Abstract
Background:
Rhinitis and conjunctivitis are often linked to asthma development through an allergic pathway. However, runny nose and watery eyes can result from non-allergic mechanisms. These mechanisms can also underlie exercise-induced wheeze (EIW), which has been associated with urgent medical visits for asthma, independent of other indicators of asthma severity or control.
Objective:
Test the hypothesis that rhinitis or watery eyes without cold symptoms (RWWC) in infancy predict development of EIW and urgent respiratory related medical visits at school-age, independent of seroatopy.
Methods:
Within a prospective birth cohort of low-income, urban children (n=332), RWWC was queried during the first year of life. Relative risks (RR) for EIW, emergency department (ED) visits and hospitalizations for asthma and other breathing difficulties at age 5–7 years were estimated with multivariable models. Seroatopy was determined at age 7.
Results:
Infant RWWC was common (49% of children) and predicted school-age EIW (RR=2.8, P<0.001), ED visits (RR=1.8, P=0.001) and hospitalizations (RR=9.8, P=0.002). These associations were independent of infant wheeze. They were also independent of birth order, an indicator of increased risk of exposure to viruses in infancy, and infant ear infections, an indicator of sequelae of upper-airway infections. The association between infant RWWC and age 5–7 ED visits was attenuated (RR=1.2, P=0.23) when age 5–7 EIW was included in the model, suggesting EIW mediates the association. Adjustment for seroatopy did not diminish the magnitudes of any of these associations.
Conclusion:
These findings suggest a non-allergic connection between infant non-wheeze symptoms and important consequences of urban respiratory health by school-age through EIW.
Keywords: Asthma epidemiology, Exercise, Pediatric Asthma
INTRODUCTION
Rhinitis and conjunctivitis are risk factors for subsequent asthma development, a connection that has been considered to occur primarily through an allergic pathway.1–3 However, non-allergic pathways, such as parasympathetic nervous system stimulation, also can cause rhinorrhea, lacrimation, and bronchial reactivity. Exercise induced bronchoconstriction (EIB), the process that underlies exercise-induced wheeze (EIW), occurs in 5–15% of the general population4,5 and 40–90% of the asthmatic population.6 EIW is thought to be related to parasympathetic nerve stimulation either by osmotic cellular changes or airway cooling.7–10 Therefore, rhinitis, watery eyes and EIW may share these physiological responses that are independent of an allergic pathway.
EIW is considered both a distinct phenotype of asthma and a symptom common among poorly controlled asthmatics.11–14 Previously, among middle-income asthmatic children living in NYC, we observed an association between EIW and urgent medical visits for asthma.15 Strikingly, this association was independent of established markers of asthma severity and control, including lower lung function and greater frequency of asthma symptoms, potentially linking an EIW phenotype to acute onset exacerbations that lead to urgent medical visits.15 Further, we found that EIW was more common among asthmatic children living in neighborhoods with greater asthma ED visits and hospitalizations, suggesting that EIW could offer some explanation for the excesses in these burdens afflicting children living in lower-income neighborhoods.15,16
Identifying early life predictors for school-age EIW development could help us better understand the early life causes of EIW and identify children who would benefit from interventions to prevent future ED visits and hospitalizations. Using a well-established, prospective birth cohort study of children born in high asthma prevalence, low-income NYC neighborhoods, we tested our hypothesis (Figure 1) that rhinitis and/or watery eye symptoms without a cold in the first year of life would be associated with subsequent development of EIW and ED visits and hospitalizations for asthma and other breathing difficulties at age 5–7 years, independent of allergic sensitization and infant wheeze.
Figure 1.

Overview of study measurements and hypothesis.
METHODS
As part of the Columbia Center for Children’s Environmental Health (CCCEH), 727 non-smoking (confirmed by cotinine) pregnant African-American or Dominican women ages 18– 35 free of hypertension, diabetes, and known HIV and who were living in Northern Manhattan and the South Bronx were enrolled between 1999 and 2006.17–19 The children were followed prospectively. This study obtained ethics approval from Columbia University’s Institutional Review Board (IRB #AAAC7096). Mothers gave informed written consent and, starting at age 7 years, children gave assent before taking part in this study.
Questionnaires
Questionnaires administered prenatally, during the third trimester and postnatally at 3, 6, 9 and 12 months included queries about the mother and child’s environmental exposures and child symptoms, including the following:
‘Rhinitis without cold’: “Does your child ever get attacks of sneezing or runny nose other than from colds?”
Watery eyes without cold: “Does your child ever get attacks of runny or itchy eyes other than from colds?”
Wheeze: “In the past 3 months, has your child had wheezing or whistling in the chest?
A child was considered to have had rhinitis or watery eyes without a cold (RWWC) or wheeze in the first year of life if their mother reported at least one episode on one of the questionnaires pertaining to that year. At ages 5, 6, and 7 years, EIW and other respiratory outcomes in the previous 12 months were ascertained using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire.20,21
A previously described Brief Respiratory Questionnaire was used to ascertain ED visits and hospitalizations for asthma or other breathing problems in the previous year using the following questions:22
ED visits: “In the last 12 months, how many times did your child have an emergency visit to a doctor, clinic or an emergency room for asthma, wheezing, cough, bronchitis, or other breathing problems?”
Hospitalization for asthma or other breathing problems: “Hospital (BRQ): In the last 12 months, how many times did your child have to stay overnight in the hospital for asthma, wheezing, cough, bronchitis, or other breathing problems?”
Seroatopy measurement
Allergen specific IgE was measured by ImmunoCap (Phadia, Uppsala, Sweden) in serum collected at age 7. Seroatopy was defined as specific IgE to dust mite, cockroach, mouse, cat, dog, trees, grass pollen, ragweed pollen or mixed mold allergens (≥0.35 IU/ml).
Statistical analysis
Analyses were restricted to children who had at least one questionnaire completed for the 1st year of life and for one year between ages 5 and 7 and who had serum IgE measured at age 7. For testing our hypothesis, symptoms in the first year of life were tested as predictors of asthma outcomes at ages 5–7 years. For prospective and cross-sectional analyses, respectively, relative risks (RR) and prevalence ratios (PR) with 95% confidence intervals (CI) were calculated using binomial regressions in generalized estimating equations (GEE). Models included a priori determined potential confounders: age, sex, race/ethnicity, income, maternal asthma, material hardship and presence of a smoker in the home. To assess the independence of these associations from an allergic sensitization pathway, seroatopy was tested in models to see if its inclusion diminished the magnitude of the association between symptoms in infancy and respiratory outcomes at school age by 10% or more. We conducted similar analyses testing for the independence from having older siblings as an indicator of increased risk of exposure to viruses in infancy and infant ear infections as an indicator of sequelae of upper-airway infections in infancy. Mediation of the association between infant RWWC and age 5–7 ED visits by EIW at age 5–7 was assessed by including EIW at age 5–7 in the model.
We conducted analyses to confirm the association we had observed between EIW and urgent medical visits in a middle-income cohort in this lower-income cohort.15 Given the study design, we also utilized the ability to examine the association prospectively, and extended our outcomes to age 9 (to examine risk associated with EIW at age 7). Analyses were restricted to the children from the main hypothesis with asthma symptoms or medication use in the past 12 months, as we did previously in our investigation of EIW and urgent medical visits among middle-income NYC children.15 Prospective analyses were conducted using repeated measures models (subjects had repeated time points) using binomial regressions in GEE models. EIW at ages 5 and/or 7 (time 1) was tested as a predictor of ED visits and hospitalizations two years later (ages 7 and/or 9, time 2). If children had data at 5, 7 and 9 years, they were included twice in the repeated measures model. Similar confounders tested with the main hypothesis were included, as well as ED visits and hospitalizations at time 1. Analyses were conducted using SPSS version 23 (Chicago, IL) and visualized in R version 3.03.
RESULTS
The demographics of the families included in the study (n=332) are described in Table 1. The children included in the analyses did not differ in demographics from those recruited for the CCCEH study but not included in the analyses because of missing data (n=395), except that mothers who reported being of African-American race were more likely than those of Dominican Republic ethnicity to be in the analyses (online Table E1). Importantly, the prevalence of “runny nose without colds”, “watery eyes without colds”, and “wheeze” in the first year of life were similar among the children included in the analyses and those not included but on whom we had symptoms data in the 1st year of life (online Table E1).
Table 1.
Demographics of study participants at birth (n=332).
| Male sex, n (%) | 155 (46.7) | |
| Mother’s age, mean (SD) | 25.3 (4.9) | |
| Mother’s race/ethnicity | African-American, n (%)A | 134 (40.4) |
| Dominican, n (%)A | 198 (59.6) | |
| Mother’s self-reported health | Asthma, n (%)B | 85 (25.6) |
| Allergy, n (%)C |
138 (41.6) | |
| Socio-demographics | No high school degree, n (%)D | 118 (36.5) |
| Receiving Medicaid, n (%) | 304 (91.6) | |
| Material hardship last 6 months, n (%)E | 139 (41.9) | |
| Domestic environment | Smoker in home, n (%)F | 122 (36.7) |
| Cockroach sightings in home, n (%) | 240G (72.7) | |
| Rodent sightings in home, n (%) | 175G (53.0) |
The mother self-identifying as being of either African-American race or Dominican Republic ethnicity was an inclusion criteria.
Mother reported either during pregnancy or on a questionnaire 3 months after the child was born that she had asthma.
Mother replied ‘yes’ to a question about having allergies asked during the first year after the child was born.
Nine of the mothers did not report whether they had completed high school.
Mother reported on the prenatal questionnaire that in the past 6 months she and her family could not afford needed food, rent, clothing or medical care or that gas/electricity was suspended because of bill non-payment.30
Non-smoking during pregnancy was an inclusion criteria.
There were two subjects that did not answer questions about cockroaches or rodents. The percentages are of the n=330 women that answered these questions.
The reports of wheeze and RWWC were common in the first year of life (Figure 2a). While there was overlap between children with these symptoms, a substantial proportion of the children had a report of RWWC without a report of wheeze (23% of cohort). Similarly, while there was overlap between the children with a report of runny nose and watery eyes, there was some discordance in these symptoms (online Figure E1). Almost all (325/332; 98%) of the children had a report of runny nose symptoms WITH a cold in the first year of life, compared with 109/332 (32%) who had a report of runny nose symptoms WITHOUT a cold. In multivariable models assessing risk factors for RWWC, only having an older sibling was statistically significantly associated with RWWC (Prevalence ratio (PR)=1.3; 95% C.I.= 1.1–1.7; P=0.023). Neither race/ethnicity, being male, having a mother with asthma, having a report of material hardship nor living in a home with a smoker in the 1st year of life were statistically significantly associated with RWWC (all P>0.05). We also evaluated potential indoor combustion sources related to household heating and cooking. A majority of the homes were heated with a radiator (91%) and had a gas stove (96%). Neither of these variables were statistically significantly associated with RWWC in infancy (P>0.8).
Figure 2. Reported symptoms (A.) Prevalence and overlap of wheeze and “runny nose and watery eyes without cold” in 1st year of life (n=332) and (B) prevalence of EIW and other respiratory outcomes from ages 5–9 years reported for the previous 12 months.

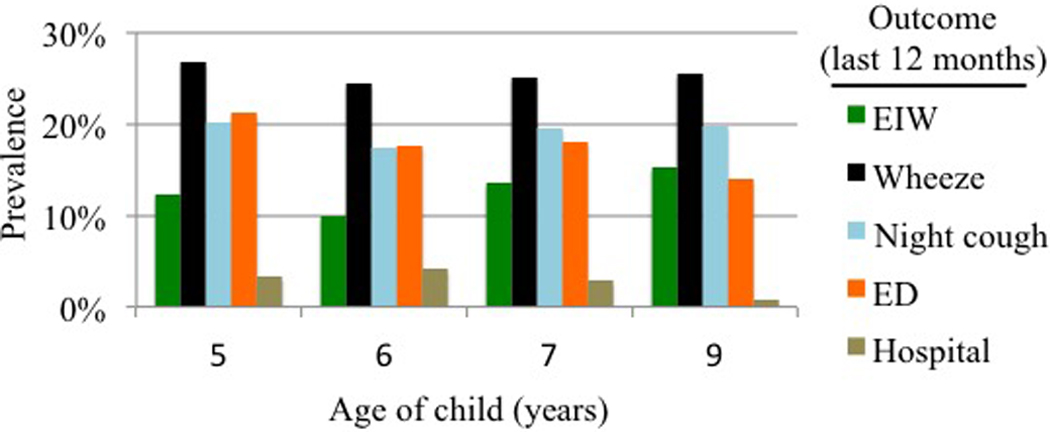
Data were available on n=300, 283, 327 and 294 children at ages 5, 6, 7 and 9, respectively.
During school age (5–7 years), EIW, wheeze, night-time cough without cold symptoms, and ED visits for asthma or other breathing problems were common (Figure 2b). Hospitalizations for asthma or other breathing problems were less common. Between ages 5–7 years, many of the children had at least one report of EIW (20%), wheeze (36%), night-time cough (34%), ED visit (29%), or hospitalization (7%).
RWWC in infancy predicting EIW at school age
In our cohort, both RWWC and wheeze in the first year of life predicted EIW at age 5–7 years in models adjusting for potential confounders (Figure 3A). Infant RWWC and wheeze also predicted school-age wheeze, night-time cough without cold, and ED visits at ages 5–7 (Figure 3B, 3C and 3D). Strikingly, hospitalizations at age 5–7 were predicted by RWWC in infancy, but not by wheeze in infancy (Figure 3E). Examining the unadjusted data, 13% (n=21/163) of the children with RWWC in infancy were hospitalized for breathing problems between ages 5–7 years, while only 1.2% (n=2/169) of the children who did not have these symptoms in infancy were hospitalized at ages 5–7.
Figure 3. Relative risks for (A.) EIW, (B.) Wheeze, (C.) Waking at night with cough without cold (D.) ED visit and (E.) Hospitalizations in the prior year at age 5–7 years with wheeze, runny nose or watery eyes in the first year of life.
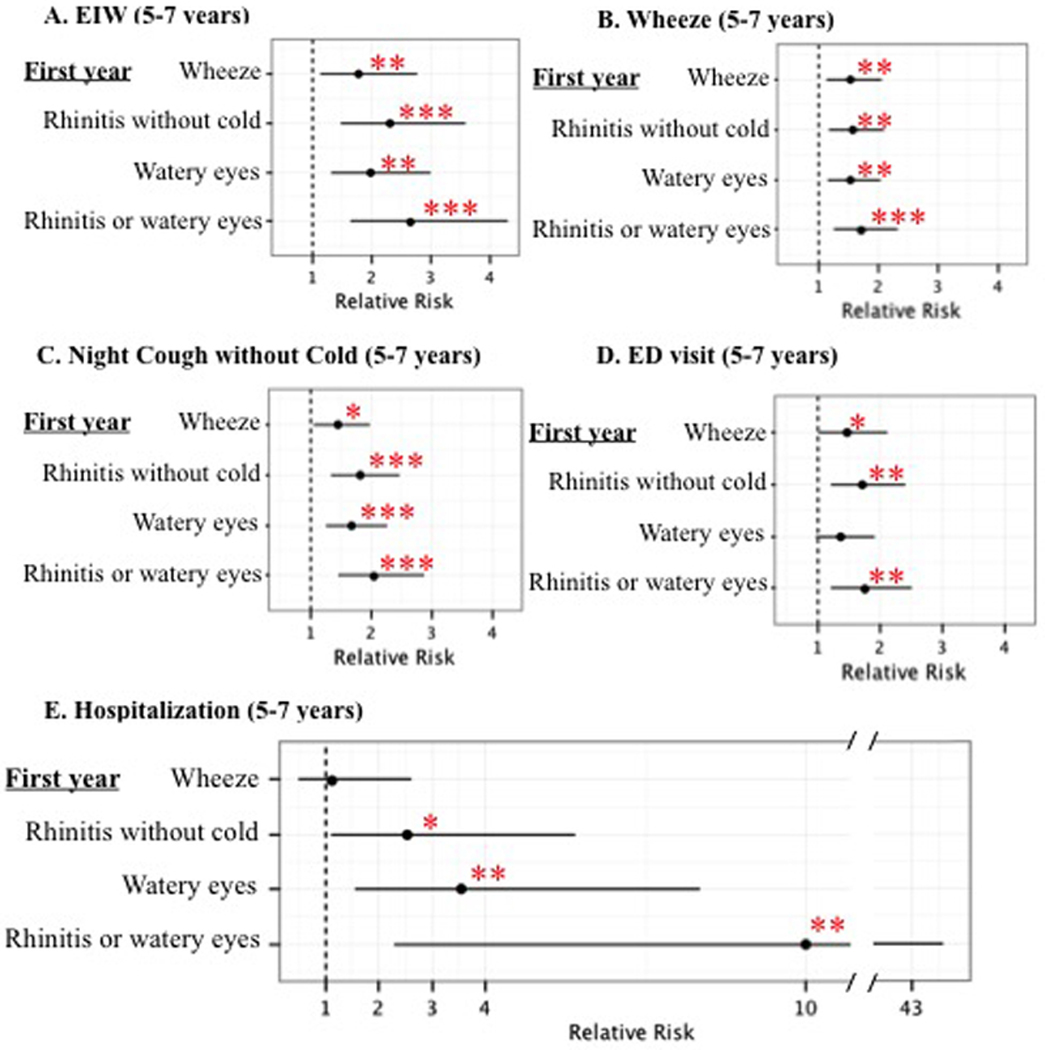
Relative risks were adjusted for sex, race/ethnicity, maternal asthma, material hardship, smoker in the home (prenatal and age 5 years). The frequencies of EIW, wheeze, waking at night with cough without cold, ED visits and hospitalization at ages 5–7 were 20%, 36%, 34%, 29% and 7%. *P<0.05, **P<0.01, ***P<0.001.
To test for the independence from infant wheeze of infant RWWC predicting school age outcomes and to compare the magnitude of risk associated with infant RWWC with that of infant wheeze, models were tested that included both of these variables, along with the same covariates included in models in Figure 3. The magnitude of the association between infant RWWC and school age EIW (RR=2.6; 95% C.I.=1.5–4.3; P<0.001) was greater than the magnitude of the association between infant wheeze and RWWC (RR=1.5; 95% C.I.=0.95–2.4; P=0.078). The 95% confidence intervals for RWWC did not overlap with the effect estimate for wheeze, nor vice-versa, indicating that the magnitude of the relative risk for RWWC was statistically significantly greater than that for wheeze. While the magnitude of the association between RWWC and ED visits (RR=1.7; 95% C.I.=1.2–2.4; P=0.005) was greater than that for wheeze (RR=1.3; 95% C.I.=0.93–1.9; P=0.11), the confidence intervals overlapped, preventing the conclusion that RWWC was a better predictor of future ED visits than wheeze. When evaluating the risk for school age hospitalizations, infant RWWC predicted hospitalizations (RR=11.1; 95% C.I.=2.5–49.8; P=0.002), but infant wheeze did not (RR=0.66; 95% C.I.=0.33–1.3; P=0.24).
Independence of association from allergic sensitization
At age 7, 47% of the children had IgE antibodies (≥0.35 IU/ml) to at least one of the 9 allergen extracts tested. In order from most to least common, children had IgE antibodies to cockroach (29% of children), mixed tree pollen (20%), cat (19%), dog (16%), mouse (15%) dust mite (15%), mixed grass pollen (9%), ragweed (11%) and mixed mold (4.2%).
To assess the independence of these associations from allergic sensitization, models testing the association between RWWC in infancy and EIW and respiratory outcomes at age 5–7 were built, including a variable for seroatopy at age 7 years. Seroatopy at age 7 was used as an indicator of being predisposed to atopy by school age. Models also included infant wheeze to show the independence of the associations with RWWC from wheeze. The inclusion of seroatopy in these models did not change the effect estimates for RWWC and subsequent EIW and respiratory outcomes (Table 2).
Table 2.
Adjusted relative risks [95% Confidence interval] for EIW and other asthma outcomes at age 5–7 with rhinitis and/or watery eyes without cold in infancy.
| Asthma outcome age 5–7 year | Frequency of outcome |
Model adjusting for covariatesA |
Model adjusting for covariatesA and seroatopyB at age 7 years |
|---|---|---|---|
| Exercise induced wheeze | 20% | 2.8 [1.7–4.5]*** | 2.8 [1.7–4.5]*** |
| Wheeze | 36% | 1.7 [1.3–2.3]*** | 1.7 [1.3–2.3]*** |
| Night-time cough without cold | 34% | 2.1 [1.5–2.9]*** | 2.1 [1.5–2.9]*** |
| Emergency department visitC | 29% | 1.8 [1.3–2.6]** | 1.8 [1.2–2.5]** |
| HospitalizationC | 7% | 9.8 [2.2–43]** | 9.8 [2.2–44]** |
P<0.05,
P<0.01,
P<0.001
All models adjusted for wheeze in the 1st years of life, sex, ethnicity/race, maternal asthma, material hardship and tobacco smoke exposure in the home.
Seroatopy was defined as measurable IgE (>0.35 IU/ml) to at least one of the allergens (dust mite, cockroach, mouse, cat, dog, grass pollen, tree pollen, ragweed pollen and mixed mold) tested at age 7 years.
ED visits and hospitalizations were reported for asthma, wheezing, cough, bronchitis, or other breathing problems
Independence of association from indicators of infant viral exposure and illness
To attempt to evaluate the independence of the association from viral illness, we used an indicator of increased risk of exposure of viruses, the presence of older siblings, and an indicator of sequelae following upper respiratory infection, ear infections reported in the 1st year of life. These variables were added to the models with seroatopy described in Table 2. Because of missing data on these two variables for 9 subjects, the sample size was smaller (n=323). The effect estimate for age 5–7 EIW with infant RWWC was the same, RR=2.8 [1.7–4.7], in models without and with the older sibling and ear infection variables. For models predicting ED visits at age 5–7, the RR for RWWC was similar in models without (RR=1.7 [1.2–2.5]) and with (RR=1.7 [1.2–2.4]) the older sibling and ear infection variables. Models predicting age 5–7 hospitalization were also similar without (RR=9.0 [2.0–41]) and with (RR=8.3 [2.0–35]) the older sibling and ear infection variables in the model.
EIW in school age predicting ED visits and hospitalizations 2 years later
We conducted analyses to confirm that the association we had observed between EIW and urgent medical visits for asthma and other breathing difficulties in a middle-income cohort 15 was present in this lower-income cohort and test for a prospective associations, which would better support the hypothesized directionality (i.e., EIW predicting ED visits). Details on which children / measures were included in the repeated measures analysis are described in online Table E2. In repeated measures analysis at school age, age 5–7 EIW predicted future (age 7–9, i.e., measured two years later) ED visits (RR 2.0 [1.2–3.2], P=0.005) and hospitalizations (RR=11 [1.2–95], P=0.034). The magnitudes of these associations were not substantially diminished when ED visits/hospitalizations at baseline or allergic sensitization variables were included in the model (Table 3).
Table 3.
Relative risk (RR) for EIW at age 5–7 predicting ED and hospitalizations 2 years later among children with asthma symptoms or taking asthma medications.
| RR for ED visitsA 2 years later (n=151 children, n=211 observations B) RR [95% CI] |
RR for hospitalizationA 2 years later (n=151 children, n=211 observationsB) RR [95% CI] |
|||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| EIW | 2.0**[1.2–3.2] | 2.0**[1.2–3.2] | 1.8*[1.1–2.8] | 10.7*[1.2–96] | 10.9*[1.2–97] | 11.9*[1.3–112] |
| Frequent Wheeze | 1.6[0.94–2.6] | 1.6[0.94–2.6] | 1.5[0.93–2.5] | 2.40.29–12] | 2.5[0.48–13] | 2.6[0.48–14] |
| Night-time cough without cold | 1.2[0.74–1.8] | 1.2[0.73–1.8] | 1.1[0.72–1.7] | 1.4[0.25–7.3] | 1.5[0.24–9.7] | 1.7[0.24–12] |
| SeroatopyC | - | 1.1[0.67–1.7] | 1.0[0.67–1.6] | - | 0.75[0.13–4.4] | 0.84[0.13–5.3] |
| Current ED visit | - | - | 1.5[0.89–2.5] | - | - | 0.64[0.045–8.9] |
P<0.05,
P<0.01
Model 1- Multivariable repeated measures GEE models adjusted for sex, race, Hispanic ethnicity, material hardship and child’s age at baseline and 2 years later. Model 2 = (Model 1) + seroatopy. Model 3 = (Model 2) + current ED visit
There were n=151 children who had asthma symptoms or taken medication in in the last year included in the prospective analyses. Among these children, there were n=117 and 104 observations at ages 5 (predicting age 7) and 7 (predicting age 9), respectively, for a total of n=211 observations (see online Table e2 for demographics).
Seroatopy was defined as measurable IgE (>0.35 IU/ml) to at least one of the allergens (dust mite, cockroach, mouse, cat, dog, grass pollen, tree pollen, ragweed pollen and mixed mold) tested at age 7 years.
EIW as a mediator of the association between RWWC and ED visits
We conducted mediation analysis to determine if school-age EIW could be in the pathway between infant RWWC predicting school-age ED. When EIW was included in the model, the association between infant RWWC and age 5–7 ED visits was substantially diminished and was no longer statistically significant (Figure 4). By contrast, including the variable, frequent wheeze at age 5–7 (as a different indicator of poor asthma control and/or increased asthma severity), the association between RWWC and ED visits was only modestly diminished. When the model was simultaneously adjusted for both EIW and frequent wheeze at age 7, the RWWC and ED visits association was similar to when only EIW (and not frequent wheeze) was included in the model.
Figure 4. Test for mediation of the association between infant RWWC and age 5–7 ED visits by age 5–7 EIW and frequent wheeze.

All relative risks adjusted for covariates described in Figure 3. The association between RWWC and ED visits was substantially diminished by inclusion of EIW in the model. *P<0.05, ***P<0.001.
DISCUSSION
Among children living in low-income, urban neighborhoods, asthma is prevalent and asthmatic children are at a much higher risk for ED visits and hospitalizations for asthma than asthmatic children in higher-income communities. While combustion byproduct exposure, pest allergen exposure and insufficient pharmacological management have been implicated in these excess morbidities,23,24 prior studies have neither satisfactorily explained these increased burdens nor developed an effective method to identify the highest risk children early in life for interventions. Our study demonstrates an association between an asthma related outcome defined by a rapid response to stimuli (EIW) and ED visits and hospitalizations among asthmatic children living in a low-income, urban community. We have connected the manifestation of EIW in children at school age to infant reports of rhinitis and/or watery eyes in the absence of a reported cold. Importantly, RWWC appears to be both independent of and more predictive than wheeze in infancy of these future respiratory morbidities and independent of an allergic pathway.
In addition to infant RWWC predicting EIW in school age children, it also predicted ED visits and hospitalizations for breathing problems. Mediation analyses indicated that this connection was primarily through EIW. Strikingly, school age children with infant RWWC were much more likely to be hospitalized for asthma or other breathing problems than children without RWWC during infancy. We acknowledge the large confidence interval for this estimate, which is the result of hospitalizations being somewhat uncommon in this general population based study (7% were hospitalized between ages 5 and 7). Still, the association was statistically significant and appeared to be a robust predictor of hospitalizations. While infant wheeze is a well-acknowledged predecessor to asthma development, the specificity of infant wheeze for future asthma is known to be low.25–27 Indeed, with this study, we found infant wheeze to be less predictive of future EIW than RWWC and not predictive of future respiratory related hospitalizations at all.
We believe the apparent independence of the association between rhinitis and later respiratory morbidity from the allergic pathway makes these findings novel. A priori, we defined our atopic population by the presence of seroatopy to common inhalant allergens at age 7. Generally, inhalant allergen persists throughout childhood, once established. Thus, our seroatopy definition should capture sensitization that had been established prior to the end of our follow-up period. While we acknowledge that would have lead us to classify children who had transient sensitization early in childhood as non-atopic, the relevance of transient early-life sensitization to inhalant allergens in relation to rhinitis or asthma has not been demonstrated.28,29 For related reasons, we limited the allergens we tested to inhalant allergens, as these are more likely to be involved in a connection between rhinitis and asthma, than food allergen sensitization.28
With this study, we built on our previous findings of a cross-sectional association between EIW and urgent medical visits by extending the generalizability of this finding to a lower-income urban population. We also strengthened the findings by observing that EIW predicted future ED visits and hospitalizations. We demonstrated a prospective association, whereby EIW predicted future ED visits and hospitalizations, even after controlling for recent ED visits or hospitalizations. This suggests that asking about EIW could be an important tool for physicians to assess risk for future urgent medical visits, in addition to traditional measures of asthma severity and control. Despite EIW being the identified risk, we have not demonstrated that exercise is the trigger leading to the ED visits or hospitalizations. Instead, EIW may identify a group of children with airway dysfunction that respond with acute bronchoconstriction to stimuli, including, but not limited to exercise.
Our hypothesis suggests a complex, physiologically linked pathway from early symptoms to later consequences. This study needs replication, but the associations appear to be robust and potentially indicate a relatively high magnitude of identifiable risk. Future studies should assess the physiologic link between infant RWWC and EIW, with autonomic nervous system development as a possible target.
This study has several limitations. First, “rhinitis and watery eye symptoms in the absence of colds” was assessed by questionnaire report by the mother. Therefore, the ‘absence of cold’ was not validated by either physician exam or testing for viral or bacterial infection. The inability to identify viral infections may have led to an over reporting of ‘symptoms without a cold’. However, with only 32% of children having a positive response to this question, it clearly discriminated from runny nose symptoms reported WITH a cold, for which virtually all of the children (i.e., 98%) had a positive response. Also, these symptoms were assessed prospectively (relative to the school age outcomes), for 3 month periods of time, limiting the recall bias associated with this type of questionnaire based study. Infants with older siblings are more likely to be exposed to viruses and ear infections develop subsequent to upper respiratory tract infections, thus providing some proxy measures for risk of exposure to viruses and a measure of children more likely to have had consequential viral infections. While we did observe a modest increase in RWWC for children with older siblings, that we did not observe any confounding when we included the presence of older siblings or infant ear infections, offers some support that the mechanism of the association between RWWC and subsequent EIW is not through an infection mechanism. Still, future work is needed to better understand what is being indicated by reported RWWC. It is also of interest that we did not observe any increased risk of RWWC with environmental tobacco smoke exposure or home combustion sources related to heating or cooking. However, given that almost all of the homes had boiler based heating and gas stoves, it is likely that we were underpowered to detect any associations with these exposures.
A second limitation was that EIW was assessed by questionnaire; EIB was not tested and a physician diagnosis of EIW was not made. This could have reduced both the specificity and sensitivity of our identification of EIW. A third limitation was that the questions about ED visits and hospitalizations were not specific for asthma, but included other breathing difficulties. Therefore, despite asthma being a major cause for ED visits and respiratory related hospitalizations in this community, we cannot limit these findings to asthma related morbidity. Another related limitation was that we did not ascertain the precipitating factors for the children’s ED visits and hospitalizations. Specifically, knowing whether these events were associated with an environmental trigger- or exercise-induced exacerbation would allow for a better understanding of whether we are identifying a specific asthma phenotype. An additional limitation of this study is the lack of generalizability. These findings may only apply to children living in lower-socioeconomic status urban United States neighborhoods. However, as this is a population that bears the excess burden of asthma ED visits and hospitalizations in urban communities, we believe these findings are of public health importance. It will be important to test for this association in other communities where urgent medical visits for asthma are common.
In conclusion, independent of early-life wheeze and allergic sensitization, infant RWWC predicted EIW and related respiratory morbidities in school age children. Our observation that these associations were independent of the development of allergic sensitization suggests a novel connection through a non-allergic mechanism. Further study of mechanism(s) and confirmation is warranted, but these findings indicate that EIW may be an important component of urban respiratory health. If confirmed, our results could provide a way to identify very young children at greater risk of asthma and other respiratory morbidity who would benefit from increased surveillance to reduce their morbidities.
Extended Data
Table E1.
Demographics of mothers during pregnancy and symptoms in the children in the first year of life among those included in the analyses (n=332) and those recruited who were excluded because of missing data (n=395).
| Included in the analyses (n=332) |
Not included in the analyses (n=395) |
Difference P value |
|
|---|---|---|---|
| Male sex, n (%) | 155/332 (46.7) | 196/395 (49.6) | 0.43 |
| Mother’s age, mean (SD) | 25.2 | 24.9 | 0.38 |
| Mother’s race/ethnicity | |||
| African-American, n (%)A | 134/332 (40.4) | 120/395 (30.4) | 0.005 |
| Dominican, n (%)A | 198/332 (59.6) | 275/395 (69.6) | 0.005 |
| Mother’s self-reported health | |||
| Asthma, n (%)B | 85/332 (25.3) | 78/395 (19.7) | 0.059 |
| Allergy, n (%)C | 138/332 (41.6) | 149/354 (42.1) | 0.89 |
| Socio-demographics | |||
| No high school degree, n (%)D | 118/332 (36.5) | 139/390 (35.6) | 0.81 |
| Receiving Medicaid, n (%) | 304/332 (91.6) | 353/391 (90.3) | 0.55 |
| Material hardship last 6 months, n (%)E | 139/332 (41.9) | 182/384 (47.4) | 0.14 |
| Domestic environment | |||
| Smoker in home, n (%)F | 122/332 (36.7) | 124/385 (32.2) | 0.20 |
| Cockroach sightings in home, n (%)G | 240/330 (72.7) | 278/389 (71.5) | 0.71 |
| Rodent sightings in home, n (%)G | 175/330 (53.0) | 216/390 (55.4) | 0.53 |
| Childs symptoms in first year of life | |||
| Wheeze | 135/332 (40.7) | 137/352 (38.9) | 0.64 |
| Runny nose without colds | 126/332 (38.0) | 133/353 (37.7) | 0.94 |
| Watery eyes without colds | 109/332 (32.8) | 106/353 (30.0) | 0.43 |
| Runny nose or watery eyes without colds | 163/332 (49.1) | 173/353 (49.0) | 0.98 |
The mother self-identifying as being of either African-American race or Dominican Republic ethnicity was an inclusion criteria.
Mother reported either during pregnancy or on a questionnaire 3 months after the child was born that she had asthma.
Mother replied ‘yes’ to a question about having allergies asked during the first year after the child was born.
Nine of the mothers did not report whether they had completed high school.
Mother reported on the prenatal questionnaire that in the past 6 months she and her family could not afford needed food, rent, clothing or medical care or that gas/electricity was suspended because of bill non-payment.
Non-smoking during pregnancy was an inclusion criteria.
There were two subjects that did not answer questions about cockroaches or rodents. The percentages are of the n=330 women that answered these questions.
Table E2.
Demographics and respiratory outcomes among the children and observations included in the repeated measures analyses testing associations between EIW at age 5–7 and ED visits and hospitalizations at age 5–9 (reported in Table 3).
| Cross-sectional analyses (n=175 children, n=339 observations) |
Prospective analyses (n=151 children, n=221 observations) |
|
|---|---|---|
| Male sex, n children (%) | 94/175 (57.1) | 82/151 (54.3) |
| Observation per child | ||
| One observation only, n children | 66 | 81 |
| Two observations, n children | 54 | 70 |
| Three observations, n children | 55 | n/a |
| Ages of observationsA | ||
| 5 years, n children | 120 | 117 |
| 7 years, n children | 116 | 104 |
| 9 years, n children | 103 | n/aA |
| Mother’s race/ethnicity | ||
| African-American, n children (%) | 62/175 (35.4) | 56/151 (37.1) |
| Dominican, n children (%) | 113/175 (64.6) | 95/151 (62.9) |
| Childs symptoms in first year of life | ||
| Wheeze, n children (%) | 90/175 (51.4) | 78/151 (51.7) |
| Runny nose or watery eyes without colds, n children (%) | 105/175 (60.0) | 94/151 (62.3) |
| Seroatopy age 7 | ||
| SeroatopyB, n children (%) | 98/175 (56.0) | 86/151 (57.0) |
| Symptoms and asthma outcomes prior year (Time 1) | ||
| EIW, n observations(%) | 126/339 (37.2) | 78/221 (35.3) |
| Night cough without cold, n observations (%) | 189/339 (55.8) | 123/221 (55.7) |
| Frequent wheeze, n observations (%) | 61/339 (18.0) | 42/221 (19.0) |
| ED visits, n observations (%) | 152/339 (44.8) | 108/221 (48.9) |
| Hospitalizations, n observations (%) | 19/339 (5.6) | 16/221 (7.2) |
| ED and hospitalizations 2 years later (Time 2) | ||
| ED visits, n observations (%) | n/a | 77/221 (34.8) |
| Hospitalizations, n observations (%) | n/a | 7/221 (3.2) |
For the prospective analyses, ages are time 1 ages. Time 1 EIW was evaluated as a predictor of time 2 ED visits and hospitalizations (2 years later).
Seroatopy was defined as measurable IgE (≥0.35 IU/ml) to at least one of the allergens (dust mite, cockroach, mouse, cat, dog, grass pollen, tree pollen, ragweed pollen and mixed mold) tested at age 7 years.
Figure E1.

Prevalence and overlap of wheeze, runny nose without cold, and water eyes without cold in 1st year of life (n=332).
Acknowledgment
Funding Sources: Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P50ES09600/R82702701, NIEHS/EPA P01ES09600/RD83214101, and NIEHS R01ES08977, R01ES013163, P50ES015905 and P30 ES009089. This publication was also made possible in part by the John and Wendy Neu Family Foundation, the New York Community Trust, the Trustees of the Blanchette Hooker Rockefeller Fund. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Abbreviations/Acronyms:
- CCCEH
Columbia Center for Children’s Environmental Health
- ED
Emergency department
- EIB
Exercise induced bronchoconstriction
- EIW
Exercise induced wheeze
- IgE
Immunoglobulin E
- ISAAC
International Study of Asthma and Allergies in Childhood
- RWWC
Runny nose or watery eyes without a cold
Footnotes
Trial registration: Not applicable
Conflicts of interest: None
References
- 1.Rzehak P, Schoefer Y, Wichmann HE, Heinrich J. A prospective study on the association between hay fever among children and incidence of asthma in East Germany. Eur J Epidemiol. 2008;23(1):17–22. [DOI] [PubMed] [Google Scholar]
- 2.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. September 20 2008;372(9643):1049–1057. [DOI] [PubMed] [Google Scholar]
- 3.Arshad SH, Kurukulaaratchy RJ, Fenn M, Waterhouse L, Matthews S. Rhinitis in 10-year-old children and early life risk factors for its development. Acta Paediatr. 2002;91(12):1334–1338. [DOI] [PubMed] [Google Scholar]
- 4.Gotshall RW. Exercise-induced bronchoconstriction. Drugs. 2002;62(12):1725–1739. [DOI] [PubMed] [Google Scholar]
- 5.Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. The Journal of pediatrics. September 2002;141(3):343–348. [DOI] [PubMed] [Google Scholar]
- 6.McFadden ER Jr., Gilbert IA. Exercise-induced asthma. N Engl J Med. May 12 1994;330(19):1362–1367. [DOI] [PubMed] [Google Scholar]
- 7.Park C, Stafford C, Lockette W. Exercise-induced asthma may be associated with diminished sweat secretion rates in humans. Chest. September 2008;134(3):552–558. [DOI] [PubMed] [Google Scholar]
- 8.Hallstrand TS, Henderson WR Jr. Role of leukotrienes in exercise-induced bronchoconstriction. Curr Allergy Asthma Rep. January 2009;9(1):18–25. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. The Journal of allergy and clinical immunology. August 2008;122(2):225–235; quiz 236–227. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. September 2000;106(3):453–459. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos NG, Arakawa H, Carlsen KH, et al. International Consensus On (ICON) Pediatric Asthma. Allergy. August 2012;67(8):976–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caillaud D, Horo K, Baiz N, et al. Exercise-induced bronchospasm related to different phenotypes of rhinitis without asthma in primary schoolchildren: the French Six Cities Study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. June 2014;44(6):858–866. [DOI] [PubMed] [Google Scholar]
- 13.Couto M, Silva D, Santos P, Queiros S, Delgado L, Moreira A. Exploratory study comparing dysautonomia between asthmatic and non-asthmatic elite swimmers. Revista portuguesa de pneumologia. Jan-Feb 2015;21(1):22–29. [DOI] [PubMed] [Google Scholar]
- 14.Carlsen KH, Anderson SD, Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. April 2008;63(4):387–403. [DOI] [PubMed] [Google Scholar]
- 15.Mainardi TR, Mellins RB, Miller RL, et al. Exercise-induced wheeze, urgent medical visits, and neighborhood asthma prevalence. Pediatrics. January 2013;131(1):e127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stingone JA, Claudio L. Disparities in the use of urgent health care services among asthmatic children. Ann Allergy Asthma Immunol. August 2006;97(2):244–250. [DOI] [PubMed] [Google Scholar]
- 17.Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. September 15 2001;164(6):995–1001. [DOI] [PubMed] [Google Scholar]
- 18.Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect. May 2004;112(6):754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. August 2003;111(10):1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. April 25 1998;351(9111):1225–1232. [PubMed] [Google Scholar]
- 21.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. March 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 22.Bonner S, Matte T, Rubin M, et al. Validating an asthma case detection instrument in a Head Start sample. J Sch Health. November 2006;76(9):471–478. [DOI] [PubMed] [Google Scholar]
- 23.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. September 9 2004;351(11):1068–1080. [DOI] [PubMed] [Google Scholar]
- 24.Habre R, Moshier E, Castro W, et al. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. Journal of exposure science & environmental epidemiology. July 2014;24(4):380–387. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. January 19 1995;332(3):133–138. [DOI] [PubMed] [Google Scholar]
- 26.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. November 15 2005;172(10):1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. November 2008;63(11):974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. September 2013;68(9):1102–1116. [DOI] [PubMed] [Google Scholar]
- 30.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. May-Jun 2004;26(3):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]


