Abstract
Preoperative portal vein embolization (PVE) induces compensatory hyperplasia of the future liver remnants (FLR), thus increasing resectability in the non-cirrhotic patients with primary liver cancer (PLC). However, it is unclear if it is similar in patients with liver cirrhosis. Therefore, the present study investigated the PVE value prior to liver resection in patients with PLC, and the liver cirrhotic effects on the compensatory hypertrophy of FLRs following PVE. In the present study, 21 patients with PLC who successfully underwent hepatic resection subsequent to PVE, were retrospectively examined. The patients were divided into a non-cirrhosis group and a cirrhosis group according to the absence or presence of cirrhosis, respectively. The FLR volume between the two groups of patients was compared. There was a significant difference in the FLR volume for all patients prior to, and 4–6 weeks following, PVE (P<0.001). PVE induced significant compensatory hypertrophy in the FLRs whether in the non-cirrhosis group (P=0.002) or cirrhosis group (P<0.001). However, no significant difference was identified between the two groups with respect to FLR volume enlargement 4–6 weeks following PVE (P=0.373). In conclusion, PVE prior to hepatectomy may promote FLR compensatory hypertrophy and an increase in the resectability of PLC tumors. No significant effects of liver cirrhosis were identified on liver lobe hyperplasia following PVE.
Keywords: liver cancer, cirrhosis, embolization, portal vein, hyperplasia
Introduction
Surgical liver resection has become a primary method for treating liver cancer in previous decades; however, a shortage in the postoperative residual liver volume has been an important factor in the surgical resection of liver cancer, and postoperative liver failure is one of the major complications (1–3) with this surgery. Numerous studies have reported that percutaneous selective portal vein embolization (PVE) prior to liver resection may effectively induce compensatory hyperplasia of the left hepatic lobe, and reduce the incidence of postoperative liver failure (4–7). There is a consensus that PVE may lead to left hepatic lobe compensatory hyperplasia in patients with liver cancer and no liver cirrhosis (8–11).
In China, the majority of patients with primary liver cancer have associated liver cirrhosis following chronic hepatitis B, and their liver function reserve is poor (12). Postoperative patients typically experience liver failure, which may result in mortality (13). A previous study revealed that there are fewer liver cirrhosis effects on compensatory hypertrophy of the left lobe subsequent to PVE (14). Therefore, the purpose of the present study was to investigate the value of preoperative PVE and the effects of liver cirrhosis on left hepatic lobe hyperplasia, which may have important clinical significance for the correct implementation of PVE and two-step hepatectomy for liver cancer. A total of 21 patients with liver cancer successfully underwent a right hepatectomy following PVE. Combined with postoperative pathological data, the effects of liver cirrhosis on the compensatory hypertrophy of future liver remnants (FLRs) subsequent to PVE were determined.
Patients and methods
Patient population
A total of 21 patients who underwent a hepatic resection following PVE without major complications between January 2010 and December 2012 were identified and retrospectively evaluated. This group included 20 males and 1 female with a mean age of 52.1±11.3 years (range, 22–64). The results of tests for the hepatitis B surface antigen were positive in 16 patients. There were 14 patients with a single lesion and 7 patients with multiple lesions in the right lobe of the liver. The mean maximum diameter of the tumors was 8.6±2.4 cm (range, 4.7–12.8). All patients were divided into non-cirrhosis (n=9, fibrosis score <3) and cirrhosis (n=12, fibrosis score ≥3) groups, according to the classification of Knodell et al (15); preoperative imaging studies, including ultrasonography (US), computed tomography (CT) or magnetic resonance imaging (MRI) and postoperative pathological data were obtained for the patients (16). The two groups were compared with baseline clinicopathological characteristics obtained prior to PVE (Table I). The present study was approved by the Ethics Research Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (Hangzhou, China), and they agreed that written informed consent was not necessary due to the retrospective nature of the present study. All data were anonymized and de-identified prior to analysis.
Table I.
Patient clinicopathological characteristics prior to PVE.
| Characteristics | Non-cirrhosis group (n=9) | Cirrhosis group (n=12) | P-value |
|---|---|---|---|
| Sex (male/female) | 9/0 | 11/1 | 1.000 |
| Age (years) | 49.3±14.0 | 54.3±8.8 | 0.337 |
| Weight (kg) | 64.3±12.1 | 62.4±7.8 | 0.664 |
| Tumor size (cm) | 9.4±2.1 | 8.0±2.6 | 0.188 |
| Tumor multiplicity (single/multiple) | 6/3 | 8/4 | 1.000 |
| Pathological type (hepatocellular carcinoma/cholangiocarcinoma) | 7/2 | 10/2 | 1.000 |
| HBsAg (+/-) | 6/3 | 10/2 | 0.611 |
| ALT (U/l) | 43.67±18.96 | 49.00±29.40 | 0.641 |
| AST (U/l) | 50.78±23.16 | 56.25±38.41 | 0.710 |
| TB (µmol/l) | 14.44±6.46 | 19.17±9.93 | 0.231 |
| Prothrombin time (sec) | 12.29±1.80 | 12.38±0.88 | 0.875 |
| Child-Pugh class (A/B/C) | 9/0/0 | 12/0/0 | n/a |
| TACE session (1/2/3) | 7/1/01 | 7/2/03 | 0.630 |
| FLR (cm3) | 447.9±86.7 | 412.4±61.3 | 0.285 |
| FLR/weight (%) | 0.70±0.07 | 0.66±0.10 | 0.374 |
PVE, preoperative portal vein embolization; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; TACE, transarterial chemoembolization; FLR, future liver remnants; n/a, not applicable.
Transcatheter arterial chemoembolization
Transarterial chemoembolization (TACE) was performed 2–4 weeks prior to PVE. Under fluoroscopic surveillance, a conventional superior mesenteric and a common hepatic arteriography were initially performed to assess the hepatic arterial anatomy, tumor burden, vascularity and portal circulation on venous-phase films. Subsequently, the tip of the catheter was selectively placed in the right hepatic artery and cisplatin and hydroxycamptothecin were slowly infused for 15 min into the hepatic artery. The infused dose of cisplatin and hydroxycamptothecin were both 2 mg/kg body weight. Subsequently, a mixture of 10–15 ml iodized oil (Lipiodol Ultrafluid; Guerbet Laboratories, Paris, France) and 20 mg epirubicin/pirarubicin was injected into the tumor-feeding arteries under fluoroscopic surveillance, followed by embolization with gelatin sponge particles (Gelfoam; Upjohn Laboratories, Kalamazoo, MI, USA).
Right PVE
PVE was performed 2–4 weeks following TACE, subsequent to the recovery of liver function (17). Under ultrasound guidance, the secondary branch of the left portal vein was percutaneously punctured with an 18-gauge PTC needle (Kyowa Hakko Co., Ltd., Nagano, Japan), and a 5F sheath (Terumo Co., Ltd., Tokyo, Japan) was introduced. Following portal venography through a 5F C2 angiographic catheter (Cordis Corporation, Miami Lakes, FL, USA), the right portal venous branches were embolized with coils using a 5F SIM2 catheter (Cordis Corporation). The right branch in proximity to the portal vein trunk (1 cm) was retained for surgical separation and stitching of the right portal vein. Repeated portal venography following embolization was performed in order to confirm complete right portal vein occlusion. Finally, the punctured passage was embolized using coils to prevent intraperitoneal hemorrhaging.
Main outcome evaluations
Liver function tests, including prothrombin time (PT) and serum levels of total bilirubin (TB), aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were performed prior to and following PVE, and prior to surgery. Hepatic contrast-enhanced CT [Brilliance-iCT, version 4.1.6.00230; Phillips Medical Systems (Cleveland), Inc., Cleveland, OH, USA] was performed prior to and 4–6 weeks subsequent to PVE in order to evaluate the degree of hypertrophy, and volumetric data was obtained from the portal phase images. Volumetric evaluations were performed using a CT analysis system for the entire liver as well as the left liver lobe (segments I–IV). The FLR volume was considered to represent the left hepatic lobe and caudate lobe as the portal vein was not embolized.
Complications following PVE included intraperitoneal hemorrhage, liver failure [defined by a prothrombin time of <50% of normal and a serum bilirubin level of >50 µmol/l on postoperative day 5 (18)], intraperitoneal hemorrhaging, gastrointestinal bleeding and biliary fistula.
Statistical analysis
Data analysis was performed using the statistical package SPSS for Windows® (version 13.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. Student's t-test, Fisher's exact test, paired sample t-test, independent sample t-test and the χ2 test were applied, where appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
All patients successfully underwent TACE treatment prior to PVE; specifically, 14, 3 and 4 patients underwent 1, 2 and 3 sessions, respectively. PVE was successfully performed in all patients 2–4 weeks following the final TACE treatment (portal vein angiography prior to and subsequent to PVE is presented in Figs. 1 and 2). The mean absolute volume of the FRL was calculated prior to and 4–6 weeks following the PVE, and increased from 427.6±73.5 to 582.6±98.3 cm3 (+37.3%), which was a statistically significant difference (P<0.001). The CT scan prior to and following PVE is presented in Figs. 3 and 4.
Figure 1.

Prior to PVE, portal venography demonstrated fluent blood flow in the main portal vein and branches. PVE, preoperative portal vein embolization.
Figure 2.
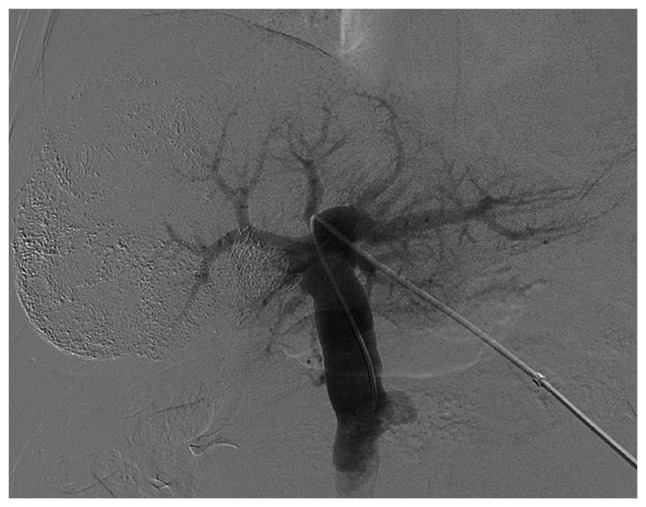
Following PVE, portal venography demonstrated that the left branch and main trunk of the portal vein remained smooth. By contrast, the right portal vein branch demonstrated total occlusion. PVE, preoperative portal vein embolization.
Figure 3.
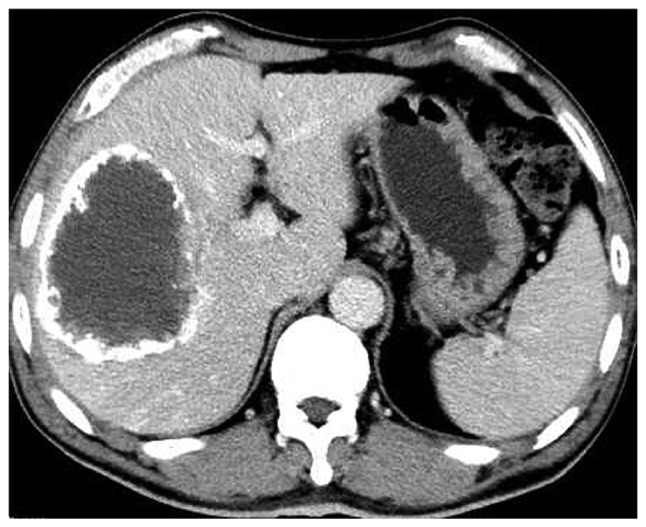
Prior to PVE, the CT scan revealed a large liver tumor in the right lobe of liver. The right hepatectomy could not be performed due to a shortage of left liver volume. PVE, preoperative portal vein embolization; CT, computed tomography.
Figure 4.

A total of 4–6 weeks following PVE, the CT scan demonstrated an increased volume of the left lobe and a decreased volume of the right lobe (FLR: From 481.1 to 670 cm3; FLR increase, 39.3%). Coils were identified in the right lobe of the liver (arrow). PVE, preoperative portal vein embolization; CT, computed tomography; FLR, future liver remnants.
In the univariate analysis, no significant differences were identified in the following factors between the groups: Sex, age, weight, size and number of tumors, pathology, hepatitis B surface antigen, ALT, AST, TB, PT, Child-Pugh class, TACE sessions, FLR and FLR/weight (P>0.05; Table I). Comparative results of the two patient groups are summarized in Table II. Following PVE (4–6 weeks), the left liver volume, as evaluated by enhanced CT, demonstrated that PVE induced significant compensatory hypertrophy whether in the non-cirrhosis group (P=0.002) or cirrhosis group (P<0.001); however, no significant difference was identified between the two groups, with respect to left liver volume enlargement 4–6 weeks following PVE (P=0.373).
Table II.
Comparison of the FLR volume prior to and following PVE.
| FLR comparison | |||
|---|---|---|---|
| Variable | Non-cirrhosis group (n=9) | Cirrhosis group (n=12) | P-value |
| FLR prior to PVE (cm3) | 447.9±86.7 | 412.4±61.3 | 0.285 |
| FLR following PVE (cm3) | 627.2±116.0 | 549.2±70.2 | 0.070 |
| Mean increase in FLR (cm3) | 179.3±145.6 | 136.8±62.6 | 0.373 |
| Mean increased percentage of FLR (%) | 45.6±47.3 | 31.1±16.1 | 0.331 |
| Each group comparison (before and after PVE) (P-value) | 0.002 | <0.001 | |
PVE, preoperative portal vein embolization; FLR, future liver remnants.
No severe complications, including intraperitoneal hemorrhage, gastrointestinal bleeding, biliary fistula or liver failure, developed one week subsequent to PVE in any of the patients. The results from the liver function tests prior to and following PVE, and prior to surgery, for the two patient groups are summarized in Fig. 5A-D. The ALT, AST, TB and PT results were similar in the groups (P>0.05).
Figure 5.
Peak values of liver function results. (A) ALT, (B) AST, (C) total bilirubin and (D) prothrombin time were similar (P>0.05) prior to PVE, following PVE and prior to surgery, in patients with non-cirrhosis liver and cirrhosis liver. Values are presented as the mean ± standard deviation. PVE, preoperative portal vein embolization; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
Radical hepatic resection is frequently contraindicated in a number of patients with liver cancer due to an increased risk for postoperative hepatic failure (1–3). PVE prior to liver resection has been proposed in order to induce atrophy of embolized lobes with compensatory hypertrophy of non-embolized FLRs, therefore preventing postoperative liver failure and improving the two-step resection rate of liver cancers (19–23). In the present study, surgical resection was not recommended for patients with large liver tumors (multiple right hepatic tumors were present in seven patients). Following PVE, the volume of the left liver (FLR) increased from 427.6±73.5 to 582.6±98.3 cm3. The mean increase in the percentage of the FLR volume induced by PVE was 37.3±33.1%, and the two-step resection rate for hepatic cancer was 100%, thereby indicating that PVE is effective for patients with liver cancer with or/and without liver cirrhosis.
For patients with liver cirrhosis, Farges et al (24) reported in a prospective study that patients with liver cirrhosis prior to partial hepatectomy benefited from PVE, and recommended performing preoperative PVE in patients with right hepatic cancer and liver cirrhosis as a routine preoperative preparation. Ogata et al (17) demonstrated that TACE combined with PVE may effectively induce hypertrophy of FLRs in patients with chronic liver disease and improve the three-year disease-free survival rate. Liu et al (14) suggested that PVE may be used as an indication for half-liver resection in patients with chronic liver injury (including cirrhosis and chronic hepatitis). In the present study, the mean percentage increase in FLR volume was significantly different in each group prior to and following PVE. However, no significant difference was identified between the two groups subsequent to PVE, demonstrating that the impact of cirrhosis on the increased liver volume following PVE was minor, but in the case selection process, the selection bias induced by the degree of cirrhosis in chronic liver disease patients may not be excluded. For example, the increased number of patients with a fibrosis score of 3 in the cirrhosis group led to the conclusion that liver cirrhosis has no obvious effects on liver lobe hyperplasia following PVE.
Regarding embolization materials, previous studies reported the use of anhydrous ethanol (25), gelatin sponges (26), isobutyl-2-cyano acrylate adhesive glue (27), coils (28), lipiodol (29), polyvinyl alcohol (PVA) particles (30), fibrin glue (22) or the combined use of two distinct embolic agents (31). Each embolic agent induced compensatory hypertrophy of non-embolized FLRs to varying degrees; however, there is no consensus regarding the best type of embolic material (27,29,30,32). Madoff et al (32) revealed that the mean percentage of FLR volume increased by 41.1% in the 4 weeks following PVE, using coils and PVA particles as embolization materials. Ji et al (29) used anhydrous ethanol and lipiodol as embolization materials to increase the FLR volume by 27% in the 4 weeks subsequent to PVE. The study by Corrêa et al (30) revealed that the left liver volume increased by 23% one month following PVE, which was attributed to the use of PVA particles. Giraudo et al (27) used isobutyl-2-cyanoacrylate adhesive glue and lipiodol as embolization agents, resulting in an FLR increase of 47.7±31.9% in the 4–8 weeks subsequent to PVE. In the present study, coils were used as embolization agents, which led to embolisms that thoroughly and maximally protected liver function. The effects of PVE on the FLR volume were significant 4–6 weeks following PVE, and no severe complications were observed, including the appearance of liver failure, severe abdominal cavity hemorrhage and gastrointestinal bleeding.
The present study also had limitations. The cirrhosis cases were not further divided into fibrosis score 3 and 4 groups according to the degree of liver cirrhosis; therefore, there may have been selection bias. In addition, the sample size was small and considering further groupings according to the degree of liver cirrhosis may decrease the sample size of each group; therefore, there may be an increase in statistical type II error (33), making the attainment of negative results increasingly likely. Future studies should be performed with larger sample sizes in order that cirrhosis cases may be further grouped based on the degree of cirrhosis. The effects of liver cirrhosis on the compensatory hypertrophy of FLRs following PVE should be further clarified, and may aid doctors in selecting appropriate patients for PVE treatment. For the optimal timing of hepatectomy subsequent to PVE, further study is required to identify the ideal embolic materials for PVE.
In conclusion, preoperative PVE may be safely and effectively performed in order to increase the rate of hypertrophy of FLRs and the probability of resection in patients with hepatic cancer with or without liver cirrhosis, which may have extensive value in clinical applications.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81371658 and 81172315/H1617), the National Major Projects of Infectious Diseases of China (grant no. 2012ZX10002-017), the National Clinical Key Subject Construction Project (General Surgery) of China (grant no. 2014ZJ01) and the Medical Health Fund of Zhejiang Province (grant no. 2013KYB097).
References
- 1.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: Analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. doi: 10.1097/00000658-200210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D, Jarnagin W, Fong Y, Blumgart LH. Extended hepatic resection: A 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. doi: 10.1016/S1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 4.Hwang S, Lee SG, Ko GY, Kim BS, Sung KB, Kim MH, Lee SK, Hong HN. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–616. doi: 10.1097/SLA.0b013e31819ecc5c. [DOI] [PubMed] [Google Scholar]
- 5.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: A meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 6.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D'Angelica M, Getrajdman GI, DeMatteo R, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 7.Yoo H, Ko GY, Gwon DI, Kim JH, Yoon HK, Sung KB, Kim N, Lee J. Preoperative portal vein embolization using an amplatzer vascular plug. Eur Radiol. 2009;19:1054–1061. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 8.Fusai G, Davidson BR. Strategies to increase the resectability of liver metastases from colorectal cancer. Dig Surg. 2003;20:481–496. doi: 10.1159/000073535. [DOI] [PubMed] [Google Scholar]
- 9.Fusai G, Davidson BR. Management of colorectal liver metastases. Colorectal Dis. 2003;5:2–23. doi: 10.1046/j.1463-1318.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, Greget M. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185:221–229. doi: 10.1016/S0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- 11.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, Lemoine A, Bismuth H. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luk JM, Liu AM. Proteomics of hepatocellular carcinoma in Chinese patients. OMICS. 2011;15:261–266. doi: 10.1089/omi.2010.0099. [DOI] [PubMed] [Google Scholar]
- 13.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–693. doi: 10.1097/00000658-200305000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Peng YH, Yang ZH, Wang G, Tang P. Preoperative portal vein embolizations increase the resectable rate of primary liver cancer. Chin J Gen Surg. 2004;19:582–583. (In Chinese) [Google Scholar]
- 15.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 16.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 18.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The ‘50–50 criteria’ on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotroneo AR, Innocenti P, Marano G, Legnini M, Iezzi R. Pre-hepatectomy portal vein embolization: Single center experience. Eur J Surg Oncol. 2009;35:71–78. doi: 10.1016/j.ejso.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Siriwardana RC, Lo CM, Chan SC, Fan ST. Role of portal vein embolization in hepatocellular carcinoma management and its effect on recurrence: A case-control study. World J Surg. 2012;36:1640–1646. doi: 10.1007/s00268-012-1522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Fu Y. Portal vein embolization before major hepatectomy. World J Gastroenterol. 2005;11:2051–2054. doi: 10.3748/wjg.v11.i14.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: Surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM, Curley SA. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–732. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: Prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization using absolute ethanol: Evaluation of its safety and efficacy. J Hepatobiliary Pancreat Sci. 2014;21:676–681. doi: 10.1002/jhbp.113. [DOI] [PubMed] [Google Scholar]
- 26.Nanashima A, Sumida Y, Abo T, Nonaka T, Takeshita H, Hidaka S, Sawai T, Yasutake T, Sakamoto I, Nagayasu T. Clinical significance of portal vein embolization before right hepatectomy. Hepatogastroenterology. 2009;56:773–777. [PubMed] [Google Scholar]
- 27.Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: A large single institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Geisel D, Malinowski M, Powerski MJ, Wüstefeld J, Heller V, Denecke T, Stockmann M, Gebauer B. Improved hypertrophy of future remnant liver after portal vein embolization with plugs, coils and particles. Cardiovasc Intervent Radiol. 2014;37:1251–1258. doi: 10.1007/s00270-013-0810-0. [DOI] [PubMed] [Google Scholar]
- 29.Ji W, Li JS, Li LT, Liu WH, Ma KS, Wang XT, He ZP, Dong JH. Role of preoperative selective portal vein embolization in two-step curative hepatectomy for hepatocellular carcinoma. World J Gastroenterol. 2003;9:1702–1706. doi: 10.3748/wjg.v9.i8.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrêa D, Schwartz L, Jarnagin WR, Tuorto S, DeMatteo R, D'Angelica M, Allen P, Brown K, Fong Y. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145:351–355. doi: 10.1001/archsurg.2010.42. [DOI] [PubMed] [Google Scholar]
- 31.Bellemann N, Stampfl U, Sommer CM, Kauczor HU, Schemmer P, Radeleff BA. Portal vein embolization using a Histoacryl/Lipiodol mixture before right liver resection. Dig Surg. 2012;29:236–242. doi: 10.1159/000339748. [DOI] [PubMed] [Google Scholar]
- 32.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: Safety and effectiveness study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 33.Giuffrida MA. Type II error and statistical power in reports of small animal clinical trials. J Am Vet Med Assoc. 2014;244:1075–1080. doi: 10.2460/javma.244.9.1075. [DOI] [PubMed] [Google Scholar]



