Abstract
We analyzed the infestation of the attached copepod species Lepeophtheirus sp on a wild population of Sciades herzbergii. The infestation and occurrence of attached copepods were related to body size, maturity and sex of host and the presence of lesions on fish skin were described. In 61 fish specimens (37 males and 24 females), total of 218 ectoparasitic copepods, including 204 mature and 14 immature stages were found. Copepods were attached to different regions of fish body without any regular pattern. The prevalence of infestation was 80.3% and intensity between 1 and 15 copepods/fish. No significant differences were found between sex or maturity and the presence of attached Lepeophtheirus sp. However, a contingency table including both sex and maturity status, and the total number of attached copepod per combined category showed a significant association. A positive correlation was found between body length of fish and intensity of infestation. Similarly, when comparing the infested fish according to group size, we found more copepods on larger individual. Attached copepods were associated with the presence of lesions visible to the naked eye. Histological analyses showed changes in cell architecture when sections of copepod-free tissues and attached copepods were compared.
Keywords: Damages, Fish, Lepeophtheirus, lesions, Sea lice, Parasitism
Graphical abstract
Highlights
-
•
The infestation of Lepeophtheirus sp is associated with skin lesions and cell damages that may increases the presence of diseases in fish.
-
•
Because the infestation of Lepeophtheirus sp is higher in the largest specimens, the structure and dynamics of the fish population may be affected.
-
•
Due to its demersal habits, S. herzbergii should contribute to the spread of infestation to their population or other animal.
1. Introduction
Parasitic copepods infect virtually all aquatic animal groups and show a staggering diversity in body form and life cycle strategies (Boxshall and Halsey, 2004). Particularly within the order Siphonostomatoida, that contains 40 families of exclusively parasitic forms, Caligidae is the richest in number of species (Boxshall, 2014). Caligid copepods are commonly known as Sea Lice and comprise 31 genera, of which, Caligus Müller, 1785 and Lepeophtheirus Von Nordmann, 1832 are the most diverse (Dojiri and Ho, 2013, Boxshall, 2014).
Sea lice are dominant ectoparasitic of farmed and wild fish populations around the world (Costello, 2006). They feed on the epithelial tissue of their hosts and may promote diseases in fish populations (Johnson et al., 2004, Venmathi et al., 2016). Sea lice also are considered as a economically important parasitic species around the world (Johnson et al., 2004, Rosenberg, 2008). Particularly, infestations of the species of Lepeophtheirus has been associated with decreased of body growth, changes in swimming behaviour, and increased of fish host morbidity by bacterial diseases in skin lesions and damages produced by attached copepods (Bui et al., 2016, Godwins et al., 2017, Llewellyn et al., 2017).
Historically, most of the studies on infestation from Caligid copepods are related to farmed fish populations in temperate zones, due to the economic costs they represent and their higher incidence at high population densities such as those occurring in aquaculture (Shepard and Gargano 2017). In contrast, fewer researches have examined copepod infestations on wild fish populations or assemblages (Todd et al., 2006, Jensen et al., 2016, Godwins et al., 2017). Similarly, in the Neotropical Regions, most of the detailed studies related to the ecological interaction between Caligid copepods and fish host refer essentially to the salmonids of the Chilean Pacific Ocean (Johnson et al., 2004, Costello, 2006). Although, the occurrence of Lepeophtheirus species have been widely recorded on different fish species in these regions (Luque et al., 2016, Morales-Serna et al., 2012, Morales-Serna et al., 2016) and particularly, they have been reported as the predominant metazoan parasites on host catfish (Luque and Tavares, 2007), the characteristics and effects of infestation are poorly known.
In this study, the infestation of an attached copepod (Lepeophtheirus sp) on a wild population of Sciades herzbergii Bloch, 1794, a Neotropical catfish species in a coastal lagoon in Northeastern Venezuela was analyzed. This fish species is endemic of South America and is located in Caribbean regions of Colombia and Venezuela and Atlantic coasts of Brazil (Cervigón, 1991, Marceniuk, 2015). S. herzbergii was selected because it is a common and one of the dominant species in the study area, as well as in estuarine waters in other locations (Cervigón, 1991, Giarrizzo and Krumme, 2007, Froese and Pauly, 2017). Also, it is part of the fishery resources in some areas like northeastern of Venezuela (Betancur et al., 2004) and Brazil (Barletta and Costa, 2009). The present study was carried out in order to describe infestation of parasitic copepod and analyze its relationships with total body length, sex, maturity and lesions on skin of the host fish.
2. Material and methods
2.1. Fish sampling and examinations
Specimens of S. herzbergii for the study were collected in July and December of 2008 in “Las Marites” lagoon, located at Southeastern coast of Margarita Island in Northeastern of Venezuela (10º53′50″- 10º55′N 63º53′54″- 63º57′20″ W). More information at this lagoon is given in Palazón et al. (1996).
Fish were transported alive to laboratory, where they were anesthetized with Benzocaine. In each fish, body surface (integument) was revised in detail for presence of attached ectoparasitic copepods.
Copepods were removed from fish by performing skin scrapings using tweezers. All the samples (scrapings) were separately placed in Petri dishes with filtered seawater and observed under a stereoscopic microscopy.
The attached copepods were divided by sex and stage of development in phase I (sexually mature) and phase II (immature). For taxonomic identification, we used the keys proposed by Boxshall and Montú (1997) and Cressey (1991).
Every fish was measured to total length with a vernier of 1 mm of precision and individuals were identified using the taxonomic keys proposed by Cervigón (1991). Sex was determined by direct observation of the primary and secondary characters of the gonads in dissected specimens (Eslava, 2004). For both sexes, the stage of maturity was discriminated in two phases (immature and mature). We grouped the individuals into two length categories to determine the intensity of infestation of ectoparasitic copepod according to size, because it is expected to higher values in larger individuals, related mainly with more surface area. We chose two sizes to have an approximate number of individuals in each one (37 small fish and 24 big fish).
2.2. Histology of the tegument of fish host
Immediately after the death of the fish, skin samples were dissected from sites with and without attached copepods. Some skin samples were fixed for 24–48 h in 10% formalin, buffered with 0.1 M phosphate buffer at pH 7.2 and others in Bouin's liquid for 24 h. The formalin-fixed samples were then washed in running water for four (4) hours to remove excess fixative, while the liquid-fixed samples were washed repeatedly with 70% ethanol. Subsequently, all the skin samples were processed until their inclusion in paraffin, in order to obtain cuts of 8 μm thick, which were stained with hematoxylin–eosin. Samples were examined in the optical microscope to compare the architecture of the skin in infested and non-infested areas and to detect and describe lesions due to the infestation.
2.3. Data analysis
Prevalence, intensity and mean intensity of infestation were calculated according to Jensen et al. (2016).
Relationships of host length and the infestation intensity of Lepeophtheirus sp. were determined using the Pearson correlation coefficient (r). While the Wilcoxon rank X2 test was used to determine the possible association between sex, sexual maturity or size length of the host with the infestation. In addition, a contingency table including both sexual aspects were created, and a X2 test with Yates’ correction for continuity to prevent overestimation of statistical significance for small dataset was implemented. In all analyses a significance level of α = 0.05 was used. Statistics were computed using the R statistical language (R Foundation for Statistical Computing).
3. Results
3.1. Infestation of copepods on host fish
All sea louses found and observed microscopically were identified as Lepeophtheirus sp based on morphological features. These copepods were attached on skin at different regions of the body of juveniles and adults of host fish, showing not a regular pattern of preference. A total of 204 copepods were sexually mature and 14 were immatures.
Prevalence of infestation was 80.3% and intensity between 1 and 15 copepods/fish with a mean value of 3.5 copepods/fish.
3.2. Host fish conditions
A total of 61 specimens S. herzbergii were analyzed with total body length between 200.00 and 389.00 mm (mean ± SD = 292.86 ± 42.06 mm). Of the total number of individuals, 37 were females with body length between 222.50 and 389.00 mm (mean ± SD = 259.41 ± 110.44 mm) and 24 males with body length between 200.00 and 372.50 mm of total length (mean ± SD = 280.60 ± 34.56 mm). Examination of the external characters of juvenile and immature adults showed no visible differences between males and females.
The analysis of the relationship between total body length of host and intensity of the infestation, showed a weak positive correlation (r = 0.328; p = 0.009).
In S. herzbergii, 52.46% of total females were infested in contrast to 27.87% of infested males. However, the Wilcoxon rank test allowed to establish that the presence of the attached Lepeophtheirus sp is independent of the sex of S. herzbergii (W = 458.5; p = 0.834) or the maturity status (W = 512.5; p = 0.464) of S. herzbergii, respectively.
On the other hand, the analysis of the contingency table including the maturity status and sex, and the presence of the attached copepod showed a significant association between both characteristics (X2 = 12.61; p = 0.004), particularly low values on immature males in contrast with the other interactions. Similarly, when comparing the infested fish according to group size, we found more copepods on larger individuals (W = 262; p = 0.0067). (Fig. 1).
Fig. 1.
Prevalence of Lepeophtheirus sp in two size groups of Sciades herzbergii.
3.3. Lesions on skin of fish host
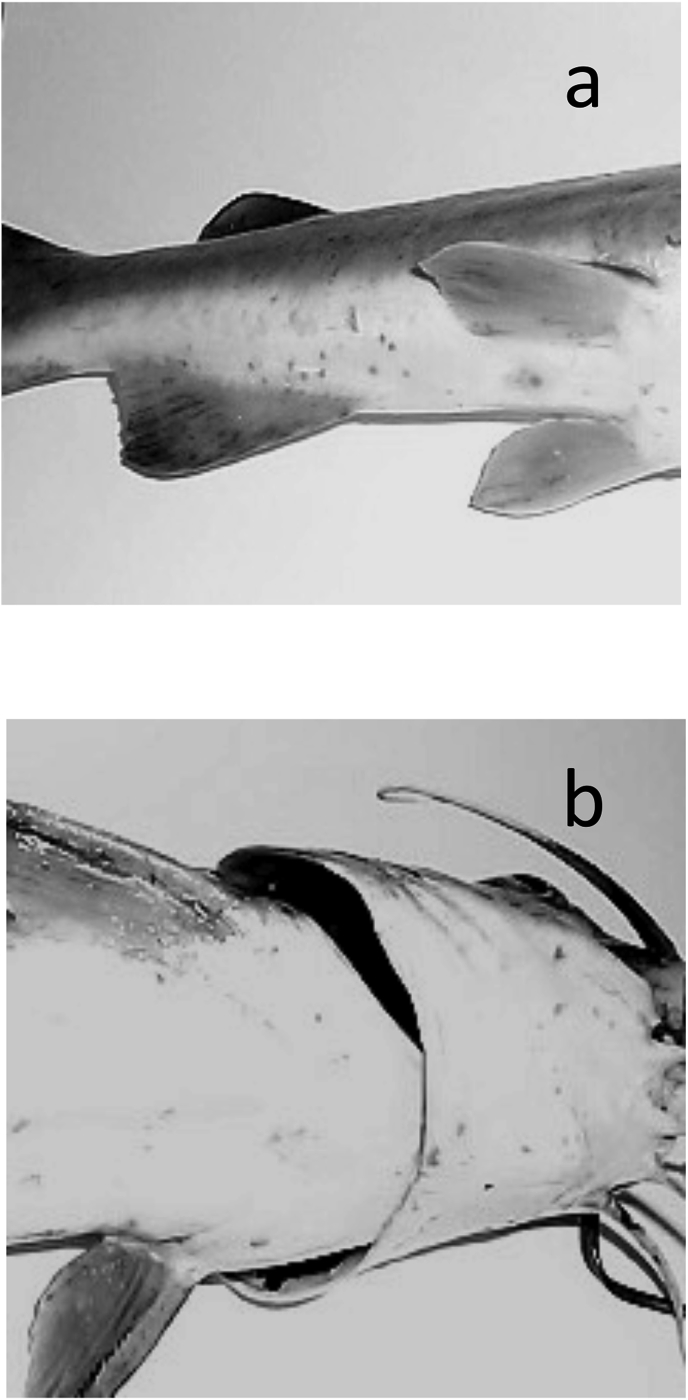
Extensive areas of skin damage in infested S. herzbergii were observed, especially when parasitic intensity was higher (N = 15). The eroded epithelial tissue was detectable with the naked eye, in the form of patches or reddish hemorrhagic areas (Fig. 2a and b).
Fig. 2.
Hemorrhagic cutaneous lesions caused by the infestation of copepods on S. herzbergii. a. Ventral view. b. Pectoral fins and mouth.
3.4. Histology of the tegument of fish host
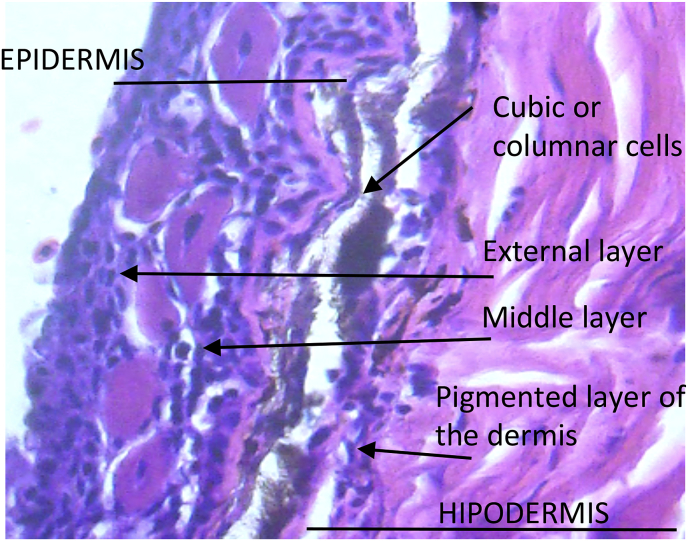
Histological analysis of the tegument of non-infested S. herzbergii shows the same three structural and functional layers of the skin of other marine teleosts: epidermis (specialized epithelium), dermis (vascularized connective tissue layer) and hypodermis or subcutaneous layer. The epidermis and dermis are separated by a basement membrane (Fig. 3).
Fig. 3.
Cross section of healthy skin of S. herzbergii, showing the different layers that make it up (Hematoxylin-eosin staining) (100X).
In detail, epidermis of non-infested fish was composed of a basal layer of cubic or columnar cells whose function is to replace those that are detached on the surface, a multi-layered medium layer of cells of variable and glandular dimensions, and an outer one formed by one or two rows of flattened surface cells parallel to the body surface. Immediately after, the layer of pigmented cells was found the dermis, composed of collagen fibers, whose main elements of the inner portion are fibroblasts (Fig. 4).
Fig. 4.
Cross section of healthy skin of S. herzbergii. Detail of the epidermis and dermis (staining with hematoxylin-eosin) (400X).
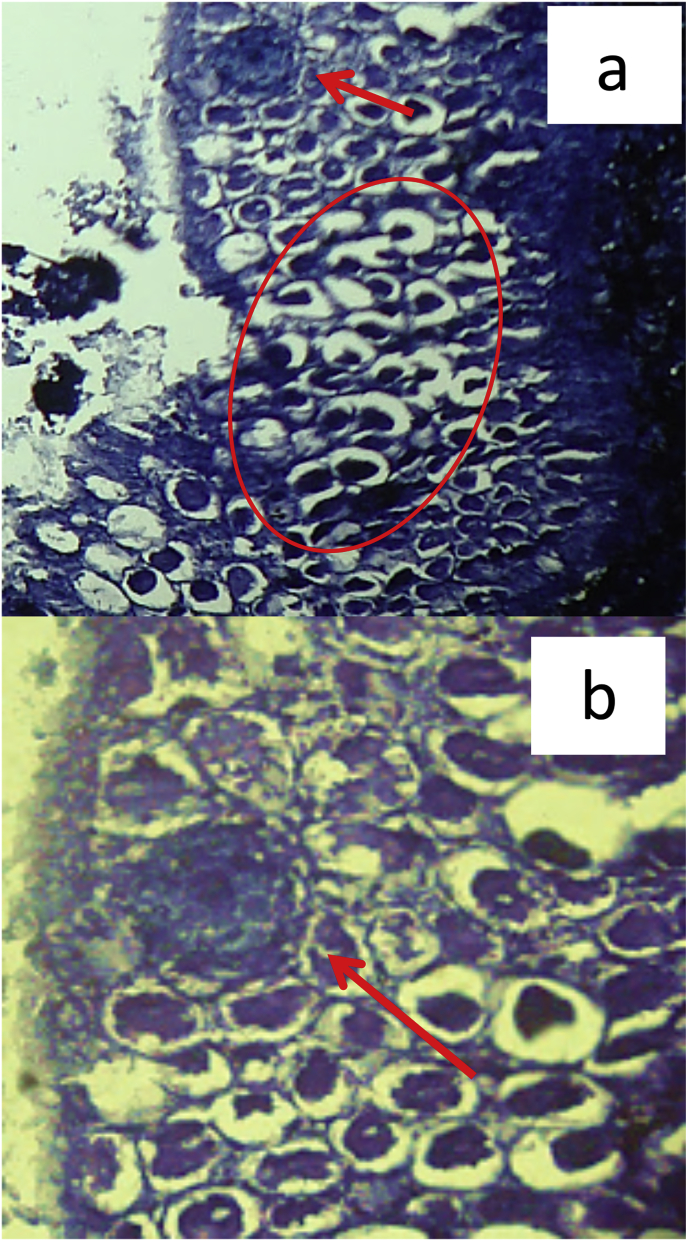
In infested fish, it was possible to detect injuries of the epithelial tissue at medium and external levels, through the presence of hypertrophic cells, modifying the architecture and normal arrangement of the tissue (Fig. 5a). In the intermediate regions of the epidermis, moderate amounts of sacculus cells round and more development with dense nuclei were observed adjacent to the affected areas (Fig. 5b). These cells of protein and/or glycoprotein nature (according to the reaction with Blue Alcán), are usually organized in a single row above the basal layer, occupying half or more thickness of the epidermis.
Fig. 5.
Transverse section of S. herzbergii skin parasitized by copepods. (Hematoxylin-eosin staining). a. Detail of the outer and middle layer of the epidermis (hyperplasia and hypertrophy) (100X). b. Sacciforme cell (400X).
4. Discussion
Evidences found of lesions in tegument tissues of host fish because of the presence of hypertrophic cells associated to infestation, may be explained as hurts resulting from mechanical injuries caused by the insertion of the pre-oral stylet or halo of adhesion of the chalimus stage of the copepod. Similar results have been reported in relation to infestations of Lepeophtheirus (Costello, 2006) on other fish hosts and other ectoparasitic copepods (Johnson et al., 2004).
Few studies have reported occurrence of Lepeophtheirus infestation on populations of Neotropical fish species (Luque et al., 2016, Morales-Serna et al., 2012, Morales-Serna et al., 2016). However, no study has described in detail lesions and evidences of histological damages on Neotropical catfish populations as such the present paper. Luque and Tavares (2007) analyzed parasitic communities on the White Sea Catfish (Netuma barba) from Brazil and only reported the general characteristics of infestations of two species of Lepeophtherius.
Lesions and damages on tissues may promote bacterial and/or virus diseases proliferation, aspect that has been reported in other fish populations (Venmathi et al., 2016). According to Johnson et al. (2004), feeding activities of ectoparasitic copepods are responsible for any primary disease that fish host develops.
The observed trend of increasing infestation intensity in larger individual is commonly found in studies of ectoparasitic copepod on catfish host (Carvallho et al., 2015) and other fish species (eg. Cañas et al. 2013). Usually, this relationship is explained because larger individual hosts had more available spaces for colonization or simply for accumulation of parasitic through the time (Bortone et al., 1978). Implications of this trend on population dynamics of host fish may be relevant. Information from other parasites relates intensity of infestation as well as co-infections effects on juveniles and reproductive ages of host that consequently alter age structure and natality rates of their population (Evans et al., 2007, Shoemaker et al., 2012, Mohamed et al., 2017).
According to Carvallho et al. (2015), because their feeding preferences, catfish can serve as intermediate, definitive or paratenic hosts of various species of parasites and contribute to expansion of infestations on intra or inter specific hosts. All these characteristics join to the high values of prevalence observed in “Las Marites” lagoon, seem to indicate that infestation of Lepeophtheirus sp could be an important factor affecting wild populations of S. herzbergii and another aquatic species in this water body. Therefore, more studies about effects of copepod parasitic on survivorship and fecundity of this fish species are requested.
Conflicts of interest
None.
Acknowledgements
Thanks to the referees for their comments and observations that contributed to improving an early version of the manuscript. We appreciate the help of Dr. E. Gómez and Dr. J. Palazón in lab works and statistical analysis. Infrastructure and laboratory facilities of the Universidad del Zulia and Universidad de Oriente from Venezuela made this research possible. Especial thanks to Prof. K. Yambay and Academic Writing Center of Escuela Superior Politécnica del Litoral from Ecuador for English grammar revisión. Rodrigo Moncayo-Estrada is supported by the Comisión de Operación y Fomento de Actividades Académicas, Instituto Politécnico Nacional and Estímulo al Desempeño Académico (EDI). Fish used in this study were catch from field surveys conducted by Universidad de Oriente, Venezuela. Fields surveys were conducted under approval and permission of the Ministry of Environment of Bolivarian Republic of Venezuela.
References
- Barletta M., Costa M. Living and non-living resources exploitation in a tropical semi-arid estuary. J. Coast. Res. 2009;56:371–375. [Google Scholar]
- Betancur R., Acero A., Mejia-Ladino L. Análisis filogenético preliminar de algunos bagres marinos (Siluriformes: Ariidae) neotropicales. Mem. Fund. La Salle Ciencias Nat. 2004;158:61–85. [Google Scholar]
- Bortone S., Bradley W., Oglesby J. The host-parasite relationship of two copepod species and two fish species. J. Fish Biol. 1978;13:337–350. [Google Scholar]
- Boxshall G., Montú M. Copepods parasitic on Brazilian coastal fisher: a handbook. Nauplius. 1997;5:1–225. [Google Scholar]
- Boxshall G., Halsey S. The Ray Society; London: 2004. An Introduction to Copepod Diversity. [Google Scholar]
- Boxshall G. Siphonostomatoida. In: Walter T.C., Boxshall G.A., editors. World of Copepods Database. Accessed through World Register of Marine Species. 2014. http://www.marinespecies.org/aphia.php?p=taxdetails&id=1104 Available from: [Google Scholar]
- Bui S., Oppedal F., Stien L., Dempster T. Sea lice infestation level alters salmon swimming depth in sea-cages. Aquac. Environ. Interact. 2016;8:429–435. [Google Scholar]
- Cañás L., Sampedro P., Fariña A., Landa J. Spatial, temporal and bathymetric distribution patterns of the parasite Chondracanthus lophii of anglerfish, Lophius piscatorius, in the northeast Atlantic. Mar. Biol. Res. 2013;9:145–156. [Google Scholar]
- Carvallho R., Takemoto R., Melo C., Jeraldo V., Madi R. Structure of parasite infracommunity of Sciades proops from the japaratuba river estuary, Sergipe, Brazil. Braz. J. Biol. 2015;75:906–913. doi: 10.1590/1519-6984.02514. [DOI] [PubMed] [Google Scholar]
- Cervigón F. Fundación Científica Los Roques; Caracas, Venezuela: 1991. Los peces marinos de Venezuela. [Google Scholar]
- Costello M. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 2006;22:475–483. doi: 10.1016/j.pt.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Cressey R. Parasitic copepods from the gulf of Mexico and Caribean sea, III: Caligus. Smithson. Contributions Zoology. 1991;497:1–53. [Google Scholar]
- Dojiri M., Ho J. Systematics of the Caligidae, copepods parasitic on marine fishes. Crustac. Monogr. Ser. 2013;18 https://doi.org/10.1163/9789004204256_014 448 pp. Available from: [Google Scholar]
- Eslava N. Universidad de Oriente; Venezuela: 2004. Alimentación y reproducción de peces. [Google Scholar]
- Evans J.J., Klesius P.H., Pasnik D.J., Shoemaker C.A. Influence of natural Trichodina sp. parasitism on experimental Streptoccus iniae or Streptococcus agalactiae infection and survival of young channel catfish Ictalurus punctatus (Rafinesque) Aquac. Res. 2007;38:664–667. [Google Scholar]
- Froese R., Pauly . 2017. FishBase.www.fishbase.org Retrieved 19th, June 2017, from. [Google Scholar]
- Giarrizzo T., Krumme U. Spatial differences and seasonal cyclicity in the intertidal fish fauna from four mangrove creeks in a salinity zone of the Curuçá estuary, north Brazil. Bull. Mar. Sci. 2007;80:739–754. [Google Scholar]
- Godwins S., Dill L., Krokosek M., Price M., Reynolds S. Reduced growth in wild juvenile sockeye salmon Oncorrhynchus nerka infected with sea lice. J. Fish Biol. 2017 doi: 10.1111/jfb.13325. Available from: DOI:10.111/jfn.13325. [DOI] [PubMed] [Google Scholar]
- Jensen A., Zydlewski G., Barker S., Pietrak M. Sea lice infestations of wild fish assemblages in North Atlantic Ocean. Transaction Am. Fish. Soc. 2016;145:7–16. [Google Scholar]
- Johnson S., Treasurer J., Bravo S., Nagayama K., Zbigniew K. Review of the impact of parasitic copepods on marine aquaculture. Zool. Stud. 2004;43:229–243. [Google Scholar]
- Llewellyn N., Leadbeater S., Garcia C., Sylvan F., Custodio M., Ang K., Powell F., Carvalho R., Creer S., Elliot J., Derome N. Parasitism perturbs the mucosal microbiome of Atlantic salmon. Sci. Rep. 2017 doi: 10.1038/srep43465. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque J., Tavares L. Checklist of copepod associated with fishes from Brazil. Zootaxa. 2007;1579:1–39. [Google Scholar]
- Luque J., Cruces C., Chero J., Paschoal F., Alves P., Da Silva A., Sánchez L., Iannocone J. Checklist of metazoan parasites of fishes from Perú. Neotropical Helminthol. 2016;10:301–375. [Google Scholar]
- Marceniuk A. Chave para identificacão dos espècies de bagres marinos (Siluriformes, Ariidae) da costa brasileira. Boletin do Instituto de Pesca. 2015;31:89–101. [Google Scholar]
- Mohamed H., Menanteau-Ledouble S., Kumar G., Abdelzaher M., El-Matbouli M. The impact of co-infections on fish: a review. Veterinary Res. 2017;47:1–12. doi: 10.1186/s13567-016-0383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Serna F., Gómez S., Pérez-Ponce G. Parasitic copepods reported from Mexico. Zootaxa. 2012;3234:43–68. [Google Scholar]
- Morales-Serna F., Medina-Guerrero R., Fajer-Avila E. Sea lice (Copepoda: Caligidae) parasitic on fishes reported from the Neotropical region. Neotropical Biodivers. 2016;2:141–150. [Google Scholar]
- Palazón J., Hernández G., Hernández J., Penoth E. Condiciones hidroquímicas de la Laguna de Las Marites, Isla de Margarita, Venezuela. Boletín del Instituto Oceanográfico de Venezuela. 1996;35:113–125. [Google Scholar]
- Rosenberg A. Aquaculture: the price of lice. Nature. 2008;451:23–24. doi: 10.1038/451023a. [DOI] [PubMed] [Google Scholar]
- Shepard S., Gargano P. Quantifying the contribution of sea lice from aquaculture to declining annual returns in a wild Atlantic salmon population. Aquac. Environ. Interact. 2017;9:181–192. [Google Scholar]
- Shoemaker C., Martins M., Xu D., Klesius P. Effect of Ichthyophthirius multifiliis parasitism on the survival, hematology and bacterial load in channel catfish previously exposed to Edwardsiella ictaluri. Parasitol. Res. 2012;111:2223–2228. doi: 10.1007/s00436-012-2988-5. [DOI] [PubMed] [Google Scholar]
- Todd C., Whyte B., McLean J., Walker A. Ectoparasitic sea lice (Lapeophtherius salmonis and Caligus elongatus) infestation of wild, adult, one Sea-winter Atlantic salmon Salmo salar returning to Scotland. Mar. Ecol. Prog. Ser. 2006;328:183–193. [Google Scholar]
- Venmathi B., Suarez-Morales E., Susumu H., Wook U. On the occurrence of caligids (Copepoda: Siphonostomatoida) in the marine plankton: a review and checklist. Zootaxa. 2016;4174:437–447. doi: 10.11646/zootaxa.4174.1.27. [DOI] [PubMed] [Google Scholar]