Abstract
Background
Post-hepatectomy liver insufficiency (PHLI) is a significant cause of morbidity and mortality after liver resection. Quantitative imaging analysis using CT scans measures variations in pixel intensity related to perfusion. A preliminary study demonstrated a correlation between quantitative imaging features of the future liver remnant (FLR) parenchyma from preoperative CT scans and PHLI. The objective of the present study was to explore the potential application of quantitative imaging analysis in PHLI in an expanded, multi-institutional cohort.
Study Design
Patients were retrospectively identified from five high-volume academic centers that developed PHLI after major hepatectomy and were matched to control patients without PHLI (by extent of resection, pre-operative chemotherapy treatment, age (±5 years), and sex). Quantitative imaging features were extracted from the FLR in the preoperative CT scan, and the most discriminatory features were identified using conditional logistic regression. %RLV was defined as follows: (FLR volume)/(total liver volume)×100. Significant clinical and imaging features were combined in a multivariate analysis using conditional logistic regression.
Results
From 2000 to 2015, 74 patients with PHLI and 74 matched controls were identified. The most common indications for surgery were colorectal liver metastases (53%), hepatocellular carcinoma (37%), and cholangiocarcinoma (9%). Two CT imaging features (FD1_4: image complexity; ACM1_10: spatial distribution of pixel intensity) were strongly associated with PHLI and remained associated with PHLI on multivariate analysis (p=0.018 and p=0.023, respectively), independent of clinical variables, including preoperative bilirubin and %RLV.
Conclusions
Quantitative imaging features are independently associated with PHLI and are a promising preoperative risk stratification tool.
Keywords: quantitative imaging, hepatic insufficiency, liver resection
INTRODUCTION
Post-hepatectomy liver insufficiency (PHLI) is a significant cause of morbidity and mortality. As the technical expertise of liver surgery and perioperative care improves, the indications for liver resection have expanded to include resections in diseased livers, extended and two-stage resections, and repeat and salvage operations. The reported incidence of PHLI ranges from 2.7% to 12.6%, and is increased in patients who have received chemotherapy and correlates directly with the extent of resection(1–9). The wide variation in reported rates is partly due to the lack of a standardized definition for PHLI and its wide clinical presentation, ranging from a transient abnormality in laboratory parameters with no change in the patient’s clinical course to fulminant liver failure and death(10).
Many approaches have been proposed to preoperatively stratify the risk of developing PHLI. Liver volumetry, calculated from pre-operative cross-sectional imaging, is commonly used to predict the remnant liver volume after major resections, with a minimum threshold of 20–25% for patients with normal livers, and 35–40% for patients with pre-existing liver disease or extensive chemotherapy exposure(4, 11–16). However, liver volume alone cannot provide an accurate quantification of the true functional capacity of the remnant liver parenchyma. The kinetic growth rate of the liver parenchymal volume after portal venous embolization (PVE) has also been demonstrated as a predictor for PHLI, however this is only applicable for this subset of preoperative patients(17, 18). The indocyanine green plasma retention rate test has also been used as a dynamic method of assessing preoperative liver function, but correlation with postoperative outcomes has been variable(19, 20).
Various clinical parameters and scoring systems have also been proposed to improve risk stratification of postoperative outcomes and morbidity. The Model for End-stage Liver Disease (MELD) was initially constructed as a predictor of mortality following transjugular intrahepatic portosystemic shunts (TIPS) and has been adopted as a measure of liver dysfunction for transplantation(21, 22). Preoperative MELD score has been identified as a predictor of PHLI, but it is limited in sensitivity and specificity, particularly when applied to patients without cirrhosis(6, 23–25). Postoperative parameters, such as the 50–50 criteria which uses prothrombin time <50% and bilirubin >50 µmol/L on postoperative day five as significant predictors of mortality, have also been used to predict PHLI but are not helpful for identifying high-risk patients prior to operation(2, 26). A robust and reliable modality of preoperative risk stratification is still needed.
We previously published the results of a preliminary study showing a correlation between quantitative imaging features of the liver on preoperative computed tomography (CT) scans and the subsequent development of PHLI(27). Quantitative imaging analysis is a technique of evaluating variations in pixel intensity, beyond what can be seen with visual inspection alone(28–32). The initial study analyzed preoperative CT scans of patients from a single institution who developed PHLI compared with matched controls who did not; the study identified two of five imaging features of the liver parenchyma that were significantly different between the 2 groups: correlation (linear dependency of gray levels on neighboring pixels) and entropy (randomness of brightness variation). However, whether these imaging features apply to multi-institutional data acquired from other CT scanners and protocols is an open question. The objective of the current study was to explore further the application of quantitative imaging analysis in PHLI in an expanded, multi-institutional cohort.
METHODS
Patient Selection
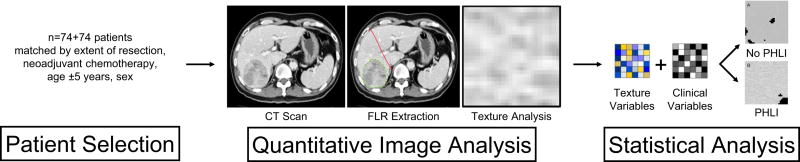
This was a multi-institution, retrospective study evaluating differences in quantitative imaging features of preoperative CT scans of patients undergoing major hepatic resection (defined as four or more segments) who developed PHLI compared with those who did not develop PHLI. The Institutional Review Board at each institution approved this study. Each participating institution is a tertiary referral center for hepatopancreatobiliary diseases and conducted an independent query of their liver resection databases for eligible patients between 2000 and 2015. Only patients with preoperative conventional portal venous phase contrast-enhanced CT scans were eligible for study inclusion. For patients who had undergone preoperative PVE, the CT scan immediately prior to PVE was used to eliminate any potential confounding effect of PVE on alterations or differences in the liver parenchyma. Patient demographic, radiographic, pathologic, laboratory, operative, and follow-up data was collected. PHLI was defined in accordance with the initial study and based on institutional experience and consensus as the occurrence of any of the following criteria within 30 days post-operatively: total serum bilirubin greater than 4.1 mg/dL without biliary obstruction or leak, international normalized ratio (INR) greater than 2.5, presence of ascites (drainage greater than 500 mL/day), or presence of encephalopathy with hyperbilirubinemia(27). Patients were matched for extent of resection, pre-operative treatment with neoadjuvant chemotherapy, age within five years, and gender (Figure 1).
Figure 1.
Study workflow illustrating patient selection and control-matching, quantitative image analysis, and statistical analysis. FLR, future liver remnant; PHLI, post-hepatectomy liver insufficiency.
Quantitative Image Analysis
Standard image processing protocol was used to extract the liver parenchyma from surrounding structures, as previously described(27). The liver, tumors, and vessels were semi7 automatically segmented from the CT scans using Scout Liver (Analogic Corporation, Peabody, MA). The actual total liver volume (TLV) was measured and the future liver remnant was identified along the pre-designated resection plane and defined as the planned remnant liver volume (pRLV)(33). The percentage remnant liver volume (%RLV) was calculated as pRLV/TLV × 100. This methodology of %RLV calculation was used to reflect only preoperatively known variables and to account for individual patient variability in TLV. The remainder of the image processing was fully automated with customized software developed for this study, adapted from the preliminary study.
A set of 255 previously-established intensity and edge-based imaging features using gray-level co-occurrence matrices (GLCM), run-length matrices (RLM), local binary patterns (LBP), fractal dimension (FD), intensity histogram (IH), and angle co-occurrence matrices (ACM) were extracted from liver parenchymal regions on CT images using Matlab R2015a(34–39).
Statistical Analysis
Data analysis was performed using IBM SPSS version 22 and R version 3.3.1. Clinical variables were evaluated for differences between the matched groups using Pearson’s chi-square test for categorical variables and the Wilcoxon signed ranks test for continuous variables. A p-value of <0.05 was considered significant. For evaluation of the imaging features, preliminary feature selection was performed using the Wilcoxon signed ranks test. Highly correlated features with correlation coefficient >0.9 were filtered, preserving only the more significant imaging features. These features were then combined with significant preoperative clinical variables in a multivariate analysis with forward selection using conditional logistic regression to determine the association between imaging features, clinical preoperative variables, and PHLI. Preoperative bilirubin was imputed for 18 patients (12%) due to missing values using multiple imputations by chained equations, which reduces the bias from missing data(40). Five imputation cycles were used and multivariate analysis was performed on each imputed data set. Results were then averaged using Rubin’s rules(41).
RESULTS
Patient Demographics and Clinical Variables
From 2000 to 2015, 74 patients were identified across five high-volume academic surgery centers that had developed PHLI following major hepatic resections and met study inclusion criteria. These 74 patients were matched with a group of control patients who did not develop PHLI. The median age of all patients was 63 years (range 28–87 years) and the majority was male (108/148, 73%). The most common indications for surgery were colorectal liver metastases (79/148, 53%), hepatocellular carcinoma (54/148, 37%), and intrahepatic cholangiocarcinoma (13/148, 9%). The most common resection performed was right hepatectomy (108/148, 73%), followed by extended right hepatectomy (35/148, 24%). Twelve patients had undergone preoperative PVE. All preoperative CTs used for quantitative image analysis were performed within 6 months of the date of surgery, with a median interval of one month. Patients with PHLI had lower %RLV (median 38.0% vs 41.6%, p=0.036) as well as higher preoperative total bilirubin than control patients (median 0.9 vs. 0.6 mg/dL, p<0.001). There were no other significant differences in demographics or presenting characteristics between patients with and without PHLI (Table 1).
Table 1.
Patient Demographics, Diagnoses, and Clinical Variables
| Characteristic | All patients, n = 148 |
Patients with PHLI, n = 74 |
Patients without PHLI, n = 74 |
p Value |
|---|---|---|---|---|
| Age, y, median (range) | 63 (28–87) | 62 (28–87) | 63 (33–82) | 0.927 |
| Sex, n (%) | 0.459 | |||
| Male | 108 (73) | 56 (76) | 52 (70) | |
| Female | 40 (27) | 18 (24) | 22 (30) | |
| Indication for operation, n (%) | 0.534 | |||
| Colorectal liver metastases | 79 (53) | 38 (51) | 41 (55) | |
| Intrahepatic cholangiocarcinoma | 13 (9) | 7 (10) | 6 (8) | |
| Hepatocellular carcinoma | 54 (37) | 27 (37) | 27 (27) | |
| Other* | 2 (1) | 2 (3) | 0 | |
| BMI, kg/m2, median (range) | 25.4 (16.5–43.3) | 25.8 (18.4–39.5) | 25.7 (16.5–43.43) | 0.681 |
| Preoperative PVE, n (%) | 12 (10) | 7 (10) | 5 (10) | 0.892 |
| Preoperative chemotherapy, n (%) | 58 (39) | 29 (39) | 29 (39) | - |
| Preoperative bilirubin, mg/dL, median (range) | 0.7 (0.1–6.1) | 0.9 (0.2–6.1) | .6 (0.1–1.7) | <0.001† |
| %RLV, median (IQR) | 39.2 (17.7–76.7) | 38.0 (17.7–74.6) | 41.6 (20.7–76.7) | 0.036† |
| Procedure, n (%) | 0.884 | |||
| Right hepatectomy | 108 (73) | 56 (76) | 53 (72) | |
| Right extended hepatectomy | 35 (24) | 16 (22) | 19 (26) | |
| Left extended hepatectomy | 4 (3) | 2 (3) | 2 (3) | |
| Positive resection margin, n (%) | 9 (6) | 5 (7) | 4 (5) | 0.731 |
| Steatosis, n (%) | 23 (16) | 12 (16) | 11 (15) | 0.663 |
| Fibrosis, n (%) | 14 (9) | 4 (5) | 10 (14) | 0.585 |
| Maximum postoperative bilirubin, mg/dL, (range) | 4.1 (0.5–53.1) | 8.3 (2.3–53.1) | 2.2 (0.5–8.1) | <0.001† |
| Maximum postoperative INR (range) | 1.8 (1.2–5.2) | 1.9 (1.2–5.2) | 1.5 (1.1–2.2) | <0.001† |
| POD5 bilirubin, mg/dL, median (range) | 2.2 (0.6–17.1) | 4.7 (0.6–17.1) | 1.5 (0.6–7.1) | <0.001† |
| POD5 INR (range) | 1.4 (1.0–4.4) | 1.4 (1.0–4.4) | 1.3 (1.0–2.1) | <0.001† |
| Postoperative encephalopathy with hyperbilirubinemia, n (%) | 6 (4) | 6 (8) | 0 | 0.012† |
| Postoperative ascites, n (%) | 16 (11) | 14 (19) | 2 (3) | 0.001† |
| Mortality, n (%) | 26 (18) | 24 (32) | 2 (3) | <0.001† |
Metastatic neuroendocrine tumor, metastatic gastrointestinal stromal tumor.
Significant.
PHLI indicates post-hepatectomy liver insufficiency; PVE, portal venous embolization; RLV, remnant liver volume; INR, international normalized ratio; POD, postoperative day.
Postoperatively, patients with PHLI had significantly higher serum bilirubin and INR on post-operative day 5 and over the course of hospital admission, and increased incidence of encephalopathy in the setting of hyperbilirubinemia, ascites, and overall mortality. Twenty-three patients who met the study definition of PHLI (31%) also met 50–50 criteria, which predict a 50% postoperative mortality rate. Of these 23 patients, 13 (57%) had a postoperative death. There was one additional patient in the control group who did not meet the study definition of PHLI but met the 50–50 criteria and survived.
Quantitative Imaging Features and Post-Hepatectomy Liver Failure
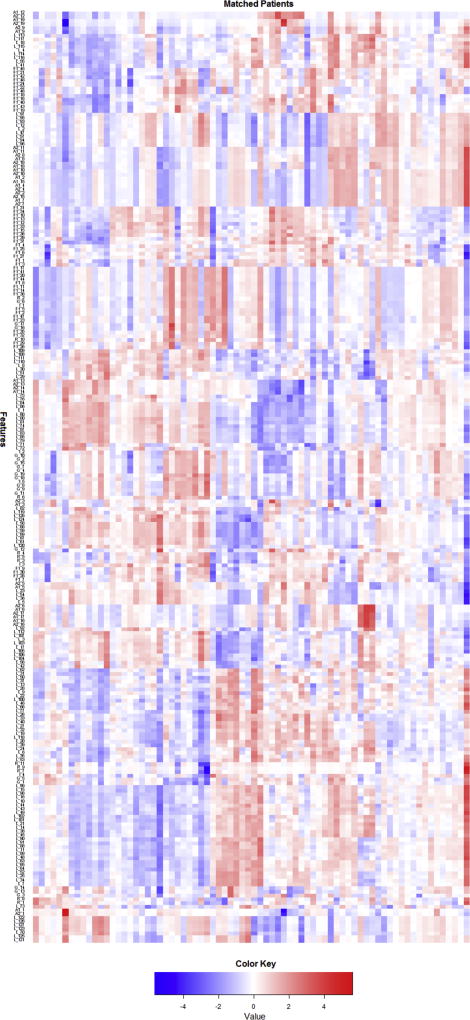
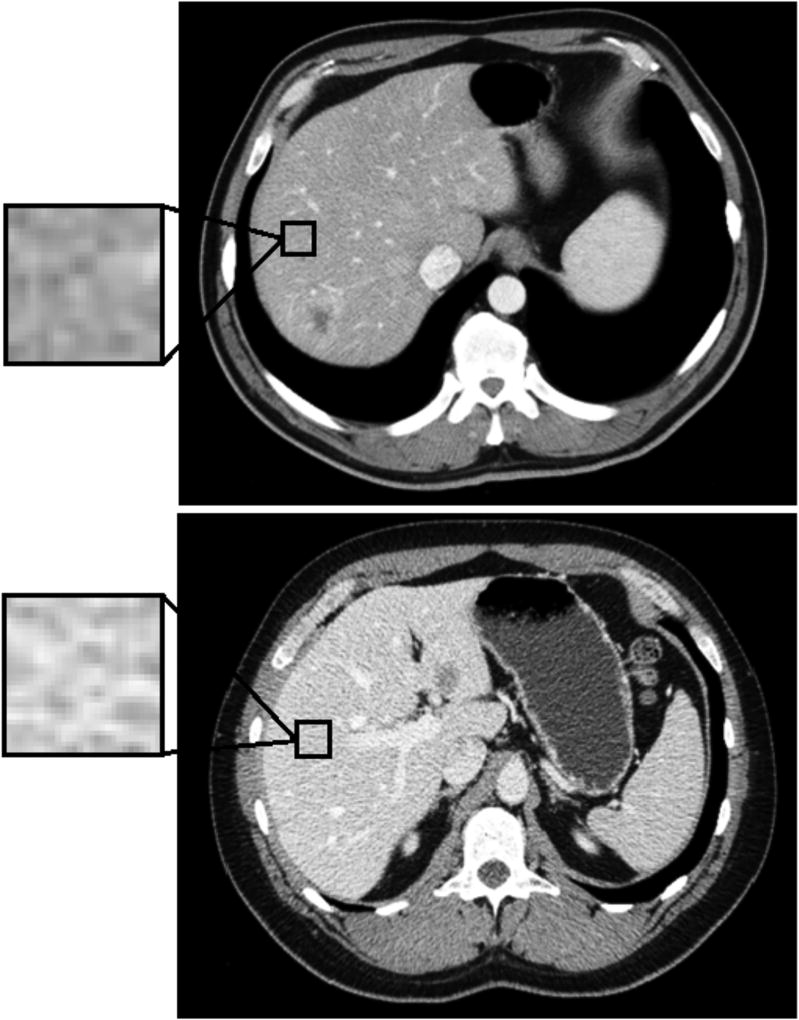
Among the 74 pairs of matched patients, 255 imaging features were extracted from the remnant liver parenchyma from the preoperative CT scan. A heat map demonstrating the relative difference in the imaging features within each matched patient pair is shown in Figure 2. The majority of the areas are red and blue, representing large relative difference (positive difference – red, negative difference - blue) in imaging features between patients with PHLI and control patients. Twenty-one imaging features out of 255 were significant in univariate analysis (including the same features reported in our earlier study)(27). When significant preoperative clinical variables were combined with these imaging features in a multivariate analysis, two imaging features along with preoperative bilirubin and calculated future RLV remained significantly associated with PHLI (Table 2). Increased values of %RLV, FD1_4, and ACM1_10 were associated with decreased occurrence of PHLI, while increased preoperative bilirubin was associated with increased occurrence of PHLI. FD1_4 is a measure of the image complexity and ACM1_10 a measure of the spatial distribution of pixel intensities within the measured field; an increased value of either measurement is a reflection of decreased heterogeneity in the image. An example from the preoperative CT scans of a matched patient pair is shown in Figure 3 (top image is from control patient, bottom image is from patient with PHLI), with a magnification view demonstrating differences at the pixel level. The patient with PHLI has a more heterogeneous appearance in the liver parenchyma, with pixel intensities ranging from nearly white to very dark grey. In comparison, the control patient has a more homogenous-appearing liver parenchyma, with majority of the pixel intensities falling within a moderate grey color. This difference is visually evident from the CT scan and is further captured and quantified by the imaging features.
Figure 2.
A heat map demonstrating normalized difference within each matched patient pair across 255 extracted imaging features.
Table 2.
Association Between Quantitative Imaging Features and Post-Hepatectomy Liver Failure
| Feature | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Preoperative bilirubin | 24.772 | 1.277–480.343 | 0.017 |
| RLV | 0.363 | 0.141–0.932 | 0.018 |
| FD1_4* | 0.410 | 0.179–0.943 | 0.018 |
| ACM1_10* | 0.495 | 0.248–0.987 | 0.023 |
These imaging features were normalized, using the following formula: (imaging feature – mean)/standard deviation.
Figure 3.
Comparison of preoperative CT scans from a pair of matched patients (left is from control patient, right is from patient with post-hepatectomy liver insufficiency). A magnification view provides a visual example of the image pixilation differences, which is quantified by imaging analysis.
DISCUSSION
The expanding indications for liver resection continue to challenge the technical limits of liver surgery. The development of PHLI remains a significant cause of postoperative morbidity and mortality and is a primary limiting factor in pursuing more extensive hepatic resections. The present study demonstrates the use of quantitative imaging features to identify differences preoperatively between patients with PHLI and those who did not. We identified a number of imaging features significantly associated with PHLI in univariate analysis that could be used for preoperative identification of PHLI. When evaluated in conjunction with clinical variables, we identified two imaging features (FD1_4, and ACM1_10) that were independently associated with PHLI. While patients with PHLI had higher preoperative bilirubin compared to control patients, this difference would not be clinically significant (median 0.9 vs 0.6 mg/dL) and a slight elevation in preoperative bilirubin would not significantly alter a patient’s treatment plan. Patients with PHLI also had lower %RLV when compared with control patients; however, the majority of patients had %RLV well above the recommended levels (median 38.0% vs 41.6%) and would have been considered appropriate candidates to proceed to resection. This demonstrates the unpredictable nature of PHLI and reflects the inadequacy of relying on these commonly used clinical parameters to assess a patient’s risk of PHLI. Quantitative imaging analysis thus appears to add incremental value to the use of standard clinical variables alone for PHLI risk stratification.
Quantitative imaging analysis has previously demonstrated significant potential as a prognostic marker in several different clinical settings. For colorectal liver metastases, imaging features representing increased tumor and surrounding liver parenchymal heterogeneity have been associated with poor patient prognosis(28, 42, 43). Changes in these imaging features during chemotherapy have also been associated with treatment response rates in both metastatic colorectal and renal cell cancers(31, 44). In the present study, we demonstrate the application of image analysis to providing a preoperative risk assessment for PHLI and identifying patients who may have inadequate liver function postoperatively despite having an acceptable %RLV and normal preoperative clinical variables. The early identification of these at-risk patients allows for preoperative preventative measures to be taken, such as preoperative portal vein embolization, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), or considering initial non-surgical therapy that may allow a less extensive resection(45, 46). These preoperative steps are critical as only supportive measures are available once PHLI occurs, and there is no effective therapy, other than liver transplantation, which is not an option for most patients.
The multi-institutional nature of this study addresses the concern that the validity and broadened application of quantitative imaging analysis may be limited by variations between different CT scanners and image acquisition parameters across institutions. While further standardization and image optimization methods can reduce the variation in quantitative imaging features, prior studies have shown the stability of these features across different scanners and protocols(47–49). Ahn et al. had previously compared image analysis results from four different CT scanners and found no significant differences in the majority of the evaluated quantitative imaging features(31). Our initial preliminary study featured only patients with CT scans performed at our institution but the present study includes patients from various institutions and continued to produce a significant association between quantitative imaging features and PHLI. This demonstrates that quantitative image analysis is a potential source of significant prognostic information despite variations in the type of CT scanner used and imaging protocols between institutions. We are also conducting an ongoing, prospective trial at our institution examining the reproducibility of quantitative imaging features under different contrast timings to further address this question.
The technique of quantitative imaging analysis offers a distinct advantage over current methods of qualitative assessment, which are prone to the subjective evaluation of each individual observer. Image analysis is also non-invasive and can be used to characterize the entire liver. The biological rationale for these quantitative imaging features remains to be precisely delineated; however, initial studies have found histologic correlates, such as with tumor grade, molecular markers of hypoxia, and liver fibrosis(30, 32, 50). In the context of PHLI, these imaging features may serve as a surrogate measure of the functional capacity of the future liver remnant. Daginawala et al. previously reported the association between quantitative imaging features of the liver parenchyma and the grade of hepatic fibrosis(32). Quantitative imaging features may reflect additional biologic and histologic patterns and future prospective studies are needed to further elucidate this relationship.
The limitations of this study include the inherent selection bias associated with a retrospective review. Some variables, such as liver steatosis and fibrosis, were not available for all patients. While both factors may affect the imaging features of the liver parenchyma, they are obtained postoperatively from the resection specimen, and are of minimal utility in constructing a preoperative model.
CONCLUSIONS
Quantitative imaging features can identify liver parenchymal changes associated with an increased risk of PHLI and are a useful adjunct to liver volumetrics in performing preoperative risk stratification among patients undergoing major liver resections.
Acknowledgments
Support for this work: This work was supported in part by NIH/NCI P30 CA008748 Cancer Center Support Grant.
Support: Dr Pak received support from the Clinical and Translation Science Center at Weill Cornell Medical Center and MSKCC (grant number UL1TR00457).
Appendix 1
Memorial Sloan Kettering Cancer Center Hepatopancreatobiliary Service Co-Authors: William R Jarnagin, MD, FACS, Peter J Allen, MD, FACS, Michael I D'Angelica, MD, FACS, Ronald P DeMatteo, MD, FACS, T Peter Kingham, MD, FACS, Vinod P Balachandran, MD, all from Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Abstract presented at the American College of Surgeons 103rd Annual Clinical Congress, Scientific Forum, San Diego, CA, October 2017.
References
- 1.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005 Feb;54(2):289–96. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucchetti A, Ercolani G, Cescon M, et al. Recovery from liver failure after hepatectomy for hepatocellular carcinoma in cirrhosis: meaning of the model for end-stage liver disease. Journal of the American College of Surgeons. 2006 Nov;203(5):670–6. doi: 10.1016/j.jamcollsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. Journal of the American College of Surgeons. 2007 May;204(5):854–62. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 62-4. [DOI] [PubMed] [Google Scholar]
- 4.Narita M, Oussoultzoglou E, Fuchshuber P, et al. What is a safe future liver remnant size in patients undergoing major hepatectomy for colorectal liver metastases and treated by intensive preoperative chemotherapy? Annals of surgical oncology. 2012 Aug;19(8):2526–38. doi: 10.1245/s10434-012-2274-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Kang DR, Lee JG, et al. Early predictor of mortality due to irreversible posthepatectomy liver failure in patients with hepatocellular carcinoma. World journal of surgery. 2013 May;37(5):1028–33. doi: 10.1007/s00268-013-1959-z. [DOI] [PubMed] [Google Scholar]
- 6.Motoyama H, Kobayashi A, Yokoyama T, et al. Liver failure after hepatocellular carcinoma surgery. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2014 Dec;399(8):1047–55. doi: 10.1007/s00423-014-1252-0. [DOI] [PubMed] [Google Scholar]
- 7.Squires MH, 3rd, Dann GC, Lad NL, et al. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2014 Oct;16(10):884–91. doi: 10.1111/hpb.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbert GS, Prussing KB, Simpson AL, et al. Early trends in serum phosphate and creatinine levels are associated with mortality following major hepatectomy. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015 Dec;17(12):1058–65. doi: 10.1111/hpb.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreatos N, Amini N, Gani F, et al. Albumin-Bilirubin Score: Predicting Short-Term Outcomes Including Bile Leak and Post-hepatectomy Liver Failure Following Hepatic Resection. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2017 Feb;21(2):238–48. doi: 10.1007/s11605-016-3246-4. [DOI] [PubMed] [Google Scholar]
- 10.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011 May;149(5):713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000 May;127(5):512–9. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 12.Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2003 Mar-Apr;7(3):325–30. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 13.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Annals of surgery. 2009 Oct;250(4):540–8. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Digestive surgery. 2012;29(1):6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 15.Shindoh J, Tzeng CW, Aloia TA, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Annals of surgical oncology. 2013 Aug;20(8):2493–500. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim CY, Park EK, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015 Feb;17(2):159–67. doi: 10.1111/hpb.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. The British journal of surgery. 2007 Nov;94(11):1386–94. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 18.Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. Journal of the American College of Surgeons. 2013 Feb;216(2):201–9. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger PM, Tamandl D, Herberger B, et al. Evaluation of chemotherapy-associated liver injury in patients with colorectal cancer liver metastases using indocyanine green clearance testing. Annals of surgical oncology. 2011 Jun;18(6):1644–50. doi: 10.1245/s10434-010-1494-1. [DOI] [PubMed] [Google Scholar]
- 20.Wakiya T, Kudo D, Toyoki Y, et al. Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-associated liver injury in patients with colorectal cancer liver metastasis. Annals of surgical oncology. 2014 Jan;21(1):167–72. doi: 10.1245/s10434-013-3203-3. [DOI] [PubMed] [Google Scholar]
- 21.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology (Baltimore, Md) 2000 Apr;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001 Jul;7(7):567–80. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Annals of surgery. 2006 Mar;243(3):373–9. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teh SH, Sheppard BC, Schwartz J, Orloff SL. Model for End-stage Liver Disease score fails to predict perioperative outcome after hepatic resection for hepatocellular carcinoma in patients without cirrhosis. American journal of surgery. 2008 May;195(5):697–701. doi: 10.1016/j.amjsurg.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 25.Fromer MW, Aloia TA, Gaughan JP, Atabek UM, Spitz FR. The utility of the MELD score in predicting mortality following liver resection for metastasis. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2016 Oct;42(10):1568–75. doi: 10.1016/j.ejso.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Balzan S, Belghiti J, Farges O, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Annals of surgery. 2005 Dec;242(6):824–8. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson AL, Adams LB, Allen PJ, et al. Texture analysis of preoperative CT images for prediction of postoperative hepatic insufficiency: a preliminary study. Journal of the American College of Surgeons. 2015 Mar;220(3):339–46. doi: 10.1016/j.jamcollsurg.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology. 2009 Feb;250(2):444–52. doi: 10.1148/radiol.2502071879. [DOI] [PubMed] [Google Scholar]
- 29.Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer imaging : the official publication of the International Cancer Imaging Society. 2013 Mar 26;13:140–9. doi: 10.1102/1470-7330.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadot E, Simpson AL, Do RK, et al. Cholangiocarcinoma: Correlation between Molecular Profiling and Imaging Phenotypes. PloS one. 2015;10(7):e0132953. doi: 10.1371/journal.pone.0132953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn SJ, Kim JH, Park SJ, Han JK. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. European journal of radiology. 2016 Oct;85(10):1867–74. doi: 10.1016/j.ejrad.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Daginawala N, Li B, Buch K, et al. Using texture analyses of contrast enhanced CT to assess hepatic fibrosis. European journal of radiology. 2016 Mar;85(3):511–7. doi: 10.1016/j.ejrad.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Simpson AL, Geller DA, Hemming AW, et al. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. Journal of the American College of Surgeons. 2014 Aug;219(2):199–207. doi: 10.1016/j.jamcollsurg.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazit L, Chakraborty J, Attiyeh M, et al. SPIE Medical Imaging; 2017. Orlando, Florida, United States: 2017. Quantification of CT images for the classification of high- and low-risk pancreatic cysts; p. 101340X. [Google Scholar]
- 35.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Transactions on Systems, Man, Cybernetics. 1973;3(6):610–21. [Google Scholar]
- 36.Tang X. Texture information in run-length matrices. IEEE Transactions on Image Processing. 1998;7(11):1602–9. doi: 10.1109/83.725367. [DOI] [PubMed] [Google Scholar]
- 37.Pietikinen M, Hadid A, Zhao G, Ahonen T. Local binary patterns for still images. Computer Vision Using Local Binary Patterns, Computational Imaging and Vision. 2011;40:13–47. [Google Scholar]
- 38.Buczkowski S, Kyriacos S, Nekka F, Cartilier L. The modified boxcounting method: Analysis of some characteristic parameters. Pattern Recognition. 1998;31:411–8. [Google Scholar]
- 39.Chakraborty J, Rangayyan RM, Banik S, Mukhopadhyay S, Desautels JEL. Statistical measures of orientation of texture for the detection of architectural distortion in prior mammograms of interval cancer. Journal of Electronic Imaging. 2012;21(3) [Google Scholar]
- 40.Van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45(3):1–67. [Google Scholar]
- 41.Rubin D. Multiple Imputation for Nonresponse in Surverys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 42.Ganeshan B, Miles KA, Young RC, Chatwin CR. In search of biologic correlates for liver texture on portal-phase CT. Academic radiology. 2007 Sep;14(9):1058–68. doi: 10.1016/j.acra.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Kato H, Kanematsu M, Zhang X, et al. Computer-aided diagnosis of hepatic fibrosis: preliminary evaluation of MRI texture analysis using the finite difference method and an artificial neural network. AJR American journal of roentgenology. 2007 Jul;189(1):117–22. doi: 10.2214/AJR.07.2070. [DOI] [PubMed] [Google Scholar]
- 44.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011 Oct;261(1):165–71. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- 45.Wicherts DA, de Haas RJ, Adam R. Bringing unresectable liver disease to resection with curative intent. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007 Dec;33(Suppl 2):S42–51. doi: 10.1016/j.ejso.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Annals of surgery. 2012 Mar;255(3):405–14. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 47.van Velden FH, Kramer GM, Frings V, et al. Repeatability of Radiomic Features in Non-Small-Cell Lung Cancer [(18)F]FDG-PET/CT Studies: Impact of Reconstruction and Delineation. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016 Oct;18(5):788–95. doi: 10.1007/s11307-016-0940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larue R, van Timmeren JE, de Jong EEC, et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: a comprehensive phantom study. Acta oncologica (Stockholm, Sweden) 2017 Nov;56(11):1544–53. doi: 10.1080/0284186X.2017.1351624. [DOI] [PubMed] [Google Scholar]
- 49.Mackin D, Fave X, Zhang L, et al. Measuring Computed Tomography Scanner Variability of Radiomics Features. Invest Radiol. 2015 Nov;50(11):757–65. doi: 10.1097/RLI.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skogen K, Ganeshan B, Good C, Critchley G, Miles K. Measurements of heterogeneity in gliomas on computed tomography relationship to tumour grade. Journal of neuro-oncology. 2013 Jan;111(2):213–9. doi: 10.1007/s11060-012-1010-5. [DOI] [PubMed] [Google Scholar]