INTRODUCTION
Evidence is accumulating on the negative short- and long-term ramifications associated with poorly mitigated vaccine injection pain in infants including high levels of infant and parental distress, dissatisfaction with the vaccination experience, development of needle fears, and future noncompliance with immunization and other health care interventions involving needles (1). The use of pain mitigation interventions is therefore clinically important and considered by the World Health Organization to be part of good immunization practice (2). At present, utilization of pain mitigation strategies during vaccination is suboptimal (3). We sought to examine parental patterns of pain mitigation strategy utilization during vaccination in infants after participation in a vaccination analgesic trial whereby parents learned about and had experience using a variety of different analgesic strategies (4). The primary research question was: What is the rate of uptake of analgesic strategies by parents during childhood vaccinations after participation in the trial?
METHODS
We conducted a prospective, observational study including parent–infant dyads who participated in a longitudinal randomized controlled trial of analgesia for infant vaccination injections. The details of the methodology for the randomized controlled trial are reported elsewhere (4). Briefly, infants were randomized to four different analgesic regimens of increasing intensity for 2-, 4-, 6- and 12-month vaccinations: 1) standard care (placebo control), 2) parent-directed educational video (video) with information about acting calm, cuddling and distracting infants, 3) video and oral sucrose solution (sucrose), and 4) video and sucrose and liposomal lidocaine cream (lidocaine). In all groups, procedural techniques to minimize pain were used including injecting without aspiration, and injecting the most painful vaccine last (4). Treatments were given in the clinics of seven participating paediatricians in a blinded manner. Randomization was performed offsite by the research pharmacy at The Hospital for Sick Children using a computer randomization table and allocation was concealed using a matched placebo for all interventions (i.e., double-dummy design) (4). At the 15-month vaccination, all infants received all the active interventions in an open manner. Parents were then informed about how to make sucrose solution and where to purchase topical anesthetics for future vaccinations as they were not available at the clinics after the 15-month vaccination.
At the 18-month vaccination, parents self-directed the analgesic interventions used, as per usual practices at the study clinics. Research assistants documented whether parents continued to use any of the pain mitigation strategies included in the trial. For the intervention ‘acting calm’, research assistants observed parent–infant interactions such as positive affect, coping-promoting behaviours and deep breathing. Parents also completed an exit survey (Box 1). The parental responses to the survey and their pattern of use of different pain mitigation strategies at the 18-month vaccination were summarized descriptively.
Box 1. Questions asked in the parent exit survey
How much stress do you feel right now about your infant getting an immunization injection on a scale of 0 to 10, where 0 is no stress and 10 is the worst stress possible?
How confident are you in your ability to manage your child’s pain today on a scale of 0 to 10, where 0 is not at all confident and 10 is completely confident?
What methods did you use to try to make your baby’s vaccine injection(s) less painful today?
Can you explain why you chose to do these things?
How satisfied are you with how well your baby’s needle pain was controlled today on a scale of 0 to 10, where 0 is not at all satisfied and 10 is the most satisfied possible?
-
Is there anything else you wanted to do but didn’t?
Sugar water and reason(s) why it was not used
Numbing cream and reason(s) why it was not used
Techniques suggested in the study video? (e.g., holding, distraction, acting calm)
What was your most favourite part of this study?
What was your least favourite part of this study?
Is there any other feedback you would like to provide to us?
RESULTS
Altogether, 130 parents participated out of 352 (44%) originally enrolled in the randomized controlled trial. Attrition occurred because the study began after the randomized controlled trial was already underway and participants were missed (n = 138), there were withdrawals from the randomized controlled trial (n = 48) and parents refused to participate (n = 36). We compared the characteristics of infants that were included and excluded, including frequency of male sex (52% versus 56%) and number of siblings (59% for both) and found no significant differences (P > 0.05).
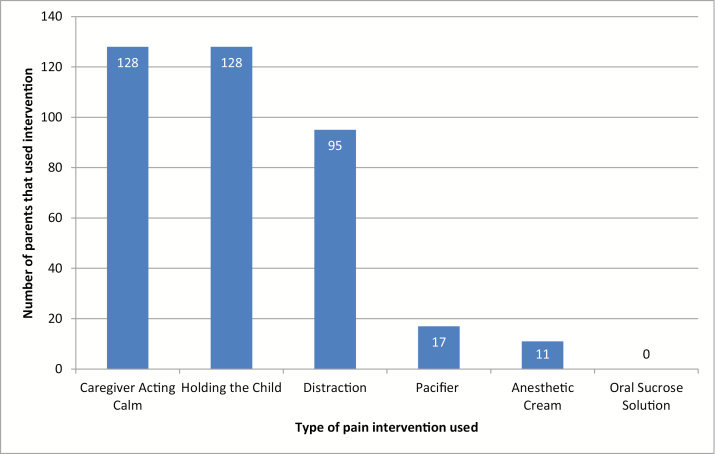
Demographic characteristics of participating infants are shown in Table 1. Altogether, 98.5% of parents held the infant close and acted calm during the injection, 73.1% distracted the infant, 13.1% used a pacifier and 8.5% used a topical anesthetic to numb the skin (Figure 1). Oral sucrose solution was never used. The mean parental satisfaction score (using verbal numerical rating scale: range 0 to 10) for pain management during vaccination was 8.7 (SD = 1.9), parental stress was 1.8 (SD = 2.5) and confidence in ability to control their infants’ pain was 7.1 (SD = 2.5).
Table 1.
Characteristics of infants (n = 130)
| Characteristic | Mean (SD) or frequency (%) |
|---|---|
| Age, months | 18.6 (1.1) |
| Sex, no. male | 68 (52) |
| Race, no. Caucasian | 66 (51) |
| Siblings, no. with 1 or more | 77 (59) |
Figure 1.
Pain management interventions used by parents during 18-month child vaccination (n = 130).
Parents who did not utilize topical anesthetics or sucrose most commonly reported that they did not find these interventions to be necessary, they did not think of them, or that they should be provided by the clinic (Table 2). The most common parental response for favourite part of the study was learning about and using methods for pain management (43.8%) and for least favourite part, it was the needle and witnessing their infant in pain (11.5%).
Table 2.
Reasons given by parents for not using sucrose solution or topical anesthetics
| Sucrose solution, n = 130* | Topical anesthetic, n = 119* | |
|---|---|---|
| Did not think it was necessary† | 59 (45.4) | 49 (41.1) |
| Forgot/didn’t think about it | 37 (28.5) | 34 (28.6) |
| Not provided by clinic/doctor/study | 13 (10) | 18 (15.1) |
| Too busy | 10 (7.7) | 8 (6.7) |
| Didn’t think it was effective | 8 (6.2) | 6 (5.0) |
| Insufficient knowledge on how to implement (i.e., make/acquire/use) | 4 (3.1) | 6 (5.0) |
| Other | 5 (3.8)‡ | 4 (3.4)§ |
| No reason given | 5 (3.8) | 5 (4.2) |
Values are frequency (%). *n = 13 parents provided more than one reason for why they did not use sucrose solution; n = 12 parents provided more than one reason why they did not use topical anesthetics; †Parents sometimes said the intervention was not necessary because the pain was minor or, alternatively, because a different pain intervention was used instead; ‡n = 3 child does not like/refuses sucrose solution; n = 2 did not know vaccination injections were to be administered on that day; §n = 3 did not know vaccination injections were to be administered on that day; n = 1 skin reaction at 15-month vaccination
DISCUSSION
These results reveal some uptake of pain mitigation interventions by parents after participation in an analgesic randomized controlled trial. Relative to other surveys of pain mitigation intervention utilization during vaccination in our community (3), these results appear qualitatively higher, suggesting a beneficial effect of participation in the trial (4).
The results also demonstrate that parents commonly used nonpharmacological methods such as holding, distracting and acting calm. Parents used pharmacological methods of controlling pain such as topical anesthetics or oral sucrose less frequently. A similar pattern of results has been observed in other studies and may reflect the feasibility of nonpharmacological methods compared to pharmacological methods and preferences of parents to employ nonpharmacological approaches (5). Parents indicated that topical anesthetics and oral sucrose solution would be utilized more if they were provided by the clinic. Making these strategies more accessible and convenient for parents to use may lead to higher utilization rates. Separately, there are data demonstrating that both sucrose and topical anesthetics can be accommodated within usual clinic waiting times and that parents are willing to pay for pain relief (4,6).
Parental satisfaction with their infants’ pain management was also qualitatively high, supporting previous research showing when pain mitigation is offered, parents report better satisfaction with medical care (7). Giving parents a role in managing their infant’s pain may give them a sense of control, which in turn, contributes to improved satisfaction with the immunization appointment. This is significant as endorsing more positive immunization experiences can promote future adherence with the immunization schedule and increased confidence in health care providers (8).
Parents were unaware of the treatments provided to their infants between 2 and 12 months, which may have contributed to their uncertainty regarding the effectiveness of the individual interventions they expressed at 18 months and lack of use of individual interventions. However, the main findings of the randomized controlled trial also demonstrated less than compelling results with respect to effectiveness of the tested interventions. Significant analgesic effects were only demonstrated for the infants allocated to the video and sucrose and lidocaine group compared to all other groups, suggesting that only the lidocaine component of the pain interventions was effective. Moreover, the magnitude of effect was modest (effect size = 0.5) (4). Detecting this difference clinically may be challenging for both parents and clinicians, and may not be visible if assessed using dichotomous markers of infant distress such as the presence or absence of infant crying.
There are some limitations worthy of discussion. Firstly, a significant proportion of parents that participated in the randomized controlled trial were excluded, which may introduce sampling bias. There was no evidence, however, of a difference in the characteristics of those that participated versus those that did not. Secondly, the lack of a control group prevents definite conclusions about the impact of prior participation in the randomized controlled trial on utilization of pain interventions. Given that the majority of parents cited using the pain strategies because they learned about them in the study and had experience with them suggests that participation in the trial positively affected uptake of interventions. For topical anesthetics specifically, all parents reported that learning about them in the study and having experience with them were the reasons for their use at the 18-month vaccination.
As a result of these findings, we recommend health care providers take an active role in facilitating pain mitigation during vaccination by educating parents about evidence-based interventions, making them available for use, and coaching parents in their use.
Acknowledgements
A Taddio declares a research grant from Pfizer, and study supplies from Natus and Ferndale. The other authors have nothing to declare.
References
- 1. Taddio A, Chambers CT, Halperin SA, et al. Inadequate pain management during routine childhood immunizations: The nerve of it. Clin Ther 2009;31(Suppl 2):S152–67. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Reducing pain at the time of vaccination. WHO position statement—September 2015. WER 2015;39:505–16 [Google Scholar]
- 3. Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012;30:4807–12. [DOI] [PubMed] [Google Scholar]
- 4. Taddio A, Pillai Riddell R, Ipp M, et al. Relative effectiveness of additive pain interventions during vaccination in infants. CMAJ. 2016. doi:10.1503/cmaj.160542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parvez E, Stinson J, Boon H, Goldman J, Shah V, Taddio A. Mothers’ beliefs about analgesia during childhood immunization. Paediatr Child Health 2010;15:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taddio A, Hogan ME, Gerges S, et al. Addressing parental concerns about pain during childhood vaccination: Is there enough time to include pain management in the ambulatory setting? Clin j Pain 2012;28:238–42. [DOI] [PubMed] [Google Scholar]
- 7. Schechter NL, Bernstein BA, Zempsky WT, Bright NS, Willard AK. Educational outreach to reduce immunization pain in office settings. Pediatrics 2010;126:e1514–21. [DOI] [PubMed] [Google Scholar]
- 8. Schempf AH, Minkovitz CS, Strobino DM, Guyer B. Parental satisfaction with early pediatric care and immunization of young children: The mediating role of age-appropriate well-child care utilization. Arch Pediatr Adolesc Med 2007;161:50–6. [DOI] [PubMed] [Google Scholar]