M0 macrophage markers are CD14 and CD68, M1 macrophage markers are CD80, TNF, IL1B, and IL6, and M2 macrophage markers are CD163, CD204, CD206, and TGFB1. Values of relative mRNA level of each marker are expressed as fold increase compared with empty vector; n = 8–10. *P < 0.05 vs. EV, #P < 0.05 vs. ApoL1-G0.
ApoL1 risk variants increased COX-2 expression.
ApoL1 risk variants induced atypical M1 macrophage polarization, which is generally associated with a proinflammatory response (9). Prostaglandins have similar effects on macropages, suggesting that ApoL1 might act by regulating prostanoid expression. Prostanoids have a common prostaglandin synthase for their production, namely COX, which has two distinct isoforms, COX-1 and COX-2. We next investigated whether ApoL1 risk variants affected the expression of COX isoforms. As shown in Fig. 4A, the gene expression of COX-2 relative to EV in ApoL1-G1 cells (4.88 ± 1.6-fold) and ApoL1-G2 cells (4.25 ± 1.5-fold) was greater than that of ApoL1-G0 (1.03 ± 0.2-fold) overexpressing THP-1 cells, whereas COX-1 expression showed no significant difference among the three variant transfected cells. Consistently, the protein expression of COX-2, but not COX-1, was also induced by overexpression of ApoL1-G1 and -G2, but not ApoL1-G0 in THP-1 cells (Fig. 4B).
Fig. 4.
ApoL1 risk variants induce cyclooxygenase (COX)-2 expression in THP-1 cells. Cultured THP-1 cells were transfected with EV or ApoL1-G0, ApoL1-G1, or ApoL1-G2 variants as indicated. A: relative COX-1 and COX-2 mRNA expression in ApoL1 transiently transfected THP-1 cells. Total mRNA was isolated and mRNA expression quantified by qRT-PCR. The average value of empty vector was used to normalize the relative mRNA expression for each cyclooxygenase. Values of relative mRNA level were expressed as fold increase compared with empty vector; n = 11–12. *P < 0.05 vs. EV; #P < 0.05 vs. ApoL1-G0. B: COX-1 and COX-2 protein expression in ApoL1 transiently transfected THP-1 cells. Cell lysates were subjected to SDS-PAGE before immunoblotting with indicated antibodies. Density of COX-1/2 normalized to β-actin was quantified by densitometry and further normalized to EV. n = 6; ns, not significant (P > 0.05).
ApoL1 risk variants increased prostanoid expression in THP-1 cells.
Since the increase in COX-2 expression likely increases the biosynthesis of prostanoids, we therefore next investigated the gene expression of prostanoid synthases in ApoL1-overexpressing THP-1 cells. As shown in Fig. 5, the response of prostanoid synthases to ApoL1 and its risk variants was not universal. ApoL1-G1 induced 3.87 ± 1.1- and 5.74 ± 0.6-fold increases in mRNA expression of PGE2 and TXA2 synthases, respectively (Fig. 5B), and ApoL1-G2 induced 4.22 ± 0.8- and 5.15 ± 0.7-fold increases in mRNA expression of PGE2 and TXA2 synthases (Fig. 5C) compared with that of EV transfected cells, whereas mRNA expression of both PGE2 (1.13 ± 0.2-fold) and TXA2 (1.38 ± 0.5-fold) synthases in ApoL1-G0 cells were similar to that of EV transfected cells (Fig. 5A). The mRNA expressions of PGI2 and PGD synthases in ApoL1-G0-, -G1-, and -G2-expressing cells were all similar compared with the EV transfection.
Fig. 5.
ApoL1 risk variants increase prostanoid mRNA expression in THP-1 cells. Cultured THP-1 cells were transfected with EV or ApoL1-G0 (A), ApoL1-G1 (B), or ApoL1-G2 (C) variants as indicated. mRNA expression of prostanoid synthases was determined by qRT-PCR. PGIS, PGI2 synthase; PGDS, PGD2 synthase; PGES, PGE2 synthase; TBXAS1, thromboxane A2 synthase. The average value of empty vector was used to normalize the relative mRNA expression for each prostanoid synthase. Values of relative mRNA level are expressed as fold increase compared with empty vector; n = 9–10. *P < 0.05 vs. EV; #P < 0.05 vs. ApoL1-G0.
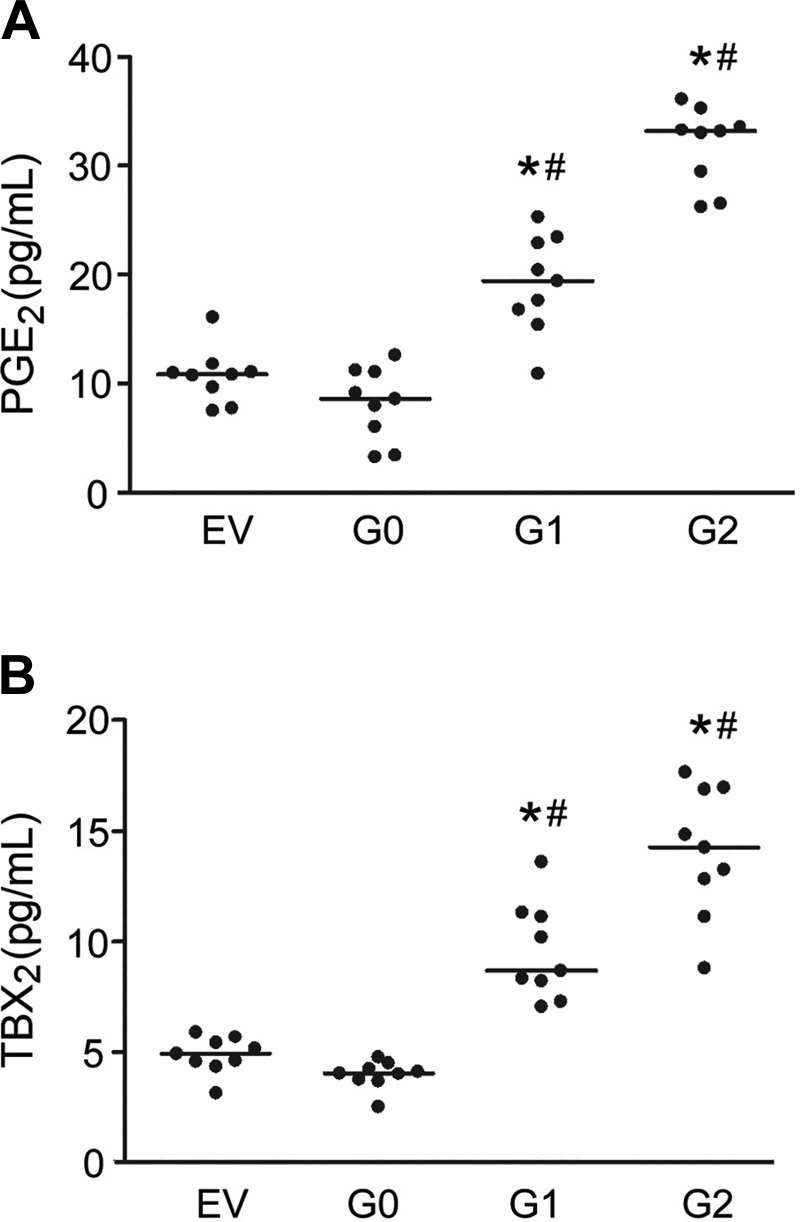
Accordingly, the amounts of PGE2 secreted into the culture medium from ApoL1-G1 (19.15 ± 4.5 pg/ml) and ApoL1-G2 (31.87 ± 3.6 pg/ml) -overexpressing cells were greater compared with ApoL1-G0 (8.16 ± 3.4 pg/ml) -overexpressing THP-1 cells (Fig. 6A). Similarly, TBX2, a stable metabolite of TXA2, had greater secretion into supernatant from ApoL1-G1 (9.54 ± 2.2 pg/ml) and ApoL1-G2 (14.08 ± 2.9 pg/ml) than that of ApoL1-G0 (3.97 ± 0.6 pg/ml) -overexpressing THP-1 cells (Fig. 6B).
Fig. 6.
ApoL1 risk variants increase prostanoid secretion in THP-1 cells. PGE2 (A) and TBX2 (B) concentrations in supernatant of ApoL1-transfected THP-1 cells were determined using respective ELISA kits. Data were summarized from 2 independent experiments; n = 9. *P < 0.05 vs. EV; #P < 0.05 vs. ApoL1-G0.
ApoL1 risk variant-induced increase in prostanoid production was inhibited by COX-2 inhibitor.
Since both COX-1 and COX-2 could contribute to the increase in the prostanoid production, we therefore investigated which COX isoform was responsible for the effect of ApoL1 risk variant-induced increase in prostanoid production in transfected THP-1 cells. CAY1040, a specific COX-2 inhibitor, but not SC-560, a specific COX-1 inhibitor, abrogated the effect of the ApoL1-G1- and -G2-induced increase in PGE2 (Fig. 7A) and TBX2 (Fig. 7B) production secreted into the culture medium. This indicates that COX-2 was the major isoform responsible for the ApoL1-induced activation of eicosanoid signaling.
Fig. 7.
ApoL1 risk variant-mediated prostanoid secretion is inhibited by a COX-2 inhibitor in THP-1 cells. PGE2 (A) and TBX2 (B) concentrations in supernatant of ApoL1-transfected THP-1 cells were determined using respective ELISA kits. SC, SC-560, a COX-1 specific inhibitor; CAY, CAY10404, a COX-2 specific inhibitor. CAY10404 but not SC-560 inhibited ApoL1-G1- and ApoL1-G2-induced increase in PGE2 and TBX2 production, suggesting a central role for COX-2 in prostanoid production in these cells in response to overexpression of ApoL1 variants. Data are summarized from 2 independent experiments; n = 8–9. †P < 0.05 vs. vehicle; *P < 0.05 vs. EV.
ApoL1 risk variant-induced secretion of TGF-β1 was COX-2 dependent.
Elevated COX-2 expression has been observed to promote TFG-β1 pathway activity in diverse pathophysiological procedures (40, 43), and so we investigated whether ApoL1 risk variant-induced COX-2 was associated with increased TGF-β1 production. Consistent with the increase in gene expression (Fig. 3, B and C), TGF-β1 protein expression was increased in both ApoL1-G1- and -G2- but not -G0-overexpressing THP-1 cells, the majority being secreted into the supernatant (Fig. 8A). TGF-β1 production was inhibited by CAY1040, a specific COX-2 inhibitor, but not by SC-560, a specific COX-1 inhibitor. These data suggest that the ApoL1 risk variant-induced increase in TGF-β1 production was mainly COX-2 dependent (Fig. 8B).
Fig. 8.
ApoL1 risk variants induce TGF-β1 secretion from THP-1 cells. TGF-β1 concentration in cell lysates or the supernatant of ApoL1-transfected THP-1 cells were determined by ELISA. A: the majority of TGF-β1 was observed in the supernatant rather than within cells in THP-1 cells with EV or with ApoL1 overexpression; n = 9. *P < 0.05 vs. EV; #P < 0.05 vs. ApoL1-G0. B: TGF-β1 levels in the supernatant of ApoL1-transfected THP-1 cells were reduced by a COX-2 inhibitor. Data are combined from 3 independent experiments. # P < 0.05 vs. ApoL1-G0; †P < 0.05 vs. vehicle.
DISCUSSION
Our data demonstrate that overexpression of ApoL1 and its risk variants in THP-1 cells resulted in an atypical M1 macrophage polarization, and only ApoL1-G1 and -G2 risk alleles induced a marked increase in COX-2 mRNA and protein expression. We also found that mRNA expression of PGE synthase (PGES) and TBXA2 synthase (TBXAS1) was significantly enhanced in response to overexpression of risk variants ApoL1-G1 and -G2 but not -G0 and that ApoL1 risk variants also enhanced the release of prostanoids PGE2 and TXB2 and TGF-β1 into the supernatant, an effect that could be completely blocked with the COX-2 selective inhibitor. These findings provide new insight into the pathophysiological role of ApoL1 risk variants in the metabolism of eicosanoid and prostanoid production, suggesting a potential link of ApoL1 risk variants in inflammatory immune responses in vivo.
ApoL1 is present in the plasma and within cells. Circulating ApoL1 is a component of high-density lipoprotein complex, which functions in lipid transport and metabolism (11, 44); intracellular ApoL1 is widely expressed in podocytes, endothelial cells, and proximal tubular cells in the kidney and other tissues and organs (42). Low levels of endogenous ApoL1 are expressed in THP-1 cells (Fig. 1A), but the function of intracellular ApoL1 in macrophages and other cells remains unknown.
Epidemiological data demonstrate that two ApoL1 risk variants are associated with progressive CKD among African Americans (5, 13, 18). In human serum, these two risk variants have trypanolytic effects on Trypanosoma brucei rhodesiense, a strain resistant to a common variant of ApoL1. The elevation of the risk of kidney diseases associated with ApoL1 risk variants may be caused by cell death (4), possibly by interfering with lysosomal trafficking, lysosomal enzyme leakage into cytosol, cytoskeleton degradation, and blocking of autophagy (4, 23). In podocytes and human coronary artery endothelial cells, interferons and polyI:C increase ApoL1 expression up to hundreds-fold, and cytotoxicity follows induction of ApoL1 (4, 23, 32); the cytotoxicity may be cell specific and ApoL1 isoform specific and also depends strongly on the overexpressed copy number of ApoL1 variants (4, 6, 34). For example, we have established HEK-293 cell lines, which stably express low levels of ApoL-G0, -G1, and -G2 without apparent toxicity (Okamoto K and Kopp JB, unpublished data).
ApoL1 risk variant effects on innate immunity combat infection by trypanosomes and may also contribute to ApoL1-associated CKD (7, 12, 26, 55). Macrophages are important in both innate and adaptive immunity. Macrophages secrete cytokines and chemokines and subsequently recruit and activate other inflammatory cells into tissues where they differentiate into M1 or M2 macrophages (14, 37). M1 macrophages induce inflammation through the proinflammatory cytokines; M2 macrophages play an anti-inflammatory role. Overexpression of ApoL1, either wild type or risk variants, induced monocyte differentiation into atypical states of M1 macrophages (Fig. 3), a proinflammatory state characterized by greatly enhanced gene expression of prototypical M1 markers CD80, TNF, IL1B, and IL6, with slightly but significantly increased gene expression of some M2 markers (Fig. 3).
Activated macrophages exist as classical activated M1 cells and alternatively activated M2 cells, M2 macrophages (27, 30, 46, 48, 56). In this study, we found that ApoL1 overexpression promoted monocyte polarization toward an atypical M1 state with expression certain M2 markers. Thus, ApoL1 risk variants, compared with ApoL1-G0, induced more IL6 mRNA (M1 marker) while also increasing M2 markers, including TGFB mRNA and protein and increasing CD163, CD204 (G2 but not G1), and CD206 RNA expression (G1 but not G2). To be sure, the differences between the -G0 variant and the risk allele variants were in many cases quantitative rather than qualitative. The M1 state is thought to be anti-inflammatory, whereas the M2 state is typically thought to suppress inflammatory responses and to repair tissue injury. Of note is the finding that risk variant-induced atypical M1 proinflammatory state could indicate that ApoL1 risk variants play a role in abnormal tissue repair and immune suppression, leading to fibrosis formation and eventual CKD (14, 19, 20, 30), although this remains speculative.
COX-1 and COX-2 catalyze the synthesis of PGH2, resulting in the production of a series of prostanoids (9). It is believed that COX-2 is rapidly induced by various stimuli and is a major contributor in the production of prostanoids and progression of inflammation (22, 41). We demonstrated that ApoL1 risk variants stimulated the increase in COX-2 gene expression in THP-1 monocytes (Fig. 6), and also increased synthesis of the prostanoids PGE2 and TXA2 (Fig. 5). The underlying mechanism that explains how ApoL1 risk variants induce COX-2 expression is not yet known. Proinflammatory cytokines, including IL-1β, TNF, and INFγ, alone or in combination, are found to upregulate COX-2 expression (41). TNF, together with EP2, induces COX-2 expression in HEK-293 cells (2). IL-1β, a cytokine secreted by M1 macrophages, is well known to stimulate COX-2 expression in various cell types including monocytes/macrophages (10, 22), while IL-1β receptor antagonists block COX-2 expression in tumor-associated macrophages (16). Whether ApoL1 indirectly through proinflammatory cytokines secreted by atypical polarized M1 macrophages or directly induces COX-2 expression through activation of transcription factor, e.g., NF-κB, needs further investigation.
PGE2 is the most abundant prostanoid detected in the kidney, and both COX-1 and COX-2 are responsible for its biosynthesis under basal condition (15). However, in cytokine-stimulated kidneys, PGE2 is generated mainly by COX-2 (15). In our study, PGE2 production was greater in ApoL1-G1 and -G2 risk variants compared with ApoL1-G0 and negative control EV-transfected cells (Fig. 5), which is consistent with the increased expression of COX-2 in risk variant transfected cells (Fig. 6), The increased PGE2 production was inhibited by a COX-2-specific inhibitor rather than a COX-1-specific inhibitor (Fig. 7), suggesting a potential role of ApoL1 risk variants as stimulators of COX-2/PGE2 expression in THP-1 cells.
TXA2 was initially believed to be generated by the COX-1 pathway (17). Recent evidence has demonstrated that COX-2 also mediates TXA2 production and kidney injury (50, 53). Tetrachlorodibenzo-p-dioxin, an environmental contaminant, induces COX-2 and subsequent TXA2 expression, which is prevented by a selective COX-2 inhibitor and a selective thromboxane receptor antagonist (52). In renal tubular epithelial and macrophage cells, hyaluronan markedly induces TXA2 mRNA and protein expression, which is inhibited by COX-2-specific inhibitors (49). In the current study, TXA2 production and COX-2 were increased in ApoL1-G1 and -G2 risk variants compared with that of ApoL1-G0- and EV-transfected cells (Figs. 5 and 6); the increased TXA2 production was inhibited by COX-2- rather than COX-1-specific inhibitors (Fig. 7), indicating a potential role of ApoL1 risk variants as stimulators of the COX-2/TXA2 pathway in THP-1 cells.
In connection with prostanoid-mediated inflammation, TGF-β1 and the COX-2 pathway affect each other. TGF-β1 induces COX-2 expression in kidney, heart, liver, and lung (41, 50, 54). In 5/6 nephrectomized rat kidneys, both COX-2 and TGF- β1 are increased, and the increased COX-2 and TGF-β1 are important in the pathogenesis of systemic hypertension and marked proteinuria in this animal model (3). In this study, we have shown that the ApoL1 risk variant-induced increase in TGF-β1 production was mainly COX-2 dependent in THP-1 monocytes (Fig. 8). Whether or not this induced increase in COX-2 and TGF-β1 production contributes to ApoL1-associated CKD needs further investigation.
In summary, we have demonstrated that ApoL1 risk variants induce atypical M1 polarization of macrophages, and we identified ApoL1 risk variants as inducers of COX-2 in THP-1 macrophages. Increased production of prostanoids and cytokines and M1 polarization of macrophages are features of CKD (1, 9, 14, 27). The current study shows COX-2, the prostanoids it generates, and cytokines including TGF-β1, are upregulated in macrophages by ApoL1 risk variants. Further studies should address the contribution of this macrophage pathway to kidney disease in animal models of ApoL1 nephropathy and in human ApoL1 nephropathies.
GRANTS
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (Z01 DK-043308), and by the Second Hospital of Dalian Medical University Start-Up Funds, Dalian, China.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L. and J.B.K. conceived and designed research; H.L., H.R., Y.W., J.R., and S.S. performed experiments; H.L., K.O., S.S., P.Q., and J.B.K. analyzed data; H.L., H.R., Y.W., P.Q., and J.B.K. interpreted results of experiments; H.L. and J.B.K. prepared figures; H.L. and J.B.K. drafted manuscript; H.L., H.R., Y.W., K.O., J.R., S.S., P.Q., and J.B.K. edited and revised manuscript; H.L., H.R., Y.W., K.O., J.R., S.S., P.Q., and J.B.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate Patrick Dummer (NIDDK, Bethesda, MD) for providing the p-Sport-ApoL1 constructs in this study, and Dr. Jurgen Heymann (NIDDK, Bethesda, MD) for editorial comments.
REFERENCES
- 1.Anders HJ. Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol 27: 2564–2575, 2016. doi: 10.1681/ASN.2016020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki T, Frȍsen J, Fukuda M, Bando K, Shioi G, Tsuji K, Ollikainen E, Nozaki K, Laakkonen J, Narumiya S. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal 10: 10, 2017. doi: 10.1126/scisignal.aah6037. [DOI] [PubMed] [Google Scholar]
- 3.Bautista-García P, Sánchez-Lozada LG, Cristóbal-García M, Tapia E, Soto V, Avila-Casado MC, Márquez-Velasco R, Bojalil R, Franco M, Herrera-Acosta J. Chronic inhibition of NOS-2 ameliorates renal injury, as well as COX-2 and TGF-beta 1 overexpression in 5/6 nephrectomized rats. Nephrol Dial Transplant 21: 3074–3081, 2006. doi: 10.1093/ndt/gfl444. [DOI] [PubMed] [Google Scholar]
- 4.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, Boustany-Kari CM, Pullen SS, Miner JH, Hu CA, Rohacs T, Inoue K, Ishibe S, Saleem MA, Palmer MB, Cuervo AM, Kopp JB, Susztak K. Transgenic expression of human ApoL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017. doi: 10.1038/nm.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney EF. Chronic kidney disease: mechanisms of ApoL1-associated renal disease. Nat Rev Nephrol 13: 62, 2017. doi: 10.1038/nrneph.2016.175. [DOI] [PubMed] [Google Scholar]
- 6.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, Freedman BI, Parks JS, Shelness GS. Biogenesis and cytotoxicity of ApoL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res 56: 1583–1593, 2015. doi: 10.1194/jlr.M059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuypers B, Lecordier L, Meehan CJ, Van den Broeck F, Imamura H, Büscher P, Dujardin JC, Laukens K, Schnaufer A, Dewar C, Lewis M, Balmer O, Azurago T, Kyei-Faried S, Ohene SA, Duah B, Homiah P, Mensah EK, Anleah F, Franco JR, Pays E, Deborggraeve S. Apolipoprotein L1 variant associated with increased susceptibility to trypanosome infection. MBio 7: e02198-15, 2016. doi: 10.1128/mBio.02198-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danilewicz M, Wagrowska-Danielwicz M. Morphometric and immunohistochemical insight into focal segmental glomerulosclerosis in obese and non-obese patients. Nefrologia 29: 35–41, 2009. doi: 10.3265/Nefrologia.2009.29.1.35.1.en.full.pdf. [DOI] [PubMed] [Google Scholar]
- 9.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15: 511–523, 2015. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiebich BL, Mueksch B, Boehringer M, Hüll M. Interleukin-1beta induces cyclooxygenase-2 and prostaglandin E(2) synthesis in human neuroblastoma cells: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. J Neurochem 75: 2020–2028, 2000. doi: 10.1046/j.1471-4159.2000.0752020.x. [DOI] [PubMed] [Google Scholar]
- 11.Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte–new perspectives offer new opportunities. Nat Rev Nephrol 10: 379–388, 2014. doi: 10.1038/nrneph.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman DJ, Pollak MR. Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 27: 204–215, 2016. doi: 10.1016/j.tem.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease. Clin Kidney J 9: 765–771, 2016. doi: 10.1093/ckj/sfw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71: 1105–1115, 2007. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 16.Hou Z, Falcone DJ, Subbaramaiah K, Dannenberg AJ. Macrophages induce COX-2 expression in breast cancer cells: role of IL-1β autoamplification. Carcinogenesis 32: 695–702, 2011. doi: 10.1093/carcin/bgr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AA, Iadarola M, Yang HY, Dionne RA. Expression of COX-1 and COX-2 in a clinical model of acute inflammation. J Pain 8: 349–354, 2007. doi: 10.1016/j.jpain.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA. ApoL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol 37: 35–42, 2017. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg F. Apolipoprotein L1 and apolipoprotein A-IV and their association with kidney function. Curr Opin Lipidol 28: 39–45, 2017. doi: 10.1097/MOL.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 21.Kurumbail RG, Kiefer JR, Marnett LJ. Cyclooxygenase enzymes: catalysis and inhibition. Curr Opin Struct Biol 11: 752–760, 2001. doi: 10.1016/S0959-440X(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 22.Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J 18: 300–310, 2004. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- 23.Lan X, Rao TK, Chander PN, Skorecki K, Singhal PC. Apolipoprotein L1 (ApoL1) Variants (Vs) a possible link between Heroin-associated Nephropathy (HAN) and HIV-associated Nephropathy (HIVAN). Front Microbiol 6: 571, 2015. doi: 10.3389/fmicb.2015.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Berre L, Hervé C, Buzelin F, Usal C, Soulillou JP, Dantal J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int 68: 2079–2090, 2005. doi: 10.1111/j.1523-1755.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Abe Y, Lee I, Shrivastav S, Crusan AP, Hüttemann M, Hopfer U, Felder RA, Asico LD, Armando I, Jose PA, Kopp JB. Increased mitochondrial activity in renal proximal tubule cells from young spontaneously hypertensive rats. Kidney Int 85: 561–569, 2014. doi: 10.1038/ki.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limou S, Dummer PD, Nelson GW, Kopp JB, Winkler CA. ApoL1 toxin, innate immunity, and kidney injury. Kidney Int 88: 28–34, 2015. doi: 10.1038/ki.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng XM, Tang PM, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Dis (Basel) 1: 138–146, 2015. doi: 10.1159/000431214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat 68-69: 165–175, 2002. doi: 10.1016/S0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motwani MP, Gilroy DW. Macrophage development and polarization in chronic inflammation. Semin Immunol 27: 257–266, 2015. doi: 10.1016/j.smim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Murray PJ. Macrophage polarization. Annu Rev Physiol 79: 541–566, 2017. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 32.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015. doi: 10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris PC, Dennis EA. A lipidomic perspective on inflammatory macrophage eicosanoid signaling. Adv Biol Regul 54: 99–110, 2014. doi: 10.1016/j.jbior.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S III, Heneghan JF, Schlöndorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR. ApoL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci USA 113: 830–837, 2016. doi: 10.1073/pnas.1522913113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olszowski T, Gutowska I, Baranowska-Bosiacka I, Piotrowska K, Korbecki J, Kurzawski M, Chlubek D. The effect of cadmium on COX-1 and COX-2 gene, protein expression, and enzymatic activity in THP-1 macrophages. Biol Trace Elem Res 165: 135–144, 2015. doi: 10.1007/s12011-015-0234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YK, Hong H, Jang BC. Transcriptional and translational regulation of COX-2 expression by cadmium in C6 glioma cells. Int J Mol Med 30: 960–966, 2012. doi: 10.3892/ijmm.2012.1052. [DOI] [PubMed] [Google Scholar]
- 37.Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov Today 22: 186–193, 2017. doi: 10.1016/j.drudis.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulnock DM, Freeman BE, Mansfield JM. Modulation of innate immunity by African trypanosomes. Parasitology 137: 2051–2063, 2010. doi: 10.1017/S0031182010001460. [DOI] [PubMed] [Google Scholar]
- 39.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986–1000, 2011. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez-Barbero A, Dorado F, Velasco S, Pandiella A, Banas B, López-Novoa JM. TGF-beta1 induces COX-2 expression and PGE2 synthesis through MAPK and PI3K pathways in human mesangial cells. Kidney Int 70: 901–909, 2006. doi: 10.1038/sj.ki.5001626. [DOI] [PubMed] [Google Scholar]
- 41.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy 46: 397–410, 2016. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 42.Samanovic M, Molina-Portela MP, Chessler AD, Burleigh BA, Raper J. Trypanosome lytic factor, an antimicrobial high-density lipoprotein, ameliorates Leishmania infection. PLoS Pathog 5: e1000276, 2009. doi: 10.1371/journal.ppat.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol 214: 405–412, 2008. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- 44.Shin HJ, McCullough PA. Focus on lipids: high-density lipoprotein cholesterol and its associated lipoproteins in cardiac and renal disease. Nephron Clin Pract 127: 158–164, 2014. doi: 10.1159/000363552. [DOI] [PubMed] [Google Scholar]
- 45.Shukha K, Mueller JL, Chung RT, Curry MP, Friedman DJ, Pollak MR, Berg AH. Most ApoL1 is secreted by the liver. J Am Soc Nephrol 28: 1079–1083, 2017. doi: 10.1681/ASN.2016040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci 72: 4111–4126, 2015. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silveira LS, Antunes BM, Minari AL, Dos Santos RV, Neto JC, Lira FS. Macrophage polarization: implications on metabolic diseases and the role of exercise. Crit Rev Eukaryot Gene Expr 26: 115–132, 2016. doi: 10.1615/CritRevEukaryotGeneExpr.2016015920. [DOI] [PubMed] [Google Scholar]
- 49.Sun LK, Beck-Schimmer B, Oertli B, Wüthrich RP. Hyaluronan-induced cyclooxygenase-2 expression promotes thromboxane A2 production by renal cells. Kidney Int 59: 190–196, 2001. doi: 10.1046/j.1523-1755.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 50.Sureshbabu A, Muhsin SA, Choi ME. TGF-β signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol 310: F596–F606, 2016. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor HE, Khatua AK, Popik W. The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol 88: 592–603, 2014. doi: 10.1128/JVI.02828-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teraoka H, Okuno Y, Nijoukubo D, Yamakoshi A, Peterson RE, Stegeman JJ, Kitazawa T, Hiraga T, Kubota A. Involvement of COX-2-thromboxane pathway in TCDD-induced precardiac edema in developing zebrafish. Aquat Toxicol 154: 19–26, 2014. doi: 10.1016/j.aquatox.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, Yang PC, Kuo ML, Jee SH. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol 129: 1016–1025, 2009. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 54.Trostel J, Garcia GE. Endogenous inhibitors of kidney inflammation. J Nephrol Res 1: 61–68, 2015. [PMC free article] [PubMed] [Google Scholar]
- 55.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, Pays A, Tebabi P, Van Xong H, Jacquet A, Moguilevsky N, Dieu M, Kane JP, De Baetselier P, Brasseur R, Pays E. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422: 83–87, 2003. doi: 10.1038/nature01461. [DOI] [PubMed] [Google Scholar]
- 56.Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int Rev Immunol 34: 82–100, 2015. doi: 10.3109/08830185.2014.969421. [DOI] [PubMed] [Google Scholar]