Abstract
Patient: Female, 40
Final Diagnosis: Sterile BPF • Primary LL & secondary HOA
Symptoms: Chronic cough • dyspnea and back pain
Medication: —
Clinical Procedure: Thoracotomy
Specialty: Surgery
Objective:
Rare disease
Background:
Leiomyomas are benign neoplasms of the smooth muscle. When found in the pulmonary system, a rare occurrence, leiomyomas can result in hypertrophic osteoarthropathy, or significant clubbing, associated with proliferation of long bone periosteum. Bronchopulmonary fistulas, or communications between the bronchial tree and pleural space, are an uncommon postoperative complication of pneumonectomies. Even more infrequent is the presence of a bronchopulmonary fistula that is determined to be sterile.
Case Report:
The patient presented in the current case report is a 40-year-old previously healthy woman who presented with a 5-year history of chronic cough, right-sided chest discomfort, and dyspnea associated with back pain, and lower leg pain. The CT scan performed on the patient revealed a mass originating from the right lower lobe. Activity at the site of the lesion, in the long bones of the upper and lower limbs, rib cage, and vertebral bones was demonstrated by a bone scan. A CT-guided biopsy was performed, and the pathology report confirmed the presence of a leiomyoma. Following a right-sided lobectomy, the resected tumor was sent for histopathology, with the results confirming the biopsy. The patient subsequently presented with a history of persistent cough associated with increased watery secretions. The CT scan revealed the presence of a bronchopleural fistula, after which the patient underwent surgical correction. All symptoms resolved, and the patient was discharged in stable condition.
Conclusions:
Here, we report on a patient who presented with 3 rare clinical findings: pulmonary leiomyoma, hypertrophic osteoarthropathy, and sterile bronchopulmonary fistula.
MeSH Keywords: Leiomyoma; Medical Oncology; Osteoarthropathy, Secondary Hypertrophic
Background
Leiomyomas are benign neoplasms of the smooth muscle, commonly present in the uterus [1]. Leiomyomas can also be found in the gastrointestinal tract, but it is exceptionally rare for the tumor to develop in the pulmonary system [2]. Leiomyomas account for 2% of all benign tumors in the pulmonary system. In the pulmonary system, leiomyomas can be localized anywhere in the trachea (16%), bronchioles (33%), and lung parenchyma (51%). The clinical presentation of the tumors varies according to their location [3]. In addition, intrathoracic neoplasms such as leiomyomas can be associated with extrapulmonary manifestations. Hypertrophic osteoarthropathy (HOA), or significant clubbing associated with proliferation of periosteum in long bones, has a wide range of prevalence and can develop in 20% of patients suffering from primary lung neoplasms [4].
Surgical resection remains the mainstay modality of treatment for leiomyoma [5]. Patients who undergo lung surgeries such as lobectomies are at risk of developing bronchopleural fistula (BPF). BPF arises when a communication is formed between the bronchial tree and pleural space. BPFs are rare, ranging from 1.5% to 28% in patients undergoing lung surgery [6]. While lung surgery remains the most common risk factor for BPFs, other risk factors include necrosis, treatment-related factors (e.g., chemotherapy and radiotherapy), tuberculosis, postoperative ventilation, and spontaneous pneumothorax [7]. BPFs can present with variable signs and symptoms such as productive cough, shortness of breath, weight loss, fever, and lethargy [7]. However, early recognition and management play a significant role in reducing the detrimental effects associated with BPF.
In this report, we present the case of a 40-year-old female patient who was found to have 3 rare entities: 1) primary lung leiomyoma, 2) secondary hypertrophic osteoarthropathy, and 3) sterile bronchopleural fistula.
Case Report
A 40-year-old previously healthy woman was referred to our care within the Department of Surgery at King Faisal Specialist Hospital and Research Center during the year 2014. She presented with a 5-year history of chronic cough, right-sided chest discomfort, and dyspnea associated with back pain, as well as lower leg pain. She reported no weight loss, no history of hemoptysis, and no tobacco smoking.
On physical examination, she was in good health, with no lymphadenopathy. However, finger clubbing and unilateral decreased air movement in the lungs were noted. Upon chest examination, air entry on the right lung field was decreased, while that on the left lung field was normal.
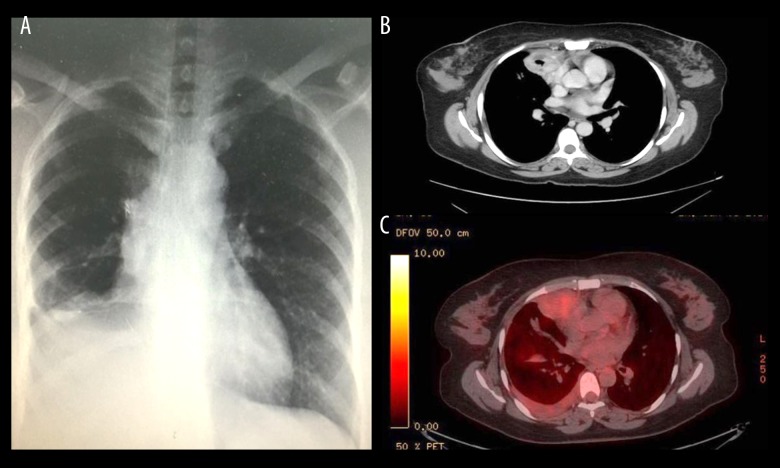
With regard to investigations, chest X-rays demonstrated a right lung mass (Figure 1A). A subsequent CT scan revealed a mass originating from the right lower lobe and extending to the mediastinum, without appreciable lymphadenopathy (Figure 1B). In addition, a PET scan was performed and showed mild activity in the right lung (Figure 1C). A bone scan showed activity at the lesion site and in the long bones of the upper and lower limbs, rib cage, and vertebral bones (Figure 2B).
Figure 1.
(A) Chest X-ray of lung mass showing leiomyoma in right lower lung field. (B) CT scan shows a mass originating from the right lower lobe extending to the mediastinum, without lymphadenopathy. (C) PET scan showed mild activity in the lung.
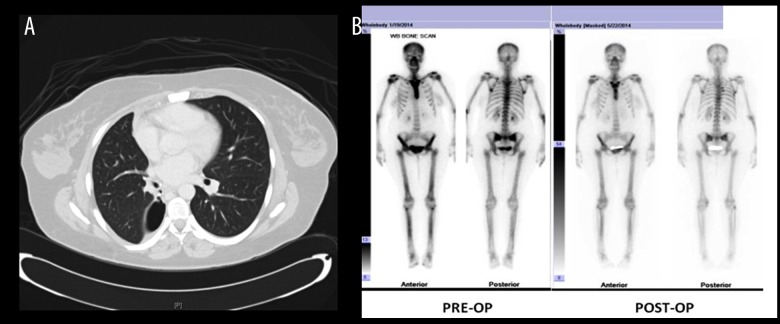
Figure 2.
(A) CT chest showing the site of the bronchopleural fistula. (B) Pre-operative bone scan showing diffuse radiotracer uptake involving the sternum, upper and mid thoracic spine, as well as both femurs and tibiae, corresponding to sclerotic changes seen on CT images. A postoperative bone scan showed interval improvement of previously noted diffuse uptake in both femora, tibia, and sternum.
A CT-guided biopsy was performed on the lung mass, and the pathology report confirmed the presence of a leiomyoma, which is a low-grade smooth muscle neoplasm. Neoadjuvant chemoradiation therapy was discussed, but due to the low-grade classification of the malignancy, the patient was advised for surgery without any neoadjuvant chemotherapy or radiotherapy.
The patient underwent right-sided lobectomy, and the resected tumor was sent for histopathology. The results of the biopsy were reported as follows: “Smooth muscle neoplasm, low grade (leiomyoma) is 13 cm in maximum dimension. No lymphovascular invasion seen. Bronchial and vascular margins are free. Three hilar lymph nodes are negative for malignancy. Adjacent lung parenchyma is unremarkable”.
The patient proceeded with an uneventful postoperative course and was followed up in the clinic. At her last clinic visit, she presented with a history of persistent cough, which was exacerbated when she was in the prone position with her head down. The cough was associated with increased watery secretions. She reported no history of fever, weight loss, or chest infection.
The patient was admitted to the hospital, and routine lab investigations were ordered. The CT scan showed the presence of a bronchopleural fistula and the patient was approved for surgery (Figure 2A). A right-sided thoracotomy was performed and the presence of a pericardial fistula, which created a communication between the pleura and the bronchial tree, was detected intraoperatively. The patient tolerated the procedure well and the chest tube was removed on postoperative day 5. Symptoms, such as persistent cough and watery secretions, resolved and the patient was discharged in stable condition. The patient was followed up with no remarkable events.
Discussion
The current case report presents 3 rare clinical entities: primary lung leiomyoma (PLL), hypertrophic osteoarthropathy (HOA), and bronchopleural fistula (BPF). Leiomyoma is a benign tumor derived from uncontrolled smooth muscle proliferation [8]. Leiomyomas are most commonly present in the uterus; however, they can develop in extrauterine sites like the esophagus and stomach [8]. Leiomyomas of the lung are exceedingly rare. Of all the benign tumors of the lung (which constitute a mere 1–5% of total lung tumors), leiomyomas account for only 2% [2]. The locations of primary lung leiomyomas include the lung parenchyma (51%), bronchus (33%), and trachea (16%). Our patient, a 40-year-old woman, presented with a primary lung leiomyoma located in the right lower lobe and extending to the mediastinum, without appreciable lymphadenopathy. The benign tumor was also found to originate from the pleura, which is uncommon for leiomyomas. The mean age of adults affected with parenchymal and bronchial leiomyomas is 35 years, which is younger than our patient. Consistent with our case is the fact that females are twice as likely to develop parenchymal leiomyomas compared to males [9].
The presentation of lung leiomyomas depends on their location, size, and degree of obstruction distal to the tumor [1,8,10,11]. Despite the considerable variability in the presentation of lung leiomyomas, not all of them are necessarily symptomatic. In fact, up to 90% of parenchymal leiomyomas show no signs or symptoms, and are detected incidentally during imaging [4,8]. In the reported case, our patient defied the odds and presented with a 5-year history of chronic cough, right-sided chest discomfort, and dyspnea, associated with back pain and lower leg pain.
The clinical features of lung leiomyoma are not restricted to pulmonary manifestations and include extrapulmonary signs and symptoms, as in our patient. In the reported case, hypertrophic osteoarthropathy (HOA), or significant clubbing, was discovered on physical examination and was supported by a bone scan. HOA arises from proliferation of the periosteum of long bones and develops in 20% of patients with primary lung neoplasms [4]. The exact pathogenesis of HOA secondary to lung disease is unknown, but it is postulated that HOA is caused by failure of the lungs to inactivate a growth factor that promotes clubbing [12]. Another possible mechanism is the failure of platelet production [13]. Since pulmonary circulation is the site of megakaryocyte fragmentation into functional platelets, endothelial interaction with these fragments could initiate local release of pro-inflammatory molecules, including vascular endothelial growth factor (VEGF). The hypothesis that VEGF plays a central role in the pathogenesis of HOA is supported by 3 main findings: 1) elevated blood levels of VEGF in patients with HOA [14–16]; 2) VEGF has a wide spectrum of actions on its target tissue, including inducing periosteal proliferation, vascular hyperplasia, endothelial activation, and edema [17,18], all of which are histopathological characteristics found in HOA [19]; and 3) surgical removal of lung tumors decreases blood levels of VEGF and tremendously reduces the signs and symptoms of HOA [20]. Additionally, HOA is not therapeutically targeted because it spontaneously resolves following treatment of the primary cause [20]. This is demonstrated by the bone scan performed on our patient post-operatively, which revealed interval improvement of previously noted abnormal diffuse radiotracer uptake in the femora, tibia, and sternum (Figure 2B).
For the treatment of primary lung leiomyomas, surgical resection of the tumor is the mainstay modality. However, given the benign nature of the tumor, more conservative choices of treatment should be considered before surgical intervention is decided upon [5]. In our case, the patient was advised to undergo surgery without neoadjuvant radiotherapy or chemotherapy. Following lobectomy of the affected right lower lobe, the postoperative phase was unremarkable except for a persistent cough that was associated with a watery discharge. Subsequent to hospital admission, a routine CT scan revealed a bronchopleural fistula (BPF).
BPFs are canalous communications between the bronchial tree and pleural space. They are relatively rare, but are associated with high morbidity and mortality [6]. BPFs most commonly arise as a postoperative complication of pneumonectomy (1.5–28% of pulmonary resections), but they can also be caused by radiotherapy and chemotherapy, a necrotic lung infection, persistent pneumothorax, and tuberculosis [21–24].
If acute, BPFs can present with purulent cough, dyspnea, hypotension, tracheal and mediastinal shifting, and pleural air leaks. Chronic BPFs may present with fibrosis of the pleura and mediastinum [6]. BPFs can be diagnostically approached through bronchoscopy, bronchography, or computed tomography (CT). CT scans have been found to be able to identify BPFs and their causes in 91% of patients [25]. Although BPF is a relatively rare complication in patients who are undergoing lobectomies (0.5%) [18], the development of a BPF that is not associated with empyema is a much rarer incident. The patient in the present report was found to have a sterile BPF, consistent with the symptomatology that included a cough with watery secretions, and no history of fever, chest infection, or weight loss. The mechanism behind the sterility of the BPF has not yet been elucidated. However, a potential cause may be the inherent antimicrobial activity of the pleural membrane. This antimicrobial activity is attributed to the presence of antibodies, complement factors, lysozymes, and alpha-defensins [26]. Another potential explanation is that the patient had a connection between 3 spaces (bronchus, pleura, and pericardium) allowing for dynamic fluid movement that may have served as a washing mechanism to prevent the growth of microbes.
Another exceedingly uncommon finding in this case was the presence of a fistulous pericardial communication with the BPF. Pericardial fistulae usually occur between the pericardium and a single other organ, such as the small intestine [27], esophagus [28], or bronchus [29]. However, in this case, a rare fistulous communication between 3 entities (the pericardial space, the pleural space, and a bronchus) led to the formation of a broncho-pleuropericardial fistula. The pericardial fistula was found during corrective surgery of the BPF.
Conclusions
Primary lung leiomyomas are exceedingly rare neoplasms of the respiratory system. They may present with hypertrophic osteoarthropathy, or significant clubbing, which is associated with lung disease. Surgical resection of leiomyomas may result in the formation of a bronchopleural fistula that can be sterile or purulent.
Footnotes
Conflict of interest
None.
References:
- 1.Awasthi A, Dubey S, Sabhikhi AK, Bal S. Primary endobronchial myxoid leiomyoma in a child: An unusual case report and review of literature. Indian J Pathol Microbiol. 2016;59(1):87–89. doi: 10.4103/0377-4929.174830. [DOI] [PubMed] [Google Scholar]
- 2.Yoon YC, Lee KS, Kim TS, et al. Benign bronchopulmonary tumors: Radiologic and pathologic findings. J Comput Assist Tomogr. 2002;26(5):784–96. doi: 10.1097/00004728-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Luthi F, Groebli Y, Newton A, Kaeser P. Cardiac and pericardial fistulae associated with esophageal or gastric neoplasms: A literature review. Int Surg. 2003;88:188–93. [PubMed] [Google Scholar]
- 4.Miller DR. Benign tumors of lung and tracheobronchial tree. Ann Thorac Surg. 1969;8(6):542–60. doi: 10.1016/s0003-4975(10)66093-6. [DOI] [PubMed] [Google Scholar]
- 5.Tamura M, Murata T, Kurumaya H, Ohta Y. Leiomyoma of an accessory tracheal bronchus. Ann Thorac Surg. 2004;78(6):2163–65. doi: 10.1016/S0003-4975(03)01500-5. [DOI] [PubMed] [Google Scholar]
- 6.Lois M, Noppen M. Bronchopleural fistulas: An overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–65. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar P, Chandak T, Shah R, Talwar A. Diagnosis and management bronchopleural fistula. Indian J Chest Dis Allied Sci. 2010;52:97–104. [PubMed] [Google Scholar]
- 8.Swarnakar R, Sinha S. Endobronchial leiomyoma: A rare and innocent tumour of the bronchial tree. Lung India. 2013;30(1):57–60. doi: 10.4103/0970-2113.106175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White SH, Ibrahim NB, Forrester-Wood CP, Jeyasingham K. Leiomyomas of the lower respiratory tract. Thorax. 1985;40(4):306–11. doi: 10.1136/thx.40.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JS, Lee M, Kim HK, et al. Primary leiomyoma of the trachea, bronchus, and pulmonary parenchyma – a single-institutional experience. Eur J Cardiothorac Surg. 2012;41(1):41–45. doi: 10.1016/j.ejcts.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zidane A, Elktaibi A, Benjelloun A, et al. Primary leiomyoma of the lung: An exceptional localization. Asian Cardiovasc Thorac Ann. 2016;24(4):393–96. doi: 10.1177/0218492316638608. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Lavin M. Digital clubbing and hypertrophic osteoarthropathy: A unifying hypothesis. J Rheumatol. 1987;14(1):6–8. [PubMed] [Google Scholar]
- 13.Dickinson CJ, Martin JF. Megakaryocytes and platelet clumps as the cause of finger clubbing. Lancet. 1987;330(8573):1434–35. doi: 10.1016/s0140-6736(87)91132-9. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe O, Arimura K, Kitajima I, et al. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/s0140-6736(96)91261-1. [DOI] [PubMed] [Google Scholar]
- 15.Silveira LH, Martinez-Lavin M, Pineda C, et al. Vascular endothelial growth factor and hypertrophic osteoarthropathy. Clin Exp Rheumatol. 2000;18(1):57–62. [PubMed] [Google Scholar]
- 16.Soubrier M, Dubost JJ, Serre AF, et al. Growth factors in POEMS syndrome: Evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum. 1997;40(4):786–87. doi: 10.1002/art.1780400430. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 18.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 19.Narayanan S, Gani VM, Sundararaju V. Primary hypertrophic osteoarthropathy with hypertrophic gastropathy. J Clin Rheumatol. 2010;16(4):190–92. doi: 10.1097/RHU.0b013e3181e04d80. [DOI] [PubMed] [Google Scholar]
- 20.Olán F, Portela M, Navarro C, et al. Circulating vascular endothelial growth factor concentrations in a case of pulmonary hypertrophic osteoarthropathy. Correlation with disease activity. J Rheumatol. 2004;31(3):614–16. [PubMed] [Google Scholar]
- 21.McManigle JE, Fletcher GL, Tenholder MF. Bronchoscopy in the management of bronchopleural fistula. Chest. 1990;97(5):1235–38. doi: 10.1378/chest.97.5.1235. [DOI] [PubMed] [Google Scholar]
- 22.Malave G, Foster ED, Wilson JA, Munro DD. Bronchopleural fistula – present-day study of an old problem: A review of 52 cases. Ann Thorac Surg. 1971;11(1):1–10. doi: 10.1016/s0003-4975(10)65404-5. [DOI] [PubMed] [Google Scholar]
- 23.Pierson DJ, Hordon CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation: A review of 39 cases. Chest. 1986;90(3):321–23. doi: 10.1378/chest.90.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Hankins JR, Miller JE, Attar S, et al. Bronchopleural fistula. Thirteen-year experience with 77 cases. J Thorac Cardiovasc Surg. 1978;76(6):755–62. [PubMed] [Google Scholar]
- 25.Ricci ZJ, Haramati LB, Rosenbaum AT, Liebling MS. Role of computed tomography in guiding the management of peripheral bronchopleural fistula. J Thorac Imaging. 2002;17(3):214–18. doi: 10.1097/00005382-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Miglioli PA, Allerberger F, Walderberg I, Hasse J. Antibacterial activity of human pleural fluid: Aone and in combination with antibiotics. Int J Antimicrob Agents. 1998;10(4):317–19. doi: 10.1016/s0924-8579(98)00053-3. [DOI] [PubMed] [Google Scholar]
- 27.Cousins C, Manhire AR. Case report: Duodeno-pericardial fistula. Clin Radiol. 1991;43(6):412–13. doi: 10.1016/s0009-9260(05)80572-0. [DOI] [PubMed] [Google Scholar]
- 28.Dennert B, Ramirez FC, Sanowski RA. Pericardioesophageal fistula associated with metallic stent placement. Gastrointest Endosc. 1997;45(1):82–84. doi: 10.1016/s0016-5107(97)70308-0. [DOI] [PubMed] [Google Scholar]
- 29.Bennett JA, Haramati LB. CT of bronchopericardial fistula: An unusual complication of multidrug-resistant tuberculosis in HIV infection. Am J Roentgenol. 2000;175(3):819–20. doi: 10.2214/ajr.175.3.1750819. [DOI] [PubMed] [Google Scholar]