Abstract
Restricted gene flow may cause positive spatial genetic autocorrelation of animal populations at fine spatial scales. The Mongolian gerbil Meriones unguiculatus is a territorial, social rodent. Territoriality may create social fences to restrict dispersal or gene flow of Mongolian gerbils to a short distance. Restricted dispersal may differentiate fine-scale spatial genetic structure of populations with increasing distances (i.e., isolation by distance [IBD]). Competition for mates and inbreeding avoidance may result in equal dispersal propensity and subsequently similar spatial genetic autocorrelation between males and females of monogamous gerbils. We genotyped 327 gerbils, live captured from 26 burrow systems on a 9-ha plot in northcentral Inner Mongolia, China, using seven microsatellite loci. Spatial genetic autocorrelation was positive within 80 m and became negative from 80 m to 200 m, suggesting restricted gene flow. Inter-group genetic and geographic distances were related positively, supporting the IBD model. Live trapping data demonstrated equal dispersal propensities of male and female gerbils. Restricted dispersal and social organization may determine fine-scale spatial population genetic structure of social rodents.
Keywords: dispersal, isolation by distance, Meriones unguiculatus, microsatellite analysis, social rodents, spatial genetic autocorrelation
Non-random spatial distributions and restricted movements of animals may result in the spatial genetic subdivision of animal populations (Chesser 1991; Roff 2002). Restricted gene flow not only differentiates population genetics between animal populations on large spatial scales (Slatkin 1987, 1993), but also determines spatial genetic structure on fine spatial scales (e.g., <1 km; Peakall et al. 2003; Double et al. 2005). On population levels, the stepping stone or isolation by distance (IBD) model predicts a positive relationship between genetic differentiation and geographic distances between populations (Wright 1943; Kimura and Weiss 1964). At fine spatial scales, restricted gene flow may result in positive spatial genetic autocorrelation (hereafter, spatial autocorrelation) in close proximity, with individuals being more genetically related within a shorter distance (Sokal and Watenburg 1983; Peakall et al. 2003). For instance, restricted gene flow results in positive spatial autocorrelation in Rattus fuscipes in a distance of less than 200 m (Peakall et al. 2003).
Natal dispersal of polygynous mammals is often male biased (Greenwood 1980). Therefore, fine-scale spatial autocorrelation may occur only in philopatric females (Peakall et al. 2003; Hazlitt et al. 2004). Dispersal of sand dune tuco-tucos Ctenomys australis is male biased, so positive spatial autocorrelation was observed only in female tuco-tucos (Mora et al. 2010). On the contrary, positive spatial autocorrelation was detected only in male superb fairy-wrens Malurus cyaneus owing to female-biased dispersal (Double et al. 2005). Therefore, sex-biased dispersal may differentiate fine-scale spatial genetic structures between the sexes within a population. We predicted that males and females would have similar spatial genetic structure if the two sexes had equal dispersal propensities.
The Mongolian gerbil Meriones unguiculatus is a social, territorial rodent, living in groups year round (Agren et al. 1989a). Territoriality may create a social fence to restrict dispersal between social groups of Mongolian gerbils within a short distance (Liu et al. 2009a). Mongolian gerbils are believed to be socially monogamous (French 1994; Solomon and Getz 1997). The theory of animal dispersal predicts equal dispersal propensities for males and females if competition for mates or inbreeding avoidance drives dispersal of monogamous mammals (Greenwood 1980). Monogamous southern pied babblers Turdoides bicolor have equal propensity to disperse and similar fine-scale spatial autocorrelation patterns between males and females (Nelson-Flower et al. 2011, 2012). Wang et al. (2011b) demonstrated genetic differentiation among social groups of Mongolian gerbils; however, they did not investigate relationships between fine-scale spatial genetic structure and dispersal of Mongolia gerbils.
In this study, we aimed to test the predictions of the following two hypotheses. First, restricted dispersal would result in positive spatial autocorrelation at fine spatial scales, with spatial autocorrelation decreasing to zero and then becoming negative with increasing distance between individuals. Second, male and female gerbils would have similar fine-scale spatial autocorrelation patterns owing to equal dispersal propensities. Positive spatial autocorrelation may enhance the fitness benefits of group living (Chesser 1991; van Staaden 1995). Therefore, the findings of this study help understand the roles of dispersal patterns and spatial variation in inter-individual genetic relatedness in the maintenance of social groups in rodents.
Materials and Methods
Study site
We conducted this study at Xima Village, an area of the steppe intermixed with cropland, in Taipusi Qi (county), Inner Mongolia, China (115°22′ E, 42°07′ N). The climate was semiarid, with average monthly temperatures ranging from –19.1°C to 21.1 °C and annual total precipitation ranging from 258 to 550 mm. Snow cover lasted from mid- or late October to early April (Liu et al. 2009b).
Our trapping plot was situated on a 9-ha grassland (300 m × 300 m). The vegetation consisted of a mixture of grasses such as Cleistogenes squarrosa and Setaria viridis; herbs such as Artemisia sieversiama, A. scoparia, and Heteropappus altaicus; and small shrubs Caragana microphylla and C. korshinskii. Leymus chinense and Corispermum mongolicum were the dominant plants.
Capture–recapture methods
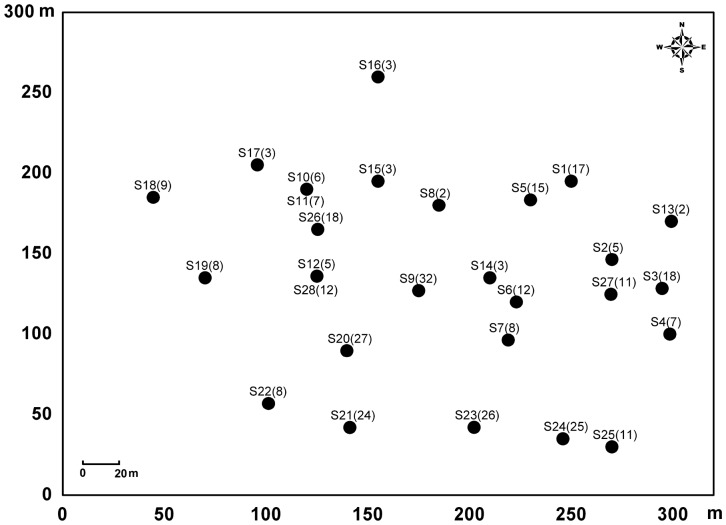
Our trapping plot encompassed 26 burrow systems of Mongolian gerbils (Figure A1). Distances between burrow systems ranged from 19.9 to 273.7 m. We live trapped Mongolian gerbils biweekly from 28 April to 21 October in 2006. Trap stations were arranged in three to four concentric circles with equal between-circle spacing at each burrow system (Liu et al. 2009b). Four to 16 trap stations were spaced equally on each circle, with one wire-mesh live trap (28 ×13 ×10 cm) being placed at each station. We placed a total of about 450 traps on our trapping plot each trapping week. Traps were baited with fresh peanuts at the time of trapping. Each trapping period lasted for three consecutive days. All captured gerbils were toe clipped at the first capture for permanent identification (ID), and were released at the sites of capture. Clipped toes were preserved in 75% ethanol for DNA extraction and microsatellite analysis. Sex, body mass, reproductive condition, trap location, and ID number were recorded for each capture. We classified Mongolian gerbils as juveniles (less than 30 g), subadults (between 30 and 50 g), and adults (>50 g). The details of our trapping methods and procedures can be found in Liu et al. (2009b). Our trapping and handling procedures followed the guidelines of the Animal Care and Use Committee of the American Society of Mammalogists (Gannon et al. 2007) and were approved by the Institutional Animal Use and Care Committee of the Institute of Zoology, Chinese Academy of Sciences.
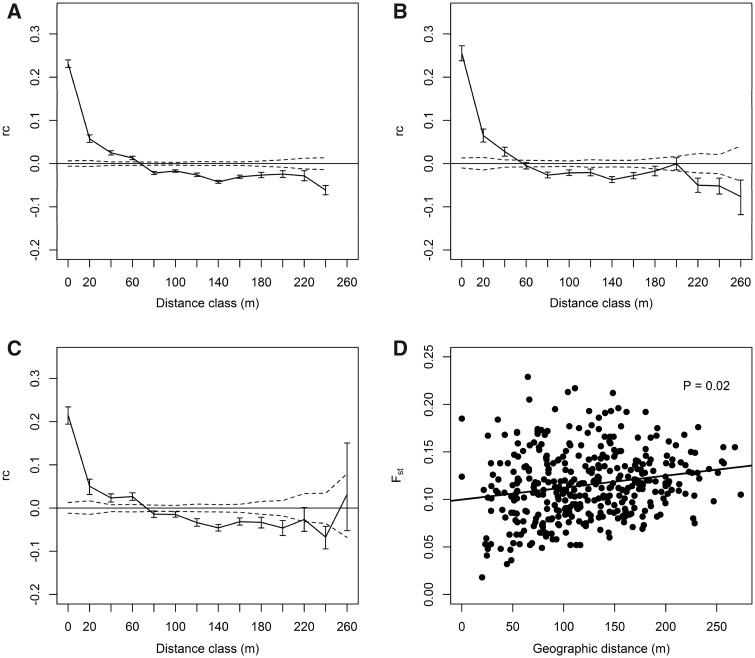
Figure 1.
Spatial genetic autocorrelations of (A) all genotyped individuals, (B) females, and (C) males of Mongolian gerbils in Inner Mongolia, China. Abscissa is the starting point of a distance class. Ordinate is spatial autocorrelation coefficient (rc) of genotypes. Two dashed lines along the x axis are the permutated 95% CIs of autocorrelations under the null hypothesis of a random distribution of genotypes in space. Vertical lines are the bootstrapped 95% CIs of mean genetic autocorrelation. Panel (D) is the relationship between pairwise inter-group Wright’s F statistic and geographic distance of Mongolian gerbils in Inner Mongolia, China.
Microsatellite analysis
We extracted nuclear DNA from clipped toe tissue samples of Mongolian gerbils using phenol–chloroform extraction methods (Sambrook and Russell 2001). We genotyped 327 gerbils from 26 burrow systems, using seven loci Mungμ1, Mungμ2, Mungμ3, Mungμ4, Mungμ5, Mungμ6, and Mungμ7 (Neumann et al. 2001; Wang et al. 2011b). All loci were in the Hardy–Weinberg equilibrium except for locus Mungμ1. No evidence suggested the linkage disequilibrium of the seven loci (Wang et al. 2011b).
Spatial autocorrelation analysis
We estimated the coordinates (x and y) of the centers of 26 burrow systems to the nearest 0.1 m using a 50 × 50 grid at a 7-m interval (Wang et al. 2011a). We used the coordinates of the center of a burrow system, at which a gerbil was captured, as the coordinates of the gerbil in spatial autocorrelation analysis (Smouse and Peakall 1999; Double et al. 2005). As a gerbil often was captured multiple times at different trap stations surrounding the center of a burrow system during our study, the center of a burrow system was equivalent to the centroid of multiple capture locations.
We used the program GENALEX 6.4 to compute the spatial autocorrelation of all 327 genotyped gerbils at the distance class sizes of 20, 30, and 40 m, respectively (Peakall et al. 2003; Peakall and Smouse 2006). We bootstrapped the 95% confidence interval (CI) of autocorrelation coefficient for each distance class with 1,000 repetitions. The bootstrap algorithm draws 1,000 random samples with replacement from the subset of pairwise genetic relatedness of a specific distance class, and estimates an empirical 95% CI (i.e., the 25th and 975th ranked bootstrapped values) for the observed autocorrelation coefficient (Peakall and Smouse 2006).
We used the permutation method to test for the significance of spatial autocorrelation with 1,000 repetitions. The permutation algorithm randomly shuffles all individuals among the geographic locations 1,000 times. The permutation test generates an empirical 95% CI (i.e., the 25th and 975th ranked permutated values) of autocorrelation coefficient under the null hypothesis of no spatial structure. If the bootstrapped 95% CI of observed autocorrelation coefficient exceeded or were less than the permutated 95% CIs under the null hypothesis in a distance range (e.g., 0–20, 20–40, or 40–60 m), we concluded that the gerbils had positive or negative spatial autocorrelation within the distance range. We also conducted spatial autocorrelation analysis for male and female subpopulations, respectively.
The distance class size of 20 m was close to the minimum distance of 19.9 m between burrow systems. Preliminary analyses showed consistent positive spatial autocorrelation among three different distance class sizes (i.e., 20, 30, and 40 m). Therefore, we used the distance class size of 20 m in the subsequent analyses.
Mantel test for the IBD model
We tested for correlation between pairwise inter-group geographic distance and pairwise inter-group Wright’s F statistic (Fst) of Mongolian gerbils using the Mantel test (Freeland 2005; Peakall and Smouse 2006). We conducted the Mantel test with the permutation option of 1,000 repetitions within GENALEX 6.4 (Peakall and Smouse 2006). If the correlation was significantly positive (P < 0.05), we concluded that the IBD model was a possible cause of observed fine-scale spatial population genetic differentiation of Mongolian gerbils.
Estimation of dispersal from trapping data
We concluded that dispersal occurred if a gerbil moved away from and did not return to a burrow system (A), at which the gerbil had been captured for more than two consecutive trapping weeks (about a breeding bout). If the dispersing gerbil arrived at another burrow system (B) and was captured at the destination B for more than two trapping weeks, we concluded that the gerbil dispersed from burrow system A to burrow system B. We calculated dispersal distance as the Euclidean distance between the centers of burrow systems A and B. Mean dispersal distances were reported with ±1 standard deviation.
Results
Spatial autocorrelation was positive within a distance range of about 0–80 m and became less clear with the increase of the distance (Figure 1A). Estimated means of autocorrelation coefficient were higher than the upper limit of the permutated 95% CI in the distance classes of 0–20, 20–40, and 40–60 m, respectively (Figure 1A). Also spatial autocorrelation was positive up to 60 and 80 m in male and female gerbils, respectively (Figure 1B, C). The Mantel test detected significant positive correlations between inter-group genetic and geographic distances (P = 0.02, Figure 1D).
We identified 24 dispersing male gerbils out of 159 captured males and 18 dispersing female gerbils out of 168 captured females during the entire study period. The proportion of dispersing individuals did not differ between males and females in a 2 × 2 contingency table test (χ2 = 0.77, df = 1, P = 0.38). Of the 24 male dispersers, there were 3 juveniles, 9 subadults, and 12 adults. Captured female dispersers included four juveniles, five subadults, and nine adults. One juvenile, two subadult, and four adult females reproduced at their dispersal destinations. The four dispersing adult females also bred before their dispersal. Average dispersal distances of male and female gerbils were 82 ± 34 m and 67.2 ± 30 m, respectively (t = 1.46, df = 40, P = 0.15). Average dispersal distance of all captured dispersers was 75.6 ± 33 m.
Discussion
Our results supported the hypothesis that restricted gene flow or dispersal would result in positive spatial genetic autocorrelation of Mongolian gerbils at fine spatial scales. Positive spatial autocorrelation was found within the average dispersal distance (<80 m). Positive spatial autocorrelation and positive relationships between genetic and geographic distances suggest that the IBD model might be a possible underlying mechanism for the fine-scale spatial genetic structure of Mongolian gerbils (Figure 1A, D). We detected positive spatial autocorrelation in both male and female gerbils, supporting the hypothesis that male and female gerbils would have similar fine-scale spatial autocorrelation patterns owing to equal dispersal propensities (Figure 1B, C).
Field observations demonstrated strong territoriality of social groups in Mongolian gerbils (Agren et al. 1989a; 1989b). Male Mongolian gerbils compete for females by combat rather than by scramble competition. The largest reproductively active males are the main defender of territories by chasing subordinates or strangers off and marking the territory borders during spring and early summer (Agren et al. 1989a). Nevertheless, all group members except for juveniles defend group territories during the food hoarding season in autumn (Agren et al. 1989b). Territoriality of social groups may increase dispersal costs with increasing aggressive behaviors (i.e., social fence) from encountered non-kin with increasing distance (Hestbeck 1982; Ostfeld 1994). Consequently, genetic relatedness may decrease exponentially in a short distance (Starrfelt and Kokko 2012). Therefore, a short dispersal distance may be sufficient for inbreeding avoidance (Ronce et al. 2001). The territoriality of social groups may restrict Mongolian gerbil’s dispersal to short distances (<80 m in this study; Agren et al. 1989b; Liu et al. 2009a), resulting in positive spatial autocorrelation. Future experimental studies are needed to investigate the effects of variation in dispersal distance and destination (or use of habitat patches) on the fine-scale spatial genetic diversity of Mongolian gerbils and to confirm the spatial genetic autocorrelation detected in this study. Group-living brush-tailed rock-wallabies Petrogale penicillata had male-biased dispersal (Hazlitt et al. 2004). Restricted dispersal of female wallabies resulted in clusters of female kin and positive spatial autocorrelation within a distance of 100 m. Therefore, spatially clustering kin and social fence may restrict gene flow to short distances in social mammals, differentiating fine-scale spatial genetic structure.
Inbreeding avoidance and local competition for mates may result in equal dispersal propensities of males and females in monogamous mammals (Greenwood 1980; Dobson and Jones 1985). Field observations indicated that male gerbils competed for reproducing females by combat. Our trapping data suggest approximately equal dispersal propensities of males and females. We also found evidence for breeding dispersal of female Mongolian gerbils, with seven adult females dispersing and reproducing before and (or) after dispersal. Additionally, we captured about two breeding pairs per burrow system in our gerbil population (Wang et al. 2011b). Competition for reproductive opportunities might take place among female group mates, so that female adults might leave their natal sites for reproductive opportunities. As a result, positive spatial autocorrelation occurred in both male and female gerbils. Likewise, positive spatial autocorrelation occurs at fine spatial scales (<500 m) in both males and females of monogamous roe deer Capreolus capreolus due to restricted dispersal of the both sexes (Bonnot et al. 2010). Furthermore, southern pied babbler, a monogamous and cooperatively breeding bird with equal propensities to disperse between males and females, had similar positive spatial autocorrelation in the both sexes (Nelson-Flower et al. 2011, 2012).
Female mammals often invest more in parental cares than males. Thus, female may benefit from competitive advantage on the familiar habitat if they stay close to natal sites, dispersing a short distance (Le Galliard et al. 2012; Blyton et al. 2015). Additionally, burrow-system-based kin groups and fitness benefits of group living may ensue from short-distanced dispersal. However, future studies are needed to investigate the behavioral mechanism (e.g., dispersal destination or habitat patches selection) for inbreeding avoidance of male gerbils that also disperse a short distance. Social organization and restricted gene flow may work in concert to shape the spatial population genetic structure of group-living mammals. Restricted dispersal and demographic benefits of genetic relatedness may lead high local abundance of gerbils, which may impose substantial impacts on the steppe ecosystems.
Acknowledgments
We thank G. Ma, T. Qiao, B. Wu, G. Wang, and T. Zhao for their assistance in field work. We are grateful to Drs Ming Li and Fuwen Wei for allowing us to use their laboratory for DNA analysis. T. D. King made helpful comments on our manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China [Grant No. 31372211] and the State Key Laboratory of Integrated Management of Pest Insects and Rodents. Partially supported by the Department of Wildlife, Fisheries and Aquaculture and the Forest and Wildlife Research Center, Mississippi State University [to G.W.].
Appendix
Figure A1.
The spatial distribution of 26 burrow systems of Mongolian gerbils on 9-ha study plot in Inner Mongolia, China. Symbols “S1”, “S2”, … , and “S26” are the identification numbers of burrow systems. Numbers in parentheses are the numbers of the genotyped gerbils captured at the burrow systems.
References
- Agren G, Zhou Q, Zhong W, 1989a. Ecology and social behavior of Mongolian gerbils Meriones unguiculatus at Xilinhot, Inner Mongolia, China. Anim Behav 37:11–27. [Google Scholar]
- Agren G, Zhou Q, Zhong W, 1989b. Territoriality, cooperation and resource priority: hoarding in the Mngolian gerbil Meriones unguiculatus. Anim Behav 37:28–32. [Google Scholar]
- Blyton MD, Banks SC, Peakall R, 2015. The effect of sex-biased dispersal on opposite sexed spatial genetic structure and inbreeding risk. Mol Ecol 24:1681–1695. [DOI] [PubMed] [Google Scholar]
- Bonnot N, Gaillard JM, Coulon A, Galan M, Cosson JF. et al. , 2010. No difference between the sexes in fine-scale spatial genetic structure of roe deer. PLoS ONE 5:e14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser RK, 1991. Influence of gene flow and breeding tactics on gene diversity within populations. Genetics 129:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson FS, Jones WT, 1985. Multiple causes of dispersal. Am Nat 126:855–858. [Google Scholar]
- Double MC, Peakall R, Beck NR, Cockburn A, 2005. Dispersal, philopatry, and infidelity: dissecting local genetic structure in superb fairy-wrens Malurus cyaneus. Evolution 59:625–635. [PubMed] [Google Scholar]
- Freeland JR, 2005. Molecular Ecology. West Sussex, UK: Wiley. [Google Scholar]
- French JA, 1994. Alloparents in the Mongolian gerbil: impact on long-term reproductive performance of breeders and opportunities for indepdendent reproduction. Behav Ecol 5:273–279. [Google Scholar]
- Le Galliard JF, Rémy A, Ims RA, Lambin X, 2012. Patterns and processes of dispersal behaviour in arvicoline rodents. Mol Ecol 21:505–523. [DOI] [PubMed] [Google Scholar]
- Gannon WL, Sikes RS, Comm ACU, 2007. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 88:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PJ, 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162. [Google Scholar]
- Hazlitt SL, Eldridge MDB, Goldizen AW, 2004. Fine-scale spatial genetic correlation analyses reveal strong female philopatry within a brush-tailed rock-wallaby colony in southeast Queensland. Mol Ecol 13:3621–3632. [DOI] [PubMed] [Google Scholar]
- Hestbeck JB, 1982. Population regulation of cyclic mammals: the social fence hypothesis. Oikos 39:157–163. [Google Scholar]
- Kimura M, Weiss GH, 1964. Stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics 49:561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang GM, Wan XR, Zhong WQ, 2009a. Effects of supplemental food on the social organization of Mongolian gerbils during the breeding season. J Zool 278:249–257. [Google Scholar]
- Liu W, Wang GM, Wang YN, Zhong WQ, Wan XR, 2009b. Population ecology of wild Mongolian gerbils Meriones unguiculatus. J Mammal 90:832–840. [Google Scholar]
- Mora MS, Mapelli FJ, Gaggiotti OE, Kittlein MJ, Lessa EP, 2010. Dispersal and population structure at different spatial scales in the subterranean rodent Ctenomys australis. BMC Genetics 11:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Flower MJ, Hockey PAR, O’ryan C, Raihani NJ, Du Plessis MA. et al. , 2011. Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behav Ecol 22:559–565. [Google Scholar]
- Nelson-Flower MJ, Hockey PAR, O’ryan C, Ridley AR, 2012. Inbreeding avoidance mechanisms: dispersal dynamics in cooperatively breeding southern pied babblers. J Anim Ecol 81:876–883. [DOI] [PubMed] [Google Scholar]
- Neumann K, Maak S, Stuermer IW, von Lengerken G, Gattermann R, 2001. Low microsatellite variation in laboratory gerbils. J Hered 92:71–74. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, 1994. The fence effect reconsidered. Oikos 70:340–348. [Google Scholar]
- Peakall R, Ruibal M, Lindenmayer DB, 2003. Spatial autocorrelation analysis offers new insights into gene flow in the Australian bush rat Rattus fuscipes. Evolution 57:1182–1195. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE, 2006. Genalex 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff DA, 2002. Life History Evolution. Sunderland (MA: ): Sinauer Associates. [Google Scholar]
- Ronce O, Oliveri I, Clobert J, Danchin E, 2001. Perspective on the study of dispersal evolution In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford, UK: Oxford University Press; 341–357. [Google Scholar]
- Sambrook J, Russell DW, 2001. Molecular Cloning: A Laboratory Manual. Cold Spring (NY: ): Cold Spring Harbor Laboratory Press. [Google Scholar]
- Slatkin M, 1987. Gene flow and the geographic structure of natural-populations. Science 236:787–792. [DOI] [PubMed] [Google Scholar]
- Slatkin M, 1993. Isolation by distance in equilibrium and nonequilibrium populations. Evolution 47:264–279. [DOI] [PubMed] [Google Scholar]
- Smouse PE, Peakall R, 1999. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Watenburg DR, 1983. A test of spatial autocorrelation analysis using an isolation-by-distance model. Genetics 105:219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, Getz LL, 1997. Examination of alternative hypothesis for cooperative breeding in rodents In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge, UK: Cambridge University Press; 199–230. [Google Scholar]
- van Staaden MJ, 1995. Breeding tactics, social structure and genetic variation in mammals: problems and prospects. Acta Theriol 3(Suppl):165–182. [Google Scholar]
- Starrfelt J, Kokko H, 2012. The theory of dispersal under multiple influences In: Clobert J, Baguette M, Benton TG, Bullock JM, editors. Dispersal Ecology and Evolution. Oxford, UK: Oxford University Press; 19–29. [Google Scholar]
- Wang Y, Liu W, Wang G, Wan X, Zhong W, 2011a. Home-range sizes of social groups of Mongolian gerbils Meriones unguiculatus. J Arid Environ 75:132–137. [Google Scholar]
- Wang Y, Liu W, Wang GM, Zhong W, Wan X, 2011b. Genetic consequences of group living in Mongolian gerbils. J Hered 102:554–561. [DOI] [PubMed] [Google Scholar]
- Wright S, 1943. Isolation by distance. Genetics 28:114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]