Abstract
Information on dimethyl nitrosamine (DMN)-induced toxicity on endocrine functions is still scanty. This study therefore investigated the outcomes of DMN-induced toxicity on endocrine (thyroid and reproductive) functions, as well as kallikrein-3 level, and effects of ascorbate treatments in male wistar rats. Thirty animals divided into six groups of five rats each were used. Group I animals were the normal control, group II animals served as vehicle control and were administered a single intraperitoneal dose of normal saline, groups III and IV were intraperitoneally injected with a single dose of 30 mg/kg DMN for 48 h, but group IV animals were post-treated orally with 5.71 mg/kg body weight (400 mg/70 kg) ascorbate for seven days, group V animals were pre-treated with same dose of ascorbate orally for seven days before intraperitoneal injection of DMN, while group VI animals were orally administered ascorbate only for seven days. Compared with control, DMN administration resulted in significant decrease (p < 0.05) in serum total cholesterol, testosterone (TST), luteinizing hormone (LH), free triiodothyronine (fT3), and kallikrein III (KLK-3) levels, as well as non-significant increase in serum thyroid stimulating hormone (TSH) level. Pre-treatment with ascorbate significantly increase LH and KLK-3 levels, while post-treatment significantly increase fT3 level. Also, pre-treatment with ascorbate significantly reduced TSH level, while there was no significant difference in TST level following ascorbate treatments. From our findings and to some extent, ascorbate demonstrates ameliorative effects against DMN-induced hormonal disruption in male wistar rats, and this may be attributed to its antioxidant property.
Keywords: Dimethyl nitrosamine, Ascorbate, Thyroid hormone, Reproductive hormone, Kallikrein-3
Highlights
-
•
DMN administration significantly decreased serum total cholesterol, testosterone (TST), luteinizing hormone (LH), free triiodothyronine (fT3), and kallikrein III (KLK-3) levels.
-
•
Pre-treatment with ascorbate significantly increases LH and KLK-3 levels.
-
•
Ascorbate post-treatment and pre-treatment significantly increased fT3 and decreased TSH levels respectively.
1. Introduction
N-nitroso compounds (NOCs) are well established carcinogens present in a vast variety of food stuffs such as smoked fish, dried malt, beer, milk products, meat products, and preserved fruit juices [1], [2], [3]. Among various NOCs, dimethyl nitrosamine (DMN) is a well-known carcinogen, mutagen, and hepatotoxin [4], [5], present in tobacco smoke, high nitrates containing water, fried meals, cosmetics, pharmaceutical agents, and agricultural chemicals [6].
DMN targets primarily the liver, which contains the necessary enzymes for its metabolic activation. Metabolism in the liver is by a microsomal membrane-bound enzyme, cytochrome P-450 2E1 [7], [8], [9]. DMN exerts carcinogenic effects and induces hepatic necrosis through metabolic activation by cytochrome P450 2E1 [10] in experimental animals. The formation of reactive oxygen species (ROS) like H2O2, superoxide anion and hydroxyl radicals (OH·) has been demonstrated during the metabolism of nitrosamines resulting in oxidative stress, which may be one of the key factors in the induction of pathological conditions such as hepatocellular necrosis, carcinogenicity, neoplastic changes, and tumor formation [11], [12], [13].
The most abundant and effective antioxidant in the human body is ascorbic acid [14]. Due to these, the present study checked the effects of treatments with ascorbate on thyroid and reproductive hormones disruption as well as kallikrein-3 level in DMN-induced toxicity in male wistar rats.
2. Materials and methods
2.1. Test substance and kits
DMN (purity ≥ 98%) used in this study were of analytical grade, product of Sigma Chemical Co., Saint Louis, MO, USA. Ascorbate (vitamin C) was purchased from Kunimed Pharmachem Limited, Lagos, Nigeria. Total cholesterol (TCHOL) kit used, is a product of Cypress Diagnostics, Langdorp, Belgium, while TST, LH, fT3, TSH, and KLK III enzyme immunoassay (EIA) test kits were products of Bio-Inteco Diagnostic Limited, Beechwood Road, England.
2.2. Experimental animals and study design
Thirty (30) male wistar albino rats of an average weight of 250 g used for this study were obtained from the animal house of the College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria. They were housed in steel metal cages in the animal house of our department and were served food and water ad libitum. Permission to use the animals was approved by the Institution's Animal Ethical Committee. After a long period of acclimatization, the rats were divided randomly into six groups (I–VI) of five animals each, and were administered as presented below:
Group I: animals served as normal control and were served food and water throughout the study.
Group II: animals served as vehicle control and were administered a single intraperitoneal dose of 6 mL/kg normal saline.
Groups III: animals were administered 30 mg/kg single intraperitoneal dose of DMN [15] for forty eight (48) hours only.
Group IV: animals were administered 30 mg/kg single intraperitoneal dose of DMN, followed by oral post-treatment with 5.71 mg/kg (400 mg/70 kg) ascorbate for seven (7) days.
Group V: animals were orally pre-treated with 5.71 mg/kg ascorbate for 7 days, followed by a single intraperitoneal dose of 30 mg/kg DMN for 48 h.
Group VI: animals were orally administered ascorbate for 7 days.
2.3. Sample collections and preparations
At the end of the experimental period, the animals were sacrificed by cervical dislocation. They were handled and used in accordance with the international guide for the care and use of laboratory animals [16]. Blood samples were collected from the abdominal artery into clean plain tubes, and were allowed to stand for 20–30 min; followed by centrifugation at 3000 rpm for 10 min. Serum was separated and aliquoted into clean 1 ml Eppendorf tubes, and stored at −18 °C until when used.
2.4. Determination of total cholesterol concentration
Serum TCHOL was determined according to the methods described in Cypress Diagnostics Kits, Langdorp, Belgium. Briefly, cholesterol esterase hydrolyzed cholesterol esters to release free cholesterol which was oxidized by cholesterol oxidase, and the resulting hydrogen peroxide (H2O2) reacted with 4-aminophenazone and phenol to form a red quinonimine dye, whose color intensity is proportional to the cholesterol concentration.
2.5. Estimations of serum levels of TST, LH, fT3, TSH, and kallikrein-3
These were done as described in Bio-Inteco Diagnostic EIA test kits, based on antibody-antigen reactions. As a result, the developed color intensities which are directly proportional to the concentrations in the test samples were measured spectrophotometrically at 450 nm using BioTek ELx800 Microplate reader (Northstar Scientific Limited).
2.6. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA), followed by least significant difference (LSD) to test for significant differences among the groups of rats using Statistical Package for Social Sciences program version 17.0. Data were expressed as mean ± standard error of mean. p values less than 0.05 were considered statistically significant.
3. Results
3.1. Effects of ascorbate treatments on serum TCHOL concentration
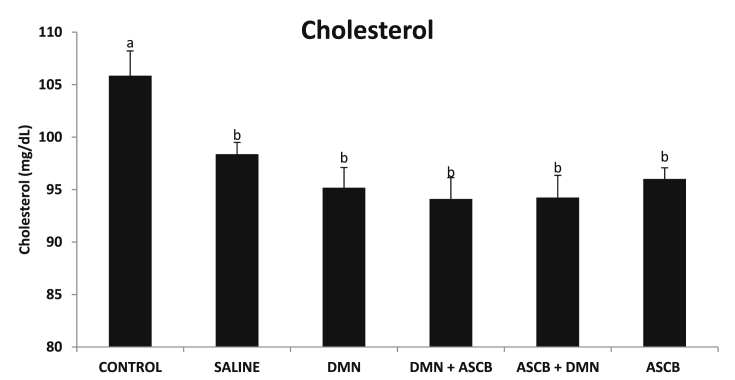
DMN administration significantly (p < 0.05) reduced the concentration of serum TCHOL concentration when compared with control (Fig. 1), while pre- and post-treatment with ascorbate did not have any significant effect (p > 0.05) on its levels (Fig. 1).
Fig. 1.
Effects of pre- and post-ascorbate treatments on total cholesterol levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
3.2. Effects of ascorbate treatments on serum LH and TST levels
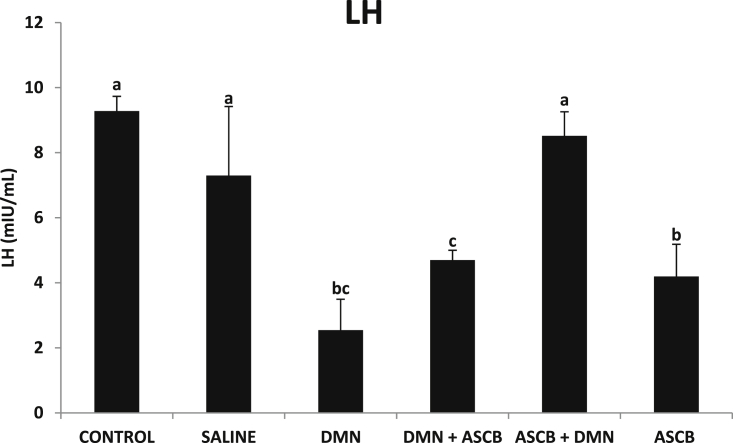
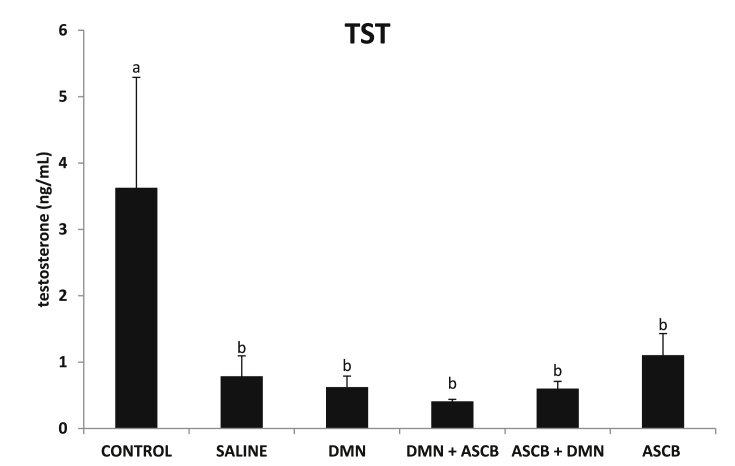
Compared with control, DMN administration resulted in significant decrease (p < 0.05) in LH (Fig. 2) and TST (Fig. 3) levels. Ascorbate pre- and post-treatments resulted into significant (p < 0.05) and non-significant increase (p > 0.05) in LH levels respectively (Fig. 2), while both forms of treatments did not have any significant (p > 0.05) effects on the TST levels (Fig. 3).
Fig. 2.
Effects of pre- and post-ascorbate treatments on LH levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
Fig. 3.
Effects of pre- and post-ascorbate treatments on TST levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
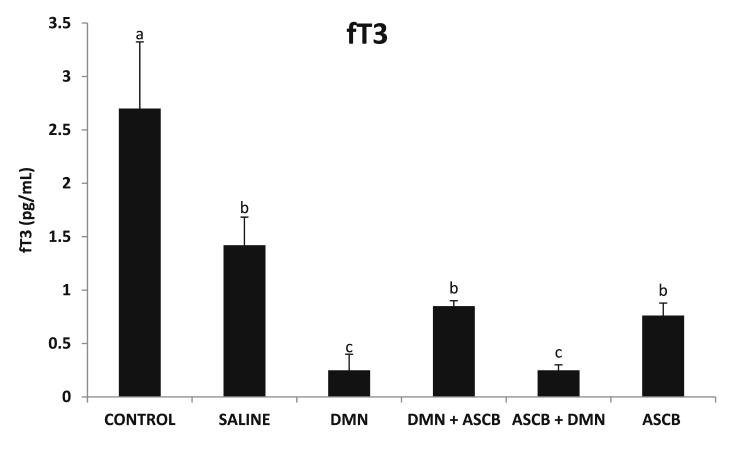
3.3. Effects of ascorbate treatments on serum TSH and fT3 levels
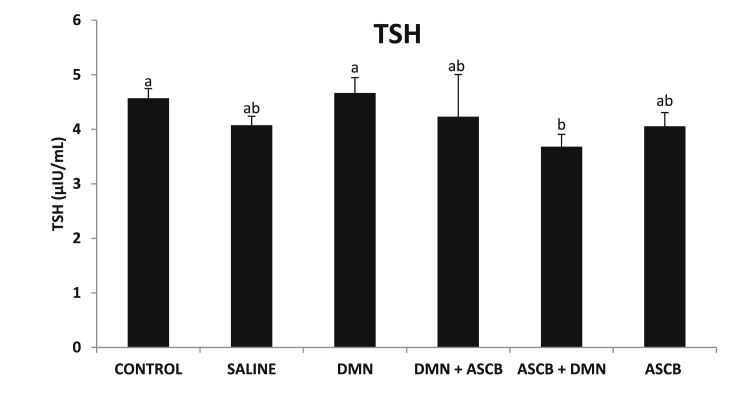
There was a non-significant increase (p > 0.05) in TSH level (Fig. 4), and a significant (p < 0.05) decrease in fT3 level (Fig. 5), accompanying DMN administration compared with control. Pre-treatment only with ascorbate significantly (p < 0.05) decrease TSH level (Fig. 4), while fT3 level was significantly (p < 0.05) increased by post-treatment with ascorbate (Fig. 5).
Fig. 4.
Effects of pre- and post-ascorbate treatments on TSH levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
Fig. 5.
Effects of pre- and post-ascorbate treatments on fT3 levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
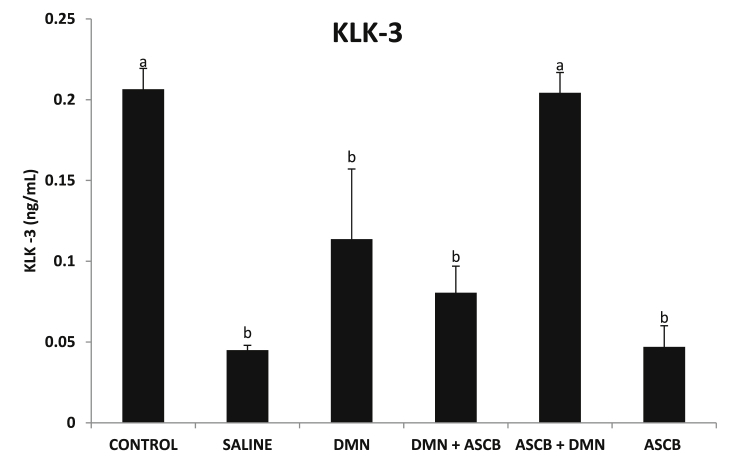
3.4. Effects of ascorbate treatments on serum KLK-3 levels
DMN administration significantly (p < 0.05) decreased in KLK-3 level when compared with control (Fig. 6), but only pre-treatment with ascorbate that was able to significantly (p < 0.05) increase the levels back to level comparable with control.
Fig. 6.
Effects of pre- and post-ascorbate treatments on KLK-3 levels in DMN- induced toxicity. Values are expressed as mean ± SEM (n = 5). Bars with different letters are significantly different (p < 0.05), while bars with similar letters are not significant (p > 0.05). DMN = dimethyl nitrosamine; ASCB = ascorbate.
4. Discussion
The present study was carried out to investigate the effects of DMN-induced toxicity on some reproductive, thyroid hormones as well as KLK-3 levels, and the role of pre- and post-ascorbate treatments in rats. One of the key functions of cholesterol in animals is to serve as a pre-cursor to the biosynthesis of steroid hormones [17]. The significant decrease in TCHOL concentrations (Fig. 1) following DMN administration may be due to alteration in the hepatic synthesis of this lipid. DMN-induced toxicity could also have altered other biological molecules that require cholesterol as precursor (e.g. bile acid), and consequently, the availability of the lipid may have been channeled to their synthesis. Some of the compounds that have also been reported to decrease serum or plasma TCHOL are sodium azide [18], and carbon tetrachloride [19].
LH is also referred to as lutrophin [20], and is produced by gonadotropic cells in the anterior pituitary gland. In females, it triggers ovulation, while in males, it stimulates leydig cells to produce TST [21]. Pituitary gland releases LH, and is controlled by actions of gonadotropin releasing hormone (GnRH). Following low levels of TST, hypothalamus releases GnRH, stimulating the pituitary gland to produce LH [22]. In this present study, DMN-induced toxicity significantly decreased serum LH level (Fig. 2), suggesting an interference of DMN with anterior pituitary, leading to lack of LH secretion. Also, it may be due to decreased secretion of GnRH by the hypothalamus, or failure of the anterior pituitary to respond to GnRH stimulation. Similar findings have been reported in rats following morphine [23], [24], [25], [26], and alcohol exposures [27], [28]. Streptozotocin-induced diabetic rats have also been reported to have low levels of LH [29]. Amelioration by ascorbate pre-treatment may be attributed to its antioxidant and cyto-protective properties against free radical damage [30], [31] that may have protected the hypothalamic–pituitary axis against free radical attack.
TST, a steroid hormone belonging to the androgen class, is found in humans and other vertebrates. 11-ketotestosterone [32] and ecdysone [33] are slightly different forms of TST found in fish and insects respectively. TST plays a major role in the development of male reproductive tissues such as testis and prostate in men. It also promotes secondary sexual characteristics including increased bone and muscle mass, and growth of body hair [34]. Also, TST is important for the prevention of osteoporosis [35], and for health and well-being [36]. Levels of TST are greater in adult male than female adults [37]. The significant decrease in serum TST (Fig. 3) obtained in this study can be attributed to the significant decrease in serum TCHOL recorded, being a precursor to steroid hormone synthesis [38]. LH stimulates leydig cells of the testes to produce TST [21], [22]. Therefore, the decreased level of serum TST recorded can be attributed to the low LH levels. Our findings are corroborated by the studies of Bazzano et al. [39] and Ochiogu et al. [40], who reported low serum TCHOL, LH and TST following monosodium l-glutamate administration in humans and West African Dwarf goats respectively. Also, Manjunath et al. [29] reported low TST and LH levels in streptozotocin-induced diabetic rats. Free radicals-induced tissue damage has been reported as a mechanism of action of DMN [12], [13]. Therefore, ineffectiveness of ascorbate treatment on serum TST levels is suggested to be due to free radical damage on testicular leydig cells [41], rendering them unresponsive to LH stimulation to produce TST.
TSH or thyrotropic hormone is a pituitary and glycoprotein hormone synthesized and secreted by thyrotrope cells in the anterior pituitary gland, regulating the endocrine function of the thyroid [42]. TSH stimulates thyroid gland to produce thyroxine (T4), and triiodothyronine (T3) which is responsible for the metabolism of virtually all tissues in the body [43]. When there is low level of thyroid hormone in the blood, high thyroid-releasing hormone (TRH) is released by the hypothalamus, so high TSH is secreted by the pituitary [44] to produce thyroid hormones [43]. Therefore, from this study, the increase in TSH level (Fig. 4), although not significant following DMN administration may be due to the low level of fT3 (Fig. 5).
T3 is a thyroid hormone that affects almost every physiological process in the body. It is circulated in blood almost completely bound to carrier proteins [45], known as thyroxine binding globulin (TBG) [45]. However, only the unbound portion of T3 (fT3) is known to be the true hormone responsible for biological actions [43]. Only 20% of thyroid hormone produced is T3, appreciable amount (85%) of the circulating T3 is therefore formed from T4 in the liver and pituitary [43]. Also, the significant decrease in fT3 level (Fig. 5) as a result DMN administration may be attributed to inability of the liver to activate T4 to T3, due to hepatic damage. DMN has been reported to have carcinogenic effects and induces hepatic necrosis in experimental animals [10]. As a component of tobacco smoke condensate and certain alcoholic beverages, DMN can induce lung, liver or renal cancers [46], [47].
PSA, also known as KLK-3 is a glycoprotein enzyme, secreted by the epithelial cells of the prostate gland. KLK-3 is needed for the ejaculate, where it liquefies semen in the seminal coagulum, enabling sperm to swim freely [48]. KLK-3 is available in serum of men with healthy prostates in small quantities, but is usually elevated in the presence of prostate cancer or other prostate disorders [49]. From this present study, there was a significant reduction in KLK-3 level (Fig. 6). Though DMN is known to be a potent hepatotoxin, mutagen and carcinogen as already reiterated, administration of DMN may not play a role in the etiology of prostate cancer.
5. Conclusion
From our findings, DMN administration led to hormonal disruption and ascorbate treatments demonstrate to some extent, degree of amelioration which may be attributed to its antioxidant [50], [51], [52] and cyto-protective properties.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Havery D.C., Fazio T. Human exposure to nitrosamines from foods. Food Technol. 1985;39(1):80–83. [Google Scholar]
- 2.Osterdahl B.C. Volatile nitrosamines in foods on the Swedish market and estimation of their daily intake. Food Addit. Contam. 1988;5(4) doi: 10.1080/02652038809373722. 587–585. [DOI] [PubMed] [Google Scholar]
- 3.Prasad M.P., Krishnaswamy K. N-nitrosamines in Indian beers. Indian J. Med. Res. 1994;100:299–301. [PubMed] [Google Scholar]
- 4.Haggerty H.G., Holsapple M.P. Role of metabolism in dimethylnitrosamine induced immunosuppression: a review. Toxicology. 1990;63:1–23. doi: 10.1016/0300-483x(90)90064-n. [DOI] [PubMed] [Google Scholar]
- 5.George J., Rao K.R., Stern R., Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138. doi: 10.1016/s0300-483x(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 6.Ajiboye T.O., Komolafe Y.O., Oloyede O.B., Ogunbode S.M., Adeoye M.D., Abdulsalami I.O., Nurudeen Q.O. Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats. Food Sci. Hum. Well. 2013;2:39–45. [Google Scholar]
- 7.Yang C.S., Tu Y.Y., Koop D.R., Coon M.J. Metabolism of nitrosamines by purified liver cytochrome P-450. Cancer Res. 1985;45:1140–1145. [PubMed] [Google Scholar]
- 8.Yoo J.S.H., Guengerich F.P., Yang C.S. Metabolism of N-nitrosodialkylamines by human liver microsomes. Cancer Res. 1988;88:1499–1504. [PubMed] [Google Scholar]
- 9.Yang C.S., Yoo J.S.H., Ishizaki H., Hong J. Cytochrome P-450IIE1: role in nitrosamine metabolism and mechanisms of regulation. Drug Metab. Rev. 1990;22:147–159. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich F.P., Kim D.H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem. Res. Toxicol. 1991;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 11.Nakae D., Kobayashi Y., Akai H., Andoh N., Satoh H., Ohashi K., Tsutsumi M., Konishi Y. Involvement of 8-hydroxyguanine formation in the initiation of rat liver carcinogenesis by low dose levels of N-nitrosodiethylamine. Cancer Res. 1997;57:1281–1287. [PubMed] [Google Scholar]
- 12.Wills P.J., Suresh V., Arun M., Asha V.V. Antiangiogenic effect of Lygodium flexuosum against N-nitrosodiethylamine-induced hepatotoxicity in rats. Chem. Biol. Interact. 2006;164:25–38. doi: 10.1016/j.cbi.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Pradeep K., Mohan C.V., Gobianand K., Karthikeyan S. Effect of Cassia fistula Linn. leaf extract on diethylnitrosamine induced hepatic injury in rats. Chem. Biol. Interact. 2007;167:12–18. doi: 10.1016/j.cbi.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Frei B.L., England L., Ames B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proceed. Nat. Acad. Sci. U.S.A. 1989;86:6377–6391. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oryan A., Eftekhari M.H., Ershad M., Panjehshahin M.R., Tabatabaei H.R. Hepatoprotective effects of whey protein isolate against acute liver toxicity induced by dimethylnitrosamine in rat. Comp. Clin. Pathol. 2011 [Google Scholar]
- 16.National Research Council . National Academy Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 17.Waterman M.R., Keeney D.S. Genes involved in androgen biosynthesis and the male phenotype. Horm. Res. 1992;38(5–6):217–221. doi: 10.1159/000182546. [DOI] [PubMed] [Google Scholar]
- 18.Somade O.T., Olorode S.K., Olaniyan T.O., Faokunla O. Quercetin, a polyphenolic phytochemical prevents sodium azide-induced extrahepatic oxidative stress in rats. Cogent Biol. 2016;2 1200798. [Google Scholar]
- 19.Wakasugi J., Tawara K., Katami K., Ikeda T., Tomikawa M. Action of malotilate on reduced serum cholesterol level in rats with carbon tetrachloride-induced liver damage. Jpn. J. Pharmacol. 1985;38:391–401. doi: 10.1254/jjp.38.391. [DOI] [PubMed] [Google Scholar]
- 20.Ujihara M., Yamamoto K., Nomura K., Toyoshima S., Demura H., Nakamura Y., Ohmura K., Osawa T. Subunit-specific sulphation of oligosaccharides relating to charge heterogeneity in porcine lutrophin isoforms. Glycobiology. 1992;2(3):225–231. doi: 10.1093/glycob/2.3.225. [DOI] [PubMed] [Google Scholar]
- 21.Louvet J.P., Mitchell H.S., Ross G.T. Effects of human chorionic gonadotropin, human interstitial cell stimulating hormone and human follicle stimulating hormone on ovarian weights in estrogen-primed hypophysectomized immature female rats. Endocrinology. 1975;96(5):1179–1186. doi: 10.1210/endo-96-5-1179. [DOI] [PubMed] [Google Scholar]
- 22.Swerdloff R.S., Wang C., Bhasin S. Developments in the control of testicular function. Bailliere's Clin. Endocrinol. Metab. 1992;6(2):451–483. doi: 10.1016/s0950-351x(05)80158-2. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel S.M., Simpkins J.W., Kalra S.P., Kalra P.S. Chronic morphine treatment induces hypersensitivity to testosterone-negative feedback in castrated male rats. Neuroendocrinology. 1985;40(1):39–44. doi: 10.1159/000124049. [DOI] [PubMed] [Google Scholar]
- 24.Cicero T.J., Adams M.L., Giordano A., Miller B.T., O'Connor L., Nock B. Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. J. Pharmacol. Exp. Ther. 1991;256(3):1086–1093. [PubMed] [Google Scholar]
- 25.Ghowsi M., Yousofvand N. Impact of morphine dependency and detoxification by methadone on male's rat reproductive system. Iran J. Reprod. Med. 2015;13(5):275–282. [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadnia H., Rezayat A.A., Hoseyni M., Sharifi N., Khajedalooee M., Rezayat A.A. Short-period influence of chronic morphine exposure on serum levels of sexual hormones and spermatogenesis in rats. Nephrourol. Mon. 2016;8(4) doi: 10.5812/numonthly.38052. e38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren J., Banan A., Keshavarzian A., Zhu Q., LaPaglia N., McNulty J. Exposure to ethanol induces oxidative damage in the pituitary gland. Alcohol. 2005;35:91–101. doi: 10.1016/j.alcohol.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Oremosu A.A., Akang E.N. Impact of alcohol on male reproductive hormones, oxidative stress and semen parameters in Sprague–Dawley rats. Mid. East Fert. Soc. J. 2015;20:114–118. [Google Scholar]
- 29.Manjunath A., Haseena S., Kusal K.D., Shaik H.S. Effect of Nigella Sativa seed and thymoquinone on reproductive parameters in sterptozotocin induced diabetic and normal male albino rats. Int. J. Intg. Med. Sci. 2016;3(3):248–252. [Google Scholar]
- 30.Kang S.A., Yang Y.J., Park H. In vivo dual effect of vitamin C on paraquat induced lung damage. Free Rad. Rev. 1998;28:93–107. doi: 10.3109/10715769809097880. [DOI] [PubMed] [Google Scholar]
- 31.Sarita N.C., Umesh M., Shruti M., Vishal S., Alka N.S. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Indian J. Clin. Biochem. 2007;22(2):101–105. doi: 10.1007/BF02913324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson R.F. Sinauer Associates; Sunderland Mass: 2005. An Introduction to Behavioral Endocrinology; p. 143. [Google Scholar]
- 33.De Loof A. Ecdysteroids: the overlooked sex steroids of insects? Males: the black box. Insect Sci. 2006;13(5):325–338. [Google Scholar]
- 34.Mooradian A.D., Morley J.E., Korewman S.G. Biological actions of androgens. Endo. Rev. 1987;8(1):1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 35.Tuck S.P., Francis R.M. Testosterone, bone and osteoporosis. Front. Horm. Res. 2009;37:123–132. doi: 10.1159/000176049. [DOI] [PubMed] [Google Scholar]
- 36.Bassil N., Alkaade S., Morley J.E. The benefits and risks of testosterone replacement therapy: a review. Ther. Clin. Risk Manag. 2009;5(3):427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torjesen P.A., Sandnes L. Serum testosterone in women as measured by an automated immunoassay and a RIA. Clin. Chem. 2004;50(3):678–679. doi: 10.1373/clinchem.2003.027565. [DOI] [PubMed] [Google Scholar]
- 38.Hinshelwood M.M. Steroidogenesis. In: Neill J.D., Knobil E., editors. vol. 4. Academic Press; New York: 1998. pp. 644–653. (Encyclopedia of Reproduction). overview. [Google Scholar]
- 39.Bazzano G., D'Elia J.A., Olson R.E. Monosodium glutamate: feeding of large amounts to man and gerbils. Science. 1970;169:1208–1209. doi: 10.1126/science.169.3951.1208. [DOI] [PubMed] [Google Scholar]
- 40.Ochiogu I.S., Ogwu D., Uchendu C.N., Okoye C.N., Ihedioha J.I., Mbegbu E.C. Serum luteinising hormone, testosterone and total cholesterol levels, libido and testicular histomorphology of male West African Dwarf goats orally or subcutaneously treated with monosodium L-glutamate. Veterinarni Med. 2015;60(5):253–260. [Google Scholar]
- 41.Dosumu O.O., Duru F.I.O., Osinubi A.A., Oremosu A.A., Noronha C.C. Influence of virgin coconut oil (VCNO) on oxidative stress, serum testosterone and gonadotropic hormones (FSH, LH) in chronic ethanol ingestion. Agric. Biol. J. N. Am. 2010;1(6):1126–1132. [Google Scholar]
- 42.Sacher R., Richard A.M. eleventh ed. F.A. Davis Company; 2000. Widmann's Clinical Interpretation of Laboratory Tests. [Google Scholar]
- 43.Wenzel K.W. Pharmacological interference with in vivo tests of thyroid function. Metabolism. 1981;30(7):717–732. doi: 10.1016/0026-0495(81)90089-5. [DOI] [PubMed] [Google Scholar]
- 44.Burger H.G., Patel Y.C. Thyrotropin releasing hormone-TSH. Clin. Endocrinol. Metab. 1977;6:831. doi: 10.1016/s0300-595x(77)80057-1. [DOI] [PubMed] [Google Scholar]
- 45.Pederson K.O. A systemic study of variables affecting protein binding of thyroxine and triiodothyronine in serum. Scand. J. Clin. Lab. Invest. 1974;34:247–255. [PubMed] [Google Scholar]
- 46.Magee P.N., Barnes J.M. Induction of kidney tumours in the rat with dimethyl-nitrosamine (N nitrosodimethylamine) J. Pathol. Bacteriol. 1967;84:19–31. doi: 10.1002/path.1700840103. [DOI] [PubMed] [Google Scholar]
- 47.Lijinsky W., Epstein S.S. Nitrosamines as environmental carcinogens. Nature. 1970;225:21–23. doi: 10.1038/225021a0. [DOI] [PubMed] [Google Scholar]
- 48.Balk S.P., Ko Y.J., Bubley G.J. Biology of prostate specific antigen. J. Clin. Oncol. 2003;21(2):383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 49.Catalona W.J., Richie J.P., Ahmann F.R., Hudson M.A., Scardino F.T., Flanigan R.C., deKernion J.B., Ratliff T.L., Kavonssi L.R., Dalkin B.L. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multi-center clinical trial of 6,630 men. J. Urol. 1994;15(5):1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 50.Dar M.A., Raina R., Mir A.H., Verma P.K., Ahmad M. Role of vitamin C against bifenthrin induced oxidative damage in lungs of wistar rats. J. Appl. Nat. Sci. 2015;8(1):346–349. [Google Scholar]
- 51.Patil J.A., Patil A.J., Govindwar S.P., Sontakke A.V. Protection of liver injury by vitamin C, E and GSH after Methomyl toxicity in rat. J. Pharm. Chem. Biol. Sci. 2016;3(4):506–517. [Google Scholar]
- 52.El-Shitany N.A., El-Desoky K. Protective effects of carvedilol and vitamin C against azithromycin-induced cardiotoxicity in rats via decreasing ROS, IL 1-β, and TNF-α production and inhibiting NF-kB and caspase-3 expression. Oxid. Med. Cell. Longev. 2016;2016:1–13. doi: 10.1155/2016/1874762. [DOI] [PMC free article] [PubMed] [Google Scholar]