Abstract
Introduction
Our previous work demonstrated that the 99mTc renal tracer, 99mTc(CO)3(FEDA) (99mTc-1), has a rapid clearance comparable in rats to that of 131I-OIH, the radioactive gold standard for the measurement of effective renal plasma flow. The uncharged fluoroethyl pendant group of 99mTc-1 provides a route to the synthesis of a structurally analogous rhenium-tricarbonyl 18F renal imaging agent, Re(CO)3([18F]FEDA) (18F-1). Our goal was to develop an efficient one-step method for the preparation of 18F-1 and to compare its pharmacokinetic properties with those of 131I-OIH in rats.
Methods
18F-1 was prepared by the nucleophilic 18F-fluorination of its tosyl precursor. The labeled compound was isolated by HPLC and subsequently evaluated in Sprague-Dawley rats using 131I-OIH as an internal control and by dynamic PET/CT imaging. Plasma protein binding (PPB) and erythrocyte uptake (RCB) were determined and the urine was analyzed for metabolites.
Results
18F-1 was efficiently prepared as a single species with high radiochemical purity (>99%) and it displayed high radiochemical stability in vitro and in vivo. PPB was 87% and RCB was 21%. Biodistribution studies confirmed rapid renal extraction and high specificity for renal excretion, comparable to that of 131I-OIH, with minimal hepatic/gastrointestinal elimination. The activity in the urine, as a percentage of 131I-OIH, was 92% and 95% at 10 and 60 min, respectively. All other organs (heart, spleen, lungs) showed a negligible tracer uptake (less than 0.4% ID). Dynamic microPET/CT imaging demonstrated rapid transit of 18F-1 through the kidneys and into the bladder; there was no demonstrable activity in bone verifying the absence of free [18F]fluoride.
Conclusions
18F-1 exhibited a high specificity for the kidney, rapid renal excretion comparable to that of 131I-OIH and high in vivo radiochemical stability. Not only is 18F-1 a promising PET renal tracer, but it provides a route to the development of a pair of analogous 18F/99mTc renal imaging agents with almost identical structures and comparable pharmacokinetic properties. These promising in vivo results warrant subsequent evaluation in humans.
Keywords: Re(CO)3([18F]FEDA), radiolabeling, PET imaging, biodistribution, renal radiotracer
1. Introduction
Radionuclide renography has made important contributions to the diagnosis and management of patients with a variety of suspected renal diseases. Chronic kidney disease is as a worldwide public health problem [1, 2] that can be ameliorated by monitoring renal function and detecting specific pathologies at an early stage. The use of 99mTc-based radiopharmaceuticals in non-invasive renal scintigraphy has been well documented [3]. Two of the most recognized technetium radiotracers for dynamic planar gamma imaging are 99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA), the only radiopharmaceutical used to measure glomerular filtration rate (GFR) [4], and 99mTc-mercaptoacetyltriglycine (99mTc-MAG3), a radiotracer that is primarily cleared by the organic anion transporter 1 (OAT1) located on the basolateral membranes of the proximal tubules [5–7]. 99mTc-MAG3 is the radiopharmaceutical of choice for the measurement of effective renal plasma flow (ERPF) [8–11] even if its clearance is only 50%–60% that of 131I-orthoidodohippurate (131I-OIH) [12, 13], the clinical radioactive standard for the measurement of ERPF [14]. However, 131I-OIH is no longer commercially available due to the higher radiation exposure and poor imaging characteristics associated with 131I [15].
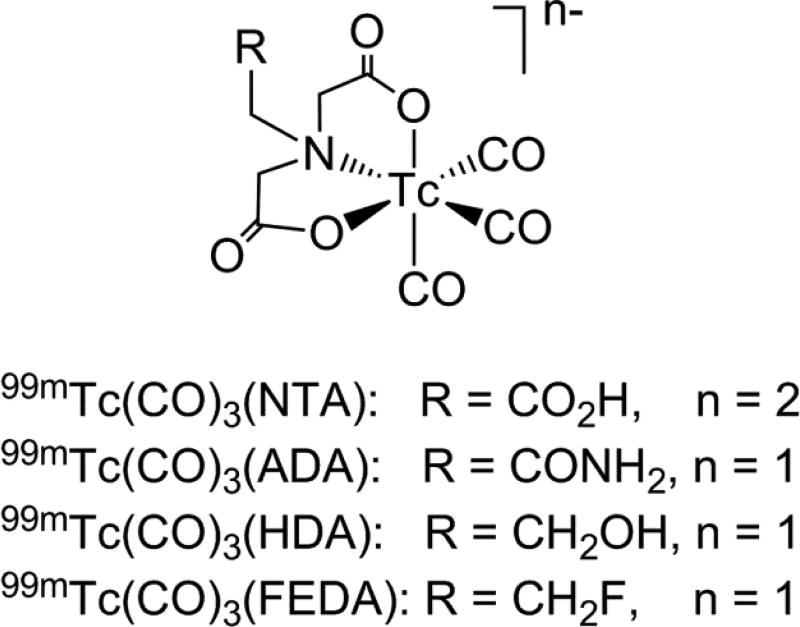
In the last decade, several new renal radiotracers based on the 99mTc-tricarbonyl core have undergone preclinical and clinical evaluation with promising results [16–23]. Among them, 99mTc-nitrilotriacetic acid (99mTc(CO)3(NTA); Figure 1) showed the greatest potential with pharmacokinetic properties in rats [17] and humans [20, 21] comparable to that of 131I-OIH. Because the presence of the charged pendant carboxylate group at physiological pH in the renal tracers has been long considered to be essential for the rapid renal extraction [24], we have recently synthesized three derivatives of 99mTc(CO)3(NTA), 99mTc(CO)3(2-acetamido)iminodiacetic acid [99mTc(CO)3(ADA)], 99mTc(CO)3(2-hydroxyethyl)iminodiacetic acid [99mTc(CO)3(HDA)] and 99mTc(CO)3(2-fluoroethyl)iminodiacetic acid (99mTc(CO)3(FEDA), 99mTc-1) (Figure 1) and have evaluated these tracers in rats to better assess the importance of a negatively charged uncoordinated group for OAT1 transporter recognition. Even though these three monoanionic 99mTc(CO)3(NTA) analogs lack the negatively charge pendant carboxylate group [22], all three demonstrated high specificity for renal excretion and pharmacokinetics in rats comparable to that of both 131I-OIH and the more hydrophilic and dianionic 99mTc(CO)3(NTA). These results support the conclusion that inner-sphere ligand properties, [99mTc(CO)3(N(CH2CO2)2)]−, shared by all four 99mTc-tricarbonyl tracers dominates the properties of the dangling group.
Figure 1.
Structures of known 99mTc-tricarbonyl renal tracers based on the iminodiacetate chelate with different pendant groups; tracers’ overall charges at physiological pH are included.
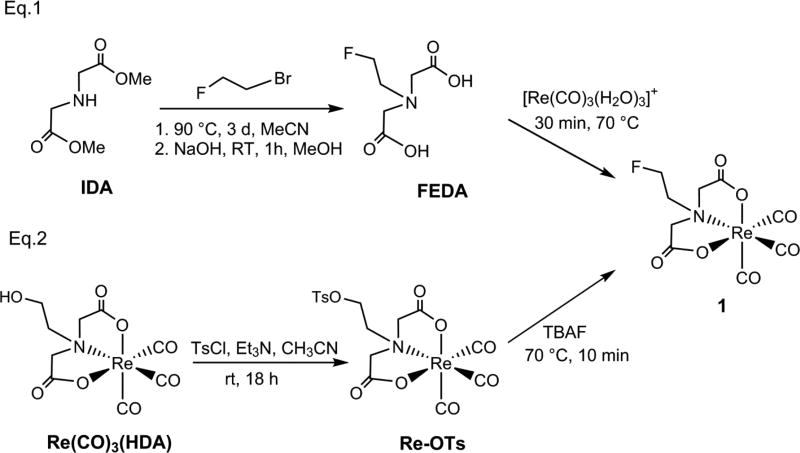
Based on our experience in the use of 99mTc/Re-tricarbonyl iminodiacetate systems in the development of renal tubular tracers [16–18, 22, 25–31], we explored the possibility of the introduction of fluorine-18 (18F) radioisotope into the rhenium-tricarbonyl complex (1, Scheme 1) in order to develop a new class of positron emission tomography (PET) renal tracers. Labeling the uncharged fluoroethyl group in 99mTc-1 (Figure 1) with 18F provides a route to the synthesis of a pair of 99mTc/18F renal tracers with almost identical structures that are likely to have similar pharmacokinetic properties. Although PET is increasingly used in early detection and treatment of many diseases, its high spatial resolution, sensitivity, and quantitative accuracy in 3-dimensional tomography make it ideal instrument for functional kidney imaging [32, 33]. Presently there are no PET radiopharmaceuticals comparable to 99mTc-MAG3 to evaluate the renal function even though several 18F derivatives of 131I-OIH have previously been reported [34, 35]. Moreover, the supply of 18F has the obvious advantage of being unaffected by possible shortages of 99mMo/99mTc generators [36, 37]. Herein, we report on the chemical synthesis, radiolabeling and preclinical evaluation of Re(CO)3(2-[18F]fluoroethyl)iminodiacetic acid (Re(CO)3([18F]FEDA, 18F-1), the PET radiolableded version of 99mTc-1, to determine if it has suitable pharmacokinetic properties for PET kidney imaging.
Scheme 1.
Synthesis of labeling precursor Re(CO)3(TsDA) (Re-OTs) and reference complex Re(CO)3(FEDA) (1) starting from the FEDA ligand (Eg.1) [22] and the labeling precursor Re-OTs (Eg.2).
2. Materials and methods
2.1. General
Re(CO)3(HDA) and the non-radioactive reference complex Re(CO)3(FEDA) (1) were synthesized as previously described [22]. All other reagent-grade chemicals and solvents were obtained from commercial suppliers and used without further purification. 1H NMR spectra were recorded on a Varian 400 MHz spectrometer, and chemical shifts are reported in δ units using the residual solvent peak as reference; electrospray mass spectrometry (ESI-MS, negative mode) was performed on a Thermo Finnigan LTQ-FT instrument. High performance liquid chromatography (HPLC) analyses of the non-radioactive Re-tricarbonyl complexes (monitored at 254 nm; Waters Breeze system) and of urine metabolites (Beckman Gold Nouveau system) were performed as previously reported [25]. Tissue/organ radioactivity was measured with an automated 2480 Wizard 2 gamma counter (Perkin Elmer) that corrects for spillover from 131I into the 99mTc window based on a prior normalization process. All animal experiments followed the principles of laboratory animal care and were conducted in compliance with the Emory Institutional Animal Care and Use Committee (IACUC).
2.2. Preparation of Re(CO)3(TsDA) (Re-OTs; rhenium-tricarbonyl-N-(2-(4-methylbenzenesulphonate)ethyl))iminodiacetic acid)
Re(CO)3(HDA) (45 mg, 0.10 mmol) was dissolved in anhydrous acetonitrile and stirred 18 h at room temperature with p-toluenesulfonyl chloride (29 mg, 0.15 mmol), triethylamine (21 µL, 0.15 mmol), and a catalytic amount of 4-N,N-dimethylaminopyridine (3 mg, 0.02 mmol). The crude product was purified by flash column chromatography on C18 silica gel (Agela Technologiest) using water:methanol (50:50). The UV-active fractions were analyzed by HPLC and those without impurities were combined and concentrated to yield the tosylate product Re-OTs as a white powder (12 mg, 20%). 1H NMR (400 MHz, D2O, pH 7): δ: 7.89 (d, 2H, J = 8.0 Hz), 7.54 (d, 2H, J = 8.0 Hz), 4.41 (t, 2H), 3.82 (d, 2H, J = 16 Hz), 3.72 (t, 2H), 3.49 (d, 2H J = 16 Hz), 2.45 (s, 3H). HRMS (M−, ESI) Calc’d for C16H15O10N187ReS: 599.99797, found: 599.99847.
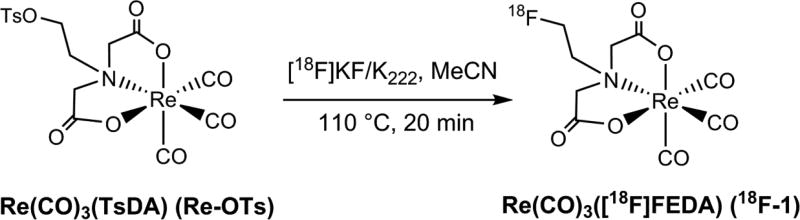
2.3. Synthesis of Re(CO)3([18F]FEDA) (18F-1; rhenium-tricarbonyl-N-(2-[18F]fluoroethyl)iminodiacetic acid)
An aqueous H18F solution (~ 1 Ci), produced by a Siemens 11 MeV RDS 111 cyclotron using the 18O(p,n)18F reaction in H218O, was transferred to a chemical processing control unit (CPCU). It was collected on an ABX QMA trap/release cartridge, released with a solution of K2CO3/H2O (0.6 mL; 1.5 mg/mL), and added to the open CPCU vessel containing a solution of K222/CH3CN (1.0 mL; 5.0 mg/mL). The [18F]fluoride mixture was dried by azeotropic distillation using additional CH3CN (3.5 mL) and a combination of heat (110°C) and a steady flow of argon to furnish the dry kryptate.
The tosylate precursor Re-OTs (3.0 mg) was dissolved in anhydrous CH3CN (1.0 mL) and added to the conical vessel containing the dry and activated K222/[18F]fluoride complex. The reaction mixture was heated at 110°C for 20 min and quenched with the high performance liquid chromatography (HPLC) solvent mixture of 0.05 M triethylammonium phosphate (TEAP) buffer (pH = 7) : abs. EtOH (80 : 20 v/v) (6 mL). The quenched reaction mixture was purified by semi-preparative HPLC using a Waters XTerra Prep RP 18 column (5µm, 19 × 100 mm) and eluted with the HPLC solvent at a flow rate of 6 mL/min (isocratic method). The fractions containing 18F-1 were collected from the semi-preparative HPLC and transferred with vacuum into one flask. They were diluted by addition of equivalent volumes of 0.05 M TEAP buffer (pH = 7). After mixing, the homogenous solution was pressure filtered with argon through an Acrodisc filter (pore size 1.0 µm) followed by a smaller filter (pore size 0.2 µm) into a 30 mL vented sterile vial to furnish the final dose solution in 10% EtOH. 18F-1 was prepared in an average decay corrected radiochemical yield (RCY) of 18% (decay corrected from the transfer of H18F(aq) to the CPCU). An aliquot (0.1 mL) of the 18F-1 solution was used to establish its chemical and radiochemical purities by analytical HPLC using a Waters Nova Pak C18 column (4 µm, 3.9 × 150 mm), a mobile phase of 0.05 M TEAP buffer (pH = 7) : MeOH (80 : 20 v/v) at a flow rate of 1 mL/min (isocratic method). Further evidence for the identity of the radiolabeled product was achieved by co-injection with the authentic standard material Re(CO)3(FEDA) (1) on the analytical HPLC column.
2.4. Biodistribution study
The biodistribution of Re(CO)3([18F]FEDA) (18F- 1) was evaluated in normal Sprague-Dawley rats with body weights ranging from 240–284 g by using dual isotope protocol. Rats were anesthetized with ketamine-xylazine (2 mg/kg of body weight) injected intramuscularly, with additional supplemental anesthetic as needed. The bladder was catheterized by use of heat-flared PE-50 tubing (Becton, Dickinson and Co.) for urine collection. Groups of six animals were injected via tail vein with 0.2 mL of a solution containing 18F- 1 (100 µCi/mL) and 131I-OIH (25 µCi/mL) in phosphate-buffered saline (PBS) pH 7.4. One additional aliquot of the 18F and 131I tracer solution (0.2 mL) for each time point was diluted to 100 mL, and three 1-mL portions of the resulting solution were used as standards.
Animals were sacrificed at 10 min and 60 min after injection. Tissues of interest, along with blood and urine, were collected and placed in counting vials. Each sample and the standards were counted for radioactivity by using an automated gamma-counter using the 18F/131I dual-label program. The percentage of the dose in each tissue or organ was calculated by dividing the activity counts in each tissue or organ by the total injected activity. The percentage injected dose in whole blood was estimated by assuming a blood volume of 6.5% of total body weight.
Two rats in the 10 min studies produced very little urine (less than 0.2 mL); these rats probably became hypotensive during anesthesia and were eliminated from the combined data analysis.
2.5. microPET imaging
Rat PET/CT imaging was conducted according to the Emory Center for Systems Imaging (CSI) Standard Operating Procedure (SOP). Quantitative whole body rat images were acquired in four Sprague-Dawley rats weighing 223–232 g using a Siemens Inveon MicroPET/CT scanner. The animals were initially anesthetized with ketamine/xylazine mixture injected intramuscularly. An acute intravenous catheter, through which the radiotracer was administered, was placed in the rat's tail vein. Animals were maintained under isoflurane anesthesia on the imaging table using a nose cone apparatus and the vaporizer set to 1–2% at a flow rate of 500–1000 mL/min during the whole experiment. Animals were positioned in the tomograph then fitted with a pulse oximeter min-clip to measure oxygen saturation and heart rate. A rectal probe was used to monitor body temperature, which was maintained by a warm air-filled blanket. A dose of approximately 0.3 mCi of Re(CO)3([18F]FEDA) in 0.2 mL of normal saline was injected via a tail vein. Dynamic PET data were acquired beginning at the time of radiotracer administration for a total duration of 30 min, followed by the acquisition of CT data over a period of 5 min. At the conclusion of the study, the rat was sacrificed prior to regaining consciousness. All data were reconstructed with OSEM3D/MAP using measured attenuation correction derived from the CT. Image data were decay-corrected to the time of injection. The time-activity curves (TAC) of kidneys and other organs (e.g. bladder, heart, liver) were ploted from the appropriate region of interest activities in each time frame. The TAC results were converted to standardized uptake values (SUV) defined by the (activity pixel value in µCi/mL)*[weight of the animal (g)]/[injected dose (µCi)].
2.6. In vivo stability, plasma protein binding and erythrocyte uptake
The bladders of two additional rats were catheterized as described for the biodistribution study. Both rats received an intravenous tail vein bolus injection of ~0.3 mCi of Re(CO)3([18F]FEDA) and urine was collected for 10 min, filtered with a 0.2 µm Millex-LG filter to remove foreign particles and analyzed by reversed phase HPLC to determine whether the complex was metabolized or excreted unchanged in the urine.
Carotid artery blood samples were obtained and the whole blood samples were placed in capillary tubes and immediately centrifuged for 15 min to determine the hematocrit. Plasma protein binding (PPB) was determined from 1 mL of plasma by ultrafiltration (Centrifree micropartition system; Amican Inc.) and calculated as PPB = [1 − (ultrafiltrate concentration/plasma concentration)] × 100. The percent erythrocytes uptake was calculated from the activity counted in the whole blood (counts/g) and packed cells (counts/g) as [(counts/g in erythrocytes × hematocrit)/counts/g in whole blood]. No correction was made for plasma trapped in the red blood cells sample. PPB and erythrocytes uptake were calculated in duplicate and the mean values reported. At the conclusion of the study, the rat was sacrificed prior to regaining consciousness.
3. Results and discussion
In this study, we report on the first in vivo biological evaluation of Re(CO)3([18F]FEDA) (18F-1) as a novel PET imaging agent that is structurally analogous to 99mTc(CO)3(FEDA) (99mTc-1). 99mTc-1 is as a promising new 99mTc renal tracer with the uncharged fluoroethyl group and with a rapid clearance comparable to that of 131I-OIH [22]. This uncharged fluoroethyl pendant group in 99mTc-1 (Figure 1) provided a means to the synthesis of the structurally analogous 18F-1 tracer. We hypothesized that a pair of 18F/99mTc renal agents with almost identical structures would likely have comparable pharmacokinetic properties.
To test our hypothesis, we had to first develop an efficient one-step labeling method for the preparation of the 18F-1 tracer. We utilized the alcohol moiety of the our recently reported Re(CO)3(HDA) complex [27] for the preparation of Re(CO)3(TsDA) (Re-OTs) as the one-step 18F labelling precursor (Scheme 1, Eq. 2). Re(CO)3(HDA) is a rhenium tricarbonyl complex with an iminodiacetate chelate coordinated in an ONO mode around the Re(CO)3 core forming two five-membered rings and with the dangling N-(2-hydroxy)ethyl group (Figure 1) [27]. The novel tosylate Re-OTs was obtained by reacting Re(CO)3(HDA) with p-toluenesulfonyl chloride in anhydrous tetrahydrofuran containing triethylamine and a catalytic amount of 4-N,N-dimethylaminopyridine at room temperature. A small aliquot of the reaction mixture was examined by analytical HPLC showing completion of the reaction at 18 h: the starting material was consumed giving rise to a single major product peak with a retention time of 23 min corresponding to the desired tosylate Re-OTs. Purification using C18 flash column chromatography yielded the tosylate precursor in 20% yield. Its structure was fully confirmed by spectroscopic and analytical methods (NMR and MS). 1H NMR spectrum of Re-OTs clearly showed two strongly coupled doublets (J = 16 Hz, AB-spin system) of the coordinated two acetate moieties verifying no change in an ONO coordination mode with two adjacent 5-membered chelate rings of the iminodiacetatic moiety of the starting Re(CO)3(HDA) complex. Also, the presence of the singlet signal at 2.45 ppm along with two doublet signals at 7.89 and 7.54 ppm attributed to the methyl group and aromatic protons of the tosyl group, respectively, confirmed the sulfonate formation by conversion of the hydroxyl group of Re(CO)3(HDA) into the tosylate in Re-OTs (Scheme 1, Eq. 2). Since the 18F labeling is almost always done at higher temperature, the Re-OTs precursor was tested for stability at 110 °C for 1 hour and found to be stable at those conditions as indicated by HPLC as no changes in its chromatogram were observed before and after the heating (see Supporting Information).
Scheme 1 also shows the synthetic routes used to synthesize the non-radioactive standard Re(CO)3(FEDA) (1). The preparation of 1 from the FEDA ligand is illustrated in Scheme 1, Eq. 1 and was conducted according to our previously reported procedure [22]; this Re complex was used as the reference compound to confirm the chemical identity of 18F-1. Furthermore, to test stability of the tosylate precursor in the settings close to 18F radiolabeling conditions, 1 was also obtained following cold fluorination of the tosylate Re-OTs with a tetrabutylammonium fluoride (TBAF) solution by heating at 70 °C (Scheme 1, Eq. 2) as confirmed by HPLC. The HPLC chromatogram showed that the peak at 23 min of the starting Re-OTs was fully replaced just after 10 min of heating by a major peak at 17 min corresponding to the target compound 1 (see Supporting Information).
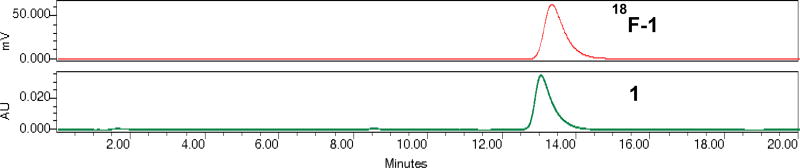
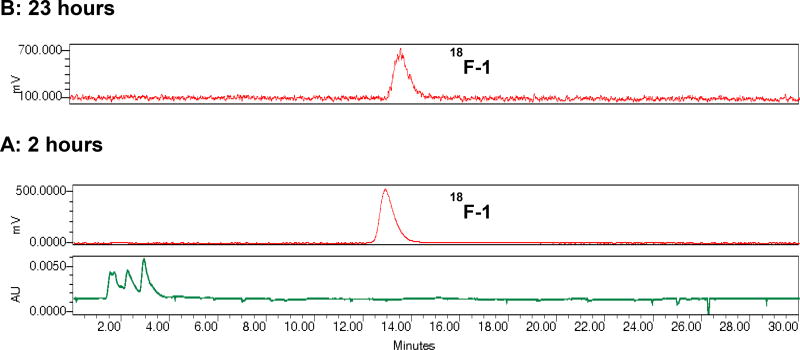
Re(CO)3(FEDA) was radiolabeled with fluorine-18 at its fluoroethyl moiety from the corresponding tosylate precursor Re-OTs using a one-step radiolabeling procedure as outlined in Scheme 2. Fluorination with the cyclotron-produced [18F]fluoride as the no-carrier-added activated K[18F]F-Kryptofix 222 complex was performed by heating the tosylate Re-OTs at 110°C for 20 minutes in anhydrous acetonitrile. The crude radiotracer Re(CO)3([18F]FEDA) (18F-1) was purified by semi-preparative HPLC and the desired pure radioactive fractions were combined. The final 18F-1 radiotracer was formulated in a 0.05 M TEAP solution (pH 7) containing 10% ethanol, collected into a sterile, pyrogen-free product vial after passing through a sterile filter, and was immediately used for biological application. 18F-1 was obtained in an average decay corrected radiochemical yield of 18% (after the HPLC analysis of the crude product) in a total synthesis time of 120 minutes from end of bombardment. This one-step one pot 18F labeling method can be readily adopted for producing clinical doses of 18F-1 under cGMP compliance. Since the specific activity of 18F-1 was not directly determined, the maximum amount of nonradioactive material in the final dose arising from the precursor is about 1 µg. On the basis of an HPLC serial dilution injection of the aqueous solution of Re(CO)3(FEDA) (1) (100 µg/mL, 10 µg/mL, 1 µg/mL, 0.1 µg/mL, and 0.01 µg/mL; starting from 0.1 µg/mL, no UV peak corresponding to 1 was observed on HPLC chromatogram) using the same analytical HPLC labeling method and a 198 mCi yield in 12 mL solution at end of synthesis, the amount of unlabeled material in the final product would not exceed 0.06 µg/mCi. Quality control HPLC of the formulated 18F-1 showed chemical and radiochemical purities greater than 99%. The identity of the radiolabeled target compound 18F-1 was confirmed by co-injection with the standard 1 on analytical HPLC (Figure 2). Both 18F-1 and 1 complexes showed similar retention times confirming the formation of the same product at the n.c.a (18F-1) and macroscopic (1) levels. The final solution of the formulated 18F-1 (pH 7) showed high in vitro stability at room temperature. No measurable decomposition was observed when samples of the formulated 18F-1 solution were analyzed by HPLC over the next 23 hours after the labeling indicating that the 18F-1 tracer did not release [18F]fluoride (Figure 3). To our knowledge, the direct radiofluorination of Re(CO)3([18F]FEDA) is the first example of the incorporation of 18F into a metal-tricarbonyl complex; we initially reported that in our abstract presentation at the 2016 Society of Nuclear Medicine and Molecular Imaging Annual Meeting [38].
Scheme 2.
Radiosynthesis of Re(CO)3([18F]FEDA) (18F-1).
Figure 2.
HPLC chromatograms of Re(CO)3([18F]FEDA) (18F-1; top, red) co-injected with Re(CO)3(FEDA) (1; bottom, green) to confirm the radiotracer identity (HPLC: 0.05 M TEAP pH 7/MeOH, 80:20 v/v, isocratic method, flow 1 mL/min).
Figure 3.
In vitro stability of Re(CO)3([18F]FEDA) (18F-1) at physiological pH was confirmed by HPLC at 2 hours (A) and 23 hours (B) after radiolabeling. The upper trace shows the radio-profile (red) and the lower trace shows the UV profile at 254 nm (green).(HPLC: 0.05 M TEAP pH 7/MeOH, 80:20 v/v, isocratic method, flow 1 mL/min).
Following the radiosynthesis and chemical and radiochemical characterization of 18F-1, we next evaluated the pharmacokinetic properties of this new radiotracer. Biodistribution studies were performed using normal rats and determined at 10 and 60 min post-injection. The pharmacokinetic properties of 18F-1 were compared to those of 131I-OIH by simultaneous intravenous administration of both 18F and 131I tracers. 131I-OIH served as an internal controlled since it is the radioactive standard for the measurement of ERPF. The results of the biodistribution studies are shown in Table 1 and are expressed as %ID per organ, blood and urine (bowel includes intestines and stomach). For comparison purposes, Table 1 includes also the previously reported biodistribution data of 99mTc(CO)3(FEDA) (99mTc-1), the 99mTc analog of 18F-1 [22].
TABLE 1.
Comparison of ex-vivo biodistribution of Re(CO)3([18F]FEDA) (18F-1) and 99mTc(CO)3(FEDA) (99mTc-1) [22] co-injected with 131I-OIH (OIH) at 10 and 60 minutes in normal ratsa. Activity concentrations are expressed as %ID ± SD in blood, urine and selected organs, and as %ID/g ± SD in bone for 18F-1.
| 10 min | 10 min | 60 min | 60 min | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| 18F-1 | OIH | 99mTc-1 | OIH | 18F-1 | OIH | 99mTc-1 | OIH | |
| Blood | 5.2 ± 0.7 | 4.5 ± 0.4 | 5.1 ± 0.9 | 5.8 ± 1.0 | 0.3 ± 0.1 | 0.8 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.4 |
| Liver | 5.6 ± 0.3 | 2.8 ± 0.6 | 5.9 ± 0.8 | 3.0 ± 0.9 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 |
| Bowelb | 2.1 ± 1.7 | 1.3 ± 0.6 | 2.9 ± 0.5 | 1.8 ± 0.2 | 3.9 ± 2.0 | 1.1 ± 0.2 | 2.9 ± 1.4 | 1.2 ± 0.5 |
| Spleen | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.1 ± 0.1 |
| Heart | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| Lung | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Kidney | 9.4 ± 2.0 | 5.2 ± 1.6 | 6.6 ± 2.1 | 4.8 ± 0.8 | 1.0 ± 0.7 | 1.0 ± 0.8 | 0.7 ± 0.2 | 0.5 ± 0.2 |
| Urine | 49.3 ± 3.7 | 54.4 ± 6.9 | 54.1 ± 7.2 | 54.2 ± 9.2 | 80.5 ± 4.4 | 84.8 ± 3.8 | 79.2 ± 6.4 | 80.2 ± 5.3 |
| %Urinec | 92 ± 14 | 100 ± 4 | 95 ± 5 | 99 ± 2 | ||||
| Bone | 0.08 ± 0.01 | 0.04 ± 0.01 | ||||||
Data are presented as mean ± SD.
18F-1 (10 min n = 4, 60 min n = 6); 99mTc-1 (10 min n = 5, 60 min n = 4).
Bowel includes intestines and stomach.
%Urine is expressed as a 18F/131I-OIH ratio for Re(CO)3([18F]FEDA) and 99mTc/131I-OIH ratio for 99mTc(CO)3(FEDA)
The data obtained for 18F-1 at 10 and 60 min time points revealed similar biodistributions for 18F-1 and 99mTc-1. Both tracers not only cleared rapidly from the blood (only ~5 % and 0.3 % of injected dose remained in the blood at 10 min and 60 min, respectively) but their blood clearance was also comparable to that of 131I-OIH at both time points (Table 1). The 18F-1 tracer demonstrated high specificity for renal excretion. Its urine activity, expressed as a percentage of 131I-OIH (% 18F/131I), was 92 ± 14 % at 10 min and 95 ± 5 % at 60 min, and it was very similar to the results obtained for its 99mTc-1 analog (% 99mTc/131I) (Table 1). The liver activity of 18F-1 decreased from 5.6 ± 0.3% ID at 10 min to 0.4 ± 0.1% ID at 60 min, while uptake in the bowel increased only slightly between these two time points (2.1 ± 1.7% ID and 3.9 ± 2.0% ID at 10 and 60 mi, respectively), suggesting that the liver activity represented blood pool activity in the liver. Liver and bowel activities of 18F-1 were analogous to those of 99mTc-1 and these values were only minimally higher than the liver and bowel activities of 131I-OIH at the same time points. The heart, lung and spleen all showed a negligible tracer uptake (< 0.4%ID). In addition, bone uptake of 18F-1 was also negligible at 10 and 60 min (< 0.1%ID/g) verifying the absence of free [18F]fluoride and supporting the tracer in vivo stability.
The biological properties of 18F-1 in rats appear to be similar to those reported for p-18F-fluorohippuran (18F-PFH) [34]. They are both exclusively excreted via the renal-urinary pathway with almost identical activities in blood (~ 0.3 %ID) and urine (~ 81% ID) at 1 h post injection. Even though their molecular structures are very different, both of these 18F renal tracers are likely transported by the same renal organic anion transporter 1 as PAH, 131-OIH and 99mTc-MAG3. One advantage of 18F-1 over 18F-PFH is its much simpler one-step one-pot radiolabeling procedure that can better facilitate the 18F-1 clinical utility. In addition, 18F-PFH was observed to have a T1/2 almost twice as great as 125I-OIH [39]. The T1/2 of 18F-1 was not directly compared with 131I-OIH in our study but the observation of comparable urine activity at both 10 and 60 minutes suggests that the T1/2 of 18F-1 is likely to be similar to that of 131I-OIH.
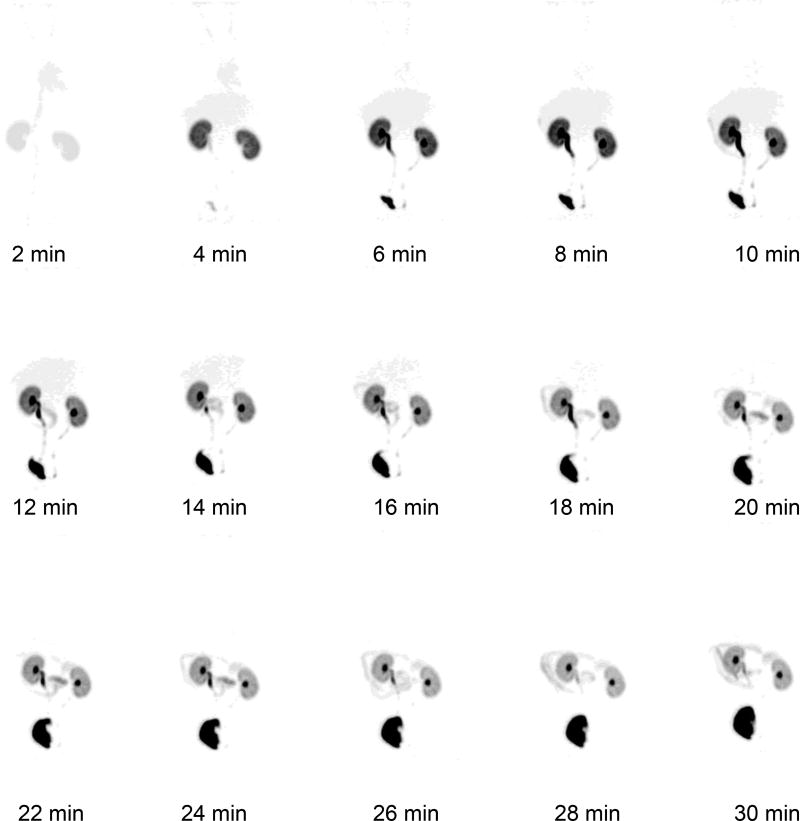
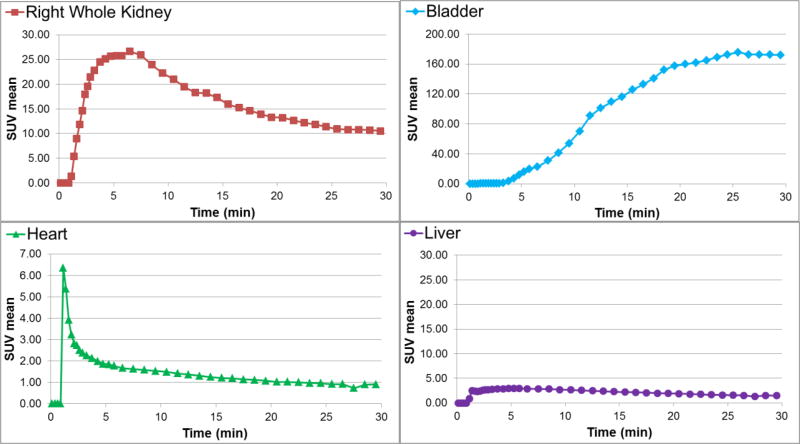
The 18F-1 tracer clearance via the renal pathway was also distinctly evident on microPET images. A PET/CT fused image at 30 min after i.v. injection of 18F-1 in rat is represented in Figure 4. There is no evidence that defluorination occurred in vivo since there was no detectable bone activity (Figure 4). Regions of interest (ROIs) from the whole organs of the kidneys, bladder, heart and liver images were measured so that the tissue accumulation of the radiotracer could be quantified. Dynamic PET imaging revealed the prominent renal uptake with fast excretion into the bladder (Figure 5), confirming that the radioactivity is almost exclusively eliminated by the renal-urinary pathway with only minimal hepatobiliary elimination. Examples of the time-activity curves (TACs) for 18F-1, resulting from the subsequent PET data analysis, are shown in Figure 6 for the right kidney, bladder, heart and liver. PET imaging and TACs of 18F-1 are consistent with the tissue distribution data described above.
Figure 4.
Fused volume rendering of PET and CT (summed 5–30 min) of a normal rat injected with Re(CO)3([18F]FEDA) (18F-1) (injected radioactivity: 0.3 mCi, anesthesia: 2% isoflurane).
Figure 5.
Representative sequence of microPET maximum intensity projection coronal images (2 min/frame, total 30 min) of Re(CO)3([18F]FEDA) (18F-1) showing rapid accumulation in the kidneys and clearance into the bladder.
Figure 6.
Representative time activity curves (TAC) in kidney, bladder, heart and liver for PET imaging studies with Re(CO)3([18F]FEDA) (18F-1) in rats.
To determine in vivo stability of 18F-1, the tracer was injected into rats and urine was collected for 10 min post-injection. HPLC analysis of urine showed only the intact parent compound 18F-1 and the absence of any metabolites thus showing that the tracer was excreted unchanged and no defluorination or radioactive degradation of 18F-1 occurred (Figure 7). Blood from the same rats was obtained to establish the plasma protein binding (PPB) and erythrocyte uptake since both of these parameters can influence the clearance of the renal tracers. In our study, both 18F-1 and 99mTc-1 tracers showed similar erythrocyte uptake of 21% vs. 20%, respectively. However, PPB of 18F-1 was 87% and was higher than PPB of its 99mTc-1 analog (61%) [22]. Regardless of differences in PPB, however, both 18F and 99mTc tracers had comparable amounts of injected activity in the urine at 10 and 60 min, and that amount was also comparable to that of 131I-OIH (Table 1).
Figure 7.
Radio-HPLC chromatograms of purified Re(CO)3([18F]FEDA) (18F-1) before injection (A) and of rat urine 10 min after i.v. injection of (18F-1) (B) (HPLC: 0.05 M TEAP pH 2.5/MeOH, gradient method [25] flow 1 mL/min).
The extraction efficiency of 18F-1 can be estimated based on the following considerations. The amount of 18F-1 appearing in the kidney as a function of time is dependent on the renal plasma flow and the extraction efficiency. Renal plasma flow was the same for both 18F and 131I tracers. The high degree of specificity for the kidney of both 18F-1 and 131I-OIH coupled with their comparable rates of urine excretion (based on the 10 and 60 min time points) indicate that they likely have very similar extraction efficiencies. It is important to note, however, that extraction efficiency is dependent of the affinity of the tracer for the tubular transport mechanism and the availability of the tracer to the transport mechanism. Availability can be modulated by red cell uptake as well as plasma protein binding and plasma protein binding affinity [40]. 18F-1 has a higher protein binding than 131I-OIH, 87% vs 44%, but a lower red cell uptake (21% vs 35%) [17]; the higher plasma protein binding of 18F-1 may result in diminished access for the tubular transport mechanism compared to 131I-OIH resulting in a lower extraction efficiency of 18F-1 whereas the lower red cell uptake of 18F-1 compared to 131I-OIH may work in the opposite direction. Furthermore, although the higher protein binding of 18F-1 may result in a diminished availability of 18F-1 to the tubular transporter, the higher protein binding of 18F-1 compared to 131I-OIH may result in a lower volume of distribution of 18F-1, greater retention of 18F-1 in the plasma and increased availability to the tubular transporter; increased availability could also compensate for a lower extraction efficiency. An accurate determination of the extraction efficiency would require a direct measurement but the more relevant clinical feature is not the extraction efficiency but the rate that the tracer appears in the urine.
4. Conclusions
An efficient, one-step radiosynthesis method was developed for the preparation of 18F-1, a new, stable, well-defined 18F-Re-tricarbonyl PET tracer with an uncharged pendant fluoroethyl group. To our knowledge, the direct radiofluorination of Re(CO)3([18F]FEDA) is the first example of the incorporation of 18F into a metal-tricarbonyl complex. 18F-1 demonstrated high in vitro and in vivo stability with no metabolic degradation. In addition, 18F-1 exhibits a high specificity for the kidney and rapid renal excretion comparable to that of 131I-OIH and its 99mTc - 1 analog in rats. The results from microPET/CT imaging studies confirm the biodistribution results. These results suggest that 18F-1 is a promising PET renal tracer that could result in a pair of analogous 18F/99mTc renal imaging agents with almost identical structures and pharmacokinetic properties.
The justification of the advantage of having a pair of analogous 18F/99mTc renal agents is twofold: (1) We would have an excellent PET tracer comparable to 131I-OIH; (2) It is often important to determine if a kidney's function is stable, improving or worsening. If one compares sequential 99mTc-DTPA and 99mTc-MAG3 studies in a single patient, for example, any difference one observes may reflect the different behavior of the different radiopharmaceuticals, not a change in the patient’s renal function; in fact, with two entirely different radiopharmaceuticals, it may be difficult to detect or to be certain of a clinically meaningful change in renal function. We hypothesize that the performance of 18F-1 will be almost identical to that of 99mTc-1; consequently, we would expect the same results in a given patient whether we used 99mTc-1 or 18F-1. 99mTc-1 is potentially available in kit form and potentially around 5 times cheaper than 18F-1. Consequently, most renal studies would likely be performed with 99mTc-1. However, if there is another shortage of 99Mo and 99mTc is not available, the patient could be studied with 18F-1 and a valid comparison made with the previous 99mTc-1 study. Or if the cost of 18F-1 comes down and PET becomes the imaging procedure of choice, 18F-1 studies could be compared with the previous 99mTc-1 studies.
These promising pre-clinical in vivo results warrant further evaluation of 18F-1 in humans.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R37 DK038842. The authors thank Eugene Malveaux for his excellent technical assistance with all animal studies. We also thank the Emory Center for Systems Imaging Radiopharmacy for the 18F production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assesment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol. 2009;182:435–44. doi: 10.1016/j.juro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AT. Radionuclides in nephrology, part 1: radiopharmaceuticals, quality control and quantitative indices. J Nucl Med. 2014;55:608–15. doi: 10.2967/jnumed.113.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaufox MD, Aurell M, Bubeck B, Fommei E, Piepsz A, Russell C, et al. Report of the radionuclides in nephrourology committee on renal clearance. J Nucl Med. 1996;37:1883–90. [PubMed] [Google Scholar]
- 5.Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Transport of 99mTc-MAG3 via rat renal organic anion transporter 1. J Nucl Med. 2004;45:80–5. [PubMed] [Google Scholar]
- 6.Eshima D, Taylor A. Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new 99mTc renal tubular function agent. Semin Nucl Med. 1992;22:61–73. doi: 10.1016/s0001-2998(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 7.Bubeck B, Brandau W, Weber E, Kalble T, Parekh N, Georgi P. Pharmacokinetics of technetium-99m-MAG3 in humans. J Nucl Med. 1990;31:1285–93. [PubMed] [Google Scholar]
- 8.O'Reilly P, Aurell M, Britton K, Kletter K, Roenthal L, Testa T. Consensus on diuresis renography for investigating the dilated upper urinary tract. Radionuclides in Nephrourology Group. Consensus Committee on Diuresis Renography. J Nucl Med. 1996;37:1872–6. [PubMed] [Google Scholar]
- 9.Esteves FP, Taylor A, Manatunga A, Folks RD, Krishnan M, Garcia EV. 99mTc-MAG3 renography: normal values for MAG3 clearance and curve parameters, excretory parameters, and residual urine volume. AJR. 2006;187:W610–W7. doi: 10.2214/AJR.05.1550. [DOI] [PubMed] [Google Scholar]
- 10.Shulkin BL, Mandell GA, Cooper JA, Leonard JC, Majd M, Parisi MT, et al. Procedure guideline for diuretic renography in children 3.0. J Nucl Med Technol. 2008;36:162–8. doi: 10.2967/jnmt.108.056622. [DOI] [PubMed] [Google Scholar]
- 11.Gordon I, Piepsz A, Sixt R. Guidelines for standard and diuretic renogram in children. Eur J Nucl Med Mol Imaging. 2011;38:1175–88. doi: 10.1007/s00259-011-1811-3. [DOI] [PubMed] [Google Scholar]
- 12.Jafri RA, Britton KE, Nimmon CC, Solanki K, Al-Nahhas A, Bomanji J, et al. Technetium-99m MAG3, a comparison with Iiodine-123 and iodine-131 orthoiodohippurate, in patients with renal disorders. J. Nucl. Med. 1988;29:147–58. [PubMed] [Google Scholar]
- 13.Taylor A, Eshima D, Fritzberg AR, Christian PE, Kasina S. Comparison of iodidne-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 1986;27:795–803. [PubMed] [Google Scholar]
- 14.Eshima D, Fritzberg AR, Taylor A. Tc-99m renal tubular function agents: current status. Semin Nucl Med. 1990;20:28–40. doi: 10.1016/s0001-2998(05)80174-6. [DOI] [PubMed] [Google Scholar]
- 15.Burbank MK, Tauxe WN, Maher FT, Hunt JC. Evaluation of radioiodinated hippuran for the estimation of renal plasma flow. Mayo Clin Proc. 1961;36:372–86. [PubMed] [Google Scholar]
- 16.Lipowska M, He H, Malveaux E, Xu X, Marzilli LG, Taylor A. First evaluation of a 99mTc-tricarbonyl complex, 99mTc(CO)3(LAN), as a new renal radiopharmaceutical in humans. J Nucl Med. 2006;47:1032–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Lipowska M, Marzilli LG, Taylor AT. 99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH. J Nucl Med. 2009;50:454–60. doi: 10.2967/jnumed.108.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipowska M, Klenc J, Marzilli LG, Taylor AT. Preclinical evaluation of 99mTc(CO)3-aspartic-N-monoacetic acid, a renal radiotracer with pharmacokinetic properties comparable to 131I-o-iodohippurate. J Nucl Med. 2012;53:1277–83. doi: 10.2967/jnumed.111.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipowska M, Klenc J, Folks RD, Taylor AT. Initial Evaluation of 99mTc(CO)3(ASMA) as a Renal Tracer in Healthy Human Volunteers. Nucl Med Mol Imag. 2014;48:216–24. doi: 10.1007/s13139-014-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor AT, Lipowska M, Marzilli LG. 99mTc(CO)3(NTA): a 99mTc renal tracer with pharmacokinetic properties comparable to those of 131I-OIH in healthy volunteers. J Nucl Med. 2010;51:391–6. doi: 10.2967/jnumed.109.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor AT, Lipowska M, Cai H. 99mTc(CO)3(NTA) and 131I-OIH: comparable plasma clearances in patients with chronic kidney disease. J Nucl Med. 2013;54:578–84. doi: 10.2967/jnumed.112.108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipowska M, Klenc J, Jarkas N, Marzilli LG, Taylor AT. Monoanionic 99mTc-tricarbonyl-aminopolycarboxylate complexes with uncharged pendant groups: Radiosynthesis and evaluation as potential renal tubular trecer. Nucl Med Biol. 2017;47:48–55. doi: 10.1016/j.nucmedbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhadwal M, Satpati D, Singhal S, Sarma HD, Venkatesh M, Banerjee S. Preparation of 99mTc(CO)3-carboxymethylthioethyl iminodiacetic acid and evaluation as a potential renal imaging agent. Curr Radiopharm. 2012;5:65–70. doi: 10.2174/1874471011205010065. [DOI] [PubMed] [Google Scholar]
- 24.Despopoulos A. A definition of substrate specificity in renal transport of organic anions. J Theor Biol. 1965;8:163–92. doi: 10.1016/0022-5193(65)90101-3. [DOI] [PubMed] [Google Scholar]
- 25.He H, Lipowska M, Christoforou AM, Marzilli LG, Taylor AT. Initial evaluation of new 99mTc(CO)3 renal imaging agents having carboxyl-rich thioether ligands and chemical characterization of Re(CO)3 analogues. Nucl Med Biol. 2007;34:709–16. doi: 10.1016/j.nucmedbio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H, Lipowska M, Xu X, Taylor AT, Carlone M, Marzilli LG. Re(CO)3 complexes synthesized via an improved preparation of aqueous fac-[Re(CO)3(H2O)3]+ as an aid in assessing 99mTc imaging agents. Structural characterization and solution behavior of complexes with thioether-bearing amino acids as tridentate ligands. Inorg. Chem. 2005;44:5437–46. doi: 10.1021/ic0501869. [DOI] [PubMed] [Google Scholar]
- 27.Klenc J, Lipowska M, Abhayawardhana PL, Taylor AT, Marzilli LG. Structure and properties of fac-[ReI(CO)3(NTA)]2− (NTA3− = trianion of nitrilotriacetic acid) and fac-[ReI(CO)3(L)]n− analogues useful for assessing the excellent renal clearance of the fac-[99mTcI(CO)3(NTA)]2− diagnostic renal agent. Inorg Chem. 2015;54:6281–90. doi: 10.1021/acs.inorgchem.5b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klenc J, Lipowska M, Taylor AT, Marzilli LG. Synthesis and characterization of fac-Re(CO)3-aspartic-N-monoacetic acid: structural analogue of a potential renal tracer, fac-99mTc(CO)3(ASMA) Eur J Inorg Chem. 2012:4334–41. doi: 10.1002/ejic.201200599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipowska M, Cini R, Tamasi G, Xu X, Taylor AT, Marzilli LG. Complexes having the fac-[M(CO)3]+ core (M=Tc, Re) useful in radiopharmaceuticals: X-ray and NMR structural characterization and density functional calculations of species containing two sp3 N donors and one sp3 O donor. Inorg Chem. 2004;43:7774–83. doi: 10.1021/ic049544i. [DOI] [PubMed] [Google Scholar]
- 30.Lipowska M, Hansen L, Marzilli LG, Taylor A. The new renal imaging agent 99mTc(CO)3(ENDAC) and the chemistry of the Re(CO)3(ENDAC) analog. J Nucl Med. 2001;42:259. [Google Scholar]
- 31.Lipowska M, He H, Xu X, Taylor AT, Marzilli PA, Marzilli LG. Coordination modes of multidentate ligands in fac-[Re(CO)3(polyaminocarboxylate)] analogues of 99mTc radiopharmaceuticals. Dependence on aqueous solution reaction conditions. Inorg Chem. 2010;49:3141–51. doi: 10.1021/ic9017568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo Z, Xia J, Mathews WB. Radiopharmaceuticals for renal positron emission tomography imaging. Semin Nucl Med. 2008;38:20–31. doi: 10.1053/j.semnuclmed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Szabo Z, Xia J, Mathews WB. Future direction of renal positron emission tomography. Semin Nucl Med. 2006;36:36–50. doi: 10.1053/j.semnuclmed.2005.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awasthi V, Pathuri G, Agashe HB, Gali H. Synthesis and in vivo evaluation of p-18F-fluorohippurate as a new radiopharmaceutical for assessment of renal function by PET. J Nucl Med. 2011;52:147–53. doi: 10.2967/jnumed.110.075895. [DOI] [PubMed] [Google Scholar]
- 35.Pathuri G, Hedrick AF, Awasthi V, Gali H. Single-step radiosynthesis and in vivo evaluation of novel fluorine-18 labeled hippurate for use as a PET renal agent. Nucl Med Biol. 2012;39:1195–201. doi: 10.1016/j.nucmedbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 36.SNMMI Newsline. NAS Report Warns of U.S. Radioisotope Shortages. J Nucl Med. 2016:19N. [PubMed] [Google Scholar]
- 37.National Academies of Sciences, Engineering, and Medicine. Molybdenum-99 for Medical Imaging. Washington, DC: The National Academies Press; 2016. [DOI] [PubMed] [Google Scholar]
- 38.Lipowska M, Jarkas N, Klenc J, Voll RJ, Goodman MM, Taylor AT. Radiosynthesis of Re(CO)3(18F-FEDA), a structural analog of 99mTc(CO)3(FEDA) and new PET tracer for renal imaging and renal function assessment. J Nucl Med. 2016;57(Suppl. 2):1066. [Google Scholar]
- 39.Pathuri G, Sahoo K, Awasthi V, Gali H. Renogram comparison of p-[18F]fluorohippurate with o-[125I]iodohippurate and [99mTc]MAG3 in normal rats. Nucl. Med. Commun. 2011;32:908–12. doi: 10.1097/MNM.0b013e32834a6db6. [DOI] [PubMed] [Google Scholar]
- 40.Eshima D, Eshima L, Hansen L, Lipowska M, Marzilli LG, Taylor A. Effect of protein binding on renal extraction of 131I-OIH and 99mTc-labeled tubular agents. J Nucl Med. 2000;41:2077–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.