Abstract
Estrogen receptors α and β (ERα and ERβ) have a unique relationship with metabotropic glutamate receptors (mGluRs) in the female rodent brain such that estradiol is able to recruit intracellular G-protein signaling cascades to influence neuronal physiology, structure, and ultimately behavior. While this association between ERs and mGluRs exists in many cell types and brain regions, its effects are perhaps most striking in the nucleus accumbens (NAc). This review will discuss the original characterization of ER/mGluR signaling and how estradiol activity in the NAc confers increased sensitivity to drugs of abuse in females through this mechanism.
Keywords: Estrogen Receptors, Drug Addiction, mGluR, Brain
Effects of estradiol outside the nucleus are often mediated by classical estrogen receptors
Today, it is widely accepted that steroid hormones can signal in ways that do not rely on receptors acting in the nucleus, including activation of receptors localized to the cellular membrane. Rapid effects of estradiol were first observed in uterine tissue over 50 years ago (Szego and Davis, 1967). Increased concentrations of cAMP induced by estradiol administration occurred within seconds, outside the timeframe that was traditionally attributed to actions of steroid hormones. These rapid effects indicated that alternate signaling mechanisms must exist, and it was hypothesized even at that time that these actions of estradiol were mediated by membrane-localized receptors. Subsequent support for this theory came from radioactive ligand binding assays that showed that synaptic plasma membranes have binding sites for estradiol (Towle and Sze, 1983), and from observations of estrogen receptors on the plasma membrane in Xenopus oocytes (Sadler et al., 1985; Sadler and Maller, 1982). The idea that estrogen receptors were physiologically active at the surface membrane was highly contentious, with these early findings often dismissed as technical artifacts. Nevertheless, the field persisted, accumulating more and more evidence of membrane-localized estrogen receptors (Chaban et al., 2003; Kelly et al., 1999; Kelly and Levin, 2001; Levin, 2001; Razandi et al., 2003, 1999).
Although several different membrane estrogen receptors have been reported (Qiu et al., 2008; Revankar et al., 2005), a large percentage of membrane-initiated steroid hormone signaling appears to be performed by a subpopulation of the same receptors that act in the nucleus. Specifically, estrogen receptors (ERs) α and β are found at the plasma membrane and are synthesized from the same transcript as their nuclear counterparts (Razandi et al., 1999). We have known that ERα and ERβ are critical for membrane signaling for at least a decade, as their genetic knockout interferes with rapid estrogen-mediated activation of the MAPK/ERK pathway (Ábrahám et al., 2004). While Ábrahám and colleagues did not examine the subcellular location where ERα and ERβ triggered MAPK signaling, the existence of these estrogen receptors at the plasma membrane has since been observed in many brain regions using a variety of techniques (Micevych and Mermelstein, 2008; Pedram et al., 2009; Razandi et al., 2003).
Though the evidence for rapid, non-nuclear initiated action of ERα and ERβ had been compelling for many years, the mechanism(s) by which these receptors signaled outside the nucleus had remained frustratingly unclear. When our lab set about to investigate the signaling pathways responsible for the effects of membrane-initiated estradiol signaling in the mid-2000s, the fundamental issue was that previous reports had typically examined second messenger signals far downstream of the membrane-initiated event. As such, the literature contained a plethora of descriptive studies examining the impact of estrogens on a wide array of cellular processes. For example, estradiol was reported to attenuate L-type calcium currents (Chaban et al., 2003; Mermelstein et al., 1996) as well as the aforementioned activation of MAPK (Gu and Moss, 1996; Lee et al., 2004; Wade and Dorsa, 2003; Zhou et al., 1996). Hence, we wanted to understand the full signaling pathways that were responsible for multiple effects of estradiol. To do so, we utilized an in vitro assay monitoring phosphorylation of the transcription factor CREB as a measure of cellular activation. We first replicated what others had reported, finding that a brief application of physiological estradiol concentrations increased CREB phosphorylation in CA3-CA1 hippocampal neurons from 1 – 2 day-old female rat pups (Boulware et al., 2005). This effect was dose dependent, rapid, and blocked by MEK inhibitors. We then went on to examine the interaction of estradiol with L-type calcium channel signaling. Increased synaptic activity leads to increased CREB phosphorylation via activation of L-type voltage-gated calcium channels, and this activation can be reproduced in cell culture by K+-mediated depolarization. In this assay, a brief stimulation of cells with 20 mM K+ robustly increases CREB phosphorylation via CaMKIV signaling (Deisseroth et al., 1996; Mermelstein et al., 2000). Pretreatment with estradiol attenuates depolarization-induced CREB phosphorylation, revealing the bidirectional effects of estradiol in its modulation of these two discrete pathways. Our experiments additionally showed that these effects of estradiol are postsynaptic, occur via membrane receptors, and, importantly, do not occur in cultures from male animals (Boulware et al., 2005).
Estrogen receptors activate mGluR signaling pathways
In order to distinguish the opposing effects of estradiol on CREB phosphorylation, we turned to a “bottom-up” approach, working backwards from CREB phosphorylation to pharmacologically isolate the two signaling pathways (Boulware et al., 2005). (The signaling pathway described below is summarized in Figure 1.) We first focused on proteins known to influence MAPK, and found that inhibition of PKC and IP3 receptors decreased estradiol-induced CREB phosphorylation. PLC inhibition similarly blocked the enhancement, without affecting estradiol-induced inhibition of L-type calcium channel effects. Because PLC, PKC, and IP3Rs are activated by Gq-coupled GPCRs (Gutkind, 2000), we hypothesized that estradiol may act through a known Gq-linked GPCR to enhance CREB phosphorylation. Our first step in addressing this hypothesis was to simply power through the catalog of pharmacological agents that act as antagonists for individual Gq-coupled receptors. With a bit of luck, the second drug we tried was LY367385, an antagonist for mGluR1a, a group I mGluR. Pretreatment of cells with this drug blocked estradiol enhancement of CREB phosphorylation without altering estradiol attenuation of L-type calcium channel signaling. These results were confirmed with a second mGluR1 antagonist. In addition, direct pharmacological activation of mGluR1a increased CREB phosphorylation, and occluded any further enhancement by estradiol. The mGluR1a agonist DHPG elicited CREB phosphorylation regardless of sex, suggesting that the sex difference in estradiol signaling lies upstream of the mGluR. This also supported the idea that estradiol was not acting directly on the mGluR, but rather relies on the ability of an estrogen receptor to activate its signaling. Indeed, we found that the ERα agonist PPT increased CREB phosphorylation, while the ER blocker ICI 182,780 eliminated the effect of estradiol. These data indicated that ERα was able to solicit mGluR1a signaling in female hippocampal neurons. We later went on to repeat these experiments in female striatal neurons, finding that the same type of ER/mGluR signaling occurs, but ERα pairs with mGluR5 instead of mGluR1a (Grove-Strawser et al., 2010).
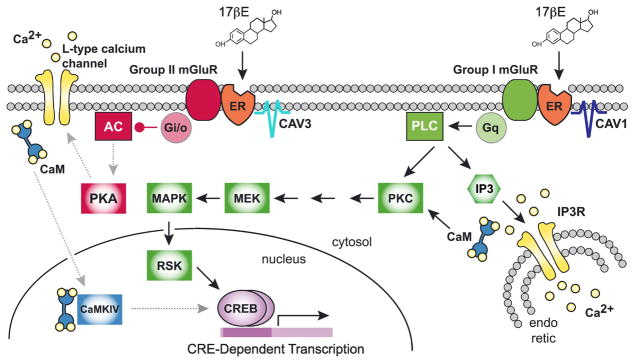
Figure 1.
17β-Estradiol (17βE) binds to membrane-localized estrogen receptors (ER) to activate distinct signaling pathways via association with Group I or Group II mGluRs. Interaction with group I mGluRs activates Gq-mediated signaling through protein lipase C (PLC) and protein kinase C (PKC), leading to intracellular calcium release and activation of MAPK/ERK. Interaction with group II mGluRs activates Gi/o-mediated inhibition of adenylyl cyclase (AC), decreasing activity (indicated by gray dashed lines) of protein kinase A (PKA), resulting in reduced L-type calcium channel currents. CAV, caveolin. IP3, inositol triphosphate. IP3R, IP3 receptor. CaM, calmodulin. MEK, MAPK/ERK kinase. MAPK, mitogen-activated protein kinase. RSK, ribosomal S6 kinase. CaMKIV, calcium/calmodulin-dependent protein kinase type IV.
Having demonstrated that Gq-coupled mGluR1a was necessary for estradiol-induced enhancement of CREB phosphorylation, we hypothesized that activation of a separate G-protein signaling pathway could explain the effect of estradiol on L-type calcium channel signaling (Boulware et al., 2005). Indeed, inhibition of group II mGluRs, which couple to Gi/o proteins to inhibit adenylyl cyclase, eliminated the effect of estradiol. Furthermore, activation of either ERα or ERβ triggered group II mGluR signaling (Figure 1). This work demonstrated specific bidirectional effects of estradiol within the same system, wherein the magnitude of concurrent excitatory input will dictate the outcome of estradiol exposure.
Since these initial studies, a greater appreciation has emerged for how estradiol can influence neuronal systems via interactions with group I mGluRs. For example, group I mGluRs can activate a variety of cellular responses, including the release of endogenous cannabinoids (Alger and Kim, 2011; Wilson and Nicoll, 2002). Estradiol has also been known to influence this system (Maccarrone et al., 2002; Scorticati et al., 2004), and these lines of evidence have recently converged. Specifically, it has been demonstrated that in female hippocampal neurons, ERα activates mGluR1a leading to endocannabinoid signaling, subsequently decreasing presynaptic GABA neurotransmission (Huang and Woolley, 2012; Tabatadze et al., 2015). This example illustrates the potential impact of ER/mGluR interaction and the importance of thoroughly understanding of the signaling mechanism.
Estradiol activation of ER/mGluR signaling underlies sex differences in addiction
Group I mGluRs
As we are particularly interested in the influence of ER/mGluR interaction on the neural circuitry underlying addiction, it is worthwhile to first take a closer look at the role of group I mGluRs independent from estradiol signaling. Group I mGluRs (mGluR1a and mGluR5) are primarily post-synaptic, located at the edge of the postsynaptic density, where they interact with Homer, Shank, and other scaffolding proteins to form a signaling complex with ionotropic receptors and downstream effectors (Verpelli et al., 2011; Xiao et al., 2000). This organization is critical for their proper function (Ronesi et al., 2012; Ronesi and Huber, 2008), which involves modulation of excitatory neurotransmission (Saugstad and Ingram, 2008). Group I mGluRs are important for synaptic plasticity (Fitzjohn and Bashir, 2008), and their activation can be involved in both LTD and LTP (Anwyl, 2009; Lüscher and Huber, 2010). Group I mGluR activity is highly associated with structural changes, particularly in dendritic spines (Vanderklish and Edelman, 2002). Many of these changes are associated with synaptic weakening or spine loss, and this modulation of spine structure may be relevant as a mechanism for synaptic refinement (Wilkerson et al., 2014).
The two group I mGluRs, mGluR1a and mGluR5, are often co-expressed, but not always to the same degree (Shigemoto et al., 1992, 1993). They are also often thought of as interchangeable, but increasing evidence suggests they have distinct and sometimes cooperative functions. For example, in the striatum and globus pallidus, mGluR5 activity regulates the outcome of mGluR1 on neuronal excitability (Kramer and Williams, 2015; Poisik et al., 2003). Even more relevant for our interests, cocaine exposure alters the influence of mGluR1 versus mGluR5 on excitatory neurotransmission (McCutcheon et al., 2011). In the context of drug abuse, mGluR5 is more thoroughly studied than mGluR1, with results showing that it is required for several drug-related behaviors, including acute locomotor responses and self-administration of cocaine (Chiamulera et al., 2001).
Estradiol enhancement of drug-induced plasticity
Because of their role in drug-induced plasticity, sex-specific modulation of group I mGluRs by estrogen receptors is an attractive explanation for the sex differences seen in addiction. On average, women begin using psychostimulants at a younger age and escalate use more quickly, which may be attributable to greater subjective effects (Segarra et al., 2010). Not only do women move through the stages of addiction more quickly than do men (Hernandez-Avila et al., 2004; Randall et al., 1999), but quitting is also harder (Carpenter et al., 2006; Lynch et al., 2002), and cravings are more intense during abstinence (Robbins et al., 1999).
Multiple lines of evidence suggest that estrogens underlie this heightened female vulnerability to addiction. First, the effects of cocaine vary across the menstrual cycle, with women reporting greater effects when estrogen levels are high, or when estrogen is exogenously administered (Evans et al., 2002; Justice and Wit, 1999). These sex differences are recapitulated in rodent models of addiction, with female rats showing faster acquisition, greater escalation, and greater reinstatement of drug use when compared to males (Anker and Carroll, 2010; Becker and Hu, 2008). Removal of endogenous ovarian hormones via ovariectomy, or blockade of estrogen receptors with antagonists, eliminates those sex differences in the behavioral responses to cocaine, while estradiol replacement restores them (Carroll et al., 2004; Jackson et al., 2006; Larson et al., 2007; Larson and Carroll, 2007; Lynch et al., 2001; Sircar and Kim, 1999). Additionally, estradiol enhances cocaine-induced locomotor sensitization in female rodents (Peris et al., 1991; Segarra et al., 2010).
When considering how estradiol may act to create sex differences in addiction, it is logical to consider its influence on dopaminergic systems, which are responsible for the acute responses to drugs of abuse, as well as on glutamatergic systems, which play a crucial role in the neuroplasticity that underlies the development of addiction. Estradiol has been found to influence the regulation and activity of the mesolimbic dopamine system (Becker and Hu, 2008, see Yoest et al., 2014 for review), providing one possible mechanism for how estradiol influences female responses to drugs of abuse. However, we and others have shown that estradiol modulation of nucleus accumbens group I mGluR signaling in females is critically important for enhancement of behaviors closely linked to addiction.
The nucleus accumbens is a critical locus for ER/mGluR signaling in the context of addiction
Specialized structure of the nucleus accumbens
The nucleus accumbens (NAc) region of the striatum carries out complicated processing required for motivated behaviors. Current models posit that glutamatergic afferents to the NAc from the hippocampus, prefrontal cortex, and amygdala provide signals needed for prediction, dopaminergic inputs from the ventral tegmental area provide reinforcement information, and GABAergic inputs engage in action selection and subsequent motor output (Lüscher and Malenka, 2011; Nestler, 2001; Sesack and Grace, 2010). The structure of the NAc is specialized in order to perform this integration. The nucleus accumbens is divided into two sub regions: the core, which is predominately interconnected with motor circuitry, and the shell, which is connected to other limbic structures. Together, these two regions control the execution of conditioned behaviors (NAc core) and their reinforcement through interaction with reward circuitry (NAc shell) (Haber, 2011; Meredith et al., 2008). Dendritic spines on medium spiny neurons (MSNs) integrate dopamine and glutamate inputs, with dopamine modulating the signal of incoming glutamatergic input (Surmeier et al., 2007). MSNs are the predominant output neurons of the striatum, and are capable of influencing motor and cognitive behaviors through projections to other brain regions (Smith et al., 2013; Yager et al., 2015). Excitatory synaptic input on to MSN dendrites is essential for generating action potential output and regulating synaptic plasticity (Britt et al., 2012; Mulder et al., 1998; O’Donnell and Grace, 1995; Papp et al., 2011; Sesack et al., 2003; Stuber et al., 2011). MSNs express GPER-1, membrane-associated ERα and ERβ, and aromatase, but express few or no nuclear ERs (Almey et al., 2012; Foidart et al., 1995; Grove-Strawser et al., 2010; Küppers et al., 2008; Küppers and Beyer, 1998, 1999; Mermelstein et al., 1996; Schultz et al., 2009; Stanić et al., 2014).
The nucleus accumbens core (NAcC) is known for its unique sex differences even outside the context of drug-induced plasticity. NAcC MSNs receive increased numbers of excitatory glutamatergic synapses in females relative to males (Sazdanović et al., 2013). Perinatal estradiol exposure in males causes the relative decrease (Cao et al., 2016). Interestingly, estradiol decreases dendritic spine density in the NAcC of adult female rats, indicating a transient decrease in putative glutamatergic input (Peterson et al., 2014; Staffend et al., 2010). Pharmacological manipulations indicate that the effects of estradiol on spines depend on mGluR signaling (Gross et al., 2016; Peterson et al., 2014), suggesting that ER/mGluR interactions are involved (Boulware et al., 2005; Grove-Strawser et al., 2010). For a summary of estradiol-mediated spine changes in the nucleus accumbens, see Table 1. Note that ER/mGluR regulation of dendritic spines is brain region specific (Gross et al., 2016). For example, while ERα functionally couples to mGluR5 within the dorsal striatum, acute estradiol treatment does not affect spines in this brain region (Peterson et al., 2014; Staffend et al., 2011). Moreover, differences between the core and shell regions of the NAc indicate different relationships between estrogen receptors and mGluRs, as well as potential differences in the effects of mGluR signaling on spines.
Table 1.
Summary of ER/mGluR signaling across subregions of the striatum.
| Brain Region | Dorsal striatum | Nucleus accumbens core | Nucleus accumbens shells |
|---|---|---|---|
| Estradiol – induced CREB phosphorylation | + | + | + |
| Estradiol – induced spine changes | − | ↓ | ↑ |
| mGlur subtype involved | mGlurR5 | mGlurR5 | mGluR1a |
It should be noted that the ability of estradiol to affect neuronal structure in order to sculpt neural circuits is not restricted to the nucleus accumbens (Ball et al., 2002; McEwen, 1980; Parducz et al., 2006; Theodosis et al., 1986). For example, estradiol treatment increases neuronal dendritic length in the ventromedial nucleus of the hypothalamus (Meisel and Luttrell, 1990). Estradiol also regulates dendritic spine density in the hippocampus in a striking manner (Gould et al., 1990; Woolley, 1998; Woolley and McEwen, 1992, 1993). There is evidence of ER/mGluR1a pairing in both of these brain regions where spines are increased (Boulware et al., 2005; Christensen et al., 2011), supporting the idea that ER/mGluR5 interactions result in spine decreases while ER/mGluR1a activity increases spine density. Furthermore, spinogenesis produced by ER/mGluR1a activity in the hypothalamic arcuate nucleus are required for female sexual receptivity, evidence that estradiol produces behaviorally-relevant changes in structure through this signaling mechanism (Christensen et al., 2011).
Nucleus accumbens ER/mGluR activity underlies estradiol enhancement of drug-related behaviors
The sex differences seen in the NAc extend to drug-induced plasticity, in that drugs of abuse can increase MSN spine density to a greater degree in females than males (Strong et al., 2017; Wissman et al., 2011a). Catherine Woolley’s group has shown that structural differences are associated with physiological differences, in that the frequency of miniature excitatory postsynaptic currents in NAc MSNs increased with cocaine in females more than males (Wissman et al., 2011b). Their work demonstrated that differences exist in excitatory synapse number per neuron rather than in presynaptic release probability, further supporting the importance of dendritic spine changes.
We have recently found evidence that ER/mGluR activity is involved in the facilitative effects of estradiol on cocaine-induced behaviors described above. Specifically, we demonstrated that mGluR5 activity is required for estradiol enhancement of cocaine-induced locomotor behavioral sensitization (Martinez et al., 2014). Importantly, this effect was specific to ambulatory behavior and did not alter other effects of estradiol replacement in ovariectomized rats, such as attenuation of weight gain. Furthermore, changes were limited to sensitized responses rather than acute responses to cocaine, indicating alterations in the ability of estradiol to influence plasticity. mGluR5 cooperates with endocannabinoid signaling to affect sensitization, as cannabinoid-1 receptor inhibition also eliminates estradiol-mediated enhancement of cocaine responses (Peterson et al., 2016). This ER/mGluR5-cannabinoid signaling mechanism is also required for estradiol potentiation of cocaine-self administration in ovariectomized rats (Martinez et al., 2016). To show this, we allowed ovariectomized rats to freely self-administer cocaine under extended access conditions for ten days. Animals receiving estradiol on a two days on/two days off schedule throughout the experiment increased their cocaine intake relative to controls. This increase was blocked when an mGluR5 antagonist was administered prior to each dose of estradiol. The mGluR5 antagonist had no effect on cocaine intake when administered on its own, without estradiol. Interestingly, these findings highlight the therapeutic potential of mGluR5 antagonism in females (Olive, 2009).
A commonality between the behaviors affected by ER/mGluR signaling is their reliance on plasticity over time. As discussed, ER/mGluR enhancement of cocaine-induced locomotor activity does not affect acute psychomotor responses but rather is restricted to effects following repeated drug exposure that lead to sensitization. This is important because this type of long-term plasticity is thought to drive the chronic, relapsing nature of addiction. For example, the development of behavioral sensitization requires plasticity within the same neural circuits that underlie incubation of craving in humans – a hallmark of addiction (Robinson and Berridge, 1993; Thomas et al., 2001; Vanderschuren and Kalivas, 2000). Unsurprisingly, ER/mGluR signaling similarly influences other aspects of addiction mediated by long-term plasticity. This can be seen in estradiol enhancement of cocaine self-administration (Martinez et al., 2016) and estradiol enhancement of cocaine-conditioned place preference reinstatement (Tonn Eisinger et al., 2017).
While ER/mGluR mediated changes in plasticity in the nucleus accumbens may explain the effects of estradiol in models of drug abuse, other behavioral effects of estradiol can be attributed to similar signaling in other brain regions. For example, ER/mGluR signaling in the hippocampus enhances learning and memory (Boulware et al., 2013; Luine and Frankfurt, 2013, 2012), and similar pathways in the dorsal root ganglion affect nociception (Chaban et al., 2011; Lu et al., 2013). Additionally, ERα-mGluR1a signaling in the arcuate nucleus of the hypothalamus underlies estradiol modulation of lordosis behavior (Christensen et al., 2011; Dewing et al., 2007). It is clear that ER/mGluR signaling provides an avenue through which estrogens can alter the plasticity state of a variety of neuronal systems to affect diverse behaviors. While this is adaptive for reproduction-related processes, it is detrimental when plastic motivational systems are usurped by drugs of abuse (Tonn Eisinger et al., 2017; Yoest et al., 2014).
ER/mGluR signaling is regulated by caveolin proteins and palmitoylation
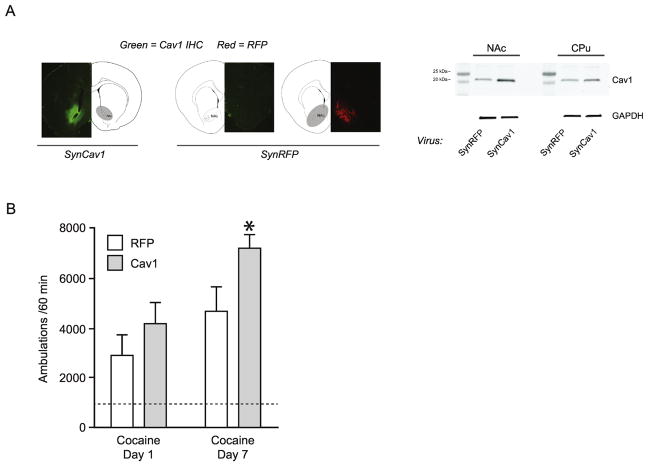
Because of the vast implications of ER/mGluR association, it is important to understand what regulates their interaction. Such regulatory mechanisms are likely to be dynamic, allowing the coupling of these receptors in not only a sex-specific manner, but also a cell-specific manner. Cell culture experiments point to two regulatory mechanisms: interaction with caveolin proteins, and palmitoylation. First, caveolin proteins – structural membrane proteins – are required to traffic ERs to the plasma membrane where they can associate with mGluRs (Boulware et al., 2007; Razandi et al., 2002). The particular caveolin isoform (there are three) determines the character of the ER/mGluR pairing. That is, ERα with mGluR1a or mGluR5, or ERα or ERβ with mGluR2/3. In this way, caveolin creates functional microdomains within the membrane, clustering receptors with their effector proteins, and providing subcellular spatial tuning (see Figure 1). In an attempt to better understand the involvement of caveolin in ER/mGluR-mediated enhancement of cocaine plasticity, and to bring our in vitro findings into an in vivo paradigm, we overexpressed Cav1 in neurons of the nucleus accumbens in ovariectomized rats (Figure 2a) and measured differences in locomotor responses to cocaine (Figure 2b). We used a dose of cocaine previously shown to not produce behavioral sensitization in ovariectomized rats without estradiol supplementation, and hypothesized that Cav1 overexpression would mimic the enhancement normally seen with estradiol. Indeed, Cav1 animals increased their locomotor responses from the first to last day of cocaine exposure, while control animals did not (Figure 2b). This indicated that Cav1 overexpression facilitated cocaine-induced plasticity.
Figure 2.
Overexpression of caveolin-1 (Cav1) in the nucleus accumbens (NAc) facilitates cocaine-mediated behavioral sensitization in female rats. (A) Immunohistochemistry confirmation of Cav1 overexpression (regulated under the synapsin (Syn) promoter) two weeks following AAV9-mediated delivery (left panel). Control animals overexpressed red fluorescent protein (RFP) (middle panels). Western blot confirmation of Cav1 overexpression in the NAc compared to caudate putamen (CPu) control region. GAPDH was used as a loading control (right panel). (B) Only animals overexpressing Cav1 exhibited behavioral sensitization between the first and seventh day of cocaine treatment (n = 9–10; *p = 0.01, paired-sample t-test between groups within day following main effect of two-way ANOVA). Dashed line indicates baseline ambulatory behavior one-day prior to cocaine administration. No differences were observed on these habituation days or in animals receiving repeated saline (data not shown).
A second source of regulation is palmitoylation – reversible post-translational lipidation. ERs must be palmitoylated in order to signal at the membrane. It is possible that neurons utilize a palmitoylation-depalmitoylation cycle to divert greater or fewer ERs to the plasma membrane, or even to integrate membrane and neuronal estradiol signaling. Future studies of membrane-associated ER regulation and signaling will need to consider the recent advances in knowledge of depalmitoylation and local palmitoylation cycles (Fukata et al., 2015, 2013, 2016; Yokoi et al., 2016). Because palmitoylation is a dynamic process, it is possible that there could be an activity-dependent component of ER palmitoylation state (Tabatadze et al., 2013). We are only just beginning to understand the extent of palmitoylation influence on signaling mechanisms that rely on membrane-tethering of otherwise soluble proteins, including ER/mGluR activity.
Finally, not only can ERs pair with different mGluRs in different brain regions, but it is becoming increasingly clear that the same mGluRs can pair with distinct downstream signaling partners to have differential effects both within and across brain regions (Gross et al., 2016; Mannaioni et al., 2001; Poisik et al., 2003; Valenti et al., 2002). In other cases, mGluR signaling may result in the same outcome, but through distinct pathways (Benquet et al., 2002; Thandi et al., 2002). These nuances in mGluR signaling are an important consideration, as they likely contribute to the varied effects of estradiol on structural plasticity. The flexibility and diversity of ER/mGluR signaling outcomes are thus conferred at multiple levels. Learning the precise mechanism that determines which mGluR an ER pairs with, and the nature of downstream effects of that mGluR, will clarify our understanding of how estradiol modulates neural systems in specific and complex ways.
Future directions and conclusions
This review has focused on estrogen regulation of female motivational behavior, as estrogens have not been found to directly affect brain regions associated with the reward pathway in male mammals (Becker, 2016; Cummings et al., 2014). Nevertheless, estrogens are known to play essential roles in avian physiology and behavior (Cornil et al., 2012), including through ER/mGluR signaling mechanisms (Seredynski et al., 2015). Moreover, ER/mGluR signaling has recently been described in the cerebellum of male mice (Hedges et al., 2018), a brain region known to affect an ever increasing variety of cognitive functions (Strick et al., 2009; Galliano and De Zeeuw, 2014). Thus, it would be premature to exclude the possibility of estrogen affecting motivated behaviors in male mammals. That said, androgen receptors (AR) are also palmitoylated by the same DHHC enzymes as estrogen receptors (Pedram et al., 2012), and thus may be trafficked to the surface membrane as well. Hence, current work is examining potential AR/mGluR signaling and its impact upon the reward circuitry in the brains of male mammals, similar to the effects of estrogens in females.
Additionally, although the membrane-localized ER signaling described in this review has generally been studied in isolation from nuclear signaling, it is becoming clearer and clearer that integration of these mechanisms must be considered (Frick, 2015). Perhaps the distinct estradiol signaling mechanisms serve as a sort of coincidence detector in various systems, whereby the convergence of rapid and slow effects promotes a certain outcome. This can be seen in the case of sexual receptivity, as both the slower and more rapid effects of estradiol are required in the hypothalamus for the normal expression of sexual receptivity in females (Kow and Pfaff, 2004). Specifically, ERα/mGluR1a signaling leads to rapid internalization of μ-opioid receptors in the medial preoptic area (Dewing et al., 2007), followed by a slower, enduring increase in dendritic spine density in the arcuate nucleus (Christensen et al., 2011). Similarly, estradiol signaling through ERα/mGluR5 affects neuronal excitability in striatal cells on the order of seconds or minutes (Grove-Strawser et al., 2010), followed by slower effects on dendritic spine plasticity (Peterson et al., 2014). These differing time courses converge to enhance motivated behaviors in females, as seen in the findings from our lab and others on estradiol facilitation of cocaine-induced plasticity. Although nuclear estrogen receptors are not found in abundance in the NAc, nuclear ERs in other reward circuitry brain regions could contribute to the effects of estrogens on the development and maintenance of addiction. Thus, it stands to reason that nuclear estradiol signaling adds another layer to the processes discussed in this review, and careful work must be undertaken to dissect these signaling mechanisms. Doing so will help explain the special nature of the ER/mGluR relationship that allows estradiol to powerfully influence neuronal physiology, structure, and, ultimately, behavior.
Highlights.
Classical estrogen receptors are responsible for non-nuclear estradiol signaling in the brain.
Estrogen receptors activate metabotropic signaling pathways to affect neuronal physiology and structure.
Estrogen receptor/metabotropic glutamate receptor signaling in the nucleus accumbens underlies sex differences in addiction.
Acknowledgments
This work was supported by NIH Grants DA035008 and DA041808 (PGM). KRTE was supported by training grant DA007234 (PGM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ábrahám IM, Todman MG, Korach KS, Herbison AE. Critical in Vivo Roles for Classical Estrogen Receptors in Rapid Estrogen Actions on Intracellular Signaling in Mouse Brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304– 315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen Receptors Are Found in Glia and at Extranuclear Neuronal Sites in the Dorsal Striatum of Female Rats: Evidence for Cholinergic But Not Dopaminergic Colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Biological Basis of Sex Differences in Psychopharmacology, Current Topics in Behavioral Neurosciences. Springer; Berlin, Heidelberg: 2010. Females Are More Vulnerable to Drug Abuse than Males: Evidence from Preclinical Studies and the Role of Ovarian Hormones; pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of Song Behavior and Avian Brain Plasticity: Multiple Sites of Action of Sex Steroid Hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The Memory-Enhancing Effects of Hippocampal Estrogen Receptor Activation Involve Metabotropic Glutamate Receptor Signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin Proteins Are Essential for Distinct Effects of Membrane Estrogen Receptors in Neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci Off J Soc Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J. Neonatal Masculinization Blocks Increased Excitatory Synaptic Input in Female Rat Nucleus Accumbens Core. Endocrinology. 2016;157:3181–3196. doi: 10.1210/en.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/S0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-Initiated Estradiol Signaling Induces Spinogenesis Required for Female Sexual Receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol., Membrane-initiated estradiol signaling regulates the central nervous system. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: Neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from Synapse to Nucleus: Postsynaptic CREB Phosphorylation during Multiple Forms of Hippocampal Synaptic Plasticity. Neuron. 1996;16:89–101. doi: 10.1016/S0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane Estrogen Receptor-α Interactions with Metabotropic Glutamate Receptor 1a Modulate Female Sexual Receptivity in Rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Bashir ZI. The Glutamate Receptors. Springer; 2008. Metabotropic glutamate receptor-dependent synaptic plasticity; pp. 509–528. [Google Scholar]
- Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280:561–574. doi: 10.1007/BF00318360. [DOI] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav, Estradiol and cognition: molecules to mind. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Sekiya A, Murakami T, Yokoi N, Fukata Y. Postsynaptic nanodomains generated by local palmitoylation cycles. Biochem Soc Trans. 2015;43:199–204. doi: 10.1042/BST20140238. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J Cell Biol. 2013;202:145–161. doi: 10.1083/jcb.201302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Murakami T, Yokoi N, Fukata M. Chapter Four - Local Palmitoylation Cycles and Specialized Membrane Domain Organization. In: Bennett V, editor. Current Topics in Membranes, Dynamic Plasma MembranesPortals Between Cells and Physiology. Academic Press; 2016. pp. 97–141. [DOI] [PubMed] [Google Scholar]
- Galliano E, De Zeeuw CI. Progress in Brain Research. Elsevier; 2014. Questioning the cerebellar doctrine; pp. 59–77. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross KS, Brandner DD, Martinez LA, Olive MF, Meisel RL, Mermelstein PG. Opposite Effects of mGluR1a and mGluR5 Activation on Nucleus Accumbens Medium Spiny Neuron Dendritic Spine Density. PLOS ONE. 2016;11:e0162755. doi: 10.1371/journal.pone.0162755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17β-Estradiol Potentiates Kainate-Induced Currents via Activation of the cAMP Cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS. Regulation of Mitogen-Activated Protein Kinase Signaling Networks by G Protein-Coupled Receptors. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- Haber SN. 11 Neuroanatomy of Reward: A View from the Ventral Striatum. Neurobiol Sensat Reward. 2011:235. [PubMed] [Google Scholar]
- Hedges VL, Chen G, Yu L, Krentzel AA, Starrett JR, Zhu JN, Suntharalingam P, Remage-Healey L, Wang JJ, Ebner TJ, Mermelstein PG. Local Estrogen Synthesis Regulates Parallel Fiber–Purkinje Cell Neurotransmission Within the Cerebellar Cortex. Endocrinology. 2018;159:1328–1338. doi: 10.1210/en.2018-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/S0039-128X(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/S1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PF, Williams JT. Cocaine decreases metabotropic glutamate receptor mGluR1 currents in dopamine neurons by activating mGluR5. Neuropsychopharmacology. 2015;40:2418–2424. doi: 10.1038/npp.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers E, Beyer C. Expression of estrogen receptor-α and β mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. doi: 10.1016/S0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Küppers E, Beyer C. Expression of aromatase in the embryonic and postnatal mouse striatum. Mol Brain Res. 1998;63:184–188. doi: 10.1016/S0169-328X(98)00279-4. [DOI] [PubMed] [Google Scholar]
- Küppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-α knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33:832–838. doi: 10.1016/j.psyneuen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor β, but not α, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Levin ER. Invited Review: Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang Q, Yu L, Lu Z, Meng S, Su D, Burnstock G, Ma B. 17β-Estradiol Rapidly Attenuates P2X3 Receptor-Mediated Peripheral Pain Signal Transduction via ERα and GPR30. Endocrinology. 2013;154:2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience, Steroid hormone actions in the CNS: the role of brain-derived neurotrophic factor (BDNF) 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol., Membrane-initiated estradiol signaling regulates the central nervous system. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Battista N, Finazzi-Agrò A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Olive MF, Carroll ME, Meisel RL, Mermelstein PG. Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro. 2016;3 doi: 10.1523/ENEURO.0140-16.2016. ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Steroid hormones and the brain: Cellular mechanisms underlying neural and behavioral plasticity. Psychoneuroendocrinology. 1980;5:1–11. doi: 10.1016/0306-4530(80)90003-7. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Luttrell VR. Estradiol increases the dendritic length of ventromedial hypothalamic neurons in female syrian hamsters. Brain Res Bull. 1990;25:165–168. doi: 10.1016/0361-9230(90)90269-6. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical Dependence of cAMP Response Element-Binding Protein Phosphorylation on L-Type Calcium Channels Supports a Selective Response to EPSPs in Preference to Action Potentials. J Neurosci. 2000;20:266. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane Estrogen Receptors Acting Through Metabotropic Glutamate Receptors: An Emerging Mechanism of Estrogen Action in Brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, da Silva FHL. Electrophysiology of the Hippocampal and Amygdaloid Projections to the Nucleus Accumbens of the Rat: Convergence, Segregation, and Interaction of Inputs. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp E, Borhegyi Z, Tomioka R, Rockland KS, Mody I, Freund TF. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Struct Funct. 2011;217:37–48. doi: 10.1007/s00429-011-0331-z. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, MacLusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: Neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroact Steroids Old Play New Game. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental Phenotype of a Membrane Only Estrogen Receptor α (MOER) Mouse. J Biol Chem. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology. 2016;110(Part A):118–124. doi: 10.1016/j.neuropharm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2014:1–8. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids, Rapid Responses to Steroid Hormones Proceedings of the 5th International RRSH Meeting; Dublin, Ireland. September 2007; 2008. pp. 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs Associate with and Regulate the Production of Caveolin: Implications for Signaling and Cellular Actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/me.16.1.100. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol Baltim Md. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER. Proximal Events in Signaling by Plasma Membrane Estrogen Receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentivesensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, Hu JH, Worley PF, Gibson JR, Huber KM. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15:431–440. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler SE, Bower MA, Maller JL. Studies of a plasma membrane steroid receptor in Xenopus oocytes using the synthetic progestin RU 486. J Steroid Biochem. 1985;22:419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- Sadler SE, Maller JL. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982;257:355–361. [PubMed] [Google Scholar]
- Saugstad JA, Ingram SL. The Glutamate Receptors. Springer; 2008. Group I metabotropic glutamate receptors (mGlu1 and mGlu5) pp. 387–463. [Google Scholar]
- Sazdanović M, Mitrović S, Živanović-Mačužić I, Jeremić D, Tanasković I, Milosavljević Z, Maliković A, Ognjanović N, Sazdanović P, Jovanović B, Jovanović J, Todorović M, Toševski J. Sexual dimorphism of medium-sized neurons with spines in human nucleus accumbens. Arch Biol Sci. 2013;65:1149–1155. [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral Vector-Mediated Overexpression of Estrogen Receptor-α in Striatum Enhances the Estradiol-Induced Motor Activity in Female Rats and Estradiol-Modulated GABA Release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorticati C, Fernández-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A, Billi S, Franchi A, McCann SM, Rettori V. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci U S A. 2004;101:11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menéndez-Delmestre R, Puig-Ramos A, Torres-Diaz YM. Estradiol: A key biological substrate mediating the response to cocaine in female rats. Horm Behav., Sex and drugs: Sex differences and hormonal effects on drug abuse. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen Receptor β Activation Rapidly Modulates Male Sexual Motivation through the Transactivation of Metabotropic Glutamate Receptor 1a. J Neurosci. 2015;35:13110–13123. doi: 10.1523/JNEUROSCI.2056-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical Substrates for Glutamate-Dopamine Interactions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female Gonadal Hormones Differentially Modulate Cocaine-Induced Behavioral Sensitization in Fischer, Lewis, and Sprague-Dawley Rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23(4):546–552. doi: 10.1016/j.conb.2013.01.026. Addiction 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2010;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of Aromatase Expression in the Adult Male and Female Mouse Brain. I Coexistence with Oestrogen Receptors α and β, and Androgen Receptors. PLOS ONE. 2014;9:e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M. Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology. 2017;121:195–203. doi: 10.1016/j.neuropharm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. J Neurosci. 2015;35:11252– 11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Smejkalova T, Woolley CS. Distribution and Posttranslational Modification of Synaptic ERα in the Adult Female Rat Hippocampus. Endocrinology. 2013;154:819–830. doi: 10.1210/en.2012-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandi S, Blank JL, Challiss R. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem. 2002;83:1139–1153. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986;17:661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids. 2017 doi: 10.1016/j.steroids.2017.11.013. [DOI] [PMC free article] [PubMed]
- Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: Differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, Di Stefano B, Mantegazza R, Broccoli V, Böckers TM. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286:34839–34850. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen Activation of Cyclic Adenosine 5′-Monophosphate Response Element-Mediated Transcription Requires the Extracellularly Regulated Kinase/Mitogen-Activated Protein Kinase Pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wilkerson JR, Tsai NP, Maksimova MA, Wu H, Cabalo NP, Loerwald KW, Dictenberg JB, Gibson JR, Huber KM. A role for dendritic mGluR5-mediated local translation of Arc/Arg3. 1 in MEF2-dependent synapse elimination. Cell Rep. 2014;7:1589–1600. doi: 10.1016/j.celrep.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid Signaling in the Brain. Science. 2002;296:678. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct. 2011a;217:181–190. doi: 10.1007/s00429-011-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011b;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-Mediated Structural and Functional Synaptic Plasticity in the Female Rat Hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat [published erratum appears in J Neurosci 1992 Oct;12(10):following table of contents] J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: Role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Cummings AJ, Becker BJ. Estradiol, Dopamine and Motivation. Cent Nerv Syst Agents Med Chem - Cent Nerv Syst Agents. 2014;14:83–89. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi N, Fukata Y, Sekiya A, Murakami T, Kobayashi K, Fukata M. Identification of PSD-95 Depalmitoylating Enzymes. J Neurosci. 2016;36:6431–6444. doi: 10.1523/JNEUROSCI.0419-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]