ABSTRACT
During infection, Mycobacterium tuberculosis colonizes macrophages or necrotic granulomas, in which low pH is one of the major challenges. The PhoPR two-component regulatory system and the cytosolic redox sensor WhiB3 both play important roles in the response to low pH by M. tuberculosis. However, whether close association exists between PhoPR and WhiB3 remains unclear. In this study, the positive regulation of whiB3 by PhoPR in mycobacteria was characterized. We observed that the expression patterns of the whiB3 gene under acidic conditions are different among mycobacterial species, suggesting that the regulation of whiB3 differs among mycobacteria. A sequence analysis of the whiB3 promoters (whiB3p) from M. tuberculosis and two closely related species, namely, M. marinum and M. smegmatis, showed that the whiB3p regions from M. tuberculosis and M. marinum contain a new type of PhoP box that is absent in the M. smegmatis whiB3p. Direct binding of PhoP to whiB3p from M. tuberculosis and M. marinum but not that from M. smegmatis was validated by in vitro protein-DNA binding assays. The direct activation of whiB3 by PhoPR under acidic conditions was further verified by reverse transcription-quantitative PCR (qRT-PCR) analysis in M. marinum. Moreover, mutating the residues important for the phosphorylation pathway of PhoPR in M. marinum abolished the activation of whiB3 expression by PhoPR under acidic conditions, suggesting that low pH triggers the phosphorylation of PhoPR, which in turn activates the transcription of whiB3. Since the PhoP box was only identified in whiB3p of pathogenic mycobacteria, we suggest that the PhoPR-whiB3 regulatory pathway may have evolved to facilitate mycobacterial infection.
IMPORTANCE The low pH in macrophages is an important barrier for infection by microbes. The PhoPR two-component regulatory system is required for the response to low pH and plays a role in redox homeostasis in Mycobacterium tuberculosis. WhiB3, a cytosolic redox-sensing transcriptional regulator, is also involved in these processes. However, there is no direct evidence to demonstrate the regulation of WhiB3 by PhoPR. In this study, we found that PhoPR directly activates whiB3 expression in response to low pH. An atypical PhoP box in the whiB3 promoters has been identified and is only found in pathogenic mycobacteria, which suggests that the PhoPR-whiB3 regulatory pathway may facilitate mycobacterial infection. This study provides novel information for further characterization of the PhoPR regulon.
KEYWORDS: Mycobacterium, transcriptional regulation, two-component regulatory systems
INTRODUCTION
The low pH in phagolysosomes of macrophages is important for host defense against infectious microbes (1). To survive within macrophages and successfully cause infection, Mycobacterium tuberculosis has developed different strategies to cope with this stress (2–4). Two-component systems (TCSs) (5) and the intracellular redox sensor WhiB3 (6, 7) are important regulators of gene expression during acid response.
TCSs are critical for signal transduction in bacteria (8). There are 12 paired TCSs in M. tuberculosis (9). PhoPR is one such system and is present in almost all Mycobacterium species (5). PhoR serves as a sensor histidine kinase and autophosphorylates a conserved histidine residue (H259 in M. tuberculosis) in response to environmental signals. The histidine-bound phosphoryl group is then transferred to a conserved aspartic acid residue (D71 in M. tuberculosis) in the receiver domain of the transcriptional regulator PhoP (10, 11), which in turn regulates over 150 genes with diverse functions (5, 12, 13). The DNA motif TCACAGC-N4-TCACAGC containing two direct repeats has been characterized as the PhoP box in PhoP-targeted promoters (14, 15). Although the PhoPR system is one of the most studied TCSs in M. tuberculosis, signals triggering the activation of PhoPR are yet to be fully characterized. Recent studies have shown that PhoP is crucial for the activation of the aprABC operon and for the survival of M. tuberculosis under acidic conditions or inside macrophages (16, 17), suggesting that PhoPR may contribute to sensing and signal transduction in an acidic environment. The deletion of aprABC results in defective mycobacterial lipid metabolism (16), indicating that lipids may also be also important for the ability to withstand acidic stress.
In addition to TCSs, WhiB3, a redox-sensing regulator containing a [4Fe-4S] cluster (18, 19), has also been reported to participate in lipid metabolism and in survival under acidic conditions (6, 7, 20). In low pH medium, the transcription of whiB3 is significantly enhanced (6), as is the transcription of the aprABC operon (16). Recently, WhiB3 was shown to be required for the maintenance of the expression of many genes, including cpsY, an extracellular polysaccharide synthase (7). Several genes involved in modulating phagosomal maturation and virulence in response to changes in pH in M. tuberculosis are also controlled by WhiB3, and the deletion of whiB3 in M. tuberculosis decreased bacterial survival at pH 4.5 (7). These data suggest that activation of whiB3 expression at low pH is important for M. tuberculosis survival during infection. However, the mechanism for the activation of whiB3 expression under acidic stress remains unclear.
In this study, we characterized the transcriptional regulation of whiB3 by PhoPR in response to low pH. We compared the expression levels of whiB3 at neutral or low pH in different mycobacterial species in the presence or absence of PhoPR. Direct binding of PhoP to the whiB3 promoter was confirmed by an in vitro protein-DNA binding assay. Interestingly, the specific regulation of whiB3 by PhoPR is only restricted to pathogenic mycobacteria, suggesting that this regulatory pathway may function during infection by pathogenic mycobacteria.
RESULTS
Expression of whiB3 is different in mycobacteria under acidic stress.
In M. tuberculosis, the expression of whiB3 is induced >10-fold at pH 4.5 (6). To determine whether similar expression of whiB3 is observed in other mycobacteria, we measured the mRNA levels of whiB3 in two closely related strains, M. smegmatis and M. marinum, under neutral (unacidified 7H9 medium, pH 6.8) and acidic (acidified 7H9 medium, pH 4.5) conditions. As shown in Fig. 1A, the expression of whiB3 was significantly upregulated in M. marinum at pH 4.5. In contrast, the expression of whiB3 in M. smegmatis was repressed under this stress condition (Fig. 1B). These results, together with results from M. tuberculosis (6), indicate that the expression of whiB3 under acidic pH conditions is different in pathogenic (M. tuberculosis and M. marinum) and nonpathogenic (M. smegmatis) mycobacterial species.
FIG 1.

Acidic stress activates the expression of whiB3 in M. marinum but not in M. smegmatis. Transcriptional levels of the whiB3 gene in M. marinum (Mma) (A) and M. smegmatis (Msm) (B) at neutral or low pH (pH 6.8 and pH 4.5, respectively). RNA levels were first normalized to either the 16S rRNA (in M. marinum) or the sigA levels (in M. smegmatis), and the relative RNA levels at pH 6.8 in these strains were normalized to 1. Mean values with standard deviations from two independent tests in duplicates are shown. *, P < 0.05.
Acid-responsive regulator PhoP binds to the promoter of whiB3 in mycobacteria.
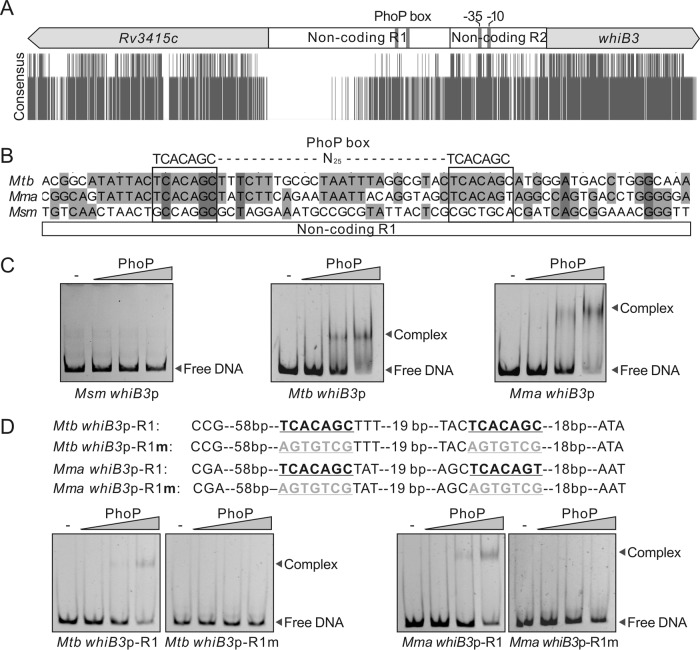
To investigate the reason for the difference in activation of whiB3 expression in M. tuberculosis, M. marinum, and M. smegmatis, we aligned the sequences of the DNA adjacent to the whiB3 genes in these three species. As shown in Fig. 2A, the locations of whiB3 and its upstream gene (Rv3415c) were highly conserved. The DNA sequences of the coding regions of these two genes were also conserved (Fig. 2A). Interestingly, the noncoding regions between the whiB3 and Rv3415c genes can be divided into two regions: the nonconserved region 1 and the relatively conserved region 2 (Fig. 2A). The promoter sequence identified in M. tuberculosis (21) is located in the relatively conserved noncoding region 2 (Fig. 2A; see also Fig. S1 in the supplemental material), suggesting that transcription initiation might be conserved in these species. The sequences of the noncoding region 1 are conserved in M. tuberculosis and M. marinum but not in M. smegmatis (Fig. S1), which is consistent with our observations that low pH only activates the expression of whiB3 in M. tuberculosis and M. marinum, suggesting that diversity in the upstream noncoding region 1 might contribute to the differences in transcriptional regulation. This region will be the focus of subsequent studies.
FIG 2.
PhoP binds to a promoter upstream of the whiB3 gene. (A) Sequence conservation of the DNA fragments adjacent to the whiB3 gene in M. tuberculosis, M. marinum, and M. smegmatis. (B) Putative PhoP box in the noncoding region of the whiB3 gene. (C) Interaction between M. tuberculosis PhoP and whiB3p from M. smegmatis, M. tuberculosis, and M. marinum tested by a gel retardation assay. Acetyl phosphate (Acp) was added at a final concentration of 100 mM in all tests. PhoP at concentrations of 0.05, 0.25, and 0.5 μM was used. (D) Effect of mutation of the putative PhoP box in whiB3p on PhoP binding. R1 represents a fragment in the noncoding region 1 upstream of whiB3p. Mutated sequences of the PhoP box are shown in gray in the upper panels.
A previous study systematically investigated the DNA binding of several transcriptional regulators, including the acid-responsive regulator PhoP, by chromatin immunoprecipitation sequencing (ChIP-seq) and identified that PhoP binds to a DNA fragment located in the noncoding R1 region upstream of the whiB3 gene in M. tuberculosis (Fig. S1) (22). In this region, we did not identify a sequence that matches the previously proposed PhoP consensus binding sequence (PhoP box) TCACAGC-N4-TCACAGC (14, 15); instead, we identified the sequence TCACAGC-N25-TCACAGC, which differs from the PhoP consensus only in the spacer length between the two direct repeats. Interestingly, the TCACAGC-N25-TCACAGC sequence is present in the noncoding R1 regions in both M. tuberculosis and M. marinum but not M. smegmatis (Fig. 2B), which led us to hypothesize that PhoP may bind to this sequence to regulate the expression of whiB3 in M. tuberculosis and M. marinum.
To test whether PhoP binds directly to the TCACAGC-N25-TCACAGC sequence in the noncoding R1 region, we performed a gel shift assay to compare the interactions of M. tuberculosis PhoP with the upstream fragment of the whiB3 gene (whiB3p) from M. smegmatis, M. tuberculosis, and M. marinum. As shown in Fig. 2C, a retardation of the whiB3p fragments from M. tuberculosis and M. marinum by phosphorylated PhoP was observed in a protein-concentration-dependent manner; however, a retardation of the whiB3p fragment from M. smegmatis was not detected.
To further confirm the direct interaction between PhoP and whiB3p, we truncated M. tuberculosis whiB3p and M. marinum whiB3p to obtain two fragments named M. tuberculosis whiB3p-R1 and M. marinum whiB3p-R1. We then performed gel retardation assays using these fragments. The results showed that phosphorylated PhoP also interacts with the truncated promoters (Fig. 2D). Furthermore, these interactions were completely inhibited when the putative PhoP box was mutated to the complementary sequences (Fig. 2D), demonstrating the specificity of the binding of PhoP with M. tuberculosis whiB3p or M. marinum whiB3p.
PhoPR activates whiB3 in M. marinum under acidic stress.
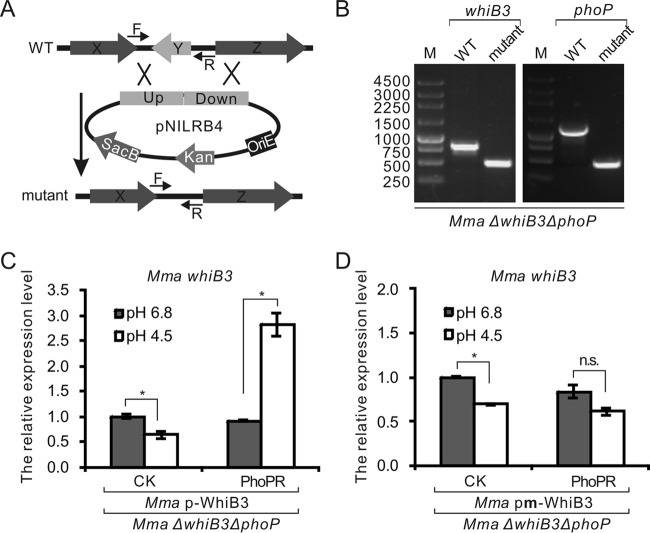
Since PhoPR has been shown to contribute to acidic stress response (17), we propose that PhoPR may be responsible for the activation of whiB3 expression under acidic stress. After verifying the binding of PhoP with M. tuberculosis whiB3p and M. marinum whiB3p in vitro, we aimed to test whether PhoP regulates the transcription of whiB3 in mycobacteria. For this purpose, we deleted the whiB3 gene from M. marinum using homologous recombination (Fig. 3A) to generate the M. marinum ΔwhiB3 strain, and then we deleted phoP from M. marinum ΔwhiB3 to obtain the M. marinum ΔwhiB3 ΔphoP strain (Fig. 3B). We next constructed a clone named pMV261-p-WhiB3, which carries the whiB3 gene from M. marinum and a 628-bp upstream fragment containing the putative PhoP box and the promoter sequence, to complement the deletion of the whiB3 gene from M. marinum. A mutation in the putative PhoP box in the noncoding region was also introduced in the pMV261-p-WhiB3 plasmid to obtain pMV261-pm-WhiB3, which was used to test whether the proposed PhoP-binding site is important for whiB3 expression. Because phoR and phoP are in the same operon in mycobacteria, and because the deletion of the phoP gene may influence the expression of phoR, we constructed a plasmid named pMV306-PhoPR, which expresses both M. marinum PhoP and PhoR from the native promoter, to complement the deletion of the phoP gene from M. marinum.
FIG 3.
PhoPR is required for the activation of whiB3 under acidic conditions in M. marinum. (A) Schematic diagram of mutant construction in mycobacteria. (B) PCR confirmation of the deletion of the whiB3 and phoP genes from M. marinum. (C and D) Expression levels of M. marinum whiB3 in M. marinum ΔwhiB3 ΔphoP strains complemented with whiB3 transcribed from the native promoter (C) or from the promoter with a mutation of the PhoP box (Mma pm) (D). Strains with or without complementation of M. marinum PhoPR were tested. PhoPR represents strains transformed with the plasmid pMV306-PhoPR and CK represents strains transformed with the plasmid pMV306. Mean values with standard deviations from two independent tests in duplicates are shown. *, P < 0.05; n.s., not significant.
The M. marinum ΔwhiB3 ΔphoP strain was cotransformed with the pMV261-p-WhiB3 or pMV261-pm-WhiB3 plasmid and pMV306-PhoPR or the control plasmid pMV306. Reverse transcription-quantitative PCR (qRT-PCR) was used to test the expression levels of the whiB3 and phoP genes. The phoP gene was successfully expressed from pMV306-PhoPR, because the mRNA level of this gene was distinctly higher in strains harboring the pMV306-PhoPR plasmid than in strains harboring the control plasmid pMV306 (see Fig. S2A and B). After being subjected to acidic stress, we observed that the expression of whiB3 was activated when PhoPR was present and native whiB3p was introduced upstream of the whiB3 gene (Fig. 3C). In the strain that lacked PhoPR complementation, the activation of whiB3 expression under acidic stress was not observed (Fig. 3C), indicating that PhoPR is required for this process. A mutation of the putative PhoP-binding site in whiB3p also diminished the activation of whiB3 expression by PhoPR (Fig. 3D), which suggests that the stimulation of whiB3 expression under acidic stress requires the binding of PhoP to whiB3p.
The activation of whiB3 by PhoPR was further confirmed by testing the expression levels of the cpsY gene, the expression of which has been reported to be activated by WhiB3 in M. tuberculosis (7). To test whether WhiB3 plays a role in maintaining the mRNA level of cpsY in M. marinum, the expression level of cpsY was examined, and a significant decrease in cpsY expression was observed in the whiB3 deletion strain, which was restored by complementation with pMV261-p-WhiB3 under both neutral and acidic pH conditions (see Fig. S3A); this result confirmed that WhiB3 regulates the expression of cpsY in M. marinum.
We next examined the mRNA levels of cpsY in the M. marinum ΔwhiB3 ΔphoP strain complemented with both WhiB3 and PhoPR. The cpsY gene was expressed at similar levels in the strain complemented with PhoPR and WhiB3 under both neutral and acidic pH conditions; however, after the treatment with a low-pH medium, the expression levels of cpsY decreased dramatically in the strain lacking phoP and in the strain containing the mutated PhoP box in the whiB3p (Fig. S3B). We noticed that the expression level of cpsY was downregulated at acidic pH in the M. marinum wild-type strain (Fig. S3A), suggesting that, in addition to PhoPR and WhiB3, some other factors may also regulate cpsY expression. Anyhow, these data indicate that PhoP regulates the expression of whiB3, which in turn controls the expression of cpsY under acidic conditions.
Phosphorylation pathway of PhoPR is important for regulating whiB3 expression.
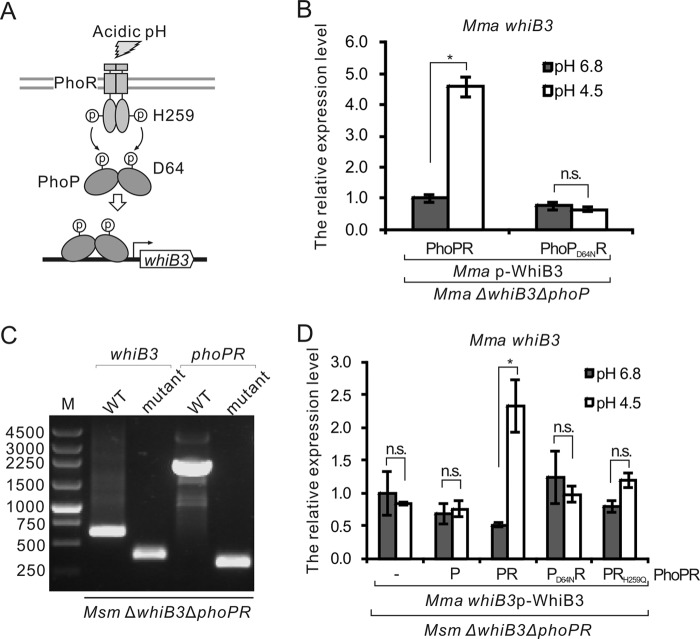
Phosphorylation cascades are essential for the sensing and transduction of external signals by two-component systems (5). In M. tuberculosis, PhoR can be autophosphorylated at the H259 site in vitro, and the phosphoryl group is rapidly transferred to the D71 site of PhoP (10, 11). The sequences of PhoP and PhoR are highly conserved in M. marinum, M. tuberculosis, and M. smegmatis (see Fig. S4). In M. marinum, PhoPR contains the phosphorylation sites D64 and H259, which correspond to D71 and H259, respectively, of M. tuberculosis PhoPR (Fig. S4).
To test whether the phosphorylation pathway is essential for the regulation of whiB3 expression (Fig. 4A), we constructed a plasmid named pMV306-PhoPD64NR, which expresses M. marinum PhoP with a D64N point mutation, to complement the deletion of the phoP gene from M. marinum. The mRNA levels of phoP in these complementary strains were similar (Fig. S2C); however, in contrast to the wild-type PhoP, PhoPD64N was unable to activate the expression of whiB3 under acidic conditions (Fig. 4B), indicating that the activation of whiB3 requires the phosphorylation of PhoP.
FIG 4.
Phosphorylation of PhoPR is important for the regulation of whiB3 expression. (A) Schematic diagram of the proposed model for the roles of the PhoPR phosphorylation pathway in the regulation of whiB3. (B) Expression levels of M. marinum whiB3 in M. marinum ΔwhiB3 ΔphoP strains complemented with wild-type M. marinum PhoPR or the phosphorylation-defective PhoPD64NR. Complementation with whiB3 transcribed from the native promoter was also performed. (C) PCR verification of the M. smegmatis ΔwhiB3 ΔphoPR strain. (D) Expression levels of M. marinum whiB3 in M. smegmatis ΔwhiB3 ΔphoPR strains complemented with M. marinum whiB3 transcribed from the native promoter. Complementation with wild-type M. marinum PhoPR or the phosphorylation-defective PhoPD64NR and PhoPRH259Q were tested. Mean values with standard deviations from two independent tests in duplicates are shown. *, P < 0.05; n.s., not significant.
To investigate the role of PhoR in the regulation of whiB3 expression under acidic stress, we tried to delete the phoR gene from M. marinum but without success. Instead, we successfully deleted both the whiB3 and phoPR genes from M. smegmatis to generate a strain named M. smegmatis ΔwhiB3 ΔphoPR (Fig. 4C). The plasmids pMV261-p-WhiB3 and pMV306-PhoPR or their derivatives, expressing proteins from M. marinum, were transformed into M. smegmatis ΔwhiB3 ΔphoPR. The expression of phoP and phoR in these complemented strains was confirmed via qRT-PCR (see Fig. S5). We next examined the mRNA levels of M. marinum whiB3 in these strains under acidic stress. As shown in Fig. 4D, the relative mRNA levels of whiB3 in the complemented strain containing wild-type M. marinum PhoPR were enhanced under acidic conditions but not in strains complemented with PhoP, PhoPD64NR, or PhoPRH259Q. These data demonstrate that the phosphorylation of PhoPR is essential for the activation of whiB3 expression under acidic conditions.
PhoP box upstream of the whiB3 gene exists only in pathogenic mycobacteria.
To analyze whether all mycobacteria contain the PhoPR-associated regulatory pathway for whiB3, we next aligned the DNA sequences adjacent to the whiB3 genes from different mycobacterial species. Consistent with our analysis for M. tuberculosis, M. marinum, and M. smegmatis, homologs of Rv3415c and whiB3 are conserved in mycobacterial species. In the noncoding regions between these two genes, the putative −35 and −10 elements for transcription initiation were identified in all of the species analyzed (Fig. 5). However, the putative PhoP box is only identified in some pathogenic mycobacteria, including M. tuberculosis complex, M. kansasii, and M. marinum (Fig. 5). These data further suggest that the specific regulation of whiB3 by PhoPR might have evolved for facilitating infection by pathogenic mycobacteria.
FIG 5.
Sequence analysis of noncoding regions upstream of the whiB3 genes in mycobacteria. Conserved sequences of the PhoP box and the −35 and −10 elements of the promoter are indicated with a gray background. Nucleotides not conserved in the PhoP box are shown in a hollow font.
DISCUSSION
Survival at low pH in macrophages is important for successful infection by M. tuberculosis (1). Transcriptional profiles of M. tuberculosis at neutral and low pH have shown that the expression of many PhoPR regulons was upregulated under acidic pH conditions, suggesting that transcriptional regulation controlled by PhoPR is important for adaption at low pH (17). Recent studies have shown that the transcriptional regulator WhiB3 is also important for acidic stress adaption, because the expression of this regulator is induced at low pH (6) and the deletion of this regulator lowered cell survival under acidic conditions (7). The relationship between these two regulators remains unclear. In this study, we introduced WhiB3 into the PhoPR regulons and confirmed that PhoPR directly regulates the transcription of whiB3 in response to low pH in pathogenic mycobacterial species. Consistent with our results, a previous transcriptome study also showed that the expression of the M. tuberculosis whiB3 gene at acidic pH is significantly reduced in a phoP mutant (23). Interestingly, the direct transcriptional regulation of whiB3 by PhoPR is restricted to some species of human-pathogenic mycobacteria.
As an important TCS, PhoPR is thought to be present and conserved in almost all mycobacterial species (5), which suggests that PhoPR plays important roles in mycobacteria. Evolutionarily conserved polymorphisms in PhoPR result in the loss of functional phenotypes (24). For example, a point mutation in PhoP is reported to be a major reason for the avirulent phenotype of the M. tuberculosis H37Ra strain compared with that of the closely related H37Rv strain (25). In addition, mutation sequences targeted by PhoPR are also widely present in mycobacterial species, for example, the regulation of whiB6 by PhoPR in M. tuberculosis (26). The PhoP box in the promoter of whiB6, encoding a regulator of the ESX-1 virulence secretion system (26, 27), was mutated in the M. tuberculosis H37Rv strain but was intact in CDC1551 and other clinical M. tuberculosis isolates. Consequently, the PhoPR exhibited different regulatory effects on whiB6 expression in M. tuberculosis H37Rv and in clinical strains (26). In this study, we found that the putative PhoP box in whiB3p is present in M. tuberculosis and M. marinum but not in M. smegmatis. An alignment of whiB3p sequences further demonstrated that the putative PhoP box is only identified in some pathogenic mycobacteria. Since pathogenic mycobacteria generally emerge after nonpathogenic species (28) and are able to interact and coevolve with hosts (29, 30), these analyses suggest that the putative PhoP box in whiB3p might be acquired during evolution, and the regulation of whiB3 by PhoPR under acidic stress may favor the survival of the bacteria during infection. In contrast, the putative PhoP box is not needed by nonpathogenic mycobacteria for survival under natural conditions.
The sequence of the putative PhoP box (TCACAGC-N25-TCACAGC) identified in whiB3p is different from the previously reported TCACAGC-N4-TCACAGC sequence (14, 15) only in the distance between the two direct repeats. Despite extensive studies of the regulons of M. tuberculosis PhoPR (31–33), the consensus PhoP-binding sequence and the mechanism by which PhoP recognizes different sequences have not been fully understood. The PhoP box TCACAGC-N4-TCACAGC was identified by a systematic evolution of ligands from a random pool of oligonucleotides containing N25 sequences (14). The newly identified PhoP box with an N25 spacer was not selected in that assay. Although ChIP-Seq of PhoP also identified the TCACAGC-N4-TCACAGC consensus sequence, several ChIP-seq signals showed no association with this box (15), suggesting a high divergence of PhoP-binding sequences. Consistent with this hypothesis, the promoter regions of several PhoP regulons have been characterized with other PhoP-binding sequences in a separate study (34). Although the spacer length of our proposed PhoP box is different from that of the previously identified PhoP box, both spacer sequences are rich in AT content (14), which suggests that, while the spacer length may be variable, AT richness might be important. In a recent study, the transcription factor RccR in Pseudomonas fluorescens was also shown to recognize two distinct sequences with identical inverted repeats separated by 3 or 14 bp (35). Furthermore, the affinity of RccR for these two binding sites is switched by its cognate ligand 2-keto-3-deoxy-6-phosphogluconate (35). Although mechanisms of PhoPR-mediated regulation of this newly proposed PhoP box warrant further study, the information provided in this study may be useful for further characterization of the mechanisms by which PhoPR regulates its targets.
Our study showed that M. marinum PhoPR can significantly enhance the expression of whiB3 under acidic conditions but not at a neutral pH (Fig. 3). PhoR has been reported to sense changes in environmental pH to first autophosphorylate and then transfer the phosphate to the regulator PhoP (10, 11). Mutations of residues important for phosphorylation in PhoR or PhoP diminished the activation of whiB3 by PhoPR under acidic conditions, suggesting that the phosphorylation of PhoP and PhoR are essential for the regulation of whiB3. The absence of the regulation of whiB3 at a neutral pH suggests that PhoPR phosphorylation may not be activated under the conditions used in this study. In a previous study, a transcriptomic comparison of wild-type M. tuberculosis and its phoP-deleted derivative showed that the whiB3 expression level was lower in the phoP-deleted strain than in the wild-type strain under normal growth conditions (15); however, these data were not further confirmed by other tests.
In addition to low pH, several other signals may also trigger the phosphorylation of PhoPR (24). These signals may be different in our study compared to those in other studies. In addition to phosphorylation, other posttranslational modifications of PhoP may also change the binding of PhoP to promoters. In Salmonella, the acetylation of K201 in PhoP inhibits the ability of PhoP to bind to DNA targets (36). In low-pH environments, PhoP can deacetylate the K201 site and regulate its targets by binding directly to the promoters (36). Protein acylation has been widely identified in M. tuberculosis (37). Further studies are required to characterize the roles of posttranslational modifications of PhoPR in mycobacteria.
Although the expression of the whiB3 gene is induced under low-pH conditions both in M. tuberculosis (6) and M. marinum (Fig. 1), target genes regulated by WhiB3 in response to acid stresses need to be identified and characterized. Recently, the first structure of the WhiB family regulator WhiB1 was elucidated (38), which will provide insightful information to characterize the regulatory mechanisms of mycobacterial WhiB3 in response to low-pH stresses.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The plasmids, bacterial strains, and oligonucleotides used in this study are summarized in Tables S1, S2, and S3 in the supplemental material, respectively. Escherichia coli strains were cultured in Luria-Bertani (LB) broth at 37°C. Mycobacterium marinum ATCC BAA-535 and its derivatives were routinely grown at 32°C in 7H9 (Difco) broth supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% oleic acid-albumin-dextrose-catalase ([OADC] Difco) or on 7H10 (Difco) agar plates supplemented with 0.2% glycerol and 10% OADC. Mycobacterium smegmatis mc2155 and its derivative strains were grown in 7H9 liquid medium supplemented with 0.2% glucose, 15 mM NaCl, 0.2% glycerol, and 0.05% Tween 80 or on 7H10 agar plates supplemented with 0.5% glycerol at 37°C. Antibiotics were used at the following concentrations: kanamycin, 20 μg/ml for mycobacteria and 50 μg/ml for E. coli; hygromycin B, 50 μg/ml for mycobacteria and 150 μg/ml for E. coli. Unacidified 7H9 broth (pH 6.8) was used as the neutral medium. For acidic treatment, mycobacterial strains were grown until the optical density at 600 nm (OD600) reached 0.3 to 0.4 and then were treated for 1 h with acidic 7H9 broth, which was acidified with hydrochloric acid to pH 4.5 as previously described (6).
Expression and purification of M. tuberculosis PhoP.
For pET28a-PhoP construction, the phoP gene with the stop codon was amplified from M. tuberculosis H37Rv genomic DNA and cloned into the pET28a plasmid between the NdeI and HindIII (TaKaRa) sites. For protein expression, the pET28a-PhoP plasmid was transformed into E. coli BL21(DE3). The expression of PhoP was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when the OD600 of cells was ∼0.5, and the cells were incubated for another 12 h at 20°C. The cells were then centrifuged and resuspended in buffer I (20 mM Tris-HCl [pH 7.9], 300 mM NaCl, 5% glycerol) with 0.2 mg/ml lysozyme. Cell lysis was carried out by ultrasonication, and the lysate was cleared by centrifugation (10,000 × g for 30 min at 4°C) and subsequently loaded onto 1 ml of Ni-nitrilotriacetic acid (Ni-NTA) resin equilibrated with buffer I. After washing with 15 ml of buffer I containing 50 mM imidazole, the proteins were eluted using buffer I containing 250 mM imidazole. Finally, the purified proteins were dialyzed in buffer I and quantified using a bicinchoninic acid (BCA) protein assay kit (Sangon Biotech, China).
Gel retardation assay.
Gel retardation assays were performed as previously described (39) with minor modifications. Briefly, DNA fragments were amplified from M. tuberculosis, M. marinum, or M. smegmatis genomic DNA. Approximately 100 ng of DNA was incubated with gradient concentrations of PhoP in 10 μl of binding buffer (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM dithiothreitol, 10% glycerol, 15 μg/ml bovine serum albumin [BSA]) at 25°C for 30 min. Acetyl phosphate (Sigma-Aldrich) at a final concentration of 100 mM was added to the reaction mixtures to phosphorylate PhoP proteins. Then, the samples were loaded onto a 6% native polyacrylamide gel and electrophoresed in 0.5× Tris-borate-EDTA (TBE) buffer.
Mutant construction and complementation.
Mutants were constructed as previously described (27, 40) with slight modifications. Briefly, for the deletion of whiB3 (MSMEG_1597) from M. smegmatis, a 750-bp upstream fragment (named MsmwhiB3-U) and a 1,127-bp downstream fragment (named MsmwhiB3-D) were amplified from M. smegmatis genomic DNA. The MsmwhiB3-U fragment was cloned into pMN252 (41) between the PmeI and SpeI sites to obtain pMN252-MsmwhiB3-U. The MsmwhiB3-D fragment was then inserted into pMN252-MsmwhiB3-D to obtain a plasmid named pMN252-MsmwhiB3-m. Subsequently, a fragment containing MsmwhiB3-U, a hygromycin resistance cassette, and MsmwhiB3-D was digested from pMN252-MsmwhiB3-m using PmeI and SwaI and ligated into the SmaI-digested pNILRB4 (42) to generate pNILRB4-MsmwhiB3-m. The resulting plasmid was used to transform M. smegmatis by electroporation, and a singly crossed strain was selected by spreading the transformed cells on a 7H10 plate containing kanamycin. The mutant was then selected by plating the singly crossed strain on plates containing 2% (wt/vol) sucrose, and the mutation was confirmed by PCR and DNA sequencing. The pML597-sacB plasmid (40) expressing the Flp enzyme was transformed into the mutant to remove the hygromycin resistance cassette located between two FRT sites. Lastly, the pML597-sacB plasmid was removed by spreading the cells on 7H10 plates containing 4% sucrose. Similar procedures were used to delete the phoPR (MSMEG_5712-MSMEG_5713) gene from the M. smegmatis ΔwhiB3 strain to obtain the ΔwhiB3 ΔphoPR strain. The deletion of the whiB3 and phoP genes from M. marinum was also conducted as described above.
For complementation of the whiB3 gene, the ORF of whiB3 with its native promoter (628 bp) from M. marinum was cloned into pMV261 (43) to obtain a plasmid named pMV261-p-WhiB3. The mutation of the putative PhoP box of the whiB3 promoter was conducted by using overlap PCR, and the mutant was inserted into pMV261 to obtain pMV261-pm-WhiB3. To complement the deletion of the phoP and phoPR genes, a fragment containing the phoPR gene and its promoter region (583 bp) was amplified from the M. marinum genome and inserted into the pMV306 plasmid (43) using a ClonExpress II one-step cloning kit (Vazyme, China) to obtain pMV306-PhoPR. A mutation in the phoP or phoR gene in this plasmid was introduced using a QuikChange II XL site-directed mutagenesis kit (Stratagene).
RNA extraction and qRT-PCR analyses.
RNA extraction and qRT-PCR analyses were performed as previously described (27, 40). Briefly, 20 ml of bacterial culture at neutral pH or subjected to acidic stress was centrifuged at 6,000 × g at 4°C for 5 min. After the pellets were ground in liquid nitrogen, RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions. The Universal SYBR green Supermix reagent (Bio-Rad) was used for the qRT-PCR. The results were normalized to 16S rRNA (or sigA where indicated) levels. The mean values and standard deviations of data from two independent experiments are shown. Data comparisons between groups were performed using Student's t tests.
Sequence analysis.
DNA sequences of the whiB3 gene and the upstream sequences from mycobacterial strains were downloaded from the NCBI genome database. Protein sequences of PhoP and PhoR were also obtained from the NCBI database. Sequence conservation was compared using ClusterX and visualized using the Jalview software.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jun Liu for providing the M. marinum ATCC BAA-535 strain and Jiaoyu Deng for providing the M. smegmatis mc2155 strain.
This work was supported by the National Natural Science Foundation of China (81271797 and 31670134) and the 135 Research Project of Wuhan Institute of Virology. Y.H. was supported by the Youth Innovation Promotion Association CAS.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00766-17.
REFERENCES
- 1.Rohde K, Yates RM, Purdy GE, Russell DG. 2007. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev 219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 2.Vandal OH, Nathan CF, Ehrt S. 2009. Acid resistance in Mycobacterium tuberculosis. J Bacteriol 191:4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouzy A, Larrouy-Maumus G, Bottai D, Levillain F, Dumas A, Wallach JB, Caire-Brandli I, de Chastellier C, Wu TD, Poincloux R, Brosch R, Guerquin-Kern JL, Schnappinger D, Sorio de Carvalho LP, Poquet Y, Neyrolles O. 2014. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog 10:e1003928. doi: 10.1371/journal.ppat.1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small JL, O'Donoghue AJ, Boritsch EC, Tsodikov OV, Knudsen GM, Vandal O, Craik CS, Ehrt S. 2013. Substrate specificity of MarP, a periplasmic protease required for resistance to acid and oxidative stress in Mycobacterium tuberculosis. J Biol Chem 288:12489–12499. doi: 10.1074/jbc.M113.456541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretl DJ, Demetriadou C, Zahrt TC. 2011. Adaptation to environmental stimuli within the host: two-component signal transduction systems of Mycobacterium tuberculosis. Microbiol Mol Biol Rev 75:566–582. doi: 10.1128/MMBR.05004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. 2006. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob Agents Chemother 50:2836–2841. doi: 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta M, Rajmani RS, Singh A. 2016. Mycobacterium tuberculosis WhiB3 responds to vacuolar pH-induced changes in mycothiol redox potential to modulate phagosomal maturation and virulence. J Biol Chem 291:2888–2903. doi: 10.1074/jbc.M115.684597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zschiedrich CP, Keidel V, Szurmant H. 2016. Molecular mechanisms of two-component signal transduction. J Mol Biol 428:3752–3775. doi: 10.1016/j.jmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parish T. 2014. Two-component regulatory systems of mycobacteria. Microbiol Spectr 2:MGM2-0010-2013. doi: 10.1128/microbiolspec.MGM2-0010-2013. [DOI] [PubMed] [Google Scholar]
- 10.Goyal R, Das AK, Singh R, Singh PK, Korpole S, Sarkar D. 2011. Phosphorylation of PhoP protein plays direct regulatory role in lipid biosynthesis of Mycobacterium tuberculosis. J Biol Chem 286:45197–45208. doi: 10.1074/jbc.M111.307447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Sinha A, Sarkar D. 2006. Transcriptional autoregulation by Mycobacterium tuberculosis PhoP involves recognition of novel direct repeat sequences in the regulatory region of the promoter. FEBS Lett 580:5328–5338. doi: 10.1016/j.febslet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol 60:312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalo-Asensio J, Mostowy S, Harders-Westerveen J, Huygen K, Hernandez-Pando R, Thole J, Behr M, Gicquel B, Martin C. 2008. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One 3:e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Wang S. 2014. DNA consensus sequence motif for binding response regulator PhoP, a virulence regulator of Mycobacterium tuberculosis. Biochemistry 53:8008–8020. doi: 10.1021/bi501019u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martin C, Cole ST. 2014. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog 10:e1004183. doi: 10.1371/journal.ppat.1004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramovitch RB, Rohde KH, Hsu FF, Russell DG. 2011. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol Microbiol 80:678–694. doi: 10.1111/j.1365-2958.2011.07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker JJ, Johnson BK, Abramovitch RB. 2014. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol Microbiol 94:56–69. doi: 10.1111/mmi.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini V, Cumming BM, Guidry L, Lamprecht DA, Adamson JH, Reddy VP, Chinta KC, Mazorodze JH, Glasgow JN, Richard-Greenblatt M, Gomez-Velasco A, Bach H, Av-Gay Y, Eoh H, Rhee K, Steyn AJ. 2016. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis. Cell Rep 14:572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JR Jr, Steyn AJ. 2007. Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc Natl Acad Sci U S A 104:11562–11567. doi: 10.1073/pnas.0700490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Crossman DK, Mai D, Guidry L, Voskuil MI, Renfrow MB, Steyn AJ. 2009. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog 5:e1000545. doi: 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shell SS, Wang J, Lapierre P, Mir M, Chase MR, Pyle MM, Gawande R, Ahmad R, Sarracino DA, Ioerger TR, Fortune SM, Derbyshire KM, Wade JT, Gray TA. 2015. Leaderless transcripts and small proteins are common features of the mycobacterial translational landscape. PLoS Genet 11:e1005641. doi: 10.1371/journal.pgen.1005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minch KJ, Rustad TR, Peterson EJ, Winkler J, Reiss DJ, Ma S, Hickey M, Brabant W, Morrison B, Turkarslan S, Mawhinney C, Galagan JE, Price ND, Baliga NS, Sherman DR. 2015. The DNA-binding network of Mycobacterium tuberculosis. Nat Commun 6:5829. doi: 10.1038/ncomms6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson BK, Colvin CJ, Needle DB, Mba Medie F, Champion PA, Abramovitch RB. 2015. The carbonic anhydrase inhibitor ethoxzolamide inhibits the Mycobacterium tuberculosis PhoPR regulon and Esx-1 secretion and attenuates virulence. Antimicrob Agents Chemother 59:4436–4445. doi: 10.1128/AAC.00719-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broset E, Martin C, Gonzalo-Asensio J. 2015. Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development. mBio 6:e01289-. doi: 10.1128/mBio.01289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JS, Krause R, Schreiber J, Mollenkopf HJ, Kowall J, Stein R, Jeon BY, Kwak JY, Song MK, Patron JP, Jorg S, Roh K, Cho SN, Kaufmann SH. 2008. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe 3:97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Solans L, Aguilo N, Samper S, Pawlik A, Frigui W, Martin C, Brosch R, Gonzalo-Asensio J. 2014. A specific polymorphism in Mycobacterium tuberculosis H37Rv causes differential ESAT-6 expression and identifies WhiB6 as a novel ESX-1 component. Infect Immun 82:3446–3456. doi: 10.1128/IAI.01824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Hu Y, Cumming BM, Lu P, Feng L, Deng J, Steyn AJ, Chen S. 2016. Mycobacterial WhiB6 differentially regulates ESX-1 and the Dos regulon to modulate granuloma formation and virulence in zebrafish. Cell Rep 16:2512–2524. doi: 10.1016/j.celrep.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 28.Tortoli E, Fedrizzi T, Meehan CJ, Trovato A, Grottola A, Giacobazzi E, Serpini GF, Tagliazucchi S, Fabio A, Bettua C, Bertorelli R, Frascaro F, De Sanctis V, Pecorari M, Jousson O, Segata N, Cirillo DM. 2017. The new phylogeny of the genus Mycobacterium: the old and the news. Infect Genet Evol 56:19–25. doi: 10.1016/j.meegid.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Bañuls AL, Sanou A, Anh NT, Godreuil S. 2015. Mycobacterium tuberculosis: ecology and evolution of a human bacterium. J Med Microbiol 64:1261–1269. doi: 10.1099/jmm.0.000171. [DOI] [PubMed] [Google Scholar]
- 30.Perrin P. 2015. Human and tuberculosis coevolution: an integrative view. Tuberculosis (Edinb) 95 Suppl 1:S112–S116. doi: 10.1016/j.tube.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Sinha A, Gupta S, Bhutani S, Pathak A, Sarkar D. 2008. PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. J Bacteriol 190:1317–1328. doi: 10.1128/JB.01074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Pathak A, Sinha A, Sarkar D. 2009. Mycobacterium tuberculosis PhoP recognizes two adjacent direct-repeat sequences to form head-to-head dimers. J Bacteriol 191:7466–7476. doi: 10.1128/JB.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalo-Asensio J, Soto CY, Arbues A, Sancho J, del Carmen Menendez M, Garcia MJ, Gicquel B, Martin C. 2008. The Mycobacterium tuberculosis phoPR operon is positively autoregulated in the virulent strain H37Rv. J Bacteriol 190:7068–7078. doi: 10.1128/JB.00712-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimino M, Thomas C, Namouchi A, Dubrac S, Gicquel B, Gopaul DN. 2012. Identification of DNA binding motifs of the Mycobacterium tuberculosis PhoP/PhoR two-component signal transduction system. PLoS One 7:e42876. doi: 10.1371/journal.pone.0042876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campilongo R, Fung RKY, Little RH, Grenga L, Trampari E, Pepe S, Chandra G, Stevenson CEM, Roncarati D, Malone JG. 2017. One ligand, two regulators and three binding sites: how KDPG controls primary carbon metabolism in Pseudomonas. PLoS Genet 13:e1006839. doi: 10.1371/journal.pgen.1006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren J, Sang Y, Tan Y, Tao J, Ni J, Liu S, Fan X, Zhao W, Lu J, Wu W, Yao YF. 2016. Acetylation of lysine 201 inhibits the DNA-binding ability of PhoP to regulate Salmonella virulence. PLoS Pathog 12:e1005458. doi: 10.1371/journal.ppat.1005458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Yang M, Wang X, Yang S, Gu J, Zhou J, Zhang XE, Deng J, Ge F. 2014. Acetylome analysis reveals diverse functions of lysine acetylation in Mycobacterium tuberculosis. Mol Cell Proteomics 13:3352–3366. doi: 10.1074/mcp.M114.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudhair BK, Hounslow AM, Rolfe MD, Crack JC, Hunt DM, Buxton RS, Smith LJ, Le Brun NE, Williamson MP, Green J. 2017. Structure of a Wbl protein and implications for NO sensing by M. tuberculosis. Nat Commun 8:2280. doi: 10.1038/s41467-017-02418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Lu P, Wang Y, Ding L, Atkinson S, Chen S. 2009. OmpR positively regulates urease expression to enhance acid survival of Yersinia pseudotuberculosis. Microbiology 155:2522–2531. doi: 10.1099/mic.0.028381-0. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Wang Z, Feng L, Chen Z, Mao C, Zhu Y, Chen S. 2016. Sigma(E)-dependent activation of RbpA controls transcription of the furA-katG operon in response to oxidative stress in mycobacteria. Mol Microbiol 102:107–120. doi: 10.1111/mmi.13449. [DOI] [PubMed] [Google Scholar]
- 41.Stephan J, Stemmer V, Niederweis M. 2004. Consecutive gene deletions in Mycobacterium smegmatis using the yeast FLP recombinase. Gene 343:181–190. doi: 10.1016/j.gene.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Balhana R, Stoker NG, Sikder MH, Chauviac FX, Kendall SL. 2010. Rapid construction of mycobacterial mutagenesis vectors using ligation-independent cloning. J Microbiol Methods 83:34–41. doi: 10.1016/j.mimet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs WR, Bloom BR. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.