Abstract
The purpose of this study was to investigate the hydrolyzation of aspirin during the process of dissolution testing for aspirin delayed-release tablets. Hydrolysis product of salicylic acid can result in adverse effects and affect the determination of dissolution rate assaying. In this study, the technique of differential spectra was employed, which made it possible to monitor the dissolution testing in situ. The results showed that the hydrolyzation of aspirin made the percentage of salicylic acid exceed the limit of free salicylic acid (4.0), and the hydrolyzation may affect the quality detection of aspirin delayed-release tablets.
Keywords: Aspirin delayed-release tablets, Drug dissolution test, Fiber-optic dissolution system, UV–vis spectrum
1. Introduction
Aspirin is widely used as an anti-rheumatic, antipyretic and anti-thrombosis drug [1], [2]. The main adverse effects produced by aspirin are gastrointestinal [3], tinnitus [4], Reye's syndrome [5] and so on. These adverse effects of aspirin result almost all from the salicylic acid.
The hydrolyzation of aspirin belongs to second order reaction [6], but with the pH value kept constant, the hydrolyzation was presumed to be pseudo-first order reaction. In recent years, some articles have reported this reaction, but there is no article reporting the effect of hydrolyzation on dissolution testing.
Salicylic acid produced by aspirin in the process of manufacturing or hydrolysis will affect the dissolution testing and assaying. Therefore, it is important to check out the limit of free salicylic acid in aspirin delayed-release tablets and other preparations. In USP30-NF-25, the limit of free salicylic acid in aspirin delayed-release tablets is not more than 4.0% of the labeled amount.
In this study we researched the hydrolyzation of aspirin during the process of dissolution test for aspirin delayed-release tablets.
2. Materials and method
2.1. Instrument
A fiber-optic drug dissolution in-situ test system (FODT) was developed by Professor JIan Chen's group of Xinjiang Medical University and Xinjiang FOCS Bio-Tech Development Co., Ltd. A UV–visible spectrophotometer (Cintra 40, GBC Scientific Equipment Pty. Ltd., Australia) was utilized for the off-line control of UV determination.
2.2. Reagents and drugs
Aspirin (Lot no. 100113-200302) and salicylic acid (Lot no. 100405-100560) reference substances were obtained from the National Institute for the Control of Pharmaceutical and Biological Products of China; aspirin enteric-coated tablets (0.1 g) were supplied by Bayer Vital GmbH (Lot no. BTA6WK5); and all other reagents were of A.R. grade; purified water was prepared in the laboratory and degassed before use.
2.3. Preparation of pH 6.8 phosphate buffer
A mixture of 0.1 M hydrochloric acid and 0.2 M tribasic sodium phosphate (3:1) was prepared; if necessary, it was adjusted with 2 M hydrochloric acid or 2 M sodium hydroxide to pH of 6.8±0.05.
2.4. Preparation of calibration solutions
Calibration solutions of aspirin and salicylic acid were prepared with pH 6.8 phosphate buffer. The aspirin calibration solutions were prepared at concentrations from 20.0 to 120.0 μg/mL. The molarity of salicylic acid solution equaled that of the labeled amount of 100 mg of aspirin delayed-release tablets diluted in 1000 mL dissolution media, the salicylic acid calibration solutions were prepared at concentrations from 15.4 to 92.4 μg/mL.
2.5. UV spectral characteristics of aspirin
Acetylsalicylic acid is stable in dry air, but gradually hydrolyzes in contact with moisture containing acetic and salicylic acids. Solutions of aspirin and salicylic acid reference substance were prepared with phosphate buffer (pH 6.8) and scanned by UV.
2.6. Linearity
A series of different concentrations of aspirin substance at 20.0%, 40.0%, 60.0%, 80.0%, 100.0% and 120.0% were prepared. The linearity of each set of standard solutions was performed to test each individual probe.
2.7. Release test
Release testing was performed with Ch.P Apparatus I, in 750 mL of pH 1.2 dissolution medium for first 2 h, and 250 mL of pH 6.8 phosphate buffer was added for the next 45 min, using probes with 5 mm path lengths. The stirring speed was 100 rpm. The detection and reference wavelengths were set at 280 nm in the acid stage and about 265 nm in the buffer stage, respectively. The real-time drug release data and profiles were obtained by FODT.
For the UV control analysis, dissolution samples were withdrawn at the end of the dissolution test and filtered using 0.80 μm filter membrane. Then we determined the amount of aspirin dissolved by UV at a large wavelength of aspirin and salicylic acid.
3. Results and discussion
3.1. UV spectral characteristics of aspirin
The absorption spectra of aspirin and salicylic acid in phosphate buffer (pH 6.8) are shown in Fig. 1. The absorption peaks were at 233 nm and 296 nm. At the wavelength of 265 nm, aspirin and salicylic acid had a different absorbance, so it affected the determination of dissolution rate. According to the UV analysis method in USP, the aspirin enteric-coated tablets were determined at 265 nm, and at the same time the absorbance of salicylic acid was observed at 265 nm as the same absorption point as that of aspirin to deduct the interference by salicylic acid.
Figure 1.
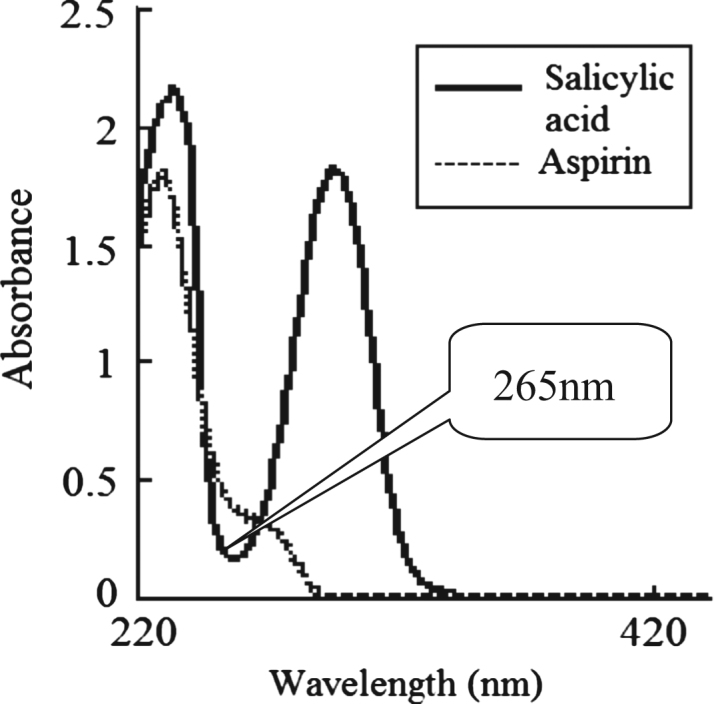
UV absorbance spectra of salicylic acids and aspirin.
3.2. Linearity
The series of standard solutions can obtain a proper absorbance range by using a probe of 5 mm path length. The calibration curve equations of aspirin are shown in Table 1.
Table 1.
Calibration curve equations of aspirin in six channels.
| Channel | Calibration curve equations | Correlation coefficient (r) |
|---|---|---|
| Channel 1 | C=0.2+295.9A | 0.9998 |
| Channel 2 | C=1.2+305.8A | 0.9996 |
| Channel 3 | C=1.0+305.8A | 0.9999 |
| Channel 4 | C=1.4+305.8A | 0.9999 |
| Channel 5 | C=0.5+294.1A | 0.9997 |
| Channel 6 | C=0.6+287.4A | 0.9998 |
| UV | C=1.5+305.4A | 0.9999 |
3.3. Release test
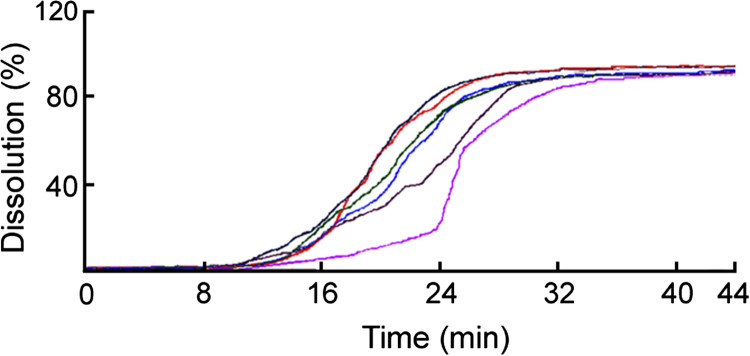
Due to the use of two dissolution media (one is the pH 1.2 medium and the other is the pH 6.8 medium), FODT had to recalibrate for the second medium [7]. Fortunately the software was compiled for delayed-release dosage form with the technique of differential spectra, and we were able to acquire a complete profile for the entire release process. So the difficulty was solved by scanning the blank twice. The dissolution profiles by the FODT are shown in Fig. 2. Table 2 shows the cumulative percentages of drug dissolved from the tablets determined by FODT and UV. There was no significant difference between the methods of FODT and UV (P>0.05).
Figure 2.
Release profiles of aspirin delayed-release tablets.
Table 2.
Comparison of the accumulative dissolution rate (%) for aspirin delayed-release tablets by FODT and UV.
| Channel | 1 | 2 | 3 | 4 | 5 | 6 | Average | RSD (%) |
|---|---|---|---|---|---|---|---|---|
| FODT | 95.8 | 91.7 | 95.8 | 94.4 | 90.9 | 92.5 | 93.5 | 2.1 |
| UV | 95.0 | 91.0 | 94.0 | 94.0 | 91.2 | 93.5 | 93.1 | 1.6 |
| SD | 0.8 | 0.7 | 1.8 | 0.4 | −0.3 | −1.0 | 0.4 | 0.9 |
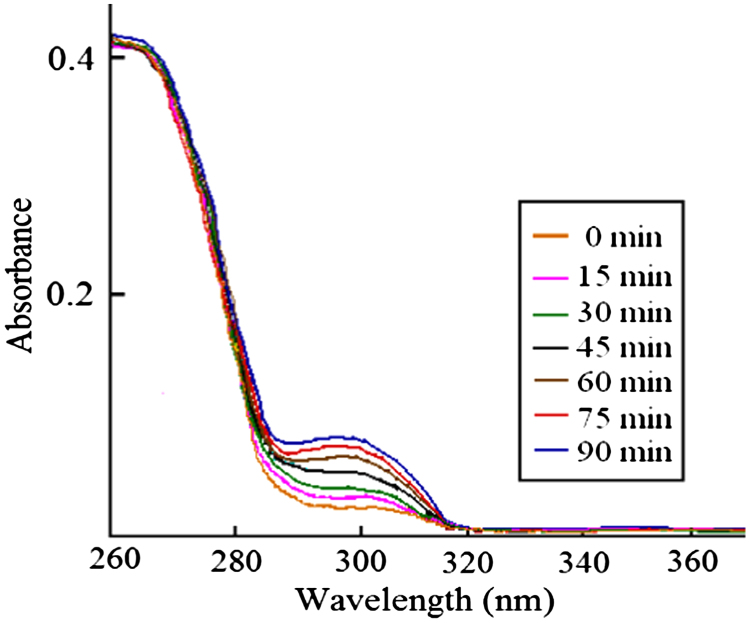
Drug dissolution–release profiles were obtained in-situ by FODT; the data acquisition interval for FODT was preset at 15 min. The absorption spectra of aspirin displayed differences. At 296 nm, absorptive changes are shown in Fig. 3. It shows that the amount of salicylic acid increased when aspirin was dissolved out constantly from the tablets.
Figure 3.
UV absorbance spectra of aspirin at different time points.
At the end of testing, we detected the absorbance of dissolution samples at 296 nm by UV and calculated the percentage of salicylic acid. The results are listed in Table 3. The hydrolyzation of aspirin made the percentage of salicylic acid exceed the limit of free salicylic acid (4.0). We can conclude that the hydrolyzation may affect the detection quality of aspirin delayed-release tablets.
Table 3.
Percentage of salicylic acid in the dissolution test for aspirin delayed-release tablets.
| Channel | 1 | 2 | 3 | 4 | 5 | 6 | Mean |
|---|---|---|---|---|---|---|---|
| Absorbance | 0.069 | 0.072 | 0.078 | 0.070 | 0.080 | 0.080 | 0.075 |
| Percentage of salicylic acid | 3.8 | 3.9 | 4.3 | 3.8 | 4.4 | 4.4 | 4.1 |
Determination of the release rate of active ingredients from drug formulations is an important procedure in pharmaceutical laboratories. Traditionally, it is analyzed by UV and HPLC. When detecting the dissolution rate of drugs which are capable of hydrolysis with traditional methods, one should quickly remove, filter and dilute samples, and then immediately detect the accumulative dissolution rate (%). Otherwise, it will affect the results of dissolution. It is time consuming and can give only little information on the dissolution behavior of drugs. FODT was proven to be useful for pharmaceutical solid formulation, productive, time saving and can acquire more information than conventional methods. It not only gives the dissolution profiles of drugs, but also gives information on absorption spectra at any time during dissolution tests, enabling us to find all changes during the tests.
Acknowledgments
We would like to acknowledge Xinjiang FOCS Bio-Tech Co., Ltd., China, for its contributions to this work. This research was supported by the Xinjiang Medical University Scientific Innovation Fund (No. XJC201129) and Xinjiang Uygur Autonomous Region Natural Science Fund (No. 2011211A041).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yan Wang, Email: wangyan1060@yahoo.com.cn.
Xin-Xia Li, Email: lxx6668@163.com.
References
- 1.Khan A., Rahman M., Islam S. Antipyretic activity of Peperomia pellucida leaves in rabbit. Turk. J. Biol. 2008;1(32):37–41. [Google Scholar]
- 2.Erkan D., Harrison M.J., Levy R. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007;56(7):2382–2391. doi: 10.1002/art.22663. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen H.T., Mellemkjaer L., Blot W.J. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am. J. Gastroenterol. 2000;95(9):2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 4.Delaney J.A., Opatrny L., Brophy J.M. Drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177(4):347–351. doi: 10.1503/cmaj.070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berges-Gimeno M.P., Stevenson D.D. Nonsteroidal anti-inflammatory drug-induced reactions and desensitization. J. Asthma. 2004;41(4):375–384. doi: 10.1081/jas-120037650. [DOI] [PubMed] [Google Scholar]
- 6.Barry E., Borer L.L. Experiments with aspirin. J. Chem. Educ. 2000;77(3):354. [Google Scholar]
- 7.Nie K., Li L., Li X.X. Monitoring ambroxol hydrochloride sustained-released tablets release by fiber-optic drug dissolution in-situ test system. Diss. Technol. 2009;17(8):14–17. [Google Scholar]