Abstract
There is a compelling link between severe brain trauma and immunosuppression in patients with traumatic brain injury (TBI). Although acute changes in the systemic immune compartment have been linked to outcome severity, the long-term consequences of TBI on systemic immune function are unknown. Here, adult male C57Bl/6 mice underwent moderate-level controlled cortical impact (CCI) or sham surgery, and systemic immune function was evaluated at 1, 3, 7, 14, and 60 days post-injury. Bone marrow, blood, thymus, and spleen were examined by flow cytometry to assess changes in immune composition, reactive oxygen species (ROS) production, phagocytic activity, and cytokine production. Bone marrow derived macrophages (BMDMs) from sham and 60-day CCI mice were cultured for immune challenge studies using lipopolysaccharide (LPS) and interleukin-4 (IL-4) models. Acutely, TBI caused robust bone marrow activation and neutrophilia. Neutrophils and monocytes exhibited impairments in respiratory burst, cytokine production, and phagocytosis; in contrast, ROS levels and pro-inflammatory cytokine production were chronically elevated at 60 days post-injury. Cultures of BMDMs from chronic CCI mice demonstrated defects in LPS- and IL-4-induced polarization when compared with stimulated BMDMs from sham mice. TBI also caused thymic involution, inverted CD4:CD8 ratios, chronic T lymphopenia, greater memory conversion, increased T cell activation, impaired interferon γ induction, and chronically elevated Th1 cytokine and ROS production. Collectively, our in-depth phenotypic and functional analyses demonstrate that TBI induces widespread suppression of innate and adaptive immune responses after TBI. Moreover, at chronic time points, TBI mice exhibit hallmarks of accelerated immune aging, displaying chronic deficits in systemic immune function.
Keywords: : chronic inflammation, immunosuppression, systemic immunity, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the primary cause of death and disability below the age of 45 years. Worldwide, TBI is responsible for nearly 10 million deaths annually, whereas ∼57 million survivors continue to cope with a diminished quality of life.1,2 A secondary infection in the hospital setting develops in more than 50% of patients with TBI, which is disproportionately higher than in those affected by stroke, burn injury, and polytrauma.3–5 The heightened risk of infection during the acute period (days to weeks) after admission is multi-factorial. In addition to surgical intervention, invasive devices, and prolonged immobilization, immune changes after TBI result from changes in autonomic function.3,6 Systemic immune changes after experimental TBI have received more limited attention; as a result, our understanding of the cellular mechanisms underlying the increased vulnerability to infection remains poor. Moreover, the long-term effects of TBI on systemic immunity are also largely unknown.
Recent data from our laboratory, and others, have shown that TBI produces progressive neurodegenerative changes associated with chronic inflammation.7–13 Aging is the single most important risk factor for neurodegenerative disease, and the gradual worsening of neuronal injury in the months after a TBI event has been theorized to accelerate the time window in which normal age-related pathology reaches clinical threshold.14–16 It is now established that age-related neurological decline is driven in part by “inflamm-aging.”17 Although several studies have identified circulating inflammatory biomarkers and leukocyte counts as prognostic indicators of TBI severity and outcome,18 it is unclear to what extent brain injury accelerates the normal inflamm-aging process.
Previous work assessed systemic immune changes in response to diffuse brain injury in mice, demonstrating complex alterations in peripheral immune cells and whole blood cytokine levels.19 A direct assessment of bone marrow responses, and more detailed analyses of myeloid and lymphocyte subsets and their functional responses have yet to be performed, however. The present study examined the pathogenesis and long-term impact of TBI on the immune system. We hypothesized that a moderate-to-severe focal contusion brain injury would have profound and region-specific effects on far reaching lymphoid tissues, resulting in temporally modulated alterations in leukocyte composition and function that persist for months after the initial traumatic event. Our data demonstrate that brain trauma induces widespread suppression of innate and adaptive immune responses in the immediate days after injury. Moreover, at two months post-injury, young mice exhibited signs of accelerated immune aging, displaying chronic deficits in systemic immune function.
Methods
Animals
Young adult male C57BL/6 mice (Taconic Biosciences, Germantown, NY; 10–12 weeks old) were housed on sawdust bedding in a specific pathogen-free facility (12 h light/dark cycle). All animals had access to chow and water ad libitum. Animal procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Maryland School of Medicine.
Controlled cortical impact (CCI)
Our custom-designed CCI injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip as described previously.20 Briefly, mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask. Mice were placed on a heated pad, and core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, a 10 mm midline incision was made over the skull, and the skin and fascia were reflected. A 5 mm craniotomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate-level CCI was induced using an impactor velocity of 6 m/sec and deformation depth of 2 mm. After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 min post-injury.
Sham animals underwent the same procedure as CCI mice except for craniotomy and cortical impact. No differences were observed among sham groups at any time point (n = 4–5/group/time point; one-way analysis of variance [ANOVA] with post hoc adjustment using the Tukey test, p > 0.05); therefore, pooled data from all sham groups are presented for each outcome measure.
Flow cytometry
Body weights were recorded immediately after mice were euthanized, and blood (200 μL) was drawn by cardiac puncture with heparinized needles. After transcardial perfusion with 60 mL of ice-cold sterile phosphate buffered saline (PBS), the spleen and thymus were removed, weighed, and processed by mechanical disruption on a 70-μm filter screen. Bone marrow was harvested from the ipsilateral femur by flushing with Roswell Park Memorial Institute (RPMI) (Lonza Group, Basel, Switzerland) medium using hydrostatic pressure. Red blood cell lysis was achieved by successive 10-min incubations with Tris-ammonium chloride (Stem Cell Technologies, Vancouver, Canada).
Splenocytes, thymocytes, and bone marrow leukocytes were washed subsequently and resuspended in a total of 5 mL of RPMI from which 500 μL was then transferred into FACS tubes. Leukocytes were washed and blocked with mouse Fc Block (eBioscience, San Diego, CA; clone 93) before staining with primary antibody-conjugated flourophores (CD45-eF450 (30-F11), CD11b-APCeF780 (M1/70), Ly6C-APC (HK1.4), Ly6G-PE (1A8), major histocompatibility complex (MHC)II- PerCP-eF710 (M5/114.15.2), CD3e-PE-Cy7 (145-2C11), CD4-FITC (GK1.5), CD8a-AF700 (53-6.7), CD44-APC (IM7), CD62L-APCeF780 (MEL-14), CD69-PE (H1.2F3), TNF-PE-Cy7 (MP6-XT22), and IL-1β-PerCP-eF710 (NJTEN3) purchased from eBioscience, whereas CD45-PerCP-Cy5.5 (30-F11), CD11b-PerCP-Cy5.5 (M/70), CD4-APC (GK1.5), and interferon (IFN)γ-PerCP-Cy5.5 (XMG1.2) were purchased from Biolegend (San Diego, CA).
For live/dead cell discrimination, a fixable viability dye, Zombie Aqua™ (Biolegend), was dissolved in DMSO according to the manufacturer's instructions and added to cells in a final concentration of 1:200. Data were acquired on a LSRII using FACsDiva 6.0 (BD Biosciences, San Jose, CA) and analyzed using FlowJo (Tree Star, San Carlos, CA). A standardized gating strategy was used to identify T cell (CD45+CD11b-CD3e+), B cell (CD45+CD11b-CD3e-MHCII+), monocyte (CD45+CD11b+Ly6C+Ly6G-), and neutrophil (CD45+CD11b+Ly6C+Ly6G+) populations as shown (Supplementary Fig. 1; see online supplementary material at ftp.liebertpub.com). Cell-specific fluorescence minus one (FMO) controls were used to determine the positivity of each antibody. Cell count estimations were performed using CountBright™ absolute counting beads (40 μL/test; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
For intracellular cytokine staining, leukocytes were collected as described above, and 1 μL of GolgiPlug containing brefeldin A (BD Biosciences) was added to 500 μL complete RPMI. Cells were subsequently stimulated with PBS or Cell Stimulation Cocktail (eBioscience; 1:500) and incubated for 2–4 h at 37°C (5% CO2). Afterward, cells were resuspended in Fc Block, stained for surface antigens, and washed in 100 μL of fixation/permeabilization solution (BD Biosciences) for 20 min. Cells were then washed twice in 500 μL Permeabilization/Wash buffer (BD Biosciences) and resuspended in an intracellular antibody cocktail containing cytokine antibodies and fixed.
For detection of reactive oxygen species, leukocytes were incubated with dihydrorhodamine (DHR) 123 (5mM; 1:500 in RPMI; Ex/Em: 500/536), a cell-permeable fluorogenic probe (Life Technologies, Invitrogen). Cells were loaded for 20 min at 37°C, washed three times with FACS buffer (without NaAz), and then stained for surface markers including viability stain. Phorbol 12-myristate 13-acetate (PMA)/ionomycin (500X) was added after loading, and respiratory burst was measured after an additional 30 min.
Phagocytic activity of myeloid cells was performed as described21 with minor modification. Briefly, yellow-green fluorescent carboxylate-modified polystyrene latex beads (1 μm mean diameter; Sigma) were added to freshly isolated cells in a final dilution of 1:100 (in RPMI). After 1 h incubation at 37°C, the cells were washed three times, resuspended in FACS buffer, stained for surface markers, and fixed in paraformaldehyde. Stimulation-induced phagocytosis was assessed by the addition of PMA/ionomycin (1:500) 30 min after incubation. Data presented in terms of mean fluorescence intensity (MFI) reflect the relative level of phagocytic activity (i.e., number of beads ingested) within a given phagocyte (i.e., bead-positive cell) population.
Preparation, culture, and treatments of bone marrow derived macrophages (BMDMs)
The BMDMs were isolated from the marrow of the femurs and tibias of age- and time-matched sham and chronically injured CCI mice (60 days post-injury; n = 5/group) as described previously.22 Briefly, after euthanasia, the marrow was flushed out into a sterile Falcon tube in Dulbecco's Modified Eagle's Medium (DMEM, Gibco Invitrogen, Carlsbad, CA) supplemented with heat-inactivated fetal bovine serum (FBS; 10%; Atlanta Biologicals, Flowery Branch, GA) and penicillin/streptomycin (1%; Gibco Invitrogen) to obtain a single cell suspension. The suspension was centrifuged (400 × g, 5 min), the supernatant was discarded, and the resulting pellet was resuspended in 20 mL of DMEM supplemented with Ladmac-conditioned media (20%). Cells were seeded in sterile cell culture flasks (T75 cm2 flasks) and were maintained in culture for a further 6 days, with media being replaced on day four.
On day six, cells were transferred to 24-well plates (0.4 × 106 cells per well) and were cultured for a further two days (n = 3 replicates/animal/group). The BMDMs were incubated in the presence both of lipopolysaccharide (LPS, 10 ng/mL; Sigma-Alrich, St. Louis, MO) and interleukin-4 (IL-4, 10 ng/mL; R&D Systems, Minneapolis, MN) for 24 h, supernatants were collected for analysis of cytokines by enzyme-linked immunosorbent assay (ELISA), and cells were harvested for analysis of markers of macrophage activation by polyacrylamide gel electrophoresis followed by Western immunoblotting.
Western blotting
Proteins were extracted using radioimmunoprecipitation assay buffer, equalized, and loaded onto 5–20% gradient gels for SDS PAGE (Bio-Rad; Hercules, CA). Proteins were transferred onto nitrocellulose membranes and blocked for 1 h in 5% milk in 1x tris buffered saline (TBS) containing 0.05 % Tween-20 (TBS-T) at room temperature. The membrane was incubated in mouse antiarginase 1 (N-20) (1:1000; BD Transduction Laboratories, San Jose, CA) or mouse anti-β-Actin (1:5000; Sigma-Aldrich) overnight at 4°C, then washed three times in TBS-T, and incubated in appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature. Membranes were washed three times in TBS-T, and proteins were visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL). Chemiluminescence was captured ChemiDoc™ XRS+ System (Bio-Rad, Pleasanton, CA), and protein bands were quantified by densitometric analysis using BioRad Molecular Imaging Software. The data presented reflect the intensity of target protein band normalized based on the intensity of the endogenous control for each sample (expressed in arbitrary units).
Cytokine analysis
Concentrations of tumor necrosis factor (TNF)α, IL-6, and IL-10 (R&D Systems, Minneapolis, MN) were measured in supernatant samples obtained from BMDMs by ELISA. Briefly, standards or samples (100 μL) were added to antibody-coated 96-well plates and incubated for 2 h at room temperature, plates were washed, and samples were incubated in detection antibody for 2 h. Plates were washed and incubated in HRP-conjugated streptavidin for 20 min at room temperature. Substrate solution (tetramethylbenzidine; Sigma-Aldrich) was added, incubation continued at room temperature in the dark for 30 min, and the reaction was stopped using H2SO4 (1M). Absorbance measurements were read at 450 nm using a Synergy HT Multi-Mode Microplate Reader (Biotek, Winooski, VT). Cytokine concentrations were calculated relative to the appropriate standard curve and expressed as pg cytokine/mL.
Statistical analyses
Data from individual experiments are presented as mean ± standard error of the mean. Multiple comparisons were assessed by one-way ANOVA analysis with the Dunnett test for multiple comparisons or two-way ANOVA analysis with Sidak or Tukey post hoc correction. Ex vivo studies were performed by an investigator blinded to surgical condition. Statistical analysis was performed using GraphPad Prism Software v. 6.0 (GraphPad Software, Inc., La Jolla, CA). p < 0.05 was considered statistically significant.
Results
Moderate-to-severe TBI induces bone marrow myelopoiesis, increased neutrophil production, and chronic T lymphopenia
To investigate the effects of moderate-to-severe focal TBI on systemic immune function, we first evaluated whether bone marrow activation was a prominent feature of the TBI response and whether changes in the de novo production of bone marrow-derived leukocytes are reflected by alterations in circulating blood leukocyte composition. We induced moderate-level CCI in adult male C57Bl/6 mice, and euthanized cohorts of injured mice at 1, 3, 7, 14, and 60 days post-injury along with time point matched sham mice (pooled) for systemic immune function analyses.
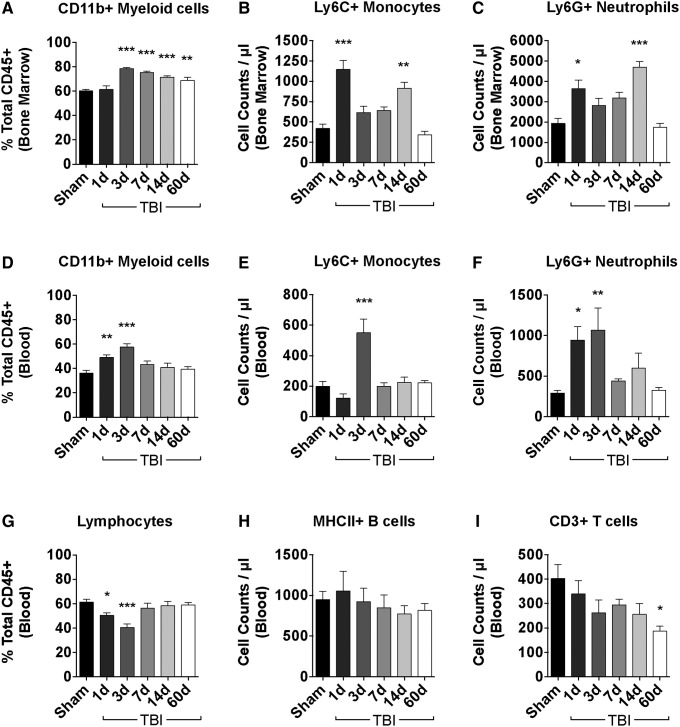
We exploited an established gating strategy to identify cellular subsets of myeloid and lymphocytic origin using flow cytometry (Supplementary Fig. 1; see online supplementary material at ftp.liebertpub.com). The percentage of CD11b+ myeloid cells was significantly increased by day three post-injury (p < 0.001) and was sustained for nearly two weeks (Fig. 1A). Cell count estimations revealed an early increase in the number of bone marrow-derived CD11b+ myeloid cells beginning at day one post-injury (p < 0.01), which remained elevated for at least two weeks, before nearly returning to baseline at day 60 post-injury (data not shown). Further analysis of bone marrow myeloid composition demonstrated a ∼two-fold increase in the number Ly6C+ monocytes and Ly6G+ neutrophils at day one post-injury, with neutrophils representing the majority of the newly produced leukocytes (Fig. 1B, C; p < 0.001 and p < 0.05, respectively).
FIG. 1.
Traumatic brain injury (TBI) induces distal bone marrow activation, increased neutrophil production, and chronic T lymphopenia. The relative leukocyte composition in the bone marrow and blood were assessed at the indicated time points after TBI. The percentage of CD11b+ myeloid cells was increased in the distal femur bone marrow acutely after TBI and remained elevated for several weeks (A). The absolute number of Ly6C+ monocyte (B) and Ly6G+ neutrophil (C) subsets was significantly increased by one day post-injury, remained elevated for weeks, but eventually normalized to baseline levels by day 60. The percentage of circulating CD11b+ myeloid cells in blood was acutely augmented by day one, reached its peak increase by day three, and returned to baseline by day seven (D). Cell count approximation of circulating leukocytes demonstrated a robust increase in the number of Ly6C+ monocytes (E) and Ly6G+ neutrophils (F) during the initial days after injury. The percentage of circulating bulk lymphocyte populations in blood is shown (G). No change in the number of MHCII+ B cells was seen (H), whereas a long-term reduction in CD3+ T cell counts was evident (I). For all experiments, n = 7–22/group. Statistical comparisons relative to sham control were determined by one-way analysis of variance with the Dunnett test. Error bars show mean standard error of the mean. *p < 0.05, **p < 0.01, and ***p < 0.001.
To determine whether TBI-induced bone marrow myelopoiesis resulted in the mobilization and egress of granulocytes into the circulation, we next assessed changes in blood leukocyte composition. The percentage of CD11b+ myeloid cells in the blood increased incrementally for the first three days after TBI (Fig. 1D). Cell count estimations of circulating blood leukocytes were performed to determine whether the proportional increase in myeloid cells was associated with an actual increase in cell number. Consistent with the de novo production of bone marrow myeloid cells, an early increase in the number of circulating neutrophils was observed at days one and three (p < 0.001) post-injury, and of circulating monocytes at day three post-injury (p < 0.001) (Figs. 1E–F). Lymphocyte counts demonstrated no change in the number of circulating MHCII+ B cells; however, a gradual, but significant, reduction in the number of CD3+ T cells was seen over the course of 60 days (Fig. 1G–I; p < 0.05). Taken together, these data suggest that a moderate-level focal TBI results in a potent distal bone marrow stimulus that spurs production and mobilization of newly generated myeloid cells into circulation, resulting in acute neutrophilia and, subsequently, chronic T lymphopenia.
TBI induces acute splenomegaly associated with an accumulation of myeloid cells
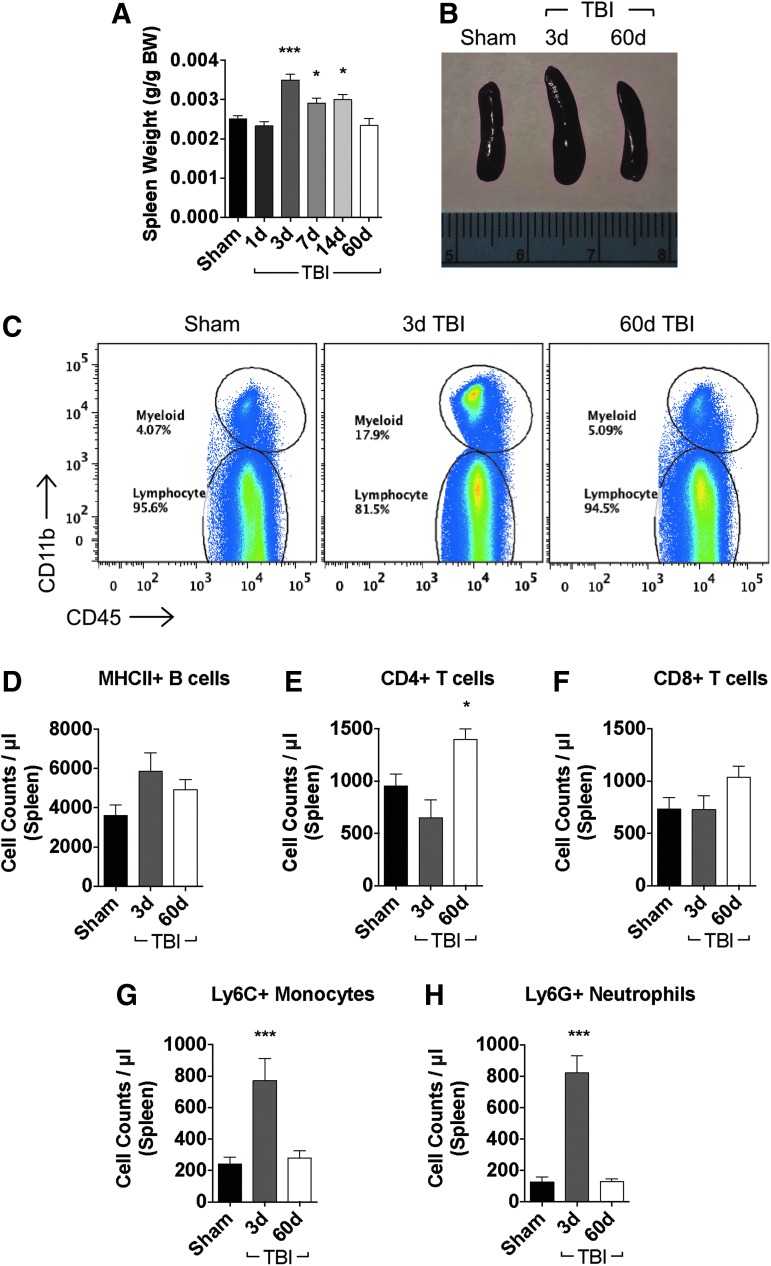
Next, we examined the effect of TBI in altering leukocyte dynamics in peripheral lymphoid tissues. Given its role in filtering, detecting, and responding to microorganisms and foreign substances in the blood, we first assessed the spleen. A significant increase in spleen weight was found at day three post-injury (Fig. 2A, B; p < 0.001) and remained elevated through day 14 (p < 0.05), before returning to baseline weight at day 60 post-injury. We then determined leukocyte counts in the spleen to better understand the disturbance in spleen size (Fig. 2C). No significant change in MHCII+ B cell or CD8+ T cell counts were detected at day three or 60 post-injury (Figs. 2D, F); however, there was a delayed and significant increase in the number of splenic CD4+ T cells at day 60 post-injury (Fig. 2E; p < 0.05). A dramatic ∼four-fold increase in splenic monocyte and neutrophil numbers was detected at day three post-injury (Fig. 2G, H; p < 0.001), but these cellular changes returned to baseline levels by day 60 post-injury. These results indicate that temporary splenomegaly is a prominent feature of the TBI systemic response and is characterized by an acute increase in the number of myeloid cells.
FIG. 2.
Moderate traumatic brain injury (TBI) results in temporary splenomegaly because of an accumulation of myeloid cells. Spleen weights were significantly increased by day three post-injury and returned to baseline size at day 60 (A; n = 7–22/group). Representative images taken from mice at acute and chronic time points after TBI are shown to illustrate changes in spleen size (B). Representative dot plots show the relative immune cell composition in the spleen after TBI (C; n = 5–10/group). Cell count data show no change in MHCII+ B cells (D). The number of CD4+ T cells was significantly increased by day 60 after TBI (E), whereas no statistical change in CD8+ T cell counts was found (F). A significant accumulation of Ly6C+ monocytes (G) and Ly6G+ neutrophils (H) was seen in the spleen at day three. Statistical comparisons relative to sham control were determined by one-way analysis of variance with the Dunnett test. Error bars show mean standard error of the mean. BW, body weight. *p < 0.05, ***p < 0.001. Color image is available online at www.liebertpub.com/neu
TBI induces thymic atrophy and chronic deficits in T cell maturation
The effects of TBI on thymic structure and composition were then examined. A dramatic decrease in thymus weight was noted at day one post-injury (60% weight loss compared with sham) (Fig. 3A, B; p < 0.001). Thymic weight loss persisted through day 60 post-injury; however, a gradual restoration in weight was observed at this latter time point (19% weight loss compared with sham). In the acute phase after TBI (day three), a similarly proportional decrease in number of T cell subsets, including CD4-CD8- (p < 0.05), CD4+CD8+ (p < 0.05), CD4+ (p < 0.001), and CD8+ (p < 0.05) T cells was found (Fig. 3C–G). Interestingly, despite complete restoration of CD4-CD8- T cell numbers and a significant rebound and elevation of CD4+CD8+ T cells in the chronic phase after TBI (day 60) (Fig. 3D, E; p < 0.001), there was a persistent loss in mature CD3+ (CD4+ and CD8+) T cell numbers through day 60 post-injury (Figs. 3F, G; p ≤ 0.05). Together our results suggest that TBI causes substantial and persistent thymic atrophy, characterized by chronic defects in T cell selection, maturation, and/or output.
FIG. 3.
Moderate traumatic brain injury (TBI) results in chronic thymic atrophy and defects in T cell maturation. Thymus weights were decreased dramatically by day one after TBI, displayed a modest rebound in weight over time, but remained significantly decreased at day 60 (A; n = 7–22/group). Representative images taken from mice at acute and chronic time points after TBI are shown to illustrate changes in thymus size (B). Representative dot plots illustrate the impact of TBI on T cell maturation in the thymus (C; n = 5–10/group). Cell count analyses reveal an acute decrease in the number of double-negative (CD4-CD8-) (D) and double-positive (CD4+CD8+) CD3+ T cells (E), and a modest decrease in CD4+ (F) and CD8+ (G) T cells. Statistical comparisons relative to sham control were determined by one-way analysis of variance with the Dunnett test. Error bars show mean standard error of the mean. BW, body weight. *p < 0.05, ***p < 0.001. Color image is available online at www.liebertpub.com/neu
Oxidative stress levels and respiratory burst potential are chronically altered after TBI
Reactive oxygen species (ROS) are key signaling molecules that play an important role in mitochondrial function, the antioxidant system, and the progression of inflammatory disorders; however, high levels of ROS production promote oxidative stress that can result in deoxyribonucleic acid damage, cellular dysfunction, and senescence.23 We began our functional assessment of the systemic inflammatory response to TBI by evaluating oxidative stress levels in leukocytes using the ROS dye DHR123. A significant rise in leukocyte-derived ROS production was found in bone marrow-derived monocyte and neutrophil populations at day three post-injury (Fig. 4A, B; p < 0.001 and p < 0.05, respectively). TBI resulted in a biphasic response whereby ROS levels were diminished below baseline at day seven post-injury before rebounding substantially and becoming highly elevated at day 60 post-injury, particularly in monocyte populations (Fig. 4B, C; p < 0.001). Circulating leukocyte-derived ROS levels were similarly increased at day three post-injury (p < 0.001), with the exception of B and T lymphocytes (Fig. 4D, E).
FIG. 4.
Chronic elevation of oxidative stress levels in leukocytes after traumatic brain injury (TBI). Basal cellular reactive oxygen species (ROS) levels were measured using dihydrorhodamine (DHR) 123. Representative histograms reveal relative ROS production of Ly6C+ monocytes and Ly6G+ neutrophils in the bone marrow after TBI: A; Ly6C+ monocytes – sham (blue, open), Ly6C+ monocytes – TBI (blue, tinted), Ly6G+ neutrophils – sham (red, open), Ly6G+ neutrophils – TBI (red, tinted), and fluorescence minus one (FMO) control (gray). Quantification of mean fluorescence intensity (MFI) values was expressed as fold-change relative to sham control and showed increased ROS production in both cell types at day three, followed by a sharp reduction by day seven (B). A considerable increase in basal oxidative stress levels can be seen at day 60 after TBI, wherein an analysis of relative cellular ROS levels reveals this effect is driven largely by increases in Ly6C+ monocyte-derived ROS (C). Representative histograms demonstrate a similar biphasic pattern of ROS production in circulating leukocytes: D; Ly6C+ monocytes – sham (blue, open), Ly6C+ monocytes – TBI (blue, tinted), Ly6G+ neutrophils – sham (red, open), Ly6G+ neutrophils – TBI (red, tinted), CD3+ T cells – sham (green, open), CD3+ T cells – TBI (green, tinted), MHCII+ B cells – sham (orange, open), MHCII+ B cells – TBI (orange, tinted), and FMO control (tinted gray). Neutrophil ROS levels were significantly depressed at days one and seven, temporarily elevated at day three, and remained substantially higher than baseline at day 60 after TBI (E). Lymphocyte ROS levels were decreased modestly in the first week after TBI; however, dramatic increases in ROS levels were observed over time. The long-term potentiation of oxidative stress levels was more prominent in myeloid cell populations than in lymphocytes (F). For all experiments, n = 5–8/group. Statistical comparisons between time points were evaluated by two-way analysis of variance with the Dunnett (B and E) and Sidak (C and F) test. Error bars show mean standard error of the mean. a.u.i.. arbitrary units of intensity. *p < 0.05, **p < 0.01, and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
Leukocytes in the blood exhibited a similar biphasic temporal pattern of ROS production as found in bone marrow. ROS levels in blood were suppressed at day seven post-injury in nearly all leukocyte subsets, but gradually increased to its highest point by day 60 post-injury, which was driven primarily by myeloid cells (Fig. 4E, F). These data suggest that TBI causes dramatic flux in leukocyte ROS production in the acute phase after TBI (up to day three post-injury) and consequently leads to a chronic elevation in oxidative stress levels at 60 days post-injury.
Respiratory burst, or the rapid release of ROS from phagocytes, is a critical component of the antimicrobial repertoire of myeloid cells and the innate immune response. ROS-mediated bacterial killing is important for preventing and managing infection and may be implicated in central nervous system (CNS) injury-induced immune suppression.24 We examined the influence of TBI on stimulus-induced respiratory burst activity in peripheral myeloid cells at acute and chronic time points using (PMA)/ionomycin challenge.25 No change in burst potential in bone marrow-derived monocytes or neutrophils was observed at day three post-injury (Fig. 5A, B). Interestingly, however, ROS generation was significantly blunted by >50% in both monocytes and neutrophils at day 60 post-injury (Fig. 5C; p < 0.001 and p < 0.001, respectively). These results suggest that, on PMA/ionomycin challenge, the microbicidal properties of phagocytes are impaired highly at chronic time points after TBI.
FIG. 5.
Chronic impairment of respiratory burst activity in phagocytes after traumatic brain injury (TBI). Respiratory burst activity was measured following phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation. Representative histograms show the relative level of ROS production in bone marrow-derived myeloid subsets as determined by dihydrorhodamine (DHR) 123 staining: A; Ly6C+ monocytes – sham (blue full, open), Ly6C+ monocytes – sham + stim (blue hatched, open), Ly6C+ monocytes – TBI (blue full, tinted), Ly6C+ monocytes – TBI + stim (blue hatched, tinted), Ly6G+ neutrophils – sham (red full, open), Ly6G+ neutrophils – sham + stim (red hatched, open) Ly6G+ neutrophils – TBI (red full, tinted), Ly6G+ neutrophils – TBI + stim (red full, tinted), and fluorescence minus one (FMO) control (gray). No change in the respiratory burst potential of Ly6C+ monocytes and Ly6G+ neutrophils was observed at day three after TBI (B). Significant impairment in reactive oxygen species (ROS) generation was found in both cell types at day 60 after TBI (C). For all experiments, n = 5–8/group. Statistical comparisons between groups were determined by two-way analysis of variance with Tukey post hoc test. Error bars show mean standard error of the mean. MFI, mean fluorescence intensity; stim, stimulation; a.u.i., arbitrary units of intensity. ***p < 0.001. Color image is available online at www.liebertpub.com/neu
Pro-inflammatory cytokine production is suppressed acutely and elevated chronically in peripheral myeloid cells after TBI
The generation of ROS also drives production of pro-inflammatory cytokines. Because inflammatory mediators serve as correlates of injury progression and are important regulators of systemic immunity, we next evaluated cytokine production levels in peripheral myeloid cells in the acute and chronic stages after TBI. Our data demonstrate a significant decrease in the percentage of TNF-expressing monocytes and neutrophils at day three post-injury (Fig. 6A, B; p < 0.001 and p < 0.05, respectively). The relative protein expression (i.e., MFI) of TNF was decreased likewise in monocytes (p < 0.05), but not neutrophils (Fig. 6C). Despite no change in the percentage of IL-1β+ myeloid cells in the bone marrow at this time, both subsets displayed marked reductions in IL-1β expression levels relative to sham control (Fig. 6D–F). Chronically, however, the percentage of IL-1β+ neutrophils in the spleen increased nearly two-fold compared with sham control (Fig. 6G, H; p < 0.05). In all, these data are consistent with the idea that TBI induces a post-acute immune suppressive state that gives rise to long-term exacerbation or dysregulation of pro-inflammatory cytokine signaling.
FIG. 6.
Traumatic brain injury (TBI) induces acute suppression and chronic upregulation of pro-inflammatory cytokine production in peripheral myeloid cells. Representative dot plots depict the expression pattern of tumor necrosis factor (TNF) in Ly6C+ monocytes and Ly6G+ neutrophils in bone marrow at day three post-injury (A). A lower percentage of TNF-positive myeloid cells was found in the acute period after TBI (B). The relative expression level of TNF-positive monocytes was reduced significantly as determined by two-way group analysis of variance (ANOVA) (C). Representative dot plots depict the expression pattern of interleukin (IL)-1β in Ly6C+ monocytes and Ly6G+ neutrophils in bone marrow at day three post-injury (D). Percentage of IL-1β-positive myeloid cells was equal in sham and TBI groups at three days post-injury (E); however, the relative expression level of IL-1β-positive Ly6C+ monocytes was significantly reduced after TBI (F). The long-term impact of TBI on myeloid cell IL-1β production in the spleen was also evaluated at day 60 post-injury (G). The percentage of IL-1β-positive Ly6G+ neutrophils was significantly increased (∼ doubled) at 60 days post-injury (H). For all experiments, n = 5–7/group. Statistical comparisons between groups were determined by two-way ANOVA with Sidak test. Error bars show mean standard error of the mean. FMO, fluorescence minus one; SSC-A, side scatter-area; MFI, mean fluorescence intensity; a.u.i., arbitrary units of intensity; n.s., not significant. *p < 0.05, **p < 0.01, and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
TBI causes acute down-regulation of MHCII expression and long-term deficits in phagocytic activity
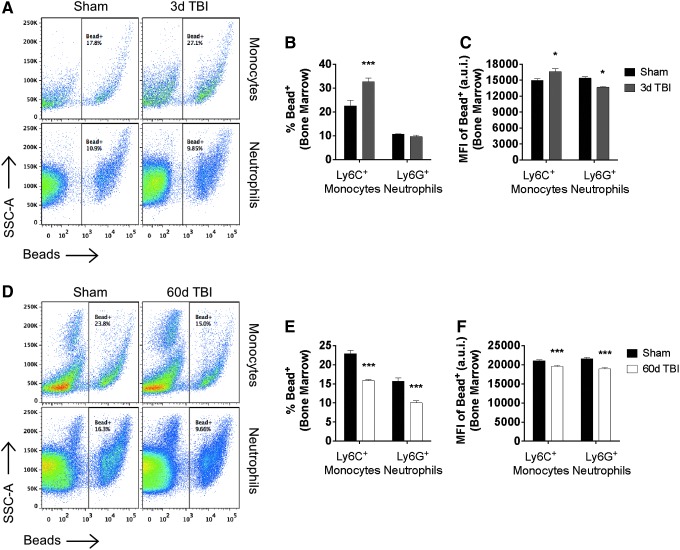
Peripheral immunosuppression is characterized by impairment in phagocyte function.26 The primary function of phagocytes is to protect the body by ingesting harmful foreign particles including bacteria. Thus, we set out to determine whether TBI alters phagocytic responses in peripheral myeloid cells. Day three after TBI was characterized by a significant increase in the percentage and number of phagocytic bone marrow-derived monocytes (p < 0.001), as demonstrated by cellular engulfment of beads (Figs. 7A, B). Conversely, neutrophils ingested significantly fewer beads per phagocyte at this time point as determined by MFI (Fig. 7C; p < 0.05). Interestingly, however, the percentage of phagocytic (i.e., bead-positive) monocytes and neutrophils was uniformly decreased at 60 days post-injury, with each exhibiting a ∼40% reduction in the number of cells capable of phagocytosing at least one bead (Fig. 7D, E; p < 0.001 and p < 0.001, respectively). The number of beads ingested by both subsets (i.e., MFI) was reduced similarly at this late time point (Fig. 7F). These results imply that the acute effects of TBI on the phagocytic potential of peripheral myeloid cells is complex, with newly generated monocytes exhibiting greater phagocytic potential that eventually becomes profoundly impaired during the chronic phase after TBI.
FIG. 7.
Traumatic brain injury (TBI) causes long-term deficits in phagocytic activity of peripheral myeloid cells. Representative dot plots show the acute effect of TBI on the phagocytic function of bone marrow-derived Ly6C+ monocytes and Ly6G+ neutrophils at day three post-injury (A). TBI-induced bone marrow activation leads to a significant increase in number of bead-positive Ly6C+ monocytes compared with sham control, but no change in the percentage of bead-positive Ly6G+ neutrophils (B). The level of phagocytic activity (i.e., number of beads per bead-positive cell) was increased similarly in Ly6C+ monocytes at day three, whereas Ly6C+ neutrophils exhibited a significant reduction in the number of beads they were capable of ingesting (C). Representative dot plots depicting the phagocytic function of bone marrow-derived Ly6C+ monocytes and Ly6G+ neutrophils at day 60 post-injury (D). The percentage of bead-positive Ly6C+ monocytes and Ly6C+ neutrophils was reduced significantly at 60 days after TBI (E), as was the level of phagocytic activity for bead-positive cells in each population (F). For all experiments, n = 5–6/group. Statistical comparisons between groups were determined by two-way analysis of variance with the Sidak test. Error bars show mean standard error of the mean. SSC-A, side scatter-area; MFI, mean fluorescence intensity; a.u.i., arbitrary units of intensity. *p < 0.05 and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
The internalization of antigen through phagocytosis and subsequent processing into peptide fragments is essential for the bidirectional communication between the innate and adaptive immune systems. Peptides are loaded into MHCII molecules and presented on the cell surface of professional antigen presenting cells (APC) where they are then recognized by T cell antigen receptors (TCR) on CD4 T cells. Both the percentage of monocytes (and monocyte-derivatives) expressing MHCII and its relative expression level were down-regulated significantly at day three post-injury, whereas at day 60, the percentage of MHCII+ cells was significantly higher compared with time-matched sham levels (Supplementary Fig. 2A–C; see online supplementary material at ftp.liebertpub.com); p < 0.01 and p < 0.01, respectively). MHCII expression was associated with higher phagocytic potential (Supplementary Fig. 2D).
Although no long-term reduction in MHCII expression was found, stimulus-induced phagocytosis was impaired profoundly in monocytes and neutrophils at day 60 post-injury (Fig. 8A–C). While PMA/ionomycin stimulation promoted neutrophil bead engulfment in sham mice, the number of neutrophils that phagocytosed any beads was surprisingly decreased at chronic time points after TBI (Fig. 8B; p < 0.001). The level of phagocytic activity in bead-positive cells was reduced similarly in both subsets after stimulation, albeit significantly more so in monocyte populations (Fig. 8C). Together these data imply that antigen presentation may be disrupted in the acute phase after TBI and is followed by long-term impairment in stimulus-driven phagocytic activity, thus, potentially preventing the internalization, processing, and presentation of bacterial antigen to cognate T cells responsible for mounting an adaptive immune response.
FIG. 8.
Traumatic brain injury (TBI) causes chronic impairment in stimulus-induced phagocytic activity. Representative histograms demonstrate chronic impairments in phagocytic function stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin in myeloid cells in the spleen at day 60 post-injury: A: Ly6C+ monocytes (blue), Ly6G+ neutrophils (red), Sham groups (open), TBI groups (tinted), vehicle (solid), stimulation (dashed), and fluorescence minus one (FMO) control (tinted gray). The percentage of bead-positive Ly6G+ neutrophils decreased significantly after stimulation compared with control, which showed an increase in bead uptake (B). The number of beads ingested by each phagocyte was impaired similarly after stimulation in myeloid cells harvested 60 days after TBI (C; n = 6/group). Statistical comparisons between time points were evaluated by two-way analysis of variance with the Sidak test (B and C). Error bars show mean standard error of the mean. MFI, mean fluorescence intensity. **p < 0.01 and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
TBI alters T cell activation and cytokine production and induces lasting changes in composition
Once an antigenic epitope has been recognized by the TCR, T cells become activated and under specific stimulatory conditions may themselves become polarized to produce immunoregulatory cytokines. Type 1 cytokine signaling (i.e., IFNγ, TNF) is protective in most infections, whereas type 2 responses assist with the resolution of cell-mediated inflammation.27 To determine the pro-inflammatory cytokine status of T cells after TBI, we probed them by PMA/ionomycin stimulation followed by intracellular staining. Whereas stimulation elicited strong IFNγ responses in both CD4+ and CD8+ T cell subsets in sham controls, relative expression levels were significantly blunted at day three post-injury (Fig. 9A, B; p < 0.001 and p < 0.001, respectively). TNF expression levels were similarly reduced at day three post-injury in CD4+ T cells (p < 0.01); however, no injury effect was seen in CD8+ T cells (Fig. 9C, D). At chronic time points, however, we observed a significant effect of TBI on IFNγ and TNF production in both CD4+ and CD8+ T cell subsets, with nearly two-fold as many T cells expressing type 1 cytokines in TBI mice after stimulation compared with time-matched sham controls (Fig. 9E–H). These results imply that type 1 T cell responses are largely suppressed in the immediate aftermath of TBI, but later become exacerbated during the chronic stages of injury.
FIG. 9.
Traumatic brain injury (TBI) induces acute suppression and chronic upregulation of Th1 cytokine production in T lymphocytes. Representative histograms illustrate the relative production levels of interferon (IFN)γ (A) and tumor necrosis factor (TNF) (C) in CD4+ and CD8+ T cell subsets in the spleen at day three post-injury (CD4+ – sham (blue full, open), CD4+ – TBI (blue full, tinted), CD4+ – sham + stim (blue hatched, open), CD4+ – TBI + stim (blue hatched, tinted), CD8+ – sham (red full, open), CD8+ – TBI (red full, tinted), CD8+ – sham + stim (red hatched, open), CD8+ – TBI + stim (red full, tinted), and FMO control (gray); vertical fiducial line included for reference). The MFI of IFNγ-positive (C) and TNF-positive (D) T cells for all groups is shown. Following stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin, IFNγ expression levels were significantly reduced in CD4+ and CD8+ T cells, whereas TNF expression was only significantly reduced in CD4+ T cells. Representative histograms illustrate the relative production levels of IFNγ (E) and TNF (F) in CD4+ and CD8+ T cell subsets at day 60 post-injury (CD4+ – sham (blue full, open), CD4+ – TBI (blue full, tinted), CD4+ – sham + stim (blue hatched, open), CD4+ – TBI + stim (blue hatched, tinted), CD8+ – sham (red full, open), CD8+ – TBI (red full, tinted), CD8+ – sham + stim (red hatched, open), CD8+ – TBI + stim (red full, tinted), and fluorescence minus one (FMO) control (gray); vertical fiducial line included for reference). The percentage of IFNγ-positive (G) and TNF-positive (H) T cells for all groups is shown. After PMA/ionomycin stimulation, IFNγ expression levels in the 60-day TBI group was significantly increased in CD8+ T cells only. There was a significant increase in the percentage of TNF-positive CD4+ and CD8+ T cells in the 60-day TBI group that was increased further after stimulation. For all experiments, n = 7/group. Statistical comparisons between stimulation groups were performed using two-way analysis of variance with the Sidak test. Error bars show mean standard error of the mean. MFI, mean fluorescence intensity; a.u.i., arbitrary units of intensity. *p < 0.05, **p < 0.01, and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
Given the chronic dysfunction in T cell cytokine production after TBI, we next ascertained whether these changes were accompanied by lasting alterations in the composition of the T cell compartment. An analysis of T cell memory subsets in the blood at day 60 post-injury revealed a significant conversion of naïve (CD44-CD62L+) CD4+ T cells into effector memory (Tem, CD44+CD62L-) populations (Fig. 10A, B; p < 0.001). No differences were seen in CD8+ memory composition (data not shown); however, the relative expression level of the activation marker CD44 was elevated significantly in these cells at day 60 post-injury (Fig. 10C; p < 0.01). Consistent with the TBI-induced generation of CD4+ Tem populations, we found a significant increase in the percentage of CD4+ T cells expressing the TCR activation marker, CD69 (Fig. 10D, E; p < 0.01). In addition, we observed a significant inversion of the CD4:CD8 ratio in blood compared with time-matched sham controls, suggesting TBI causes long-term impairment of the cell-mediated immunity (Fig. 10F; p < 0.05). Collectively, these data indicate that TBI leads to a profound rearrangement of the T cell compartment, evidenced by increased TCR activation and memory conversion, and a marked reduction in circulating T helper cells.
FIG. 10.
Long-term changes in T cell memory phenotype, T cell antigen receptors (TCR) activation state, and CD4:CD8 ratios after TBI. Chronic alterations in the circulating T cell compartment were assessed after TBI. Representative dot plots depict the relative proportion of naïve (CD44-CD62L+), central memory (CD44+CD62L+), and effector memory (CD44+CD62L-) populations in CD4+ T cell subsets (A). The percentage of naïve, central memory, and effector memory CD4+ (B) T cells was quantified (n = 9–15/group). The mean fluorescence intensity (MFI) of CD44+ T cells were quantified (C). A representative dot plot shows CD69 expression on T cell subsets at day 60 after TBI (D). The percentage of CD69-positive CD4+ T cells was significantly increased after TBI (E; n = 5/group). The CD4:CD8 ratio in the blood was significantly inverted at day 60 after TBI (F). Statistical comparisons between stimulation groups were performed using two-way analysis of variance with the Sidak test. Error bars show mean standard error of the mean. SSC-A, side scatter-area. *p < 0.05, **p < 0.01, and ***p < 0.001. Color image is available online at www.liebertpub.com/neu
Long-term macrophage dysfunction induced by TBI
The long-term dysfunction in myeloid and lymphocyte responses after TBI suggests that brain trauma induces permanent changes in peripheral immune cells. Whether these changes are dependent on the environmental milieu (e.g., extrinsic factors generated by TBI) or reflect intrinsic, epigenetic imprinting in sympathetic stress-activated bone marrow progenitors is not clear. To address this question, we harvested bone BMDMs from chronically injured (day 60 post-injury) and time-matched sham control mice and cultured them in vitro for one week. The BMDMs were stimulated with either LPS or IL-4, and pro-inflammatory and anti-inflammatory markers were assessed.
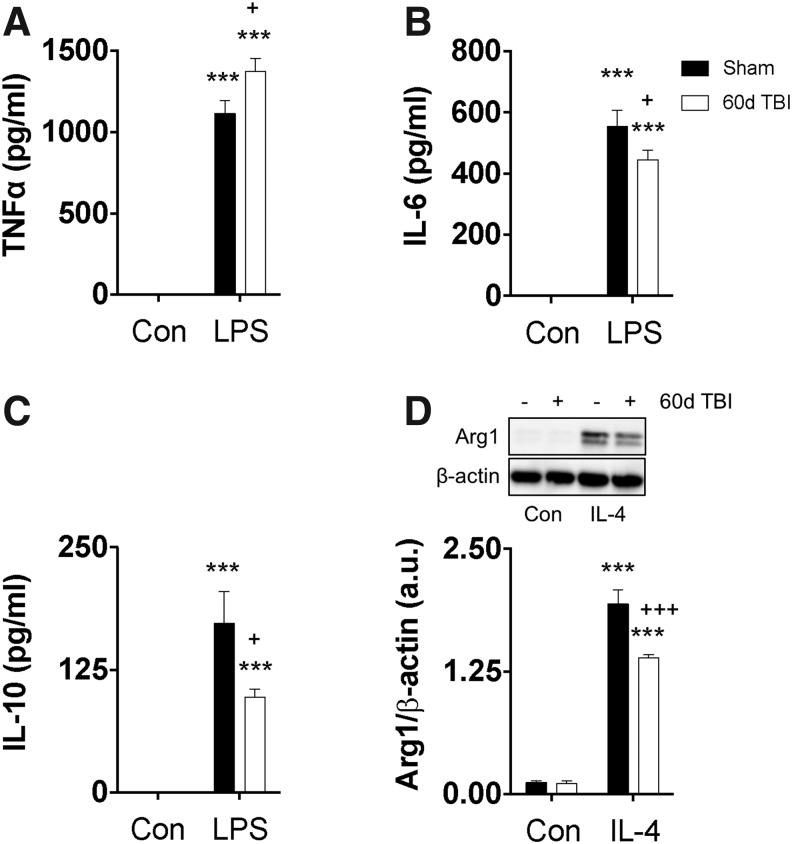
Notably, there were significant polarization deficits in BMDMs isolated from mice with chronic TBI when compared with BMDMs from time-matched sham control mice. Specifically, chronic TBI BMDMs exhibited comparably greater secretion of TNF (p < 0.05) and lower concentrations of IL-6 (p < 0.05) and IL-10 (p < 0.05) after LPS stimulation when compared with sham control BMDMs (Fig. 11A–C). Whole cell protein expression for the anti-inflammatory and alternative marker, Arg1, demonstrated that in chronic TBI BMDMs, Arg1 was significantly decreased (p < 0.001) after IL-4 stimulation when compared with Arg1 expression levels in sham BMDMs (Fig. 11D). Thus, our data suggest that bone marrow-derived macrophages from mice with chronic TBI exhibit long-lasting, or permanent, changes in inflammatory gene expression, which may be regulated by underlying epigenetic mechanisms.
FIG. 11.
Traumatic brain injury (TBI)-induced chronic bone marrow dysfunction persists in the absence of brain injury or environmental cues. Bone marrow-derived macrophages were harvested from age-matched sham and TBI mice at day 60 and cultured in vitro to assess polarization dynamics after either lipopolysaccharide (LPS) or interleukin (IL)-4 stimulation. Supernatants were examined by enzyme-linked immunosorbent assay, and secreted protein concentrations for tumor necrosis factor (TNF) (A), IL-6 (B), and IL-10 (C) were quantified under control and LPS-stimulated conditions. Representative images of Western blots and subsequent densitometry quantification showing relative protein expression of Arg1/β-actin (D) in whole cell lysate under control and IL-4–stimulated conditions. For all experiments, n = 5/group. Statistical comparisons between stimulation groups were performed using two-way analysis of variance with the Sidak test. Error bars show mean standard error of the mean. a.u. arbitrary unit. *p < 0.05 and ***p < 0.001.
Discussion
This study demonstrates that TBI has profound and persisting effects on systemic immunity that show similarities to changes associated with normal aging. TBI caused long-term dysfunction in oxidative stress levels, respiratory burst, phagocytosis, cytokine production, and polarization responses, together with increased thymic involution, lymphopenia, memory conversion, TCR activation, and CD4:CD8 inversion. These changes occurred in parallel with robust activation of neuroinflammatory pathways in the injured brain (data not shown), which have been shown to drive secondary neurodegeneration at distant sites (e.g.. hippocampus and thalamus), and result in cognitive impairments and long-term neurological dysfunction in mice with TBI.7,11,28 The marked and sustained changes in systemic immune function after injury may explain the high rates of infection and infection-related mortality after brain trauma.5,29–31 Further, the apparent effect of TBI to accelerate immune aging suggests that survivors may have increased senescence and age-related immune dysfunction longer term.
Brain injury can elicit a robust stress-induced hematopoietic response via sympathetic activation of bone marrow.32,33 The bone marrow response is evidenced by acutely elevated neutrophil counts in the blood of humans and mice after TBI. Despite previous reports demonstrating increased neutrophil number during the first two days after TBI,34,35 few studies have examined the change in composition of bone marrow leukocytes.
Our study is the first to demonstrate evidence for early robust bone marrow activation after TBI, with significantly increased myelopoiesis observed by day one in distal bone marrow. These early increases in de novo neutrophil and monocyte production were followed subsequently by elevation in circulating blood myeloid cell counts, indicating sustained mobilization and egress of cells from bone marrow. This bone marrow myelopoiesis after TBI appeared to be biphasic, with a second increase at day 14. Given the large time gap between day 14 and day 60, at which time bone marrow myeloid cell counts normalize to baseline levels, it is unknown what effect this delayed myelopoiesis may have on the progression of injury and/or recovery. Although myelopoiesis returned to baseline levels by day 60, functional analyses demonstrates that these cells were dysregulated fundamentally with regard to basal and stimulus-induced activation.
Neutrophils are the first line of defense against rapidly dividing bacteria, fungi, and yeast and, thus, are instrumental in promoting resistance to infection. We demonstrated profound and temporal changes in neutrophil function after TBI. Previous studies have shown substantial increases in NOS2, COX2, and NOX2 in leukocyte homogenates from patients with TBI at 24 h.36 Although some studies suggest that ROS production is significantly higher in neutrophils of patients with TBO at 24 h,35,36 others indicate that ROS production is reduced by day nine after TBI.37,38 Our data are consistent with such previous work, showing increased ROS levels at day three but subsequent reduction by day seven. Although the biological importance of this demonstrated impairment in respiratory burst activity is not known, we can only speculate that such an aberrant physiological response would not be as effective in killing microbes.
This is evidenced further in patients with an inherited immunodeficiency in NADPH oxidase (NOX2)-dependent phagocytes known as chronic granulomatous disease that exhibit deficits in oxidative burst, as measured by DHR123, and show an increased susceptibility to infection.39,40 We also showed long-term impairments in the phagocytic activity of both neutrophil and monocyte populations after TBI. Similar deficiencies in the phagocytosis of Escherichia coli have been demonstrated in patients with TBI for up to two weeks.36,37
Pathways that link the brain and immune system are disrupted after injury, resulting in systemic immune dysfunction, termed CNS injury-induced immune deficiency syndrome.6 Such changes have been described primarily in experimental stroke models41–43 and less well examined after head injury. The present studies revealed profound deficits in innate immune cell function within the first week after TBI. These changes include reduced TNF and IL-1β production in monocytes, as well as decreased neutrophil phagocytosis, lower basal ROS production, and decreased MHCII expression.
There was an association between MHCII expression on APCs and Th1 cytokine production levels in CD4 T cells at both acute and chronic stages after TBI, although its impact on antigen presentation capacity will need to be examined in greater detail. Human leukocyte antigen-antigen D related expression on peripheral blood monocytes has been shown to correlate with impaired TNF responses in sepsis, and low expression correlates with higher risk and severity of surgical infection.44,45 Increased circulating and spleen-trafficking/borne myeloid cells were also found at these early time points. Although it is not clear whether the accumulation of myeloid cells in the spleen is because of de novo monocyte production in situ or augmented levels of trafficking myeloid cells, these findings differ from those in experimental stroke models (i.e., middle cerebral artery occlusion), which induce splenic atrophy and lymphocyte apoptosis.46
TBI also caused significant alterations in adaptive immunity, with marked thymic atrophy and a striking depletion of all T cell subsets early after injury. Long-term deficits in CD4+ and CD8+ T cell selection were also found. The chronic reduction in the thymic output of mature naïve T cells is consistent with an earlier study,19 as well as our finding that TBI causes long-term T lymphopenia. Clinically, the reported frequency of T cells in the blood of patients with head trauma is below control group levels early after the insult; however, levels gradually normalized during hospitalization.47 A study has shown that 65% of patients with severe TBI show T lymphopenia on admission, with reductions in T cell number associated with worse neurological outcome and an increase in pulmonary infection.48 Acutely, we found a blunted induction of IFNγ production by both CD3+ subsets after TBI. IFNγ plays an important role in promoting Th1 responses that may affect progression of brain injury.49–51 These experiments used an artificial T cell stimulation protocol (i.e., PMA/ionomycin), and while future studies will require more physiologically relevant stimuli that involve TCR ligation to better understand T cell functional responses, biological differences in cytokine production were nevertheless evident after TBI.
We also demonstrated exaggerated chronic Th1 cytokine production in both CD4+ and CD8+ subsets and a significant increase in the percentage of CD69+ effector memory T cells. The decrease in overall circulating T cell numbers and subsequent increase in the frequency of memory populations suggests that the TCR repertoire may shrink in the chronic phase of TBI, leading to a reduced capacity to respond to novel pathogenic antigens. The expression of CD69, an early activation marker of TCR ligation, at chronic periods after TBI, indicates a low level of persistent antigenic stimuli/exposure, possibly driven by chronic leakiness of the gut.52 While it is important to note that CD69 expression can be induced without cognate antigen recognition, we observed that CD69+ T cells exclusively had memory phenotype. Finally, our finding that circulating CD4:CD8 ratios were inverted chronically after TBI indicates deterioration of the immune state.53 Alteration in the CD4:CD8 ratios in rats subjected to repetitive mild TBI was similarly demonstrated at four weeks.54 Taken together, these data collectively suggest there are adaptive immune changes during the chronic phase of TBI that reflects diminished cell-mediated immunity.
Injured mice exhibited long-term dysfunction in bone marrow-derived myeloid responses, including deficits in stimulus-driven phagocytosis, oxidative burst, and cytokine production. These changes occurred gradually over a period of 60 days after TBI and are reminiscent of normal aging phenotypes.55–57 Importantly, when cultured under defined in vitro conditions, bone marrow-derived macrophages from chronically injured mice still demonstrated significant stimulus-induced polarization deficits, implying these changes are cell-intrinsic and potentially permanent. The question remains as to how cellular dysfunction is maintained stably across generations of relatively short-lived myeloid cells if it is not driven chronically by extrinsic factors in the injury environment. Emerging data point to epigenetic regulation of innate immune responses, and that perturbation of these epigenetic mechanisms can drastically alter normal macrophage function and contribute to pathologies ranging from obesity and autoimmunity to neurodegenerative diseases.58,59 Therefore, moderate-to-severe TBI may induce epigenetic changes in bone marrow progenitors that are inherited and propagated over time, resulting in long-term systemic immune dysfunction. Further investigation into underlying epigenetic mechanisms and the potential use of histone deacetylase inhibitors to target systemic immune dysfunction after TBI is clearly needed to address this open question.
Conclusion
We provide strong evidence for chronic and persistent innate and adaptive immune dysfunction after TBI. These changes were demonstrated in multiple leukocyte subsets and characterized by acute immune suppression and chronic immune impairment. Moreover, TBI in young mice induces long-term changes in leukocyte composition, function, and response to secondary challenge, similar to alterations found in aging immune senescence phenotypes. We have identified several key hallmark features underlying systemic immune impairment after TBI. Future studies will be required to unravel the mechanisms behind TBI-induced immune dysfunction and, subsequently, how changes in leukocyte behavior affect brain injury and long-term neurological outlook.
Supplementary Material
Acknowledgments
We would like to acknowledge Ethan Glaser, B.Sc., for his technical assistance, and Dr. Xiaoxuan Fan, Ph.D., and Karen Underwood, B.Sc., of the University of Maryland Greenebaum Comprehensive Cancer Center Flow Cytometry Facility for support with flow cytometry studies. We would also like to thank Dr. Nevil Singh, Ph.D., for helpful discussion. This work was supported by National Institutes of Health grants R01NS082308 (D.J. Loane), R01NS037313 (A.I. Faden), and F32NS105355 (R.M. Ritzel), and The National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center P30-AG028747 (D.J. Loane).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 [PubMed] [Google Scholar]
- 2.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 3.Hazeldine J., Lord J.M., and Belli A. (2015). Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front. Neurol. 6, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alharfi I.M., Charyk Stewart T., Al Helali I., Daoud H., and Fraser D.D. (2014). Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J. Neurotrauma 31, 452–458 [DOI] [PubMed] [Google Scholar]
- 5.Scott B.N., Roberts D.J., Robertson H.L., Kramer A.H., Laupland K.B., Ousman S.S., Kubes P., and Zygun D.A. (2013). Incidence, prevalence, and occurrence rate of infection among adults hospitalized after traumatic brain injury: study protocol for a systematic review and meta-analysis. Syst. Rev. 2, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisel C., Schwab J.M., Prass K., Meisel A., and Dirnagl U. (2005). Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786 [DOI] [PubMed] [Google Scholar]
- 7.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith C., Gentleman S.M., Leclercq P.D., Murray L.S., Griffin W.S., Graham D.I., and Nicoll J.A. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C. (2013). Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 35–44 [DOI] [PubMed] [Google Scholar]
- 11.Kabadi S.V., Stoica B.A., Byrnes K.R., Hanscom M., Loane D.J., and Faden A.I. (2012). Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J. Cereb. Blood Flow Metab. 32, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 14.Cole J.H., Leech R., Sharp D.J., and Alzheimer's Disease Neuroimaging Initiative. (2015). Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 77, 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith D.H., Johnson V.E., and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood R.L. (2017). Accelerated cognitive aging following severe traumatic brain injury: A review. Brain Inj. 31, 1270–1278 [DOI] [PubMed] [Google Scholar]
- 17.Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., and De Benedictis G. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 [DOI] [PubMed] [Google Scholar]
- 18.Woodcock T. and Morganti-Kossmann M.C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwulst S.J., Trahanas D.M., Saber R., and Perlman H. (2013). Traumatic brain injury-induced alterations in peripheral immunity. J. Trauma Acute Care Surg. 75, 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., and Burns M.P. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritzel R.M., Patel A.R., Grenier J.M., Crapser J., Verma R., Jellison E.R., and McCullough L.D. (2015). Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett J.P., Henry R.J., Villapol S., Stoica B.A., Kumar A., Burns M.P., Faden A.I., and Loane D.J. (2017). NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J. Neuroinflammation 14, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Bazhin A.V., Werner J., and Karakhanova S. (2013). Reactive oxygen species in the immune system. Int. Rev. Immunol. 32, 249–270 [DOI] [PubMed] [Google Scholar]
- 24.El-Benna J., Hurtado-Nedelec M., Marzaioli V., Marie J.C., Gougerot-Pocidalo M.A., and Dang P.M. (2016). Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol. Rev. 273, 180–193 [DOI] [PubMed] [Google Scholar]
- 25.Vernon P.J., Schaub L.J., Dallelucca J.J., Pusateri A.E., and Sheppard F.R. (2015). Rapid detection of neutrophil oxidative burst capacity is predictive of whole blood cytokine responses. PLoS One 10, e0146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosmann M. and Ward P.A. (2013). The inflammatory response in sepsis. Trends Immunol. 34, 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spellberg B. and Edwards J.E., Jr (2001). Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 32, 76–102 [DOI] [PubMed] [Google Scholar]
- 28.Byrnes K.R., Loane D.J., Stoica B.A., Zhang J., and Faden A.I. (2012). Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflammation 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesinger M.R., Kumar R.G., Wagner A.K., Puyana J.C., Peitzman A.P., Billiar T.R., and Sperry J.L. (2015). Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J. Trauma Acute Care Surg. 78, 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selassie A.W., Fakhry S.M., and Ford D.W. (2011). Population-based study of the risk of in-hospital death after traumatic brain injury: the role of sepsis. J. Trauma 71, 1226–1234 [DOI] [PubMed] [Google Scholar]
- 31.Suz P., Vavilala M.S., Souter M., Muangman S., and Lam A.M. (2006). Clinical features of fever associated with poor outcome in severe pediatric traumatic brain injury. J. Neurosurg. Anesthesiol. 18, 5–10 [DOI] [PubMed] [Google Scholar]
- 32.Courties G., Herisson F., Sager H.B., Heidt T., Ye Y., Wei Y., Sun Y., Severe N., Dutta P., Scharff J., Scadden D.T., Weissleder R., Swirski F.K., Moskowitz M.A., and Nahrendorf M. (2015). Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 116, 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denes A., McColl B.W., Leow-Dyke S.F., Chapman K.Z., Humphreys N.E., Grencis R.K., Allan S.M., and Rothwell N.J. (2011). Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J. Cereb Blood Flow Metab. 31, 1036–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhind S.G., Crnko N.T., Baker A.J., Morrison L.J., Shek P.N., Scarpelini S., and Rizoli S.B. (2010). Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J. Neuroinflammation 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junger W.G., Rhind S.G., Rizoli S.B., Cuschieri J., Baker A.J., Shek P.N., Hoyt D.B., and Bulger E.M. (2013). Prehospital hypertonic saline resuscitation attenuates the activation and promotes apoptosis of neutrophils in patients with severe traumatic brain injury. Shock 40, 366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y., Liu P., Guo F., Zhang Z.Y., and Zhang Z. (2013). Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One 8, e68963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks W., Gołąbek-Dropiewska K., Bryl E., Dudek R., Wieruszewski J., Stasiak M., Witkowski Z., Lasek J., Pawłowska J., and Jóźwik A. (2013). Immunomonitoring in patients with early moderate and severe head trauma. Cent. Eur. J. Immunol. 38, 494–499 [Google Scholar]
- 38.Wolach B., Sazbon L., Gavrieli R., Broda A., and Schlesinger M. (2001). Early immunological defects in comatose patients after acute brain injury. J. Neurosurg. 94, 706–711 [DOI] [PubMed] [Google Scholar]
- 39.Chen Y. and Junger W.G. (2012). Measurement of oxidative burst in neutrophils. Methods Mol. Biol. 844, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gennery A. (2017). Recent advances in understanding and treating chronic granulomatous disease. F1000Res. 6, 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emsley H.C. and Hopkins S.J. (2010). Post-stroke immunodepression and infection: an emerging concept. Infect. Disord. Drug Targets 10, 91–97 [DOI] [PubMed] [Google Scholar]
- 42.Meisel C., Prass K., Braun J., Victorov I., Wolf T., Megow D., Halle E., Volk H.D., Dirnagl U., and Meisel A. (2004). Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke 35, 2–6 [DOI] [PubMed] [Google Scholar]
- 43.Liesz A., Hagmann S., Zschoche C., Adamek J., Zhou W., Sun L., Hug A., Zorn M., Dalpke A., Nawroth P., and Veltkamp R. (2009). The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke 40, 2849–2858 [DOI] [PubMed] [Google Scholar]
- 44.Winkler M.S., Rissiek A., Priefler M., Schwedhelm E., Robbe L., Bauer A., Zahrte C., Zoellner C., Kluge S., and Nierhaus A. (2017). Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFalpha response: a diagnostic tool for immunosuppression? PLoS One 12, e0182427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheadle W.G., Hershman M.J., Wellhausen S.R., and Polk H.C., Jr (1991). HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am. J. Surg. 161, 639–645 [DOI] [PubMed] [Google Scholar]
- 46.Offner H., Subramanian S., Parker S.M., Wang C., Afentoulis M.E., Lewis A., Vandenbark A.A., and Hurn P.D. (2006). Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 176, 6523–6531 [DOI] [PubMed] [Google Scholar]
- 47.Smrcka M., Mrlian A., and Klabusay M. (2005). Immune system status in the patients after severe brain injury. Bratisl. Lek. Listy. 106, 144–146 [PubMed] [Google Scholar]
- 48.Mazzeo A.T., Kunene N.K., Gilman C.B., Hamm R.J., Hafez N., and Bullock M.R. (2006). Severe human traumatic brain injury, but not cyclosporin a treatment, depresses activated T lymphocytes early after injury. J. Neurotrauma 23, 962–975 [DOI] [PubMed] [Google Scholar]
- 49.Lozano D., Gonzales-Portillo G.S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., and Borlongan C.V. (2015). Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 11, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz G., Arumugam T.V., Stokes K.Y., and Granger D.N. (2006). Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113, 2105–2112 [DOI] [PubMed] [Google Scholar]
- 51.Seifert H.A., Leonardo C.C., Hall A.A., Rowe D.D., Collier L.A., Benkovic S.A., Willing A.E., and Pennypacker K.R. (2012). The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab. Brain Dis. 27, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma E.L., Smith A.D., Desai N., Cheung L., Hanscom M., Stoica B.A., Loane D.J., Shea-Donohue T., and Faden A.I. (2017). Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 66, 56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luz Correa B., Ornaghi A.P., Cerutti Muller G., Engroff P., Pestana Lopes R., Gomes da Silva Filho I., Bosch J.A., Bonorino C., and Bauer M.E. (2014). The inverted CD4:CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation 21, 206–212 [DOI] [PubMed] [Google Scholar]
- 54.Bai R., Gao H., Han Z., Ge X., Huang S., Chen F., and Lei P. (2017). Long-term kinetics of immunologic components and neurological deficits in rats following repetitive mild traumatic brain injury. Med. Sci. Monit. 23, 1707–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brubaker A.L., Palmer J.L., and Kovacs E.J. (2011). Age-related dysregulation of inflammation and innate immunity: lessons learned from rodent models. Aging Dis. 2, 346–360 [PMC free article] [PubMed] [Google Scholar]
- 56.Linehan E. and Fitzgerald D.C. (2015). Ageing and the immune system: focus on macrophages. Eur. J. Microbiol. Immunol. (Bp) 5, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikolich-Zugich J. (2014). Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J. Immunol. 193, 2622–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perkins D.J., Patel M.C., Blanco J.C., and Vogel S.N. (2016). Epigenetic mechanisms governing innate inflammatory responses. J. Interferon Cytokine Res. 36, 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amit I., Winter D.R., and Jung S. (2016). The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol. 17, 18–25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.