Abstract
Objective
The effects of diets high in refined grains on biliary and colonic bile acids have been investigated extensively. However, the effects of diets high in whole versus refined grains on circulating bile acids, which can influence glucose homeostasis and inflammation through activation of farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (TGR5), have not been studied.
Materials and Methods
We conducted a secondary analysis from a randomized controlled crossover feeding trial (NCT00622661) in 80 healthy adults (40 women/40 men, age 18–45 years) from the greater Seattle Area, half of which were normal weight (BMI 18.5–25.0 kg/m2) and half overweight to obese (BMI 28.0–39.9 kg/m2). Participants consumed two four-week controlled diets in randomized order: 1) a whole grain diet (WG diet), designed to be low in glycemic load (GL), high in whole grains, legumes, and fruits and vegetables, and 2) a refined grain diet (RG diet), designed to be high GL, high in refined grains and added sugars, separated by a four-week washout period. Quantitative targeted analysis of 55 bile acid species in fasting plasma was performed using liquid chromatography tandem mass spectrometry. Concentrations of glucose, insulin, and CRP were measured in fasting serum. Linear mixed models were used to test the effects of diet on bile acid concentrations, and determine the association between plasma bile acid concentrations and HOMA-IR and CRP. Benjamini-Hochberg false discovery rate (FDR) < 0.05 was used to control for multiple testing.
Results
A total of 29 plasma bile acids were reliably detected and retained for analysis. Taurolithocholic acid (TLCA), taurocholic acid (TCA) and glycocholic acid (GCA) were statistically significantly higher after the WG compared to the RG diet (FDR<0.05). There were no significant differences by BMI or sex. When evaluating the association of bile acids and HOMA-IR, GCA, taurochenodeoxycholic acid, ursodeoxycholic acid (UDCA), 5β-cholanic acid-3β,12α-diol, 5-cholanic acid-3β-ol, and glycodeoxycholic acid (GDCA) were statistically significantly positively associated with HOMA-IR individually, and as a group, total, 12α-hydroxylated, primary and secondary bile acids were also significant (FDR<0.05). When stratifying by BMI, chenodeoxycholic acid (CDCA), cholic acid (CA), UDCA, 5β-cholanic acid-3β, deoxycholic acid, and total, 12α-hydroxylated, primary and secondary bile acid groups were significantly positively associated with HOMA-IR among overweight to obese individuals (FDR <0.05). When stratifying by sex, GCA, CDCA, TCA, CA, UDCA, GDCA, glycolithocholic acid (GLCA), total, primary, 12α-hydroxylated, and glycine-conjugated bile acids were significantly associated with HOMA-IR among women, and CDCA, GDCA, and GLCA were significantly associated among men (FDR<0.05). There were no significant associations between bile acids and CRP.
Conclusions
Diets with comparable macronutrient and energy composition, but differing in carbohydrate source, affected fasting plasma bile acids differently. Specifically, a diet characterized by whole grains, legumes, and fruits and vegetables compared to a diet high in refined grains and added sugars led to modest increases in concentrations of TLCA, TCA and GCA, ligands for FXR and TGR5, which may have beneficial effects on glucose homeostasis.
Keywords: bile acid, insulin resistance, FXR, dietary patterns, whole grains, feeding study
1. Introduction
Bile acids are synthesized by hepatocytes from cholesterol and facilitate the absorption of fat and fat-soluble vitamins [1–3]. These primary bile acids are conjugated with glycine or taurine before secretion into the duodenum via the bile duct, and are ultimately converted into secondary bile acids by gut microbes [1–3]. In humans, 90% of the biliary bile acid pool consists of the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA), and the secondary bile acid deoxycholic acid (DCA), and their conjugates [4]. Other secondary bile acids, e.g. lithocholic acid (LCA) and ursodeoxycholic acid (UDCA), are present in low amounts (<5%) [4]. Reabsorption of bile acids takes place in the terminal ileum and colon where they undergo enterohepatic circulation [1] with only ~5% excreted in feces [2, 3].
Hepatic primary bile acid synthesis is homeostatically regulated, due to the cytotoxic properties of bile acids [4]. Synthesis is negatively regulated through bile acid activation of farnesoid X receptor (FXR) which leads to inhibition of CYP7A1 and CYP8B1, enzymes that facilitate hepatic bile acid synthesis [1, 2]. Because FXR is activated differently by each bile acid (CDCA > DCA > LCA > CA) [2, 3], alterations in bile acid pool composition may lead to different rates of bile acid production and secretion. Differences in the gut microbiome community may also affect bile acid profiles as several steps in microbial metabolism alter both the composition and availability of secondary bile acids [5].
Previous evidence suggests that diets characterized by high intakes of red meat, fat and refined grains, and low intakes of dietary fiber, are strongly correlated with the incidence of obesity and metabolic syndrome, and higher colonic concentrations of secondary bile acids [6]. However, less is known about circulating plasma bile acid concentrations and their capacity to be modified by diet. Diabetic and insulin-resistant individuals show increased circulating total bile acids, and insulin resistance is positively associated with taurine conjugated and 12α-hydroxylated bile acid concentrations in plasma [2]. Hepatic glucose homeostasis appears to be affected by activation of the bile acid nuclear receptor FXR, which alters the expression of genes involved in glycogen synthesis, glycogenolysis, and gluconeogenesis. In addition to repressing bile acid synthesis, binding of FXR leads to inhibition of hepatic glucose production [2, 7]. Bile acids also act as ligands for the G protein-coupled bile acid receptor 1 (GPBAR1, more commonly known as TGR5), which is predominantly activated by secondary bile acids [8]. This receptor can regulate glucose homeostasis through stimulation of glucagon-like peptide-1 (GLP1) secretion in ileal L-cells [2, 3, 7], which is relevant because this incretin acutely stimulates insulin secretion and contributes to longer-term β-cell health [7]. TGR5 is present in different organs and tissues throughout the body, and in addition to glucose regulation, it modulates inflammation by reducing phagocytosis and inhibiting pro-inflammatory cytokine production [9]. Aberrations of these actions, i.e., by changes in the bile acid milieu, may contribute, in part, to the development of metabolic syndrome [9].
The aim of this study was to determine the effects of a diet high in whole grains, legumes, and fruits and vegetables, as compared to a diet high in refined grains and added sugars, on plasma bile acids in healthy adults, using samples from a completed randomized, controlled crossover feeding trial. We hypothesized that each diet would affect plasma bile acids differently. As an exploratory aim, we also evaluated the association between post-intervention bile acid concentrations and insulin resistance and high sensitivity C-reactive protein (CRP), a marker of inflammation. The results of this study may contribute to a better understanding of the protective properties of consuming a diet rich in whole grains, legumes, and fruits and vegetables, and may also provide insight into the association of bile acids and risk of metabolic dysregulation.
2. Materials and Methods
2.1. Study Design
We conducted an ancillary study using data from the Carbohydrate and Related Biomarkers (CARB) study: a randomized, controlled, crossover feeding trial, conducted from June 2006 to July 2009 [10]. The CARB study (registered at http://www.clinicaltrials.gov/ as NCT00622661) aimed to evaluate the effects of two different dietary patterns on biomarkers related to cancer risk and metabolic dysregulation. The study was double blinded for both participants and outcome assessors. While participants knew they were consuming two different diets, they were unaware of what the specific differences were, i.e., that the diets differed in carbohydrate source and type. To maintain blinding during analyses, laboratory personnel and statisticians were only provided with binary class labels (0,1). The Institutional Review Board and the Clinical Trial Office of the Fred Hutchinson Cancer Research Center (Fred Hutch) reviewed and approved all procedures. Written informed consent was provided by all participants. Power calculations were conducted for the parent study for primary trial outcomes.
2.2. Study Population
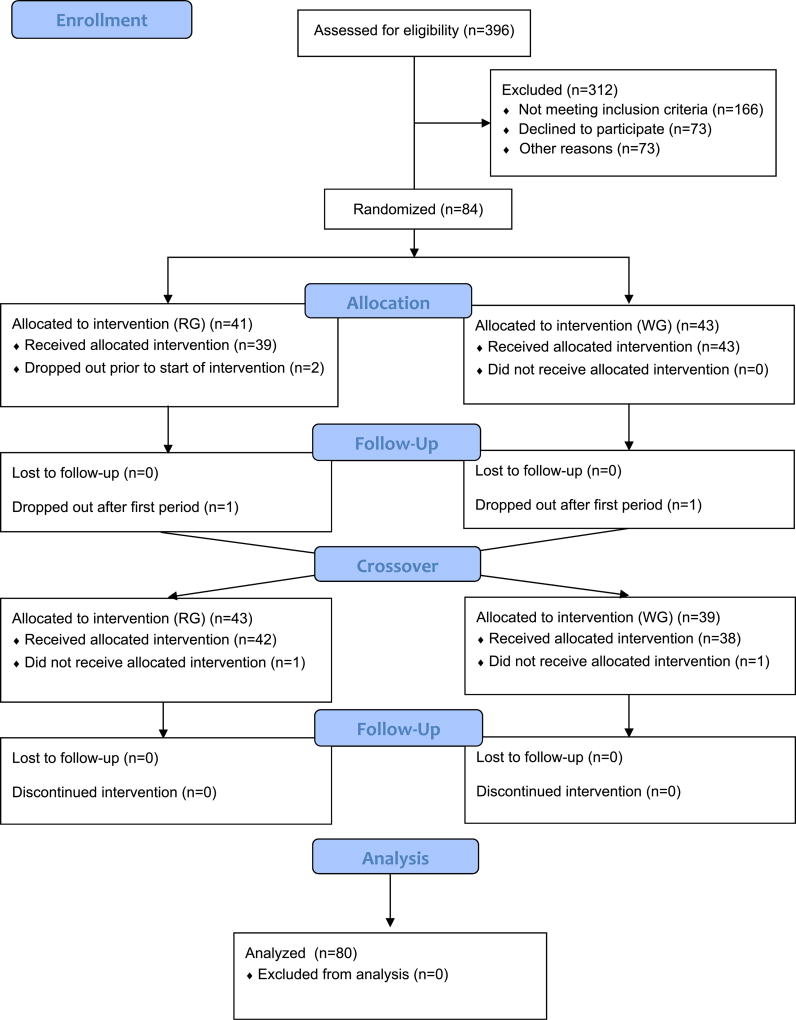
Complete details on recruitment and study design have been published previously [10]. Briefly, healthy adults between the ages of 18 and 45 years were recruited from the Greater Seattle area. Of the 84 randomized participants, those with complete biospecimen data (n=80) were included in this study. Half of the participants were female and half were male, with equal numbers of normal weight (body mass index (BMI) 18.5–25.0 kg/m2) and overweight/obese (BMI 28.0–40.0 kg/m2). Individuals with a BMI of 25.1–27.9 kg/m2 were excluded in order to provide sufficient contrast between the normal and overweight/obese groups. Computer-generated block-randomization was applied on BMI, sex and race/ethnicity. The study manager allocated each new recruit to the next assignment. Exclusion criteria included: the use of prescription medications (including oral contraceptives), use of tobacco, consumption of more than two alcoholic drinks per day, a fasting blood glucose >100 mg/dl determined as part of study screening, and an inability to consume study foods due to a food allergy, a medical condition or restrained dietary habits [11]. At baseline, all participants underwent body composition assessment using a whole-body dual-energy X-ray absorptiometry (DXA) scan (GE Lunar DPX-Pro, GE Healthcare, Milwaukee, WI), had height (to the nearest 0.5 cm) and weight (to the nearest 0.5 kg) measured using a wall-mounted stadiometer and calibrated digital platform scale, respectively, and completed questionnaires on demographic characteristics, usual physical activity and medical history.
2.3. Study Diets
The participants were randomized to a eucaloric 4-week diet, which was either designed to be low in glycemic load (GL), high in whole grains, legumes, fruits and vegetables, referred to as the whole grain (WG) diet, or designed to be high in GL, high in refined grains and added sugars, referred to as the refined grain (RG) diet. After a 4-week washout, participants crossed-over to the other diet. Of the 84 randomized participants, 43 were randomly allocated to the WG diet during the first diet period followed by the RG diet during the second diet period, and 41 to the opposite sequence. All foods were prepared in the Fred Hutch Human Nutrition Laboratory. The majority of study foods were consumed at Fred Hutch, under staff supervision. Compliance was evaluated through post-meal leftover food records for meals consumed at Fred Hutch, and self-reported food consumption check lists for foods taken home. These evaluations indicated that adherence to the diets was >98% [12]. Participants were instructed to eat only the foods provided for the study for both diet intervention periods. Both diets were provided as seven-day recurring menus using ProNutra™ (Viocare, Inc. Princeton, N.J.). Complete details on diet menus and consumption have been published previously [10]. Each participant’s calorie level provided was computed based on the Mifflin equation [13] and was designed to be weight maintaining. Weight was measured thrice weekly with energy adjustments as necessary to ensure that weight was maintained throughout both diet periods. Diet composition differed primarily in carbohydrate type and quality, which necessarily altered fiber content (56 g/d for the WG vs 25 g/d for the RG diets for the reference diet of 2400 kcal/d). Both diets were eucaloric, provided adequate amounts of all required and essential nutrients, and were comparable in macronutrient composition (as percent of total energy) [12].
2.4. Sample Collection, Inflammation Markers, Glucose and Insulin Measurements
Blood samples were collected at the beginning and end of each four-week feeding period after a 12-hour overnight fast, and were processed and stored at −80 °C. Serum concentrations of CRP, glucose and insulin were measured as part of the parent trial at four time points, before and at the end of each diet period using standard assays as described previously [10, 12]. We used the homeostasis model assessment (HOMA-IR) as our measurement of insulin resistance, dividing the product of fasting insulin and fasting glucose by a normalizing factor [14].
2.5. Bile Acid Measurements
Fasting plasma bile acid concentrations were determined from archived blood specimens collected at the beginning of the first feeding period and at the end of each feeding period. All samples were stored at −80°C and only never-thawed samples were used. A total of 55 bile acids were analyzed, plus five stable isotope (deuterium)-labeled internal standards for concentration determination. The 55 bile acid standard compounds were purchased from Sigma-Aldrich (Saint Louis, MO) and Steraloids, Inc (Newport, RI). Internal standards (cholic acid-2,2,4,4-D4 (CA-D4), lithocholic acid-2,2,4,4-D4 (LCA-D4), deoxycholic acid-2,2,4,4-D4 (DCA-D4), glycocholic acid-2,2,4,4-D4 (GCA-D4), and glycochenodeoxycholic acid-2,2,4,4-D4 (GCDCA-D4)) were obtained from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA). Purities ~ >95–99% for non-labeled standards and > 98% for isotope-labeled compounds. Solvents acetonitrile, methanol, ammonium acetate, and acetic acid (LC-MS grade) were obtained from Fisher Scientific (Pittsburgh, PA).
Standard operating procedures from the Northwest Metabolomics Research Center were followed to prepare plasma samples for liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. In brief, aliquots of plasma (100 µL) were mixed with a 10 µL solution of five internal standards (CA-D4, LCA-D4, DCA-D4, GCA-D4, and GCDCA-D4, 10 µM each) and methanol (500 µL), and then vortexed for 10 s. Samples were then stored at −20 °C for 20 min for metabolite extraction, followed by sonication in an ice bath for 10 min, and centrifugation at 14,000 RPM for 15 min at 4 °C. Supernatants (500 µL) were vacuum-dried in a clean Eppendorf tube, and reconstituted in MeOH/H2O (1:1, v/v) to 100 µL. A set of 90 µL calibration samples were also prepared at four different concentrations of each bile acid around its expected concentration range. The bile acids were grouped into sets of five, such that 44 samples were prepared (11 solutions times four concentrations), and 10 µL of the mixture of five deuterated internal standards (10 µM) were added. Then these solutions were vacuum-dried and reconstituted in MeOH/H2O (1:1, v/v) to 100 µL.
Each prepared sample (2 µL) was injected into LC-MS (Waters Acquity I-Class UPLC TQS-micro MS, Waters, Milford, MA) for analysis using negative ionization mode. The MS system was equipped with an electrospray ionization source. Chromatographic separation was performed on a Waters XSelect HSS T3 column (2.5 µm, 2.1 × 150 mm), with a flow rate of 0.3 mL/min, auto-sampler temperature of 4 °C, and column compartment temperature of 40 °C. The mobile phase was composed of two solvents: 5 mM ammonium acetate in H2O with 0.1% acetic acid (A) and acetonitrile with 0.1% acetic acid (B). After 1 min of isocratic elution of 75% of solvent A, it decreased to 5% at t=15 min. Composition was then maintained at 5% for 10 min, followed by an increase to 75% at t=25. The total experimental time for each injection was 40 min.
Using multiple-reaction-monitoring (MRM) mode, targeted data were acquired. All 55 bile acids and five stable isotope-labeled internal standards MRM transitions were monitored in the negative mode. Metabolite identities were validated by spiking mixtures of standard compounds. TargetLynx software (Waters, Milford, MA) was used to integrate extracted MRM peaks. Absolute bile acid concentrations (µM) were calculated by first constructing calibration curves using the 44 solutions of bile acids as described above plus blank samples. Concentrations of the bile acids were then calculated from the peak areas in the samples and the slope of the calibration curve [15, 16].
2.6. Statistical Analysis
We used 26 blinded quality control samples to determine the coefficient of variation (CV) for each bile acid, which ranged from 9% to 150%. Bile acids with very low signal or CVs >30% were excluded. Of the 55 bile acids measured, 29 were retained for analysis. A total of 24 zero values across the 29 bile acids and 240 samples were imputed with a value equivalent to half of the lowest concentration measured per bile acid. As in previous studies [17–19], bile acids were grouped by summing bile acids into the following categories: total, primary, secondary, glycine conjugated, taurine conjugated and 12α-hydroxylated (CA, DCA and their conjugates) bile acids. All bile acids and group values were log-transformed to approximate a normal distribution.
Using a linear mixed model, we first tested the diet effects on each plasma bile acid concentration individually, adjusted for age, sex, BMI, baseline bile acid measurement, batch, diet period and sequence. Additionally we performed stratified analyses by sex, BMI and body fat percentage. A priori subgroup analyses were planned to evaluate the effects of intervention by adiposity. We first assessed the effects of diet stratified by BMI as per recruitment. However, as BMI alone is a poor indicator of body fatness [20], we subsequently analyzed diet effects based on body fat percentage classification as determined by DXA. Body fat mass was classified as high for females >32.0% and males >25.0 [21, 22]. Of note, even though we had recruited equal numbers of normal weight vs. overweight/obese participants by BMI, a larger number (i.e., 51 for high body fat versus 40 for overweight to obese BMI) were categorized into a high adiposity group when using body fat mass. As point estimates were similar, we present results based on BMI.
For our exploratory analyses we evaluated the association between each individual bile acid concentration and each bile acid group abundance, and insulin resistance expressed as HOMA-IR, and the inflammation marker CRP. Values below the limit of detection (LOD) for CRP (n=29 of 160 measures) were imputed as half the measured concentration. Log-transformed values were used for these analyses; we applied the same model as our first analysis with the exclusion of adjusting for baseline values, and additionally adjusting for study diet. Results are presented as unadjusted absolute concentrations (µM) for ease of interpretation. All statistical analyses were performed using Stata (v15.1; StataCorp, College Station, TX, USA). Benjamini-Hochberg false discover rate (FDR) <0.05 for 29 tests (primary analysis), and 35 tests (exploratory analyses) was applied [23].
3. Results
3.1. Effects of diet on plasma bile acid concentrations
Characteristics of the 80 participants stratified by sex and BMI are given in Table 1. When evaluating the effects of diet, plasma concentrations of six bile acids were different between the two diets at a nominal p <0.05: taurolithocholic acid (TLCA), taurocholic acid (TCA), glycocholic acid (GCA), taurochenodeoxycholic acid (TCDCA) and glycochenodeoxycholic acid (GCDCA) were higher after the WG diet compared to the RG diet, while hyocholic acid (HCA) was lower (Table 2). Three of these, TLCA, TCA and GCA, satisfied the more stringent FDR <0.05. We did not find any significant differences when bile acids were stratified by sex, BMI or body fat (data not shown).
Table 1.
Characteristics of Carbohydrate and Related Biomarkers (CARB) study participants (n=80) stratified by sex and body mass indexa
| Men (n=40) |
Women (n=40) |
Normal weight b (n=40) |
Overweight to obese b (n=40) |
|
|---|---|---|---|---|
| Age, (yrs) | 31.0 (8.3) | 28.2 (5.9) | 26.5 (6.4) | 32.7 (8.6) |
| BMI, (kg/m2) | 27.5 (5.9) | 27.3 (6.0) | 22.3 (1.6) | 32.6 (3.7) |
| Body fat %c | 25.7 (9.8) | 39.7 (9.5) | 25.4 (9.1) | 40.1 (9.1) |
| Baseline HOMA-IR | 2.2 (1.9) | 2.4 (2.0) | 1.8 (1.2) | 3.5 (2.2) |
| Baseline plasma bile acid groups (µM) d | ||||
| Σ Total bile acids | 7.5 (4.8) | 7.2 (4.3) | 6.9 (4.2) | 7.8 (4.8) |
| Σ 12α-hydroxylated bile acids | 3.6 (3.0) | 3.4 (2.7) | 3.1 (2.5) | 3.9 (3.1) |
| Σ Primary bile acids | 3.6 (3.3) | 3.3 (3.0) | 3.5 (3.1) | 3.3 (3.2) |
| Σ Total secondary bile acids | 4.0 (2.4) | 3.9 (2.0) | 3.4 (1.6) | 4.5 (2.6) |
| Σ Total taurine conjugated | 0.7 (0.7) | 1.0 (1.0) | 0.9 (1.0) | 0.8 (0.7) |
| Σ Total glycine conjugated | 1.9 (1.8) | 2.0 (1.1) | 1.8 (1.1) | 2.1 (1.8) |
| Race/Ethnicity, n | ||||
| Non-Hispanic white | 16 | 19 | 14 | 21 |
| Hispanic | 13 | 7 | 11 | 9 |
| African-American | 7 | 10 | 8 | 9 |
| Other e | 4 | 4 | 7 | 1 |
Mean (SD) unless otherwise indicated.
Body mass index; ≤18.5 BMI ≤ 25.0 kg/m2 for normal weight; 28.0 ≤ BMI ≥ 39.9 kg/m2 for overweight to obese.
Body fat % based on DXA scan.
Mean plasma concentrations at Day 0 of the first diet intervention.
Native Hawaiian / Pacific Islander, Asian.
Table 2.
Intervention effects of a whole grain (WG) versus a refined grain (RG) diet on individual plasma bile acids (n=80)
| Baseline a | RG diet a | WG diet a | p-value b | |
|---|---|---|---|---|
| Primary bile acids | µM | µM | µM | |
| Taurocholic acid | 0.26 (0.45) | 0.20 (0.23) | 0.44 (0.92) | <0.001 c |
| Glycocholic acid | 0.51 (0.52) | 0.42 (0.54) | 0.77 (1.75) | <0.001 c |
| Taurochenodeoxycholic acid | 0.22 (0.22) | 0.15 (0.15) | 0.21 (0.31) | 0.04 |
| Glycochenodeoxycholic acid | 0.49 (0.35) | 0.38 (0.47) | 0.32 (0.02) | 0.04 |
| Chenodeoxycholic acid | 0.89 (1.05) | 0.66 (0.80) | 0.63 (0.97) | 0.44 |
| Cholic acid | 1.02 (1.83) | 0.46 (0.52) | 0.56 (1.15) | 0.50 |
| Secondary bile acids | ||||
| Taurolithocholic acid | 0.00 (0.00) | 0.003 (0.002) | 0.01 (0.01) | <0.001 c |
| Hyocholic acid | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.03 |
| Glycohyodeoxycholic acid | 0.29 (0.30) | 0.24 (0.25) | 0.24 (0.52) | 0.06 |
| Tauro-ursodeoxycholic acid | 0.02 (0.02) | 0.02 (0.02) | 0.02 (0.02) | 0.10 |
| Ursodeoxycholic acid | 0.02 (0.03) | 0.82 (0.62) | 0.78 (0.67) | 0.15 |
| Hyodeoxycholic acid | 0.86 (0.60) | 0.19 (0.25) | 0.19 (0.34) | 0.16 |
| Tauro-Ω muricholic acid | 0.20 (0.21) | 0.02 (0.02) | 0.02 (0.04) | 0.16 |
| Deoxycholic acid | 0.94 (0.66) | 0.89 (0.67) | 0.85 (0.74) | 0.17 |
| Taurodeoxycholic acid | 0.21 (0.26) | 0.16 (0.17) | 0.20 (0.27) | 0.23 |
| Glycoursodeoxycholic acid | 0.07 (0.06) | 0.06 (0.06) | 0.06 (0.12) | 0.30 |
| Glycolithocholic acid | 0.54 (0.68) | 0.03 (0.01) | 0.03 (0.01) | 0.37 |
| Glycodeoxycholic acid | 0.23 (0.22) | 0.43 (0.54) | 0.49 (0.61) | 0.41 |
| Isolithocholic acid | 0.04 (0.02) | 0.05 (0.03) | 0.05 (0.04) | 0.44 |
| 5β-Cholanic acid-3β, 12α-diol | 0.25 (0.36) | 0.25 (0.22) | 0.24 (0.22) | 0.45 |
| 9(11), (5β)-Cholenic acid 3α-ol-12-one | 0.03 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.50 |
| Tauro-α muricholic acid | 0.05 (0.04) | 0.06 (0.07) | 0.05 (0.05) | 0.52 |
| Taurohyocholic acid | 0.02 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.58 |
| Murocholic acid | 0.04 (0.02) | 0.32 (0.63) | 0.34 (0.68) | 0.61 |
| Taurohyodeoxycholic acid | 0.02 (0.02) | 0.04 (0.03) | 0.04 (0.04) | 0.71 |
| 5-Cholenic acid-3β-ol | 0.06 (0.07) | 0.02 (0.01) | 0.02 (0.01) | 0.78 |
| Glycohyocholic acid | 0.05 (0.04) | 0.06 (0.06) | 0.06 (0.06) | 0.84 |
| Lithocholic acid | 0.02 (0.01) | 0.04 (0.02) | 0.04 (0.02) | 0.90 |
| 3α-Hydroxy-12 ketolithocholic acid | 0.03 (0.02) | 0.02 (0.01) | 0.02 (0.01) | 1.00 |
Mean plasma bile acid concentrations (standard deviation) at baseline and end of each dietary intervention.
P values derived from linear mixed models comparing the two intervention diets at the end of treatment on individual bile acids, adjusted for baseline bile acid concentrations, diet sequence, diet period, batch, age, sex and body mass index (kg/m2).
Statistically significant with Benjamini-Hochberg (FDR < 0.05 for 29 tests).
3.2. Association between plasma bile acids concentrations and HOMA-IR
When examining the association between plasma bile acids and HOMA-IR, GCA, taurodeoxycholic acid (TDCA), ursodeoxycholic acid (UDCA), 5β-cholanic acid-3β,12α-diol, 5-cholenic acid-3β-ol, and GDCA, and the sum of 12α-hydroxylated, primary, and secondary bile acids were all positively associated with HOMA-IR (FDR<0.05; Table 3). Within strata of BMI, we detected a significant, positive association between CDCA, CA, UDCA, 5β-cholanic acid-3β,12α-diol, and the sum of total, primary, secondary and 12α-hydroxylated bile acids and HOMA-IR among the overweight to obese group (FDR<0.05; Table 3). When stratifying by sex, GCA, CDCA, TCA, CA, UDCA, GDCA, glycolithocholic acid (GLCA), total, primary, 12α-hydroxylated, and glycine-conjugated bile acids were significantly positively associated with HOMA-IR among women, and CDCA, GDCA, and GLCA were significant among men (FDR<0.05; Table 3). Lastly, no significant associations were found between plasma bile acid concentrations and CRP (data not shown).
Table 3.
Associations between plasma bile acid concentrations and HOMA-IR among all study participants, and stratified by BMI and sex.
| All (n=80) |
Normal weight a (n=40) |
Overweight to obese a (n=40) |
Men (n=40) |
Women (n=40) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Primary bile acids | µM b | p-valuec | µM b | p-valuec | µM b | p-valuec | µM b | p-valuec | µM b | p-valuec |
| Glycocholic acid | 0.60 (1.30) | <0.001 d | 0.74 (1.76) | 0.04 | 0.46 (0.52) | 0.02 | 0.65 (1.64) | 0.82 | 0.55 (0.84) | <0.01 d |
| Taurochenodeoxycholic acid | 0.18 (0.24) | <0.01 d | 0.23 (0.32) | 0.05 | 0.13 (0.11) | 0.11 | 0.16 (0.27) | 0.25 | 0.20 (0.21) | 0.25 |
| Taurocholic acid | 0.32 (0.68) | 0.03 | 0.24 (0.31) | 0.02 | 0.40 (0.91) | 0.14 | 0.32 (0.79) | 0.01 | 0.32 (0.56) | 0.01 d |
| Glycochenodeoxycholic acid | 0.43 (0.68) | 0.16 | 0.56 (0.91) | 0.03 | 0.32 (0.25) | 0.04 | 0.43 (0.75) | 0.45 | 0.44 (0.60) | 0.02 |
| Chenodeoxycholic acid | 0.65 (0.89) | 0.42 | 0.65 (0.94) | 0.12 | 0.64 (0.83) | <0.001 d | 0.80 (0.99) | <0.01 d | 0.50 (0.73) | 0.01 d |
| Cholic acid | 0.51 (0.89) | 0.91 | 0.52 (1.00) | 0.19 | 0.49 (0.76) | <0.001 d | 0.52 (0.55) | 0.04 | 0.50 (1.13) | <0.01 d |
| Secondary bile acids | ||||||||||
| Ursodeoxycholic acid | 0.80 (0.65) | <0.001 d | 0.65 (0.58) | 0.66 | 0.95 (0.67) | <0.001 d | 0.85 (0.71) | 0.10 | 0.76 (0.06) | 0.01 d |
| 5β-Cholanic acid-3β, 12α-diol | 0.24 (0.22) | <0.01 d | 0.22 (0.20) | 0.17 | 0.26 (0.24) | <0.01 d | 0.27 (0.22) | <0.01 | 0.21 (0.22) | 0.32 |
| 5-Cholenic acid-3β-ol | 0.02 (0.01) | <0.01 d | 0.02 (0.01) | 0.52 | 0.02 (0.01) | 0.78 | 0.02 (0.01) | 0.08 | 0.02 (0.01) | 0.13 |
| Glycodeoxycholic acid | 0.46 (0.58) | 0.01 d | 0.44 (0.63) | 0.23 | 0.48 (0.512 | 0.03 | 0.40 (0.42) | <0.01 d | 0.52 (0.69) | <0.01 d |
| Glycolithocholic acid | 0.03 (9.01) | 0.02 | 0.03 (0.01) | 0.10 | 0.03 (0.01) | 0.05 | 0.03 (0.01) | <0.01 d | 0.03 (0.01) | <0.01 d |
| 9(11),(5β)-Cholenic acid-3α-ol-12-one | 0.04 (0.02) | 0.02 | 0.04 (0.02) | 0.49 | 0.05 (0.02) | 0.12 | 0.05 (0.02) | 0.62 | 0.04 (0.02) | 0.93 |
| Taurodeoxycholic acid | 0.02 (0.23) | 0.03 | 0.18 (0.26) | 0.12 | 0.17 (0.19) | 0.12 | 0.14 (0.15) | 0.05 | 0.22 (0.28) | 0.05 |
| Tauro-α muricholic acid | 0.05 (0.06) | 0.03 | 0.07 (0.06) | 0.34 | 0.04 (0.05) | 0.97 | 0.04 (0.04) | 0.31 | 0.07 (0.07) | 0.31 |
| Taurohyocholic acid | 0.01 (0.01) | 0.05 | 0.01 (0.01) | 0.39 | 0.00 (0.00) | 0.88 | 0.00 (0.00) | 0.45 | 0.01 (0.01) | 0.84 |
| Glycohyodeoxycholic acid | 0.24 (0.41) | 0.16 | 0.30 (0.54) | 0.17 | 0.19 (0.19) | 0.05 | 0.27 (0.52) | 0.21 | 0.22 (0.24) | 0.21 |
| Glycoursodeoxycholic acid | 0.06 (0.10) | 0.22 | 0.07 (0.12) | 0.10 | 0.05 (0.04) | 0.15 | 0.07 (0.12) | 0.28 | 0.05 (0.05) | 0.28 |
| Murocholic acid | 0.33 (0.65) | 0.27 | 0.35 (0.72) | 0.83 | 0.30 (0.56) | 0.03 | 0.36 (0.71) | 0.80 | 0.03 (0.59) | 0.08 |
| Hyodeoxycholic acid | 0.19 (0.30) | 0.34 | 0.20 (0.35) | 0.69 | 0.18 (0.23) | 0.04 | 0.21 (0.35) | 0.73 | 0.17 (0.23) | 0.73 |
| Hyocholic acid | 0.01 (0.01) | 0.37 | 0.01 (0.01) | 0.43 | 0.01 (0.01) | 0.22 | 0.01 (0.01) | 0.96 | 0.01 (0.01) | 0.96 |
| 3α-Hydroxy-12 ketolithocholic acid | 0.02 (0.01) | 0.39 | 0.02 (0.01) | 0.17 | 0.02 (0.01) | 0.82 | 0.02 (0.01) | 0.93 | 0.02 (0.01) | 0.27 |
| Lithocholic acid | 0.04 (0.02) | 0.50 | 0.04 (0.02) | 0.85 | 0.04 (0.02) | 0.11 | 0.04 (0.02) | 0.62 | 0.04 (0.02) | 0.62 |
| Taurolithocholic acid | 0.01 (0.01) | 0.59 | 0.01 (0.01) | 0.35 | 0.01 (0.01) | 0.31 | 0.01 (0.01) | 0.21 | 0.00 (0.00) | 0.24 |
| Tauro-Ω muricholic acid | 0.02 (0.03) | 0.63 | 0.03 (0.04) | 0.85 | 0.01 (0.02) | 0.27 | 0.02 (0.03) | 0.26 | 0.03 (0.03) | 0.26 |
| Deoxycholic acid | 0.87 (0.70) | 0.67 | 0.71 (0.63) | 0.62 | 1.03 (0.74) | <0.001 d | 0.92 (0.78) | 0.06 | 0.82 (0.62) | 0.02 |
| Glycohyocholic acid | 0.06 (0.06) | 0.69 | 0.08 (0.07) | 0.70 | 0.04 (0.04) | 0.89 | 0.06 (0.05) | 0.82 | 0.07 (0.07) | 0.67 |
| Isolithocholic acid | 0.05 (0.04) | 0.72 | 0.04 (0.03) | 0.67 | 0.05 (0.04) | 0.32 | 0.05 (0.04) | 0.72 | 0.04 (0.02) | 0.72 |
| Taurohyodeoxycholic acid | 0.04 (0.03) | 0.86 | 0.05 (0.04) | 0.23 | 0.03 (0.02) | 0.77 | 0.04 (9.04) | 0.31 | 0.04 (0.03) | 0.73 |
| Tauro-ursodeoxycholic acid | 0.02 (0.02) | 0.94 | 0.02 (0.03) | 0.17 | 0.02 (0.01) | 0.67 | 0.02 (0.02) | 0.99 | 0.02 (0.02) | 0.99 |
| Bile acid groups | ||||||||||
| Σ Total bile acids | 6.46 (4.87) | <0.01 d | 6.69 (5.77) | 0.03 | 6.24 (3.78) | <0.001 d | 6.74 (5.34) | 0.12 | 6.19 (4.37) | <0.01 d |
| Σ 12α-hydroxylated bile acids | 2.93 (2.87) | <0.01 d | 2.99 (3.53) | 0.04 | 2.88 (2.02) | <0.001 d | 2.94 (3.01) | 0.23 | 2.92 (2.73) | <0.001 d |
| Σ Total primary bile acids | 2.69 (3.26) | <0.01 d | 3.10 (4.21) | 0.02 | 2.28 (1.84) | <0.01 d | 2.87 (3.79) | 0.16 | 2.50 (2.64) | <0.01 d |
| Σ Total secondary bile acids | 3.78 (2.47) | <0.01 d | 3.59 (2.47) | 0.13 | 3.96 (2.47) | <0.01 d | 3.87 (2.61) | 0.08 | 3.68 (2.33) | 0.03 |
| Σ Total glycine conjugated | 1.89 (2.52) | 0.03 | 2.21 (3.26) | 0.04 | 1.56 (1.37) | 0.04 | 1.90 (2.81) | 0.76 | 1.87 (2.20) | <0.01 d |
| Σ Total taurine conjugated | 0.82 (1.13) | 0.09 | 0.99 (1.48) | 0.05 | 0.65 (0.59) | 0.30 | 0.73 (1.22) | 0.67 | 0.29 (1.04) | 0.10 |
Normal weight: 18.5 ≥ BMI ≤ 25.0 kg/m2. Overweight to obese: 28.0 ≤ BMI ≤ 39.9 kg/m2.
Mean plasma bile acid concentrations (standard deviation).
P values from linear mixed models evaluating the association of HOMA-IR and bile acids adjusted for study diet, diet sequence, diet period, batch, age, sex and BMI, except for stratified analyses by BMI and sex, which omit these variables, respectively.
Statistically significant after Benjamini-Hochberg (FDR < 0.05 for 35 tests). All statistically significant associations were positively associated.
4. Discussion
In this secondary analysis from a randomized, controlled crossover feeding study, we found significantly higher plasma concentrations of the secondary bile acid TLCA and primary bile acids TCA and GCA after the WG diet compared to the RG diet. Bile acid pools modulate TGR5 and FXR activity [2, 5, 7], and in turn influence bile acid metabolism (FXR), glucose homeostasis, and immune-regulation (FXR and TGR5) [2, 7, 9]. TLCA is one of the most potent ligands for TGR5, a stimulator of incretin secretion and inhibitor of NF-κB signaling [5, 9, 24, 25]. TCA and GCA can also activate TGR5, but to a lesser extent [24]. Therefore, the modest increases in bile acids observed after the WG diet, particularly TLCA, may be beneficial.
Few intervention studies have assessed the effects of diet on plasma bile acids, and none have evaluated the effects of glycemic load dietary patterns, specifically. A small (n=10), short-term crossover feeding trial found that a high-fat, high-protein diet significantly increased plasma CA (27%), and to a lesser extent CDCA and DCA concentrations, compared to a control or high-fat, low-protein diet [26]. Another study in 16 participants examined post-prandial plasma bile acid response [27] to isocaloric beverages consisting primarily of carbohydrate (as glucose), protein or lipid [27]. The high-lipid beverage increased total plasma bile acids, whereas the high-carbohydrate beverage had little effect. These findings suggest that macronutrient quantity, particularly dietary fat, modulates plasma total bile acid concentrations. While we observed differences in specific bile acids between the two diets differing in carbohydrate type, total plasma bile acid concentrations did not differ, possibly because total macronutrient content was similar.
Differences in concentrations of specific bile acids in response to the test diets may reflect, in part, shifts in gut microbial metabolism. Gut microbiota can alter bile acid pools directly through taurine or glycine deconjugation, catalyzed by microbes carrying the bile salt hydrolase gene, and through subsequent 7α-dehydroxylation of deconjugated species [2, 5]. Deconjugation of bile acids can also affect the circulating bile acid pool indirectly as these bile acids are less easily reabsorbed [28]. Increased plasma concentrations of TLCA after the WG diet may be due to increased 7α-dehydroxylation. Reasons for increased concentrations of the primary bile acids GCA and TCA are less clear; however, fecal bile acid excretion with different amounts and types of fibers may alter plasma concentrations [29, 30]. Our two diets differed substantially in mean fiber content (28 g/d on RG versus 56 g/d on RG), which can alter gut microbial diversity and activity [31]. It is likely that gut microbial metabolism is contributing to these bile acid differences, and we plan to investigate this in future analyses.
Considering that insulin secretion, glucose homeostasis and immune responses may be affected by bile acids through TGR5 and FXR activation [2], we explored the association between plasma bile acids and insulin resistance, and CRP. We reported previously that the WG diet compared to the RG diet did not reduce HOMA-IR or CRP [10, 12]. However, in the present analysis, several individual bile acids and groups, including 12α-hydroxylated bile acids, were significantly positively associated with HOMA-IR even after applying a stringent FDR, particularly among overweight to obese individuals, and among women, who tend to have higher adiposity than men [32]. Several cross-sectional studies have investigated the association between circulating peripheral bile acids and insulin resistance, however comparison of these to our study is difficult due to differences in study populations and their lack of standardized diets. In one study, a significant positive association was found between 12α-hydroxylated bile acids and insulin resistance [17]. The authors speculated that insulin resistance disrupts the ability of insulin to inhibit production of 12α-hydroxylated bile acids. In obese individuals with and without nonalcoholic steatohepatitis, the primary bile acids CA and CDCA, as well as 12α-hydroxylated bile acids, were positively associated with HOMA-IR and fasting insulin, respectively [33]. Several other studies comparing individuals across the continuum of insulin resistance, from healthy individuals to patients with type 2 diabetes (T2DM), have also reported differences in plasma bile acid profiles, although results varied [18, 19, 34]. While we found higher HOMA-IR associated with higher total bile acids, we found no associations with taurine conjugated bile acids, and glycine conjugated species were only associated with HOMA-IR in women. Taken together, our findings, along with previous investigations, show that total circulating bile acids, and specifically 12α-hydroxylated bile acids (CA, DCA, and their conjugates), are consistently positively associated with insulin resistance.
Plasma bile acid profiling in the context of a randomized, controlled crossover feeding study is novel. The crossover study design is an important strength, as it enabled testing of effects of two dietary patterns, with each person acting as their own control. Our study population included both men and women, and normal and overweight to obese individuals, allowing for sub-group analyses. Further, the controlled diets reduced confounding effects of diet determining the association between bile acids and insulin resistance, given that all participants consumed the same diets. Use of HOMA-IR can be considered a limitation because of inaccurate values in individuals with severely impaired or absent β-cell function [14], but considering that individuals with fasting blood glucose >100mg/dL and/or a history of diabetes were excluded, this is unlikely to affect our results. Another potential limitation is the use of fasting rather than post-prandial bile acids. A longitudinal study of intra-individual variation in serum bile acids indicated that circulating bile acids exhibit substantial diurnal variability [35]. However, all blood draws were collected in the morning after a 12 hour overnight fast, reducing within-person variation. Finally, the parent study was not designed to address the impact of the diets on circulating bile acid concentrations, nor the association between bile acids and HOMA-IR or fasting CRP. Therefore, our ability to detect effects or associations was limited by a sample size that was not estimated to detect the associations in this particular analysis, and by the characteristics of the study population. This was particularly true for CRP, which was below the limit of detection in ~20% of our younger, healthy population.
4.1. Conclusions
In conclusion, we found that diets with comparable macronutrient composition but differing carbohydrate type and quality, affect circulating plasma bile acids differently. Specifically, higher TLCA, GCA and TCA concentrations were observed after the WG compared to the RG diet. Given that these bile acids activate TGR5 and FXR, which play a role in glucose homeostasis, these roles may contribute, in part, to the beneficial effects of a dietary pattern characterized by whole grains, legumes, and fruits and vegetables.
Supplementary Material
Figure 1.
Highlights.
Three plasma bile acids increased after a whole versus refined grain diet pattern.
There were significant positive associations between bile acids and HOMA-IR.
Diet patterns varying in carbohydrate type affect plasma bile acids differently.
Acknowledgments
Funding: Work supported by National Institutes of Health [R01 CA192222, U54 CA116847, P30 CA015704], UMCN Student Grant [160699] University Fund Nijmegen.
Abbreviations
- ASBT
apical sodium-dependent bile acid transporter
- BMI
body mass index
- CA
cholic acid
- CA-D4
cholic acid-2,2,4,4-D4
- CARB
Carbohydrate and Related Biomarkers
- CDCA
chenodeoxycholic acid
- CRP
C-reactive protein
- CV
coefficient of variation
- DCA
deoxycholic acid
- DCA-D4
deoxycholic acid-2,2,4,4-D4
- DXA
dual-energy X-ray absorptiometry
- FDR
false discovery rate
- FXR
farnesoid X receptor
- GCDCA
glycochenodeoxycholic acid
- GCDCA-D4
glycochenodeoxycholic Acid-2,2,4,4-D4
- GCA
glycocholic acid
- GCA-D4
glycocholic acid-2,2,4,4-D4
- GDCA
glycodeoxycholic acid
- GLCA
glycolithocholic acid
- GLP1
glucagon-like peptide-1
- GPBAR1/TGR5
G protein-coupled bile acid receptor 1
- HCA
hyocholic acid
- HOMA-IR
homeostasis model assessment-insulin resistance
- LCA
lithocholic acid
- LCA-D4
lithocholic Acid-2,2,4,4-D4
- LC-MS
liquid chromatography tandem mass spectrometry
- MRM
multiple-reaction-monitoring
- RG
refined grain
- TCDCA
taurochenodeoxycholic acid
- TCA
taurocholic acid
- TDCA
taurodeoxycholic acid
- TLCA
taurolithocholic acid
- UDCA
ursodeoxycholic acid
- WG
whole grain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
BNRG, SLN, DR, MK, JWL conceived the study; SLN, TWR, AS, MK, MAJH, PDL, MLN, DR and JWL designed the study and obtained funding; YS, MLN, and JWL participated in protocol design, participant recruitment and data collection; HG, DW, and DR conducted the assays; BNRG, SLN, TR, and AS and analyzed the data; BNRG, SLN, MK interpreted the data; BNRG and SLN wrote the manuscript, and all authors provided critical revisions. All authors read and approved the final manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Mazuy C, Helleboid A, Staels B, Lefebvre P. Nuclear bile acid signaling through the farnesoid X receptor. Cell Mol Life Sci. 2015;72:1631–50. doi: 10.1007/s00018-014-1805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:573–83. doi: 10.1016/j.bpg.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–98. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–58. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 5.Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21:702–14. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Gadaleta RM, Garcia-Irigoyen O, Moschetta A. Bile acids and colon cancer: Is FXR the solution of the conundrum? Mol Aspects Med. 2017;56:66–74. doi: 10.1016/j.mam.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Taoka H, Yokoyama Y, Morimoto K, Kitamura N, Tanigaki T, Takashina Y, et al. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. 2016;7:260–70. doi: 10.4239/wjd.v7.i13.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: From basic research to clinical application. Digestive and Liver Disease. 2014;46:302–12. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–72. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–74. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24:1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 12.Runchey SS, Pollak MN, Valsta LM, Coronado GD, Schwarz Y, Breymeyer KL, et al. Glycemic load effect on fasting and post-prandial serum glucose, insulin, IGF-1 and IGFBP-3 in a randomized, controlled feeding study. Eur J Clin Nutr. 2012;66:1146–52. doi: 10.1038/ejcn.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 14.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 15.Raftery D. Mass spectrometry in metabolomics: methods and protocols. New York: Humana Press; 2014. [Google Scholar]
- 16.Sarafian MH, Lewis MR, Pechlivanis A, Ralphs S, McPhail MJ, Patel VC, et al. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2015;87:9662–70. doi: 10.1021/acs.analchem.5b01556. [DOI] [PubMed] [Google Scholar]
- 17.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–91. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent RP, Omar S, Ghozlan S, Taylor DR, Cross G, Sherwood RA, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50:360–4. doi: 10.1177/0004563212473450. [DOI] [PubMed] [Google Scholar]
- 19.Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–51. doi: 10.1210/jc.2013-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32(Suppl 3):S56–9. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457–65. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 22.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 24.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 25.Arlia-Ciommo A, Piano A, Svistkova V, Mohtashami S, Titorenko VI. Mechanisms underlying the antiaging and anti-tumor effects of lithocholic bile acid. Int J Mol Sci. 2014;15:16522–43. doi: 10.3390/ijms150916522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bortolotti M, Kreis R, Debard C, Cariou B, Faeh D, Chetiveaux M, et al. High protein intake reduces intrahepatocellular lipid deposition in humans. Am J Clin Nutr. 2009;90:1002–10. doi: 10.3945/ajcn.2008.27296. [DOI] [PubMed] [Google Scholar]
- 27.Morton GJ, Kaiyala KJ, Foster-Schubert KE, Cummings DE, Schwartz MW. Carbohydrate feeding dissociates the postprandial FGF19 response from circulating bile acid levels in humans. J Clin Endocrinol Metab. 2014;99:E241–5. doi: 10.1210/jc.2013-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–99. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampe JW, Slavin JL, Melcher EA, Potter JD. Effects of cereal and vegetable fiber feeding on potential risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:207–11. [PubMed] [Google Scholar]
- 30.Gunness P, Michiels J, Vanhaecke L, De Smet S, Kravchuk O, Van de Meene A, et al. Reduction in circulating bile acid and restricted diffusion across the intestinal epithelium are associated with a decrease in blood cholesterol in the presence of oat beta-glucan. FASEB J. 2016;30:4227–38. doi: 10.1096/fj.201600465R. [DOI] [PubMed] [Google Scholar]
- 31.Hullar MA, Lancaster SM, Li F, Tseng E, Beer K, Atkinson C, et al. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24:546–54. doi: 10.1158/1055-9965.EPI-14-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuente-Martin E, Argente-Arizon P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: It is not only a question of quantity and distribution. Adipocyte. 2013;2:128–34. doi: 10.4161/adip.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legry V, Francque S, Haas JT, Verrijken A, Caron S, Chavez-Talavera O, et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J Clin Endocrinol Metab. 2017;102:3783–94. doi: 10.1210/jc.2017-01397. [DOI] [PubMed] [Google Scholar]
- 34.Sun W, Zhang D, Wang Z, Sun J, Xu B, Chen Y, et al. Insulin resistance is associated with total bile acid level in type 2 diabetic and nondiabetic population: a cross-sectional study. Medicine (Baltimore) 2016;95:e2778. doi: 10.1097/MD.0000000000002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner C, Othman A, Saely CH, Rein P, Drexel H, von Eckardstein A, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6:e25006. doi: 10.1371/journal.pone.0025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.