Abstract
Streptococcus mutans, the primary aetiological agent of dental caries, is one of the major bacteria of the human oral cavity. The pathogenicity of this bacterium is attributed not only to the expression of virulence factors, but also to its ability to respond and adapt rapidly to the ever-changing conditions of the oral cavity. The two-component signal transduction system (TCS) CovR/S plays a crucial role in virulence and stress response in many streptococci. Surprisingly, in S. mutans the response regulator CovR appears to be an orphan, as the cognate sensor kinase, CovS, is absent in all the strains. We found that acetyl phosphate, an intracellular phosphodonor molecule known to act in signalling, might play a role in CovR phosphorylation in vivo. We also found that in vitro, upon phosphorylation by potassium phosphoramide (a high-energy phophodonor) CovR formed a dimer and showed altered electrophoretic mobility. As expected, we found that the conserved aspartic acid residue at position 53 (D53) was the site of phosphorylation, since neither phosphorylation nor dimerization was seen when an alanine-substituted CovR mutant (D53A) was used. Surprisingly, we found that the ability of CovR to act as a transcriptional regulator does not depend upon its phosphorylation status, since the D53A mutant behaved similarly to the wild-type protein in both in vivo and in vitro DNA-binding assays. This unique phosphorylation-mediated inhibition of CovR function in S. mutans sheds light on an unconventional mechanism of the signal transduction pathway.
Keywords: CovR, Two component regulator, Streptococcus mutans
Introduction
As the primary aetiological agent of dental caries, Streptococcus mutans predominantly colonizes the human oral cavity [1, 2]. The bacterium has various mechanisms that allow it to survive and successfully colonize the oral cavity. It metabolizes the dietary carbohydrates of the host to produce glucan, an extracellular sticky polysaccharide, which is necessary for anchoring to the tooth surface and the formation of dental plaque [3]. S. mutans can also ferment carbohydrates ingested by the host and secrete lactic acid as a byproduct [4]. As a result of this, there is a steep fall in pH in dental plaque, which leads to demineralization of the tooth enamel and the development of dental caries. The bacterium is able to survive and grow under this extreme acidic condition owing to its ability to induce a protective acid tolerance response [5]. The pathogenicity of this bacterium is attributed not only to the expression of virulence factors, but also to its ability to respond and adapt rapidly to the ever-changing conditions of the oral cavity, including the availability of nutrients, various stresses and fluctuations of temperature and pH.
In bacteria, two-component signal transduction systems (TCSs) have evolved to sense and respond to changes in the internal and external environment [6]. TCSs consist of a membrane-embedded sensor kinase that responds to an environmental stimulus by auto-phosphorylation of a conserved histidine residue. The phosphoryl group is subsequently transferred to the cognate response regulator. The response regulator usually contains a receiver domain (REC) with a conserved aspartic acid (Asp) residue, which is the phosphorylation target and an effector domain (EF). The EF domain is activated upon phosphorylation of the aspartic acid residue [6–10]. This alteration of the phosphorylation status of the response regulator leads to structural changes that affect dimerization, binding to the target DNA and gene expression [6]. Therefore, TCSs act as an efficient system to coordinate gene expression in response to alterations in the environmental signals. Since TCSs are indispensable for the successful pathogenesis of many human pathogens [11, 12], novel antimicrobials targeting TCSs are presently becoming attractive weapons to combat pathogens [13, 14].
Generally, streptococci encode at least 14 TCSs that play vital roles in bacterial adaptation, stress tolerance response, virulence factor production and biofilm formation [15, 16]. Among these, CovR/S is one of the most important and widely studied TCSs in streptococci [17–25]. CovR/S was originally identified in S. pyogenes (group A streptococcus; GAS), where it either directly or indirectly controls the expression of nearly 15 % of the genes, many of which are responsible for pathogenesis and stress survival [26, 27]. CovR is also essential for the expression of virulence-related genes in S. agalactiae (group B streptococcus; GBS) and S. dysgalactiae (group C streptococcus; GCS) [21, 22, 28]. As in GAS, CovR/S regulates approximately 6 % of the genes in GBS, either directly or indirectly [22]. In both GAS and GBS, CovR primarily acts as a repressor of the target genes under its regulon, including its own expression [20, 22, 29–31]. However, CovR also functions as a transcriptional activator and in GBS nearly half of the CovR regulon genes are activated by CovR [22]. In most cases, CovR binds directly to the promoter regions of the target genes and regulates their expression directly; however, in some cases CovR regulates gene expression indirectly by involving another regulator [20, 22, 29–32]. In S. mutans, CovR regulates approximately 6.5 % of the genome, both as a transcriptional repressor and as an activator [33]. It represses the expression of some key genes involved in virulence, such as gtfB/C (glucosyltransferase B/C), gbpC (glucan-binding protein C), and also auto-regulates its own expression [17, 18, 34]. CovR also activates the expression of numerous genes, including SMU.1882 and bac operon (bacitracin synthetase), which are well studied [33, 35].
CovR belongs to the OmpR/PhoB family of response regulators [23]. Multiple mechanisms have been proposed to explain the modulation of CovR activity [23]. In GAS, the cognate sensor kinase CovS activates CovR by phosphorylating the conserved Asp residue on CovR. However, CovS, under certain stress conditions, also dephosphorylates CovR and thereby suppresses its repressor activity [26]. Recently, it has been shown that in GAS an orphan sensor kinase, RocA, also phosphorylates CovR at the conserved Asp residue [36]. Furthermore, in both GAS and GBS, CovR activity is modulated by a eukaryotic-like serine/threonine kinase (STK), which phosphorylates a conserved threonine (Thr) residue that is present in the REC domain [37, 38]. The phosphorylation of the Thr residue inhibits CovR-mediated gene transcription in vivo [37, 38]. In vitro, low-molecular-weight phosphodonors, such as acetyl phosphate or carbamoyl phosphate, are also capable of activating CovR by phosphorylating the Asp residue [23].
We have previously shown that CovR in S. mutans appears to be an orphan since the cognate CovS was absent in all the S. mutans strains [18]. Recent studies have shown that some response regulators can be activated in a phosphorylation-independent manner [39–41]. In vitro studies have also demonstrated that some OmpR/PhoB family response regulators can form dimers even in the absence of any-high energy phosphor donor [42, 43], indicating that an active form of dimers exists without phosphorylation. Likewise, the response regulator PhoP from Mycobacterium tuberculosis can bind to the target promoters in the unphosphorylated form [44, 45]. It is likely that phosphorylation of the REC domain is not always necessary to directly induce DNA binding of the EF domain. It seems that phosphorylation only facilitates dimerization of the REC domains to bring the EF domains closer [46].
In this work, we investigated the molecular mechanisms underlying the activation of orphan CovR in S. mutans. We found that acetyl phosphate, a well-known signalling molecule, might play a role in the phosphorylation of CovR in vivo, and might serve as a link between the central metabolism and environmental signal transduction. We also found that the ability of CovR to act as a transcriptional regulator does not depend upon its phosphorylation status, since the phosphorylation of the conserved Asp is not necessary for DNA binding and gene expression. This unique phosphorylation-independent activation of CovR in S. mutans is quite different from the activation of its homologues that have been studied in other groups of streptococci, and hence presents a mechanism that questions the so-called ‘indispensable’ role of phosphorylation in the activation of response regulators of TCSs.
Methods
Strain construction
The ackA gene along, with the 0.4 kb region on its upstream and downstream, was amplified using the primers up-ackA-F and dn-ackA-R. Similarly, pta, along with the 0.4 kb regions on either side of it, was amplified using the primers up-pta-F and dn-pta-R (for details of all the primers, please refer to Table S1, available in the online version of this article). The amplicons were then cloned into pGEM-T Easy vector (Promega, USA) to create pIBT10 and pIBT12 (see Table 1 for details of all the strains and plasmids). To delete about 95 % of pta and ackA, inverse PCRs were performed on pIBT10 and pIBT12, respectively, using the primers listed in Table S1. The inverse PCR products were ligated to a loxP-Km cassette [47]. Next, the deleted genes with an inserted loxP-Km cassette were PCR-amplified using the universal M13 forward and reverse primers. Cultures of competent S. mutans UA159 were transformed with the amplicons to allow homologous recombination and allelic replacement. The transformants were selected on THY agar plates supplemented with kanamycin. Finally, the Km cassette was excised with the help of pCrePA as described previously [47]. The S. mutans strains with deleted pta and ackA were designated as IBST6 and IBST12, respectively. Similarly, to create a double mutant strain of S. mutans lacking both pta and ackA, the PCR-amplified product of deleted ackA with the loxP-Km cassette was transformed into IBST6. The steps mentioned above were repeated to create IBST16, a clean double mutant lacking the pta-ackA genes. PCR and DNA sequencing were used to verify the resultant clean double mutant strain.
Table 1. List of bacterial strains used in this study.
| Strain | Genotype or description | Source |
|---|---|---|
| S. mutans | ||
| UA159 | Wild-type, serotype c | Laboratory stock |
| IBST6 | UA159 ∆pta | This study |
| IBST12 | UA159 ∆ackA | This study |
| IBST16 | UA159 ∆pta∆ackA | This study |
| IBS10 | UA159 ∆covR | Biswas and Biswas [17] |
| Plasmids | ||
| pGEM-T Easy | Cloning vector, Ampr | Promega, USA |
| pIBT10 | pta along with the 0.4 kb region on its upstream and downstream cloned into pGEM-T Easy, Ampr | This study |
| pIBT12 | ackA along with the 0.4 kb region on its upstream and downstream cloned into pGEM-T Easy, Ampr | This study |
| pCrePA | Contains cre under promoter of pagA and a temperature-sensitive replicon, Emr | Laboratory stock |
| pASK-IBA43+ | Expression plasmid, Ampr | IBA, Germany |
| pIB81 | pASK with His-CovR, Ampr | This study |
| pIB86 | pASK with His-CovR D53A, Ampr | This study |
| pIB87 | pASK with His-CovR D53E, Ampr | This study |
| pIB184Km | Shuttle vector for protein expression in S. mutans, Kmr | Biswas et al. [16] |
| pIBE20A | pIB184Km with CovR from S. mutans, Kmr | This study |
| pIBF1 | pIB184Km with D53ACovR from S. mutans, Kmr | This study |
| pIBF2 | pIB184Km with D53E CovR from S. mutans, Kmr | This study |
| pIBE26A | pIB184Km with CovR from GAS, Kmr | This study |
| pIBE27A | pIB184Km with CovR from GBS, Kmr | This study |
| pIBE28A | pIB184Km with D53A CovR from GAS, Kmr | This study |
| pGhost9TR | pGhost9 derivative, shuttle vector with a thermo-stable replicon, Emr | Maguin et al. [78] |
| pIB121 | pIB107 with PgbpC, Kmr | Biswas et al. [34] |
| pIB602 | pIB107 with PSMU.1882, Kmr | Chong et al. [35] |
| pIBT8 | pGhost9TR with PgbpC-gusA, Emr | This study |
| pIBT9 | pGhost9TR with PSMU.1882-gusA, Emr | This study |
Construction of reporter plasmids and glucuronidase (Gus) assays
The promoter regions of gbpC and SMU.1882, along with the gusA gene, were amplified from the vectors pIB121 and pIB602 [34, 35], respectively, using the primers Hind-pIB107-F and Pst-pIB107-R. The amplicons were digested with HindIII and PstI and then cloned into HindIII-PstI-digested pGhost9TR. The pGhost9TR vector harbouring PgbpC-gusA was named pIBT8, whereas that carrying PSMU1882-gusA was named pIBT9. To investigate the role of CovR in the expression of gbpC and SMU.1882, wild-type and ΔcovR strains complemented with various forms of CovR were transformed with the reporter plasmids, pIBT8 and pIBT9. Similarly, to study the role of the phosphorylation of CovR in regulating the expression of gbpC and SMU.1882, ΔackA strain (IBST12), Δpta strain (IBST6) and ΔackA-Δpta strain (IBST16) were also transformed with pIBT8 and pIBT9. β-glucuronidase (Gus) assays were performed according to the procedure described previously [17].
Construction of covR derivatives for complementation and purification
A covR ORF was amplified using the primers listed in Table S1 and the chromosomal DNA of wild-type UA159 as a template. The amplicon was digested with EcoRI and XhoI and cloned into EcoRI-XhoI-digested vector pASK-IBA43+ (IBA, Germany) to generate pIB81. E. coli DH5α cells were transformed with pIB81 to express recombinant His-tagged CovR. For overexpression of His-CovR, cultures were grown to an OD600 of 0.6 and then anhydrotetracycline was added to a final concentration of 0.2 µg ml−1 before the cultures were further grown at 26 °C for 4 h. His-CovR was then purified using a Ni-nitrilotriacetic acid (NTA) column (Qiagen) with a standard protocol and then dialyzed overnight at 4 °C against buffer containing 50 mM Tris-Cl (pH 8.2), 50 mM NaCl and 10 % glycerol. Using site-directed mutagenesis with pIB81 as template, pIB86 and pIB87 were constructed, which have mutations of Asp to Ala (D53A) and Asp to Glu (D53E), respectively, at the fifty-third residue of CovR. E. coli DH5α cells harbouring pIB86 and pIB87 were induced as described above to purify His-tagged mutant forms of CovR.
The covR gene was amplified from the chromosomal DNA of UA159 with the help of the primers BamHI-covR-F10 and Eco-sal-covR-R10, digested with BamHI and EcoRI, and cloned into pIB184Km to generate pIBE20A. The plasmid pIBE20A was further used as a template to construct pIBF1 and pIBF2, which have mutations of Asp to Ala and Asp to Glu, respectively at the fifty-third residue of CovR. Similarly, the primers Bam GAS CovR F and Xho GAS CovR R were used to amplify wild-type and the D53A mutant form of GAS CovR using the plasmids pJRS996 and pJR29, respectively, as templates [26]. The amplicons were digested with BamHI and XhoI and cloned into pIB184Km to generate pIBE26A and pIBE28A, respectively.
Detection of CovR phosphorylation in vivo and in vitro
The strains were grown in 10 ml of THY to OD595=0.5 and harvested by centrifugation. Cell lysates were prepared and care was taken to keep them chilled to minimize spontaneous dephosphorylation. The cells were first resuspended in 1.0 ml of cold 50 mM Tris pH 7.0 and 8 µl of Protease Inhibitor Cocktail Set III, EDTA-free (Calbiochem). Cell suspensions were then transferred to chilled Lysing Matrix B tubes (MP Biomedicals) and lysed with five consecutive runs of 30 s each at a speed of 6 m s−1. The lysed cells were then centrifuged at 10 000 g for 1 min at 4 °C. About 15 µg of protein as determined by Bradford assay (BioRad) was loaded on the gel for each sample. As phosphorylation controls, lysates were boiled at 100 °C for 3 min and then kept on ice after mixing with β-mercaptoethanol and sample buffer. Boiling at 100 °C for 3 min removed the heat-labile Asp phosphorylation. The samples were electrophoresed on a polyacrylamide gel containing acrylamide-Phos-tag ligand (Wako Pure Chemicals). The gel contained a 10 % resolving solution [10 % (w/v) 29 : 1 acrylamide::N,N’-methylene-bis-acrylamide; 350 mM Tris-Cl (pH 6.8); 0.1 % SDS; 75 µM Phos-tag acrylamide; and 150 µM Zn(NO3)2] and a 4 % stacking solution [4 % (w/v) 29 : 1 acrylamide::N,N’-methylene-bis-acrylamide; 350 mM Tris-Cl (pH 6.8); 0.1 % SDS]. Electrophoresis was carried out at 150 V at 4 °C for 1 h 30 min in MOPS running buffer (pH 8.0) (0.1 M Tris-Cl, 0.1 M MOPS, 0.1 % SDS and 5 mM sodium metabisulphite). The gels were washed with transfer buffer containing 1 mM EDTA for 15 min to remove Zn2+ and then with transfer buffer to remove EDTA. Finally, the phosphorylated and nonphosphorylated forms of CovR were detected by standard Western blotting using anti-CovR antibodies as described previously [48].
To study the phosphorylation of CovR in vitro, ~5 µg of purified His-CovR, His-CovR D58A or His-CovR D53E were incubated in phosphorylation buffer containing 50 mM Tris-Cl (pH 7.4), 50 mM KCl, 20 mM MgCl2, 1 mM DTT and 20 mM potassium phosphoramidate (PAM) for 30 min at room temperature and then resolved by a native 8 % polyacrylamide gel run under denaturing conditions. His-tagged wild-type and D53A mutant forms of CovR were cross-linked after treatment with PAM, with the help of 1 % formaldehyde at room temperature for 10 min. Similarly, His-CovR was heated after incubation in the presence of PAM. Formaldehyde cross-linked and heated samples were then run on a 10 % SDS-polyacrylamide gel.
Semi-quantitative and real-time RT-PCR analyses
Semi-quantitative (sq) RT-PCR was used to quantify the level of expression of gbpC and SMU.1882 in wild-type (UA159/pIB184 Km), ΔcovR (IBS10/pIB184 Km) and various complementing strains. RNA samples were prepared from the S. mutans cultures grown to the mid-exponential phase (70 Klett Units) using a previously described protocol [34]. RNA samples were quantified using a UV spectrophotometer. The sqRT-PCR analyses were performed with a two-tube RT PCR system. We used 1 µg of RNA for first-strand cDNA synthesis (1 h incubation at 42 °C using SuperScript II reverse transcriptase (Invitrogen, CA, USA). The reaction was terminated by incubating the tubes at 70 °C for 15 min, followed by treatment with RNaseH (Invitrogen) at 37 °C for 20 min. After purification using a PCR purification column (Qiagen), the concentration of the cDNA samples was determined using a UV spectrophotometer. We used 5 ng of cDNA as a template for PCR amplification using the gene-specific primers listed in Table S1. The amplicons were electrophoresed on a 1 % agarose gel, photographed and quantified using Doc-It-LS (UVP) software. The gyrA served as an internal control to ensure that equal amounts of RNA were being used in each sqRT-PCR reaction. Using the cDNA prepared above, real-time PCR was performed on a LightCycler instrument (Roche Diagnostics Applied Science) using the same primers as for the sqRT-PCR. The reactions were performed in 20 µl final volume consisting of 10 ng cDNA, 1X Power SYBR Green PCR Master Mix (Applied Biosystems) and 0.5 µM of each sense and antisense primer pair. The cycling conditions were as follows: 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min with fluorescent measurement at the end of each cycle and a melting curve ramp. The housekeeping gene gyrA served as an internal control to normalize the expression level between samples. The melting curve and quantitative analyses were conducted by using LightCycler analysis software version 1.1, following the manufacturer's instructions (Roche Diagnostics Applied Science).
Biolayer interferometry (BLI)
BLI was used to detect the binding of wild-type and mutant forms of CovR to the promoters of gbpC and SMU.1882. [49]. The PCR-amplified promoter regions of gbpC and SMU.1882 were biotinylated on one strand at the 5′ end. Biotinylated DNAs, prepared in binding buffer, were loaded on hydrated streptavidin biosensors for 5 min. A subsequent washing (2 min) by exposing the biosensor tip to the binding buffer was performed to remove any unbound excess DNA. Next, association was performed for 5 min by exposing the biosensor tip to a 0.5 µM solution of CovR (wild-type or mutants) prepared in the binding buffer. The dissociation of the DNA–protein complex was assessed by exposing the complex bound to the biosensor tip to binding buffer for 2 min.
Results
The Asp53 (D53) residue is required for phosphorylation of S. mutans CovR
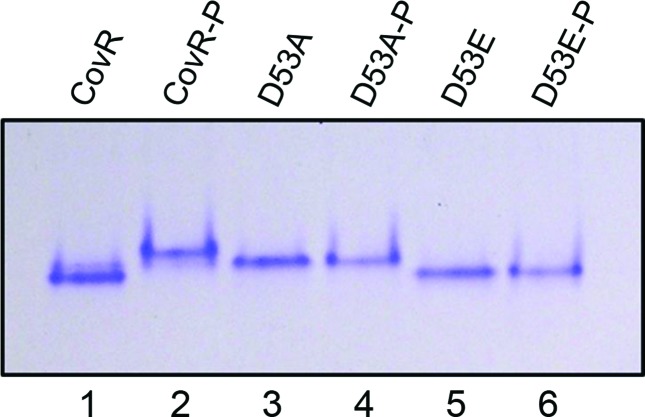
Homologous CovR from GAS, which has an identity of around 75 % with CovR from S. mutans, is phosphorylated at the aspartic acid (D) residue present at position 53 [50]. Hence, using site-directed mutagenesis, we generated a mutant CovR (CovR D53A) of S. mutans, where the D53 residue was mutated to alanine. Similarly, another mutant (CovR D53E) was generated in which the aspartic acid residue was mutated to glutamic acid. This latter mutant (CovR D53E) mimicked the phosphorylated aspartate residue [51–53]. Purified wild-type CovR, CovR D53A and CovR D53E were incubated in the presence or absence of potassium phosphoramidate (PAM) for 30 min and then separated on a native 8 % polyacrylamide gel under nondenaturing conditions (Fig. 1). PAM is a high-energy phosphodonor that is able to phosphorylate the aspartate residues of response regulators [54, 55]. The phosphorylated wild-type CovR migrated more slowly than the nonphosphorylated protein. Although CovR D53A migrated more slowly than the nonphosphorylated wild-type CovR, no difference in migration was observed between the potassium phosphoramidate-treated samples and the untreated samples (Fig. 1). Similarly, no difference in electrophoretic mobility was observed between the potassium phosphoramidate-treated samples and the untreated CovR D53E samples, while the migration was almost the same as that of nonphosphorylated wild-type CovR. This indicates that the D53 residue is necessary for phosphorylation of CovR.
Fig. 1.
Phosphorylation of CovR occurs at Asp53 (D53). CovR, CovR D53A and CovR D53E proteins were purified from E. coli and ~5 µg each protein was incubated with or without potassium phosphoramidate (PAM; 20 mM) for 30 min at room temperature for phosphorylation. The samples were then loaded on a native 8 % polyacrylamide gel under non-denaturing conditions and run at 120 V for 3 h 30 min. The gels were washed and stained with PageBlue (Thermo Scientific). The experiments were repeated at least twice and a representative gel is shown. Samples denoted as ‘P’ are PAM-treated (lanes 2, 4 and 6).
Phosphorylation of CovR at D53 leads to its dimerization
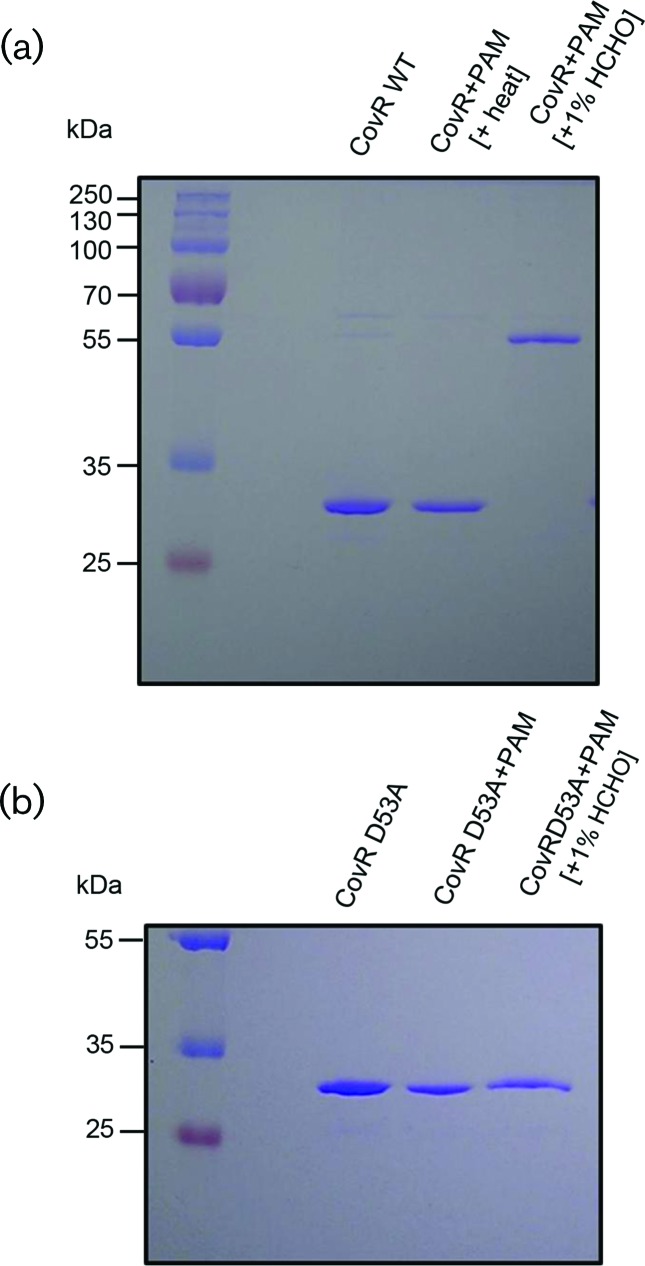
It has been seen that in GAS (S. pyogenes) the binding of CovR to several promoters is enhanced by the phosphorylation of purified CovR [20, 29, 31, 50]. Moreover, the phosphorylated CovR is also capable of repressing transcription from Pcov and Psag in vitro [30, 31]. Again, upon phosphorylation, purified His-tagged CovR is reported to form multimers in solution [29]. Therefore, we wanted to explore the oligomerization status of phosphorylated CovR in S. mutans. After incubation with PAM, purified wild-type CovR and mutant CovR (CovR D53A) were cross-linked using 1 % formaldehyde and then subjected to SDS-PAGE. Formaldehyde reacts with the amino groups of the N-terminus and side-chains of arginine, cysteine, histidine and lysine residues, leading to intramolecular cross-links [56, 57]. After phosphorylation, wild-type CovR was successfully cross-linked by formaldehyde and showed retarded mobility (Fig. 2a). The phosphorylated oligomer had nearly twice the mass of the nonphosphorylated wild-type CovR. No such retarded mobility was seen when the mutant CovR D53A was used (Fig. 2b). Since CovR D53A has Ala residue at position 53, it was not phosphorylated and hence could not form dimers. Moreover, the phosphorylation at the D53 residue was extremely heat labile, as the electrophoretic mobility of heated wild-type phosphorylated CovR was same as that of nonphosphorylated CovR (Fig. 2a, middle lane).
Fig. 2.
Dimerization of CovR. (a) Wild-type (WT) S. mutans CovR protein was purified from E. coli and then phosphorylated with PAM. Phosphorylated CovR protein (~5 µg) was then either heat treated at 90 °C for 3 min or directly incubated with 1 % formaldehyde without heat treatment. The samples were then separated on a 10 % SDS-polyacrylamide gel. (b) S. mutans D53A CovR protein (~5 µg) was purified from E. coli after incubation with either PAM or PAM plus 1 % formaldehyde. The samples were separated on a 10 % SDS-polyacrylamide gel. Experiments were repeated at least thrice and a representative gel is shown.
In vivo phosphorylation of CovR
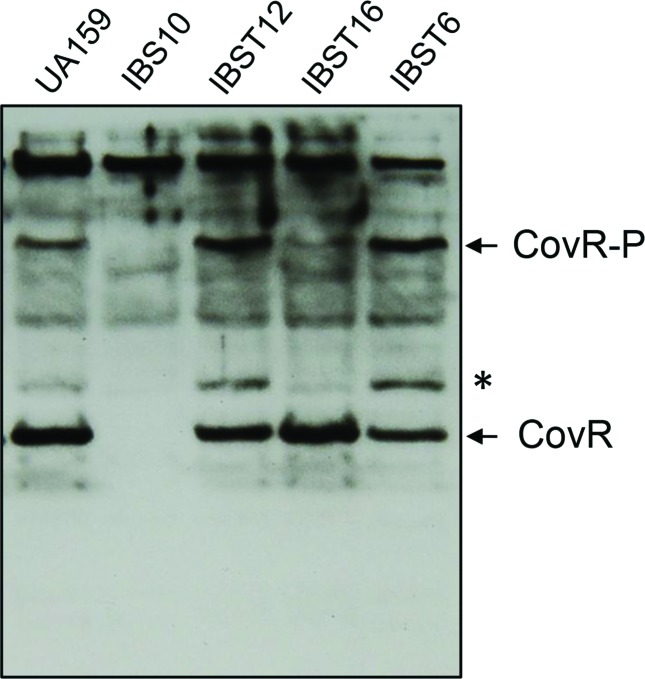
S. mutans carries the gene pta encoding the phosphotransacetylase responsible for converting acetyl coenzyme A (acetyl-CoA) to acetyl phosphate, which in turn is converted to acetate by acetate kinase (encoded by ackA), resulting in the production of ATP [58]. Acetyl phosphate has been shown to act as a signalling molecule, as it can donate phosphate for the TCSs. Thus, acetyl phosphate links the central metabolism to the environmental sensing and signal transduction system [59–62]. Since CovR in S. mutans is an orphan regulator, we wanted to study whether acetyl phosphate serves as a potential phosphodonor in phosphorylating CovR. A double mutant strain of S. mutans (IBST16) was constructed in which the Pta–AckA pathway was deleted. Cell lysates prepared from wild-type (UA159), ∆covR (IBS10), ∆pta (IBST6), ∆ackA (IBST12) and ∆pta∆ackA (IBST16) strains were subjected to Phos-tag SDS-PAGE. After electrophoresis, phosphorylated and non-phosphorylated CovR were detected by Western blot analysis using an anti-CovR antibody [48]. The analysis revealed the presence of a slow migrating phosphorylated CovR form in the wild-type (UA159), in addition to the non-phosphorylated CovR (Fig. 3). These two bands were absent in the ∆covR strain. In addition to these two bands, we also observed a band that migrated more slowly than the non-phosphorylated CovR, but faster than the phosphorylated species (Fig. 3, marked with an asterisk); this band was absent in the ∆covR strain. Surprisingly, we found that the quantity of phosphorylated CovR species increased in both the ∆pta (IBST6) and ∆ackA (IBST12) strains as compared to the wild-type UA159. On the other hand, in the double-deleted ∆pta∆ackA strain (IBST16) nonphosphorylated CovR was abundant, and the phosphorylated and intermediated species were drastically reduced. Recently, it has been shown that in S. mutans, deletion of either ∆ackA or ∆pta∆ackA leads to increased accumulation of acetyl phosphate concentration in the cell [63], which may be available for CovR phosphorylation. Since aspartate phosphorylated species are heat-labile, we further confirmed that CovR phosphorylation effected through heating of the samples resulted in the abolition of the signal corresponding to phosphorylated CovR (Fig. S1). We note that the slower migrating band (Fig. 3, asterisk) also appears to be heat-labile (Fig. S1). Taken together, our results suggest that acetyl phosphate might play a role as a phosphodonor to CovR in vivo. However, we cannot rule out other small-molecule phosphodonors at this point.
Fig. 3.
Detection of phosphorylated CovR in vivo. Western blot using an anti-CovR antibody of lysate wild-type (UA159), ΔcovR (IBS10), ΔackA (IBST12), Δpta (IBST6) and ΔackA Δpta (IBST16) samples separated on a 10 % phos-tag-SDS-polyacrylamide gel. Prior to loading, lysates derived from cultures grown to mid-exponential phase were incubated with β–mercaptoethanol and 3× SDS sample buffer (New England Biolabs) on ice. CovR-specific bands are indicated on the right. Note that a CovR-specific band (indicated by an asterisk, *) accumulated in the ΔackA and Δpta single mutants. The experiments were repeated at least thrice and a representative gel is shown.
Differential gene expression by CovR
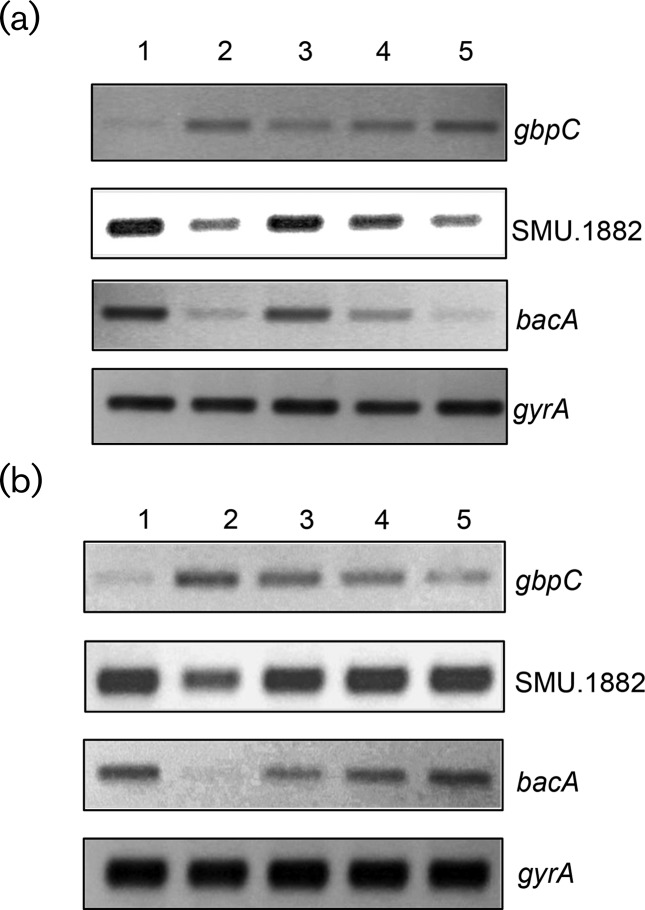
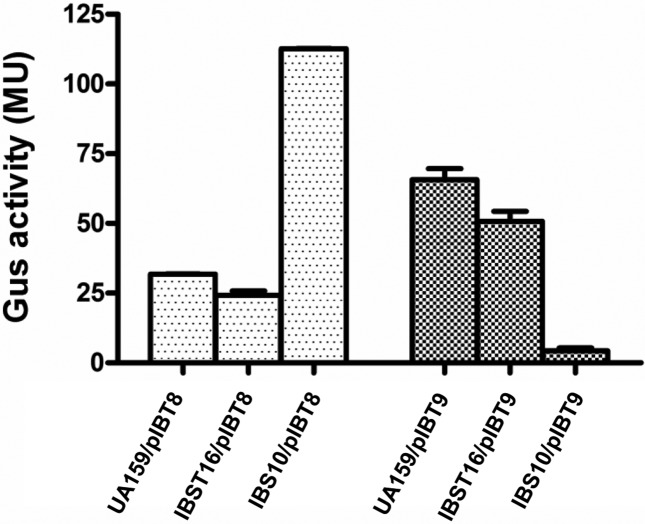
To understand whether the phosphorylated or nonphosphorylated form of CovR was the active moiety responsible for regulating gene expression, semi-quantitative (sq) and real-time RT-PCR analyses were performed. In S. mutans, CovR has been shown to act as both a transcriptional activator and a repressor. CovR represses the expression of various genes responsible for virulence, such as gtfB/C (glucosyltransferase B/C) and gbpC (glucan-binding protein C) [17, 34]. On the other hand, CovR also activates the expression of various genes, including SMU.1882 and bacA [33, 35]. Wild-type S. mutans (UA159/pIB184Km), its isogenic ∆covR derivative (IBS10/pIB184Km) and various covR-complementing strains were grown to mid-exponential phase (70 Klett Units) and harvested. RNA samples were isolated and used for sqRT-PCR and real-time RT-PCR. The gyrA gene was used as a control to ensure that equal amounts of RNA were being used in the RT-PCRs. As shown in Fig. 4(a), lower levels of transcripts of gbpC were observed in the wild-type and ∆covR strains complemented with either the wild-type CovR or the CovR D53A mutant, as compared to the ∆covR strain and the ∆covR strain complemented with the CovR D53E mutant. Similarly, the transcription levels of the SMU.1882 and bacA in the wild-type and the ∆covR strains complemented with the wild-type CovR or the CovR D53A mutant were higher than those of the ∆covR strain and the ∆covR strain complemented with the mutant CovR D53E. We verified this observation by using real-time RT-PCR (Fig. S2). CovR D53A behaved like the wild-type, whereas CovR D53E behaved like the ∆covR strain. To further confirm our observation, we constructed two reporter plasmids in which the promoter regions of gbpC (PgbpC) and SMU.1882 (PSMU.1882) were transcriptionally fused with the gusA gene to generate pIBT8 and pIBT9 plasmids, respectively. These plasmids were introduced into the wild-type, ∆covR and ∆covR complemented with the wild-type or mutant CovR derivatives. When we measured the Gus activities from these strains, we found that both the wild-type CovR and CovR D53A complemented the ΔcovR mutant, while the CovR D53E failed to complement (Fig. S3). Taken together, our data suggest that the nonphosphorylated CovR (D53A) is the active form, as it behaved like the wild-type CovR. On the other hand, the phosphorylated CovR (D53E) behaved like the ΔcovR strain.
Fig. 4.
Differential gene expression by CovR. Semi-quantitative RT-PCR analysis of gbpC, SMU.1882 and bacA in the wild-type (UA159/pIB184Km), ΔcovR (IBS10/pIB184Km) and various covR complementing strains (IBS10/Smu CovR, IBS10/Smu CovR D53A, IBS10/Smu CovR D53E, IBS10/GAS CovR and IBS10/GAS CovR D53A). RNA was isolated at the mid-exponential phase of growth and sQRT-PCR was performed as described in the text. The gyrA gene was included as an internal control to ensure that equal amounts of RNA were loaded per lane. The experiments were repeated at least twice, with two independent RNA isolations. (a) Lane 1, UA159/pIB184Km; lane 2, IBS10/pIB184Km; lane 3, IBS10/Smu CovR; lane 4, IBS10/Smu CovR D53A; lane 5, IBS10/Smu CovR D53E. (b) Lane 1, UA159/pIB184Km; lane 2, IBS10/pIB184Km; lane 3, IBS10/Smu CovR; lane 4, IBS10/GAS CovR; lane 5, IBS10/GAS CovR D53A. Note that lanes 4 and 5 contain CovR from GAS.
Although the CovR family proteins share a high degree of homology, the mode of CovR activation appears to vary. While CovR activity could be modulated by Ser/Thr kinase, the conserved Thr 65 residue, which is the target for phosphorylation, is absent in S. mutans CovR (Fig. S4). To verify whether heterologous CovR proteins could complement the S. mutans ∆covR strain, we selected CovR from S. pyogenes (GAS) and S. agalactiae (GBS). We also generated a GAS CovR mutant where the conserved Asp residue was replaced with Ala (GAS CovR D53A). As shown in Fig. 4(b), ∆covR strains complemented with wild-type CovR from GAS (GAS CovR) or the mutant CovR from GAS (GAS CovR D53A) show lower levels of gbpC transcripts as compared to the ∆covR strain. Likewise, ∆covR strains complemented with GAS CovR or GAS CovR D53A show higher levels of SMU.1882 and bacA transcripts as compared to the ∆covR strain. Thus, the D53A mutant forms of CovR from both S. mutans and GAS are able to complement the ∆covR strain. To further confirm this finding, we decided to carry out a real-time RT-PCR analysis. The real-time RT-PCR data were congruent with our previous findings and confirmed the observation that the D53A mutant CovR from S. mutans and GAS are indeed capable of complementing the ∆covR strain to the wild-type condition (Fig. S2). Similar to the GAS CovR, we found that GBS CovR is also capable of complementing the S. mutans ∆covR strain for activation of the SMU.1882 and bacA transcription and repression of the gbpC transcription (Fig. S2, data not shown).
Effect of the Pta–AckA pathway on CovR mediated gene expression
To study the role of Pta–AckA pathway on gene expression, we made use of the reporter plasmids pIBT8 and pIBT9. These plasmids were introduced into the wild-type, ∆covR and ∆ackA∆pta strains. We then measured the Gus activity from these strains by growing them until the mid-exponential phase. As shown in Fig. 5, we found that the activities for both the repressed (PgbpC) and the activated (PSMU.1882) promoters for the wild-type and the ∆ackA∆pta double mutant strains were similar (Fig. 5). On the other hand, as expected, the expression of PgbpC increased and the expression of PSMU.1882 decreased in the ∆covR as compared to the wild-type. Taken together, these results suggest that CovR activity was not altered the in ∆ackA∆pta double mutant strain.
Fig. 5.
CovR-mediated gene expression in the AckA–Pta pathway mutant. Expression of PgbpC (a) and PSMU.1882 (b) in the wild-type (UA159), ∆covR (IBS10) and ∆ackA∆pta (IBST16) strains. The cultures were grown in THY broth at 37 °C and harvested at the mid-exponential phase, while Gus activity was measured as described in the text. The values shown are units of glucuronidase activity (with standard errors of the mean of experiments repeated at least twice).
Binding of CovR to promoter regions of both activated and repressed genes
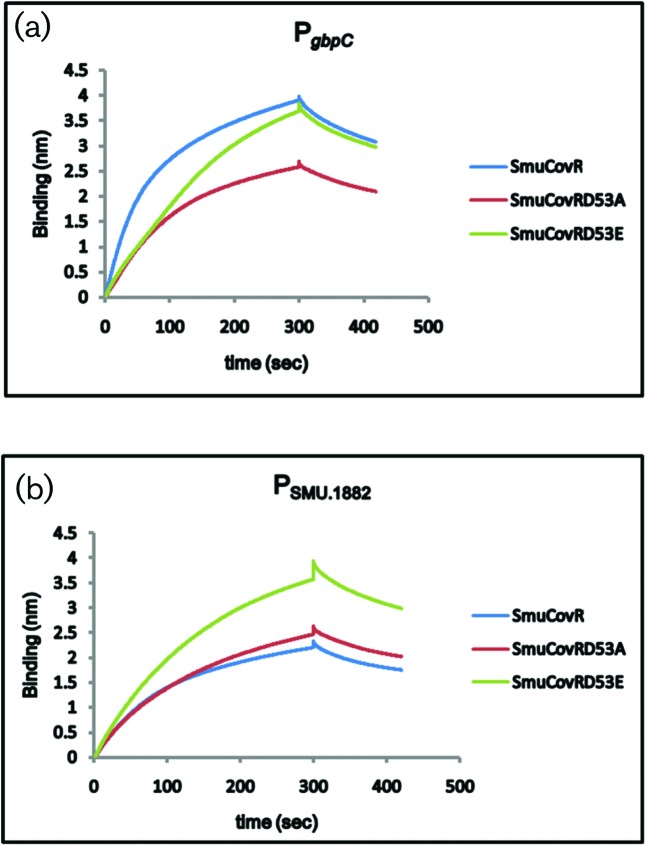
To compare the binding ability of the wild-type CovR and the CovR mutants, we used BLI technology and biotinylated promoter regions of gbpC and SMU.1882 (Fig. 6). We found that both the wild-type and the mutants were able to bind to both of the promoters. However, while the association constants (ka) for the wild-type CovR and CovR D53A were very similar (1.6⋅104 and 1.6⋅104, respectively), the ka value for CovR D53E was lower (ka=5.4⋅103) for the gbpC promoter. On the other hand, the ka value for the binding to SMU.1882 by wild-type CovR was nearly twice that for the CovR D53A (9.7⋅103 vs 4.9⋅103). CovR D53E’ s ka value for the SMU.1882 promoter was the lowest (2.1⋅103). The dissociation constants for all the proteins for these promoters were very similar (between 2.1–3.1⋅10−3). Surprisingly, we found that GAS CovR was unable to bind to these promoters in vitro, although GAS CovR was able to complement the ∆covR strain (data not shown).
Fig. 6.
Differential binding of wild-type and mutant CovR. Biotinylated promoter regions of gbpC and SMU.1882 were immobilized on streptavidin biosensors and then exposed to 0.5 µM CovR, CovR D53A and CovR D53E proteins purified from E. coli as described in the text. The reactions were carried out in binding buffer [20 mM Tris, 100 mM NaCl, 0.01 mM DTT, 5 % glycerol (v/v), 1 mM EDTA, 0.01 mg ml−1 BSA, 5 mM MgCl2 and 10 µg ml−1 poly (dI-dC) at pH 7.5] for a period of 5 min to allow association followed by 2 min exposure to binding buffer to allow dissociation.
Discussion
Among the various TCS systems of streptococci, CovR/S is the most widely studied, since this TCS plays a key role in stress adaptation and virulence. While the regulons that are controlled by CovR in various streptococci, including GAS, GBS and S. mutans, have been well established, the molecular mechanisms of CovR activation are still perplexing and highly complex. For S. mutans, the mechanisms of CovR activation remained unexplored. Except for a few cases, CovR is associated with the cognate sensor kinase CovS. CovS functions both as a kinase and as a phophatase, similar to the EnvZ histidine kinase that activates OmpR [26]. Thus, CovS could provide the phosphate for the conserved Asp residue of CovR as well as remove the phosphate group from CovR to modulate the activity. However, CovR in S. mutans is an orphan response regulator, since the cognate CovS sensor kinase is absent in the genomic locus that encodes CovR [18]. This is true for all of the publicly available genomes of S. mutans, indicating that this organism has indeed adopted a lifestyle in which CovS function is dispensable. In this work, we wanted to understand how CovR activity is modulated in S. mutans.
We found that in S. mutans CovR is indeed phosphorylated in the cell. The phosphate group for this CovR phosphorylation seems to be at least in part donated by the intracellular acetyl phosphate. This is because when we deleted both the ackA and pta genes to inactivate the acetyl phosphate accumulation, we found that CovR phosphorylation was drastically reduced (Fig. 3). We also found that inactivation of either the ackA or the pta genes (single mutant) leads to an increase in CovR phosphorylation (Fig. 3). While this is surprising, a recent report by Kim and colleagues [63] suggested that the AckA–Pta pathway is not the major contributor to acetyl phosphate accumulation in S. mutans, at least not under anaerobic conditions. Indeed, these investigators observed that the deletion of the ackA gene leads to the accumulation of nearly eightfold increased quantities of acetyl phosphate in the cell [63]. However, these authors found very little difference in acetyl phosphate accumulation between the wild-type and either pta-deletion or ackA-pta double deletion mutants. Furthermore, these authors suggested that acetyl phosphate accumulation in S. mutans is complex and occurs through an alternative pathway involving SMU.1299, a putative acetate kinase. While we speculate that acetyl phosphate is the key phosphor donor for CovR in the cell, we cannot rule out other small-molecule phosphor donors, including ATP.
To correlate the level of CovR phosphorylation with the in vivo gene expression, we measured the promoter activity of the PgbpC and PSMU.1882 promoters. Our results indicate that phosphorylation does indeed render CovR inactive, as we found increased activity for PgbpC and decreased activity for PSMU.1882 in the single mutant strains (either ackA or pta) in which we observed increased phosphorylated CovR species (Fig. S3, data not shown). This was surprising, since it has been suggested that in other streptococci phosphorylated CovR is the active form [20, 50, 64]. However, our results suggest that nonphosphorylated CovR is indeed the active form, since we found that the CovR D53A mutant was able to functionally complement the ΔcovR mutant. Furthermore, the CovR D53E mutation, which is predicted to mimic the phosphorylated CovR form, could not complement the ΔcovR mutation. Towards this end, we previously showed that the binding of CovR to promoters of gtfB/C was not enhanced upon phosphorylation [17]. Therefore, we believe that in S. mutans nonphophorylated CovR is active and involved in gene expression. However, at present we do not know the exact role of CovR phosphorylation in gene transcription.
We found that the phosphorylation of CovR leads to dimerization of the protein. The dimerization of response regulators often modulates DNA binding affinity, however many response regulators can bind DNA without phosphorylation, including the well-studied OmpR (CovR belongs to the OmpR family), PhoP and SsrB [65–67]. In addition to dimerization, phosphorylation also alters the specificity of promoter binding [68]. Our CovR D53A was unable to form dimers and, since the mutant was able to complement the ΔcovR phenotypes, we speculate that the active form is the monomer. Of note, we also found that both the CovR D53A and CovR D53E mutants bound to the target promoters, albeit with different affinities as compared to the wild-type CovR. Recently, it was demonstrated that in the GAS the CovR D53E mutant, which is also a monomeric protein, was able to complement some, but not all of, the CovR-mediated gene expression [69]. These authors suggested that in GAS, CovR-mediated gene regulation occurs through two modes: dimerization-dependent and dimerization-independent. It seems that in S. mutans the dimerization-independent mode is the active mode that is involved in gene regulation.
We also observed that CovR from both GAS and GBS was able to complement S. mutans covR deficiency successfully (Fig. 4 and data not shown). This was a surprising finding, since in both GAS and GBS the active species is the phosphorylated CovR, which is also a dimer. To confirm that phosphorylation is indeed not necessary, we used the D53A mutant of GAS CovR. As expected, this mutant was also able to complement the S. mutans covR deficiency (Fig. 4). Heterologous complementation was also an unexpected finding, because it suggests that CovR protein might recognize some common structural features on the target promoter other than the mere sequence motif. Of note, the exact sequence to which CovR binds to is still unknown. Although GAS CovR is generally reported to bind to the hexanucleotide motif ATTARA [20], it can bind to other regions that are devoid of this motif. This motif was believed to be important for in vitro binding conditions, but not in vivo conditions [32]. Similarly, GBS CovR is proposed to bind a nonanucleotide motif TATTTTAA [22]. We previously suggested that S. mutans CovR might recognize a sequence motif that is different from those recognized by its homologues in GAS and GBS [17]. We also suggested that S. mutans CovR might bind to complex consensus sequences or recognize a unique structural feature in DNA [33]. Alternatively, it is possible that an intermediate accessory protein might be involved with the actual DNA binding, and upon binding it recruits/interacts with CovR. Although we do not have any direct evidence for this mechanism, it is important to point out that – at least for the bacA operon – we showed that CovR interacts directly with the nucleoid protein HLP, which acts as an anti-silencer for the operon and displaces it from the promoter to activate gene expression [70].
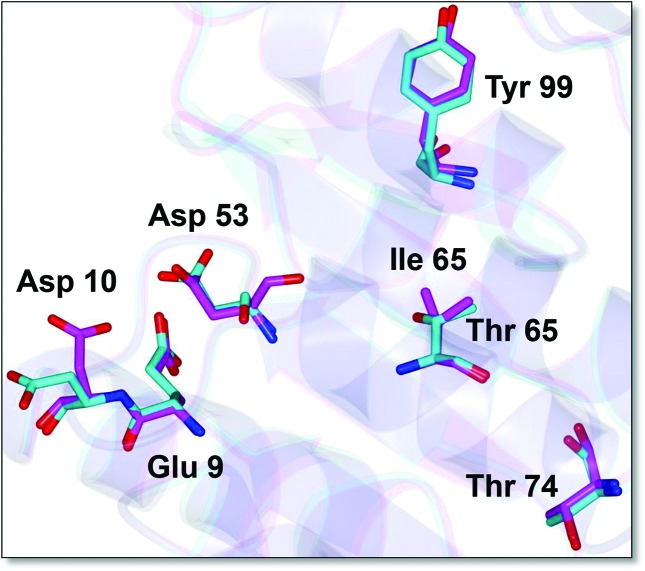
CovR homologues display a high degree of sequence similarity (Fig. S4). Recently, the crystal structure for the C-terminal DNA-binding domain of GAS CovR was determined (PDB ID: 3RJ;[71]). However, the structure of the full-length CovR is not currently available. To gain an insight into the differences between GAS and S. mutans CovR we used this structure and the I-TASSER program (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) to model and compare the two CovR proteins. Both of the models superimpose well with one another. The predicted structure for the receiver domain (REC) is shown in Fig. 7. The CovR REC domain, like other typical REC domains, contains a doubly wound α/β fold with five-stranded parallel β-sheets flanked by two α-helices on one side and three α-helices on the other side (Fig. 7, not shown). The CovR REC domain also contains the conserved ‘acidic-triad’ residues, as well as the ‘Y/T-coupling’ residues (Fig. 7). These Y/T-coupling residues are necessary for the repositioning of the quaternary structure during dimer formation [72]. While these critical residues are well conserved in CovR, we found that S. mutans CovR lack the threonine residue at position 65 (T65) (see Figs S4 and7). This residue is the target for phosphorylation by the Ser/Thr kinase and phosphorylation of T65 inactivates CovR [36, 37, 66]. In S. mutans, the T65 residue has been naturally replaced with isoleucine (Fig. S4). We also found that in two other oral streptococci (S. sobrinus and S. criceti), the 65 position is occupied by metheonine. The exact significance of this replacement is currently unknown and in S. mutans Ser/Thr kinase has no effect on CovR-mediated gene expression.
Fig. 7.
Putative structure of the receiver domain (REC) of S. mutans CovR. The N-terminal REC domain of S. mutans CovR was superimposed on the GAS CovR crystal structure (PDB ID: 3RJP). The structures were computed with I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) using the full-length sequences of CovR proteins. The key residues that constitute the ‘acidic triad’ (Glu9, Asp10 and the phosphor-accepting Asp53) are shown. Also shown are the ‘Y/T-coupling’ residues (Th74 and Try99). The residues shown in magenta belong to S. mutans CovR, while the residues shown in cyan are the GAS CovR.
While the majority of TCSs are considered as paired systems containing both the response regulator and the cognate sensor kinase encoding genes adjacent to each other, orphan unpaired response regulators are also highly abundant [73]. Some of these orphan response regulators are activated by non-cognate sensor kinases through cross-talk mechanisms [74, 75], while others are constitutively activated without the phosphorylation [66, 76]. Most often these latter orphan regulators contains a Glu (instead of Asp) at position 53 [76]. However, for Streptococcus pneumoniae RitR, which is a close homologue of CovR and an orphan, the Asp53 residue is replace with an Asn residue. The activity of RitR is regulated by Ser/Thr kinase and not by Asp phosphorylation [77]. In this work we showed that for some response regulators, such as CovR, multiple activation mechanisms exist, depending on the species. While in GAS or GBS, CovR is activated by Asp phosphorylation, in S. mutans CovR is active without phosphorylation. In fact, in S. mutans phosphorylation might inactivate CovR and this phosphorylation may occur in the cell through acetyl phosphate, the level of which varies during growth and under stress conditions.
Funding information
This study was supported by an award (DE022660) from the NIDCR (NIH) to I. B.
Acknowledgements
We thank Dr Vijay Pancholi (OSU) for the anti-CovR antibody. We also thank Dr Ruth Silvermann (UNC) for providing us with potassium phosphoramidate (PAM) and Dr Scott Lovell (KU) for help with the modelling.
Conflicts of interest
The authors declare there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: GAS, group A streptococcus; GBS, group B streptococcus; PAM, Potassium phosphoramidate; REC, receiver domain; SMU, Streptococcus mutans; TCS, two-component signal transduction system.
Five supplementary figures and one supplementary table are available in the online version of this article.
Edited by: M. Vickerman and J. Stulke
References
- 1.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas JA. Virulence properties of Streptococcus mutans. Front Biosci. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson J, Hamilton I. Textbook of Clinical Cariology. 1994. Metabolic activity of oral bacteria; pp. 71–88. [Google Scholar]
- 5.Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 6.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 7.Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 9.Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitrophanov AY, Groisman EA. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006;4:705–709. doi: 10.1038/nrmicro1478. [DOI] [PubMed] [Google Scholar]
- 12.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh Y, Eguchi Y, Watanabe T, Okamoto S, Doi A, et al. Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr Opin Microbiol. 2010;13:232–239. doi: 10.1016/j.mib.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Worthington RJ, Blackledge MS, Melander C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Future Med Chem. 2013;5:1265–1284. doi: 10.4155/fmc.13.58. [DOI] [PubMed] [Google Scholar]
- 15.Ajdić D, Mcshan WM, Mclaughlin RE, Savić G, Chang J, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci USA. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas I, Drake L, Erkina D, Biswas S. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J Bacteriol. 2008;190:68–77. doi: 10.1128/JB.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas S, Biswas I. Regulation of the glucosyltransferase (gtfBC) operon by CovR in Streptococcus mutans. J Bacteriol. 2006;188:988–998. doi: 10.1128/JB.188.3.988-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong P, Drake L, Biswas I. Modulation of covR expression in Streptococcus mutans UA159. J Bacteriol. 2008;190:4478–4488. doi: 10.1128/JB.01961-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham MR, Smoot LM, Lei B, Musser JM. Toward a genome-scale understanding of group A Streptococcus pathogenesis. Curr Opin Microbiol. 2001;4:65–70. doi: 10.1016/S1369-5274(00)00166-1. [DOI] [PubMed] [Google Scholar]
- 20.Federle MJ, Scott JR. Identification of binding sites for the group A streptococcal global regulator CovR. Mol Microbiol. 2002;43:1161–1172. doi: 10.1046/j.1365-2958.2002.02810.x. [DOI] [PubMed] [Google Scholar]
- 21.Graham MR, Smoot LM, Migliaccio CA, Virtaneva K, Sturdevant DE, et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc Natl Acad Sci USA. 2002;99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, et al. CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 23.Churchward G. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol Microbiol. 2007;64:34–41. doi: 10.1111/j.1365-2958.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- 24.Gryllos I, Grifantini R, Colaprico A, Jiang S, Deforce E, et al. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol Microbiol. 2007;65:671–683. doi: 10.1111/j.1365-2958.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang SM, Ishmael N, Dunning Hotopp J, Puliti M, Tissi L, et al. Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. J Bacteriol. 2008;190:1956–1965. doi: 10.1128/JB.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalton TL, Scott JR. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J Bacteriol. 2004;186:3928–3937. doi: 10.1128/JB.186.12.3928-3937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton TL, Collins JT, Barnett TC, Scott JR. RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J Bacteriol. 2006;188:77–85. doi: 10.1128/JB.188.1.77-85.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner K, Malke H. Dual control of streptokinase and streptolysin S production by the covRS and fasCAX two-component regulators in Streptococcus dysgalactiae subsp. equisimilis. Infect Immun. 2002;70:3627–3636. doi: 10.1128/IAI.70.7.3627-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AA, Engleberg NC, Dirita VJ. Repression of virulence genes by phosphorylation-dependent oligomerization of CsrR at target promoters in S. pyogenes. Mol Microbiol. 2001;40:976–990. doi: 10.1046/j.1365-2958.2001.02441.x. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Gusa AA, Scott JR, Churchward G. Binding of the global response regulator protein CovR to the sag promoter of Streptococcus pyogenes reveals a new mode of CovR-DNA interaction. J Biol Chem. 2005;280:38948–38956. doi: 10.1074/jbc.M506121200. [DOI] [PubMed] [Google Scholar]
- 31.Gusa AA, Scott JR. The CovR response regulator of group A streptococcus (GAS) acts directly to repress its own promoter. Mol Microbiol. 2005;56:1195–1207. doi: 10.1111/j.1365-2958.2005.04623.x. [DOI] [PubMed] [Google Scholar]
- 32.Gusa AA, Froehlich BJ, Desai D, Stringer V, Scott JR. CovR activation of the dipeptide permease promoter (PdppA) in Group A Streptococcus. J Bacteriol. 2007;189:1407–1416. doi: 10.1128/JB.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dmitriev A, Mohapatra SS, Chong P, Neely M, Biswas S, et al. CovR-controlled global regulation of gene expression in Streptococcus mutans. PLoS One. 2011;6:e20127. doi: 10.1371/journal.pone.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas I, Drake L, Biswas S. Regulation of gbpC expression in Streptococcus mutans. J Bacteriol. 2007;189:6521–6531. doi: 10.1128/JB.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong P, Chattoraj P, Biswas I. Activation of the SMU.1882 transcription by CovR in Streptococcus mutans. PLoS One. 2010;5:e15528. doi: 10.1371/journal.pone.0015528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller EW, Danger JL, Ramalinga AB, Horstmann N, Shelburne SA, et al. Regulatory rewiring confers serotype-specific hyper-virulence in the human pathogen group A Streptococcus. Mol Microbiol. 2015;98:473–489. doi: 10.1111/mmi.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W-J, Walthers D, Connelly JE, Burnside K, Jewell KA, et al. Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Mol Microbiol. 2009;71:1477–1495. doi: 10.1111/j.1365-2958.2009.06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kant S, Agarwal S, Pancholi P, Pancholi V. The Streptococcus pyogenes orphan protein tyrosine phosphatase, SP-PTP, possesses dual specificity and essential virulence regulatory functions. Mol Microbiol. 2015;97:515–540. doi: 10.1111/mmi.13047. [DOI] [PubMed] [Google Scholar]
- 39.Ma S, Selvaraj U, Ohman DE, Quarless R, Hassett DJ, et al. Phosphorylation-independent activity of the response regulators AlgB and AlgR in promoting alginate biosynthesis in mucoid Pseudomonas aeruginosa. J Bacteriol. 1998;180:956–968. doi: 10.1128/jb.180.4.956-968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong E, Lee HM, Ko H, Kim DU, Jeon BY, et al. Structure of an atypical orphan response regulator protein supports a new phosphorylation-independent regulatory mechanism. J Biol Chem. 2007;282:20667–20675. doi: 10.1074/jbc.M609104200. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz D, Salinas P, Lopez-Redondo ML, Cayuela ML, Marina A, et al. Phosphorylation-independent activation of the atypical response regulator NblR. Microbiology. 2008;154:3002–3015. doi: 10.1099/mic.0.2008/020677-0. [DOI] [PubMed] [Google Scholar]
- 42.Bachhawat P, Swapna GV, Montelione GT, Stock AM. Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure. 2005;13:1353–1363. doi: 10.1016/j.str.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toro-Roman A, Mack TR, Stock AM. Structural analysis and solution studies of the activated regulatory domain of the response regulator ArcA: a symmetric dimer mediated by the alpha4-beta5-alpha5 face. J Mol Biol. 2005;349:11–26. doi: 10.1016/j.jmb.2005.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S, Sinha A, Sarkar D. Transcriptional autoregulation by Mycobacterium tuberculosis PhoP involves recognition of novel direct repeat sequences in the regulatory region of the promoter. FEBS Lett. 2006;580:5328–5338. doi: 10.1016/j.febslet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Sinha A, Gupta S, Bhutani S, Pathak A, Sarkar D. PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. J Bacteriol. 2008;190:1317–1328. doi: 10.1128/JB.01074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon S, Wang S. Structure of the response regulator PhoP from Mycobacterium tuberculosis reveals a dimer through the receiver domain. Biochemistry. 2011;50:5948–5957. doi: 10.1021/bi2005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee A, Biswas I. Markerless multiple-gene-deletion system for Streptococcus mutans. Appl Environ Microbiol. 2008;74:2037–2042. doi: 10.1128/AEM.02346-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal S, Agarwal S, Pancholi P, Pancholi V. Role of serine/threonine phosphatase (SP-STP) in Streptococcus pyogenes physiology and virulence. J Biol Chem. 2011;286:41368–41380. doi: 10.1074/jbc.M111.286690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Concepcion J, Witte K, Wartchow C, Choo S, Yao D, et al. Label-free detection of biomolecular interactions using BioLayer interferometry for kinetic characterization. Comb Chem High Throughput Screen. 2009;12:791–800. doi: 10.2174/138620709789104915. [DOI] [PubMed] [Google Scholar]
- 50.Gusa AA, Gao J, Stringer V, Churchward G, Scott JR. Phosphorylation of the group A Streptococcal CovR response regulator causes dimerization and promoter-specific recruitment by RNA polymerase. J Bacteriol. 2006;188:4620–4626. doi: 10.1128/JB.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horstmann N, Saldaña M, Sahasrabhojane P, Yao H, Su X, et al. Dual-site phosphorylation of the control of virulence regulator impacts group a streptococcal global gene expression and pathogenesis. PLoS Pathog. 2014;10:e1004088. doi: 10.1371/journal.ppat.1004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siam R, Marczynski GT. Glutamate at the phosphorylation site of response regulator CtrA provides essential activities without increasing DNA binding. Nucleic Acids Res. 2003;31:1775–1779. doi: 10.1093/nar/gkg271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delauné A, Dubrac S, Blanchet C, Poupel O, Mäder U, et al. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect Immun. 2012;80:3438–3453. doi: 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuster M, Silversmith RE, Bourret RB. Conformational coupling in the chemotaxis response regulator CheY. Proc Natl Acad Sci USA. 2001;98:6003–6008. doi: 10.1073/pnas.101571298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creager-Allen RL, Silversmith RE, Bourret RB. A link between dimerization and autophosphorylation of the response regulator PhoB. J Biol Chem. 2013;288:21755–21769. doi: 10.1074/jbc.M113.471763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metz B, Kersten GF, Hoogerhout P, Brugghe HF, Timmermans HA, et al. Identification of formaldehyde-induced modifications in proteins: reactions with model peptides. J Biol Chem. 2004;279:6235–6243. doi: 10.1074/jbc.M310752200. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman EA, Frey BL, Smith LM, Auble DT. Formaldehyde crosslinking: a tool for the study of chromatin complexes. J Biol Chem. 2015;290:26404–26411. doi: 10.1074/jbc.R115.651679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warner JB, Lolkema JS. CcpA-dependent carbon catabolite repression in bacteria. Microbiol Mol Biol Rev. 2003;67:475–490. doi: 10.1128/MMBR.67.4.475-490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukat GS, Mccleary WR, Stock AM, Stock JB. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci USA. 1992;89:718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mccleary WR, Stock JB. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem. 1994;269:31567–31572. [PubMed] [Google Scholar]
- 61.Wolfe AJ, Chang DE, Walker JD, Seitz-Partridge JE, Vidaurri MD, et al. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol Microbiol. 2003;48:977–988. doi: 10.1046/j.1365-2958.2003.03457.x. [DOI] [PubMed] [Google Scholar]
- 62.Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol. 2007;189:5574–5581. doi: 10.1128/JB.00564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JN, Ahn SJ, Burne RA. Genetics and physiology of acetate metabolism by the Pta-Ack pathway of Streptococcus mutans. Appl Environ Microbiol. 2015;81:5015–5025. doi: 10.1128/AEM.01160-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin WJ, Walthers D, Connelly JE, Burnside K, Jewell KA, et al. Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Mol Microbiol. 2009;71:1477–1495. doi: 10.1111/j.1365-2958.2009.06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Head CG, Tardy A, Kenney LJ. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J Mol Biol. 1998;281:857–870. doi: 10.1006/jmbi.1998.1985. [DOI] [PubMed] [Google Scholar]
- 66.Desai SK, Winardhi RS, Periasamy S, Dykas MM, Jie Y, et al. The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. Elife. 2016;5 doi: 10.7554/eLife.10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W, Hulett FM. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J Bacteriol. 1997;179:6302–6310. doi: 10.1128/jb.179.20.6302-6310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kenney LJ. Structure/function relationships in OmpR and other winged-helix transcription factors. Curr Opin Microbiol. 2002;5:135–141. doi: 10.1016/S1369-5274(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 69.Horstmann N, Sahasrabhojane P, Yao H, Su X, Shelburne SA. Use of a phosphorylation site mutant to Identify distinct modes of gene repression by the control of virulence regulator (CovR) in Streptococcus pyogenes. J Bacteriol. 2017;199:e00835-16. doi: 10.1128/JB.00835-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biswas I, Mohapatra SS. CovR alleviates transcriptional silencing by a nucleoid-associated histone-like protein in Streptococcus mutans. J Bacteriol. 2012;194:2050–2061. doi: 10.1128/JB.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horstmann N, Sahasrabhojane P, Suber B, Kumaraswami M, Olsen RJ, et al. Distinct single amino acid replacements in the control of virulence regulator protein differentially impact streptococcal pathogenesis. PLoS Pathog. 2011;7:e1002311. doi: 10.1371/journal.ppat.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zschiedrich CP, Keidel V, Szurmant H. Molecular mechanisms of two-component signal transduction. J Mol Biol. 2016;428:3752–3775. doi: 10.1016/j.jmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ortet P, Whitworth DE, Santaella C, Achouak W, Barakat M. P2CS: updates of the prokaryotic two-component systems database. Nucleic Acids Res. 2015;43:D536–D541. doi: 10.1093/nar/gku968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Espinosa J, Boyd JS, Cantos R, Salinas P, Golden SS, et al. Cross-talk and regulatory interactions between the essential response regulator RpaB and cyanobacterial circadian clock output. Proc Natl Acad Sci USA. 2015;112:2198–2203. doi: 10.1073/pnas.1424632112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Shu D, Chen L, Jiang W, Lu Y. Cross-talk between an orphan response regulator and a noncognate histidine kinase in Streptomyces coelicolor. FEMS Microbiol Lett. 2009;294:150–156. doi: 10.1111/j.1574-6968.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 76.Maule AF, Wright DP, Weiner JJ, Han L, Peterson FC, et al. The aspartate-less receiver (ALR) domains: distribution, structure and function. PLoS Pathog. 2015;11:e1004795. doi: 10.1371/journal.ppat.1004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ulijasz AT, Falk SP, Weisblum B. Phosphorylation of the RitR DNA-binding domain by a Ser-Thr phosphokinase: implications for global gene regulation in the streptococci. Mol Microbiol. 2009;71:382–390. doi: 10.1111/j.1365-2958.2008.06532.x. [DOI] [PubMed] [Google Scholar]
- 78.Maguin E, Prévost H, Ehrlich SD, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1111/j.1365-2958.2008.06532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.