Abstract
The Dictyostelium genome encodes only two MAPKs, Erk1 and Erk2, and both are expressed during growth and development. Reduced levels of Erk2 expression have been shown previously to restrict cAMP production during development but still allow for chemotactic movement. In this study the erk2 gene was disrupted to eliminate Erk2 function. The absence of Erk2 resulted in a complete loss of folate and cAMP chemotaxis suggesting that this MAPK plays an integral role in the signaling mechanisms involved with this cellular response. However, folate stimulation of early chemotactic responses, such as Ras and PI3K activation and rapid actin filament formation, were not affected by the loss of Erk2 function. The erk2− cells had a severe defect in growth on bacterial lawns but assays of bacterial cell engulfment displayed only subtle changes in the rate of bacterial engulfment. Only cells with no MAPK function, erk1−erk2− double mutants, displayed a severe proliferation defect in axenic medium. Loss of Erk2 impaired the phosphorylation of Erk1 in secondary responses to folate stimulation indicating that Erk2 has a role in the regulation of Erk1 activation during chemotaxis. Loss of the only known Dictyostelium MAPK kinase, MekA, prevented the phosphorylation of Erk1 but not Erk2 in response to folate and cAMP confirming that Erk2 is not regulated by a conventional MAP2K. This lack of MAP2K phosphorylation of Erk2 and the sequence similarity of Erk2 to mammalian MAPK15 (Erk8) suggest that the Dictyostelium Erk2 belongs to a group of atypical MAPKs. MAPK activation has been observed in chemotactic responses in a wide range of organisms but this study demonstrates an essential role for MAPK function in chemotactic movement. This study also confirms that MAPKs provide critical contributions to cell proliferation.
Keywords: MAPK, Erk2, Erk1, Dictyostelium, Chemotaxis
Graphical abstract

1. Introduction
Mitogen activated protein kinases (MAPKs) are components of many eukaryotic signal transduction pathways [1–4]. These proteins generally function downstream of protein kinase cascades that include MAPK kinases (MAP2Ks) and MAPK kinase kinases (MAP3Ks). Once activated, MAPKs phosphorylate and regulate a wide variety of proteins throughout the cell. Mitogens, chemoattractants, and other extracellular signals can stimulate MAPK pathways and lead to changes in cell growth, movement, gene expression, and differentiation [3, 5]. While the interactions and functions of some MAPKs have been well documented, many members of this regulatory protein family remain uncharacterized [6]. Sequence similarities and functional roles have provided the basis to organize the family of mammalian MAPKs into subfamilies such as the ERKs (extracellular signal regulated kinases), p38 MAPKs, pJNKs (c-Jun N-terminal kinases), and other smaller groups but not all of these groups are present in other eukaryotes [5]. Some MAPKs are known to have redundant functions (e.g., mammalian Erk1/Erk2) and others can have common activators and substrates but promote different cellular responses (e.g., yeast Fus3 and Kss1 in regulating mating responses and filamentous growth, respectively) [4, 7, 8]. The signaling pathways that use MAPKs can be quite varied but the activation mechanism of most characterized MAPKs includes a dual phosphorylation of residues in a highly conserved motif (TXY) within a catalytic domain [5]. This activation is typically mediated by MAPK kinases (MAP2Ks; also known as Meks) that are capable of phosphorylating both serine/threonine and tyrosine residues [9]. However, a group of atypical MAPKs does not appear to be phosphorylated by conventional MAP2Ks [5, 10, 11]. MAPKs have been found in all eukaryotic kingdoms and appear to be present in all free-living eukaryotes suggesting these proteins regulate basic cellular processes in eukaryotes [12, 13].
Simple eukaryotic organisms have been particularly useful for the characterization of MAPK function and specificity. These eukaryotes are typically amenable to both genetic and biochemical analysis and they tend to have relatively few MAPKs. The yeast Saccharomyces cerevisiae has 5 MAPKs and the soil amoeba Dictyostelium discoideum has only 2 MAPKs compared to the 13 MAPKs found in mammals [3, 4]. MAPKs in yeast have been associated with responses to mating pheromones, starvation, osmotic stress, and cell wall stress [4]. The two MAPKs in Dictyostelium, designated as Erk1 and Erk2 (also referred to as ErkA and ErkB, respectively), play important roles in the developmental life cycle that is initiated by the loss of nutrients [3]. Starved Dictyostelium aggregate using a relayed intercellular cAMP signal and the multicellular mounds undergo morphogenesis to form a slug and then a fruiting body consisting of a spore mass on top of a stalk [14, 15 ]. Cells lacking Erk1 aggregate into small mounds that have accelerated morphogenesis and the overexpression of Erk1 results in large aggregates that have delayed morphogenesis indicating that Erk1 function can inhibit developmental progression [16–18]. Genetic analysis of Erk2 function has been extensive but limited to the characterization of a leaky erk2 allele in which Erk2 expression is reduced but not eliminated [17, 19–23]. A reduction of Erk2 expression results in cells with insufficient external cAMP signaling to allow cell aggregation in clonal populations but cells retain the ability to chemotax to cAMP [19, 23, 24]. In the presence of wild-type cells, the reduced Erk2 expression mutant can co-aggregate because of the cAMP provided by wild-type cells [19, 22, 23]. The deficiency in cAMP signaling of this mutant can also be suppressed by the loss of the cAMP-specific phosphodiesterase, RegA, allowing the double mutant to undergo and complete multicellular development [20].
Stimulation of Dictyostelium with the chemoattractants folate or cAMP results in a rapid phosphorylation of Erk2 that is followed by the phosphorylation of Erk1 as the level of phosphorylated Erk2 decreases [17, 25]. Folate stimulation of Erk2 phosphorylation requires the folate receptor, Far1, and its coupled G protein, Gα4 [21, 26]. However, cAMP stimulation of Erk2 phosphorylation only requires a cAMP receptor, cAR1 or cAR3, and appears to be independent of G protein function, at least the function of Gα2 and Gβ subunits [27, 28]. The basis for this distinction remains to be determined and the proteins that transduce the signals from the receptor to the MAPK are not well characterized [29]. The Dictyostelium genome encodes only a single MAP2K, MekA (also known as Mek1), based on sequence similarity to characterized orthologs [16]. Cells lacking MekA form small aggregates with accelerated development, similar to phenotype observed for erk1− cells. Previous studies have also suggested that Erk1 but not Erk2 kinase activity is dependent on MekA function [16, 27, 30].
In this study the function of Dictyostelium Erk2 was investigated through the creation of an erk2− gene disruption mutant, resulting in a complete loss of Erk2 function. This erk2− mutant was analyzed for growth and developmental phenotypes including chemotaxis and phagocytosis. A double MAPK mutant, erk1−erk2−, was also created and analyzed. In addition, the dependence of Erk1 phosphorylation on Erk2 function was examined in response to chemotactic stimulation. The results of these analyses suggest that Erk2 is essential for chemotactic movement and stimulation of Erk1 phosphorylation. Erk2 function was also found to be important for early phagocytic responses and, together with Erk1, Erk2 contributes to axenic growth. Similarities of the Dictyostelium Erk2 with the mammalian MAPK15 (also known as Erk8) were investigated to assess the possible role of Erk2 as an atypical MAPK.
2. Material and Methods
2.1. Strains and development
All of the Dictyostelium strains used in this study were derived from the parent axenic strain KAx-3 except for the noted loci. Axenic strains have been derived from wild-type strains through mutations, including those at the NF-1 locus [31, 32]. The JH10 thymidine auxotrophic strain, disrupted at the thyA locus (also designated thy1), has been previously described [33]. The erk1−, erk1−thyA−, and mekA− strains have been previously described [17, 18]. Phenotypic comparisons of the MAPK mutants were done with KAx-3 cells due to the auxotrophic requirements of the JH10 strain. Dictyostelium were grown in HL-5 axenic medium (with or without thymidine supplement) or on lawns of Klebsiella aerogenes on SM+/3 agar plates [34]. For the analysis of plaque growth rate cells were mixed with bacteria and plated at a low density on SM+/3 plates to allow the formation of plaques from single cells. DNA constructs and vectors were inserted into Dictyostelium using electroporation as previously described [35]. For developmental analysis cells were harvested from axenic medium by centrifugation and washed in phosphate buffer (12mM NaH2PO4 adjusted to pH 6.1 with KOH). Cells were plated on nonnutrient plates (1.5% agar in phosphate buffer) from suspensions of 5 × 107 cells/ml or less. For chimeric development, clonal populations were mixed at indicated ratios prior to plating cells on nonnutrient plates. Fluorescent images were detected and recorded using fluorescence microscopy.
2.2. Recombinant DNA constructs and amplifications
A genomic fragment containing the thyA gene excised with BamHI was inserted into the unique BglII sites of an erk2 cDNA to disrupt the erk2 open reading frame. The erk2∷thyA construct was excised at flanking sites with XhoI and XbaI and electroporated into thyA− or erk1−thyA− cells to disrupt the erk2 locus. Erk1 and Erk2 expression vectors utilizing the act15 promoter were created using the pTX-GFP2 vector (replacing the GFP with MAPK sequence) as previously described [22]. The pTX-GFP2 vector was also used to label strains with GFP as previously described [36]. An Erk2 expression vector conferring blasticidin resistance was created by transferring a pact15:GFP2:erk2 SpeI fragment into a pBluescript SK- vector containing a blasticidin resistance gene at the PstI site [37]. This vector was linearized at a unique SphI site near the amino-terminal coding region of erk2 and integrated into the erk2∷thyA locus of erk2− cells through a single homologous recombination event. This knock-in construct regenerated a complete erk2 open reading frame downstream of the endogenous erk2 promoter. Verification of integration events into the erk2 locus was conducted using PCR amplification. PCR primer sequences and binding locations are described in supplementary figures (Fig. S1).
2.3. Chemotaxis Assays
Above agar chemotaxis assays were performed as previously described [22]. Cells were grown in fresh axenic medium 24 h prior to harvesting, washing and suspension in phosphate buffer at 2 × 107 cells/ml for Dictyostelium. Droplets (< 1 μl) of cell suspensions were spotted on nonnutrient agar plates and then 1 μl of chemoattractant was spotted approximately 2 mm from the cell droplet. Images of the cells were recorded immediately after the plating of the cells and chemoattractant and recorded again 2.5 – 3 h later. The agar surface near the cell droplet was scarred with a needle to allow the early and late images to be aligned so that the original cell droplet perimeter could be overlaid on the later image. Cell movement toward the chemoattractant source was determined by measuring the distance from the original cell droplet perimeter to the leading edge of migrating cells. Chemotaxis was analyzed using a dissecting microscope (Nikon SMZ2). Videos were created using time-lapse photography with 20s intervals between images for 33 min. ImageJ with MTrackJ plugin software was used to trace cell migration tracks and determine the migration distance for selected cells.
2.4. Analysis of bacterial cell engulfment
Dictyostelium engulfment of bacteria in suspension cultures was conducted as previously described [26]. Axenic Dictyostelium cells were washed and resuspended at 1×106 cells/ml in phosphate buffer. Live K. aerogenes bacteria were labeled with pHrodo Red (Thermo Scientific) and incubated with Dictyostelium in phosphate buffer at a 100:1 ratio at 22°C in shaking cultures (150 rpm). At indicated times, Dictyostelium cells were centrifuged and suspended in buffer containing 50 mM Tris pH 8.8 and 150 mM NaCl to quench the fluorescence of non-engulfed bacteria. Dictyostelium and bacteria were distinguished through forward and side scatter (FSC and SSC) and the engulfment of bacteria was indicated by the level of fluorescence detected using FACSort flow cytometer (BD Bioscience) with Cell Quest software (v. 3.3). Data analysis was conducted using FlowJo (v. 10.0.8; Tree Star). Quantification of engulfed bacteria number per Dictyostelium cell was analyzed using confocal microscopy. Dictyostelium were plated in four well chambers (Lab-Tek) and incubated with pHrodo labeled K. aerogenes in phosphate buffer. After 15 min, phosphate buffer was replaced with basic buffer to stop engulfment and quench extracellular bacteria fluorescence for imaging.
2.5. Reporter protein translocation
Reporter protein translocation was measured as previously described [26]. Cells expressing PHCRAC-GFP, RBD-GFP or LimEΔcoil-GFP were harvested, washed with phosphate buffer prior to plating in four well chambers (Lab-Tek). A Zeiss Laser Scanning Microscope 880 with a 60x, 1.3 NA Plan-Neofluar objective lens was used to acquire time-lapse images every 2 seconds. Cells were exposed to a final concentration of 100 μM folic acid to induce PHCRAC-GFP, RBD-GFP or LimEΔcoil-GFP translocation from cytosol to plasma membrane. Confocal images were used to determine the temporal-spatial intensity changes of PHCRAC-GFP or LimEΔcoil-GFP. Fluorescence intensity at the plasma membrane was measured over time and normalized to the first frame when folate was added. At least ten cells were quantified for each strain.
2.6. Immunoblot analysis of MAPKs
For analysis of MAPK abundance, cells were harvested from axenic medium, washed in phosphate buffer and lysed by mixing with SDS-PAGE loading buffer on ice. Immunoblot analysis of Erk2 protein was conducted using an affinity-purified Erk2 antiserum as the primary antibody. This antiserum was generated in rabbits using the ERK2 peptide ERKKQTNPTKPD (containing a cysteine residue at the amino terminus for attachment procedures) as an antigen and the peptide was also used for the affinity purification (Genscript). The analysis of MAPK phosphorylation was conducted as previously described [17]. Cells were grown in shaking cultures to mid log phase (~3 × 106 cells/ml) and then harvested by centrifugation. Cells were washed once in phosphate buffer and suspended at 1 × 108 cells/ml. Starved cells were shaken in a conical tube for 3–5 hours with pulses of 100nM cAMP every 15 min except for the 15 min prior to an assay. For analysis of cAMP stimulation, cells were stimulated with 100 nM cAMP and cell samples were collected and lysed at the indicated time by mixing with SDS-PAGE loading buffer on ice. Cell extracts were subjected to immunoblot analysis using a rabbit monoclonal antibody phospho-p42/44 MAPK (#4370, Cell Signaling Technology). For folate stimulation of MAPK phosphorylation, cell suspensions were shaken for 1 h in phosphate buffer prior to stimulation with 50 μM folate. A secondary anti-rabbit antibody conjugated to horseradish peroxidase (HRP) and bioluminescence reaction was used for detection of the primary antibodies. In some blots the biotinylated mitochondrial 3-methylcrotonyl-CoA carboxylase α (MCCC1) was used as a gel loading control and this protein was detected using HRP-Streptavidin as previously described [38].
2.7. MAPK Ortholog analysis
MAPK sequences were identified in sequence databases using UniProt and BLASTp searches using default parameters in the non-redundant protein sequences database (NCBI). Molecular phylogenetic analysis was conducted in MEGA7 using the Maximum Likelihood method based on the JTT matrix-based model [39, 40]. The percentage of replicate trees in which the associated proteins clustered together in the bootstrap test (1000 replicates) are shown next to the branches [41]. Only branches corresponding to partitions reproduced in more than 50% of the bootstrap replicates are labeled. Each tree is drawn to scale, with branched lengths measured in the substitutions per site.
3. Results
3.1. Disruption of the erk2 gene
Previous analyses of Dictyostelium Erk2 function have used erk2 mutants that contain an insertion mutation near the erk2 open reading frame [17, 19–23]. This allele was originally created through restriction enzyme-mediated insertion (REMI) mutagenesis and the allele has been recapitulated in axenic strains through homologous recombination. This allele has a significant reduction of erk2 expression that leads to inadequate cAMP production for the aggregation stage of development but only subtle changes in chemotactic responses to either cAMP or folate [24]. Although this allele results in developmental defects, activated Erk2 can still be detected [17]. For clarity this allele will now be referred to as erk2RE (reduced expression) to distinguish it from an erk2− allele described in this report that does not produce functional Erk2 protein. Given the apparent importance of Erk2 in Dictyostelium development we created and verified an erk2− strain that contains the erk2 open reading frame disrupted with the auxotrophic marker gene thyA (Fig. 1A and S1A). Dictyostelium discoideum has only two MAPKs, Erk1 and Erk2, and so we also created a strain with no MAPKs by disrupting the erk2 gene in a strain already containing a disrupted erk1 gene. Both erk2− and erk1−erk2− strains were transformed with Erk2 expression vectors for complementation and the erk1−erk2− strain was also transformed with an Erk1 expression vector. Levels of Erk2 protein in mutants and complemented strains were verified by immunoblot analysis using antiserum generated against an Erk2 peptide (Fig. 1C). Wild-type and complemented erk2− mutants displayed a band at approximately 42 kDa (predicted size of Erk2) and this band was absent in erk2− and erk1−erk2− strains.
Figure 1. Disruption and knock-in complementation of the erk2 locus.

A) Homologous recombination of the erk2∷thyA fragment (see Materials and Methods for construction) with the erk2 locus. The location of primer binding sites (arrows) used for PCR verification of recombination are shown. Open rectangles represent the erk2 open reading frame, the closed rectangle represents the open reading from of an adjacent gene and the thick black line represents the thyA genomic fragment. B) Knock-in of an Erk2 expression vector with blasticidin resistance into the disrupted erk2∷thyA locus at the SphI site. Hashed lines represent sequences not shown to reduce the size of image. Description of PCR products and primer sequences are described in Fig. S1. C) Immunoblot of Erk2 protein in wild-type (WT), erk2−, and erk1−erk2− strains and in mutant strains complemented with Erk2 expression vector (Erk2). Lysates of cells grown in axenic medium were analyzed for Erk2 protein by immunoblot analysis. Coomassie staining of the gel was used as a lane loading control.
3.2. erk2− cells have growth defects on bacterial lawns
When grown on bacterial lawns, cells with the erk2 gene disruption displayed a slow plaque growth rate and no multicellular development compared to wild-type cells (Fig. 2A). Transformation of the erk2− cells with Erk2 expression vectors (both extrachromosomal and integrating) using the relatively constitutive act15 promoter resulted in a rescue of the plaque growth rate and, in some transformants, a rescue of multicellular development was also observed. The lack of multicellular development in some transformants might be due to the heterologous overexpression of Erk2 because these vectors can also result in aggregation deficient phenotypes in wild-type cells. To express Erk2 from its endogenous promoter an Erk2 expression vector conferring blasticidin resistance was linearized within the erk2 open reading frame and integrated into the erk2∷thyA locus upstream of the gene disruption site (Fig. 1B, Fig. S1). This knock-in integration of an erk2 vector resulted in cells with a single copy of a complete erk2 open reading frame downstream of the endogenous erk2 promoter. Erk2 expression from the endogenous promoter provided a more efficient rescue of both plaque growth rate and multicellular development. Cells with both MAPK gene disruptions, erk1−erk2−, also had a slow plaque growth phenotype but this phenotype was more extreme than that of cells with only the disruption of the erk2 gene. Reduced plaque growth rates have also been observed with other Dictyostelium mutants, particularly those defective in responding to folate. Two mutants with defects in folate responses, far1− and gα4− strains, displayed plaque growth rates slower than wild-type cells but faster than erk2− cells suggesting that Erk2 likely functions in cellular processes other than folate responses. To assess whether the slow plaque growth phenotypes of erk2− and erk1−erk2− are due to general growth defects the mutants were analyzed for cell proliferation in shaking cultures of axenic medium. Interestingly, the erk2− mutants had proliferation characteristics similar to that of complemented cells and wild-type cells (Fig. 2B). However, erk1−erk2− cell proliferation was much slower under these conditions but complementation of the erk2− allele in this strain rescued this defect suggesting that only a loss of both MAPKs has an impact on proliferation in axenic medium. The erk1-erk2− cell proliferation defect was not the result of unusual cytokinesis mechanisms because the distribution of single and multinucleated cell particles was similar to the other strains (Fig. S2).
Figure 2. Dictyostelium growth.

A) Growth of MAPK mutants and wild-type cells on bacterial lawns. Individual strains were mixed with bacteria and plated on SM+/3 plates as described in the Materials and Methods section. Images of plaques were captured 5 days later. All images are the same magnification. B) Growth of MAPK mutants and wild-type cells in shaking cultures of axenic medium. Wild-type (WT), erk2−, erk1−erk2−, and the erk2 mutant strains complemented Erk2 expression vectors (Erk2) were inoculated into shaking cultures of HL-5 axenic medium and cells concentrations were determined using a hemacytometer at the indicated times. Each data point represents 4 counts of at least 100 cells. Error bars represent standard deviation in multiple counts.
3.3. Loss of Erk2 impairs bacterial engulfment
Engulfment of bacteria by Dictyostelium is mediated in part by the folate receptor Far1 and stimulation of this receptor also activates Erk2 [26]. To determine the potential role of Erk2 in bacterial phagocytosis, we used a flow cytometry analysis to quantitatively compare the engulfment of pHrodo-labeled Klebsiella aerogenes by wild-type, erk2−, and other mutant strains. Cells lacking Erk2 or Far1 displayed a similar delay in the initial engulfment in the phagocytosis of the fluorescently-labeled bacteria in suspension cultures compared to wild-type cells. The phenotypic similarity between erk2− and far1− cells suggests that Erk2 plays a role in Far1-mediated bacterial phagocytosis (Fig 3A and B). This delayed uptake of bacteria was also consistently observed when cells were monitored by confocal microscopy (Fig 3C and D). The number of engulfed bacteria in erk2− cells was less than wild-type cells. This defect was rescued by complementation with the Erk2 expression vector. After the initial delay, the rate of erk2− bacterial engulfment was similar to that of wild-type cells implying that other mechanisms might contribute to the slow plaque growth rate on bacterial cell lawns. The lower fluorescence intensity of pHrodo-bacteria in erk2− cells suggests a potential defect in phagosomal maturation.
Figure 3. Engulfment of bacteria.

A) Wild-type (WT), far1−, and erk2− strains and erk2− mutant complemented with Erk2 expression vector (Erk2) were mixed with pHrodo-labelled live bacteria and analyzed at indicated times for the percentage of pHrodo-positive cells. B) Graphical representation of data from (A). C) Images of engulfed bacteria in cells after 15 min. D) Quantitation of bacterial cell uptake into cells. The engulfed bacterial number in each cell was measured and plotted.
3.4. Erk2 is required for folate chemotaxis
Reduced plaque growth rates can potentially result from defects in the ability of cells to properly forage for bacteria at the perimeter of the plaque. Dictyostelium forage for bacteria primarily using the folate receptor and downstream G proteins as a mechanism to facilitate chemotactic movement [42, 43]. The erk2− cells displayed a defect in folate chemotaxis similar to that of far1− and gα4− mutants when analyzed in an above agar assay (Fig. 4A and S3). This defect is rescued by the presence of the erk2 gene. In the absence of folate stimulation erk2− cells did not migrate as far as wild-type and the complemented erk2− cells suggesting Erk2 function contributes to cell motility, directionality, and/or other mechanisms (e.g., cell repulsion) associated with cell dispersal. The erk1−erk2− cells also lacked chemotaxis to folate but these cells did not disperse from the initial cell droplet as much as the erk2− cells suggesting that the loss of both MAPKs has a detrimental impact on cell dispersal. Time-lapsed videos of erk2− cells showed migratory paths typical of random movement compared to the more directed movement paths observed for wild-type cells or complemented mutants in the presence of folate (Fig. 4B, C and S4–8). The average path lengths for erk2− or erk1-erk2− cells were comparable but substantially less than that of wild-type or complemented cells. This difference in individual cell path lengths suggests that chemotactic cell movement is compromised in the MAPK mutants. The reduced cell dispersal of the double MAPK mutant compared to the erk2− mutant was surprising given that erk1− cells do not have a defect in folate chemotaxis (Fig. S9). The basis of this cell dispersal defect is not known but the defect was also observed in cAMP chemotaxis assays (Fig. 6C) and during drug selection of transformants (Fig. S10). It is possible that both MAPKs might have overlapping contributions to cell movement. The requirement of Erk2 for folate chemotaxis indicates that this MAPK plays an important role in the foraging of Dictyostelium to bacteria.
Figure 4. Chemotaxis of MAPK mutants to folate.

A) Above-agar chemotaxis assay images for wild-type (WT), erk2−, and erk1−erk2− strains and erk2− mutants complemented with Erk2 vector (Erk2) after 2.5 h exposure to droplets of 100 μM folate. A) Relative movement of wild-type (WT), erk2−, erk1−erk2−, gα4− and far1− strains and MAPK mutants complemented with Erk2 expression vector (Erk2) toward folate (filled bars) and relative movement in the absence of folate (open bars). Values indicate maximum distance of cell migration toward the source of folate or migration in any direction in the absence of folate. Error bars represent the standard deviation of the error. B) Cell migration paths of select cells were mapped over a 30 min period using time-lapse photography as described in the Materials and Methods section. All images are the same magnification. C) Graphical representation of the average path lengths in arbitrary units (a.u.) from (B). Error bars represent standard deviation. Student’s unpaired t-test comparing to WT, P<0.0001 (*).
3.5. Loss of Erk2 does not affect folate detection and early signaling events
Chemoattractant sensing in Dictyostelium is mediated in part by the rapid activation of Ras proteins, phosphoinositide 3-kinases (PI3Ks), and actin polymerization [44, 45]. Fluorescent reporters that bind to activated Ras (RBD-GFP), phosphorylated inositol lipids (PHCRAC-GFP), and actin filaments (LimEΔcoil-GFP) can assess these cellular responses through the translocation of the reporter to the plasma membrane [46–48]. All of these responses typically begin within a few seconds of chemoattractant stimulation and prior to the activation of Erk2 suggesting these responses occur independently of Erk2 function. When expressed in erk2− cells, these reporters translocated to the membrane with kinetics and amplitudes similar to that observed for wild-type or complemented erk2− cells (Fig. 5A–C). This observation is also consistent with previous studies that suggest MAPK activation might occur in a parallel signaling pathway [49, 50]. Therefore, the loss of Erk2 function does not significantly impact early chemotactic responses to folate.
Figure 5. Early chemotactic signaling in response to folate.
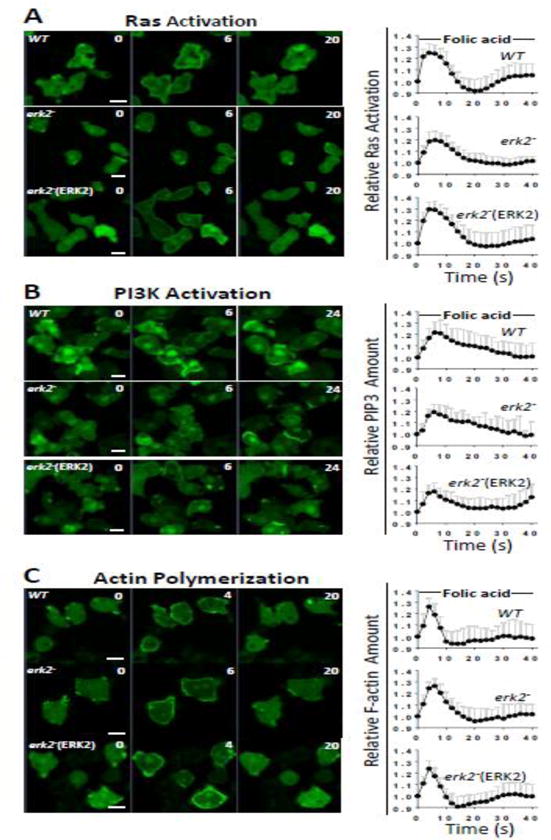
Translocation of Ras, PI3K, and actin filament reporters in wild-type (WT), erk2− cells (erk2−), and complemented erk2− cells (Erk2) in response to folate stimulation was assayed as described in the Materials and Methods section. A) Translocation of the Ras activation reporter RBD-GFP to the membrane. B) Translocation of the PI3K activation reporter PHCRAC-GFP to the membrane. C) Translocation of the actin filament reporter LimEΔcoil-GFP to the membrane. Graphs indicate relative intensity of fluorescence at the membrane and 1 represents the intensity at the start of the response. Error bars represent standard deviation. All images are the same magnification and scale bar represents 5 μm.
3.6. Erk2 is required for development and cAMP chemotaxis
The erk2− and erk1−erk2− mutants failed to aggregate when synchronously starved on nonnutrient agar but the expression of Erk2 in these mutants restored multicellular development similar to that of wild-type and erk1− cells, respectively, including the small aggregate and accelerated development characteristic of erk1− development (Fig. 6A). Earlier studies have shown that the aggregation defect of mutants with reduced Erk2 expression can be rescued by the presence of wild-type cells in a chimeric population because the erk2RE mutants retain the ability to chemotaxis to cAMP [22, 23]. In contrast, the erk2− or erk1−erk2− cells do not co-aggregate with wild-type cells as indicated by lack of cell elongation and the absence of these cells in aggregation streams (Fig. 6B). This observation suggests that the mutants do not respond to wild-type cAMP signaling or produce an inhibitory mechanism to the cAMP-mediated aggregation of wild-type cells. The erk2− cells were also analyzed in above-agar cAMP chemotaxis assays. Cells lacking Erk2 were not capable of chemotaxing to cAMP but chemotaxis could be restored by complementation with the Erk2 expression vector (Fig. 6C). Both the lack of cAMP chemotaxis and the inability to co-aggregate with wild-type cells suggest that the failure of erk2− mutants to undergo multicellular development is due to a chemotaxis defect and not just a defect in cAMP production.
Figure 6. Development and cAMP chemotaxis.

A) Wild-type (WT), erk2−, and erk1-erk2− mutants and mutants complemented with Erk2 expression vector (Erk2) developed on nonnutrient plates for 13 h. All images are the same magnification. B) A GFP vector was used to label erk2−, erk1−erk2−, and wild-type (WT) cells. Labeled cells (GFP) were mixed in a 1:9 ratio with unlabeled wild-type cells and and cell droplets (1×107 cells/ml) plated for development on nonnutrient agar plates. Images of aggregation streams were taken at 12 h. All images are the same magnification. C) Above-agar cAMP chemotaxis assay. After 4 h of starvation in shaking phosphate buffer cells were plated on nonnutrient plates near droplets of 100 μM cAMP. Images of cells were taken at 0 h and 2.5 h and distance was measured of the leading edge of cells toward the source of cAMP. Migration distances under 100 μm are typical for random movement in the absence of exogenous cAMP. Error bars represent the standard deviation of the error. Student’s unpaired t-test comparing to WT, P<0.0001 (*).
3.7. Loss of Erk2 impairs Erk1 activation in folate chemotactic response
We had previously shown that reduced levels of Erk2 in erk2RE cells did not impact the phosphorylation of Erk1 in the secondary response to folate stimulation [17]. Given the importance of Erk2 in chemotaxis, the phosphorylation of Erk1 in response to folate was examined in the erk2− cells and found to be absent, indicating a dependence on Erk2 function (Fig. 7A). However, a low level of phosphorylated Erk1 could be detected in erk2− cells suggesting that Erk2 function is only required for the burst of phosphorylated Erk1 as a secondary response to chemotactic stimulation. Erk1 activity has been previously shown to be dependent on MekA, the only known MAP2K in Dictyostelium, but a requirement of MekA for the phopshorylation of Erk1 had not been demonstrated [16]. The stimulation of mekA− cells with folate or cAMP resulted in the phosphorylation of Erk2 but not Erk1 indicating that MekA only regulates Erk1 and not Erk2 (Fig. 7B). This result implies that the phosphorylation of Erk2 must be facilitated through a mechanism that does not require a conventional MAP2K.
Figure 7. Phosphorylation of MAPKs.

A) After 50 μM folate stimulation erk2− and wild-type (WT) were lysed at times indicated and analyzed for the phosphorylation of MAPKs by immunoblots using phospho-MAPK specific antibodies (upper panel). Coomassie blue stained gel as loading control (lower panel). B) Phosphorylation of MAPKs in mekA− cells in response to folate or cAMP. Cells were stimulated with either 50 μM folate or 100 nM cAMP and then analyzed for phosphorylation of the MAPKs as described in (A) (upper panel). Detection of CCCM using HRP-streptavidin as a loading control (lower panel).
3.8. Erk2 sequence is related to atypical MAPKs
A phylogenetic analysis of the Dictyostelium MAPKs with other eukaryotic MAPKs suggests that the Dictyostelium Erk1 belongs to a group of MAPKs that is found in a wide variety of eukaryotes (Fig. 8). This group contains prototypical MAPKs in yeast (e.g., Fus2) and mammals (e.g., ERK1/2) that have been characterized extensively. In contrast, the Dictyostelium Erk2 shares more sequence similarity to a group of MAPKs that includes the mammalian MAPK15 (also referred to as Erk8). This group of kinases has been previously referred to as atypical MAPKs because typical MAP2Ks have not been identified as the activators of these MAPKs [5, 10, 11]. This atypical regulation is consistent with the Dictyostelium Erk2 belonging to this group of MAPKs. Orthologs of the Dictyostelium Erk2 exists in other amoebae and in animals where cell movement plays important roles but not in fungi where cell movement is absent. The fungal MAPKs (e.g., Aspergillus nidulans, AnMAPK) that are most closely related to the Dictyostelium Erk2 belong to other MAPK groups suggesting the evolution of organisms without cell movement did not require this group of atypical MAPKs.
Figure 8. Phylogenetic analysis of MAPKs.

All known MAPKs in human (Hs), yeast/Saccharomyces cerevisiae (Sc), and Dictyostelium discoideum (Dd) were used to construct the phylogenetic tree using MEGA7 as described in the Materials and Methods. Selected MAPKs with similarity to atypical human MAPK15 (Erk8) from Drosophila melanogaster (Dm) and Acanthamoeba castellanii (Ac) were also included in the tree. A BLAST search of fungal genomes using the Dictyostelium Erk2 protein as the query yielded only MAPKs with similarities to the human Erk1/2 group such as the one representative MAPK included from Aspergillus nidulans (An).
4. Discussion
This study of erk2 gene disruption mutants has revealed the essential role of the Dictyostelium Erk2 in chemotaxis to folate and cAMP whereas previous studies of erk2RE mutants had implied only a subtle role in chemotaxis (Fig. 9). While required for chemotaxis, Erk2 function does not impact early chemotactic responses such as Ras and PI3K activation and early actin filament assembly. Previous studies have shown levels of Erk2 activation in rasC− mutants to be the same as wild-type cells in response to cAMP and only reduced by half in response to folate suggesting Erk2 regulation occurs through a parallel signaling pathway [49, 50]. The requirement of Erk2 function for two different chemotactic responses suggests that this MAPK plays an integral role in general chemotactic responses and could possibly be important for other cell fates that involve chemotactic movement. The rapid activation of Erk2 in response to chemoattractants argues that Erk2 function is necessary for cell movement in response to chemoattractants rather than being a general requirement for all cell movement. However, erk1−erk2− mutants show a strong defect in cell dispersal suggesting both MAPKs have overlapping contributions to cell movement in the absence of an exogenous stimulus. The role of Erk2 function in chemotactic responses is clearly different than that of Erk1 function. In an earlier study, erk1− cells have been described as impaired with respect to cAMP responses but these cells can aggregate and complete all other phases of development in clonal populations [17, 18]. However, erk1− cells typically form smaller aggregates with precocious development suggesting that developmental signaling is aberrant. Folate chemotaxis and foraging capabilities of erk1− cells are comparable to that of wild-type cells (Fig. S9). The phenotypic differences between erk1− and erk2− cells in foraging and multicellular development suggest that the two Dictyostelium MAPKs regulate different cellular processes even though they appear to have overlapping contributions to cell movement.
Figure 9. Model of Erk2 mediated signaling pathways.

Multiple chemoattractant stimulated pathways lead to the activation of Erk2 and downstream cellular responses such as chemotaxis and Erk1 activation. Early chemotactic responses such as Ras and PI3K activation and actin filament formation are not dependent on Erk2 function. Like mammalian atypical MAPKs, the activation of Erk2 does not require the only known MAP2K in Dictyostelium. Folate but not cAMP responses require G protein function for Erk2 activation.
How Erk2 mediates chemotaxis independent of early chemotactic responses remains a major question because little is known about the regulators and targets of atypical MAPKs. Genetic evidence suggests Erk2 is a negative regulator of the cAMP-specific phosphodiesterase, RegA, and therefore Erk2 function could indirectly lead to increased cAMP-dependent protein kinase (PKA) activity [20]. However the loss or over-expression of RegA does not eliminate chemotaxis, suggesting other downstream regulatory proteins exist. Epp2, a protein phosphorylated in an Erk2-dependent manner, is important but not essential for cAMP chemotaxis and cAMP production [51]. The primary structure Epp2 has so far not provided clues as to the function of this protein. Typical MAPKs are known to phosphorylate and regulate other protein kinases and transcription factors but such downstream regulatory proteins have not yet been reported in Dictyostelium [5]. Erk2 function could potentially regulate the expression of genes that facilitate chemotaxis but such function would not address the role of Erk2 activation during chemotaxis.
MAPKs in other organisms have been associated with the regulation of cell proliferation and so MAPK pathways have been a focus for understanding and treating cancerous growth [1, 52–54]. The association of MAPK function with cell growth and proliferation in mammalian systems has been largely based on the activation of MAPKs downstream of receptor tyrosine kinases and Ras proteins that drive these processes [5, 52, 55–59]. The compromised proliferation of the Dictyostelium double MAPK mutant indicates that the MAPKs are important but not essential for proliferation. Some synergy may exist between the erk1− and erk2− gene disruptions because no proliferation defects have been noted for strains carrying one or the other mutant alleles implying Erk1 and Erk2 signaling pathways might have some overlap in the regulation of cell proliferation. While the basis of the erk1−erk2− proliferation defect remains to be determined, this proliferation phenotype supports early assertions that MAPK signal transduction might be a good target for inhibiting cell proliferation. Dictyostelium growth and proliferation are typically intertwined with finding nutrient sources but the cell proliferation defect of erk1−erk2− mutants in axenic suspension cultures occurs in the absence of cell migration. However it is possible that nutrient uptake in Dictyostelium suspensions could include cellular processes related to those important for cell migration.
The roles of Erk2 and Erk1 function in Dictyostelium development are quite different and possibly oppositional. The loss of Erk2 function blocks development at the aggregation stage and the loss of Erk1 function can accelerate developmental progression [17]. Therefore it is interesting that both MAPKs become activated in response to cAMP and folate stimulation. The rapid phosphorylation of Erk2 and then later phosphorylation of Erk1, as Erk2 becomes dephosphorylated, indicates a temporal distinction in the regulation of these MAPKs. The timing of Erk1 phosphorylation correlates with the adaptation to the stimulus and therefore Erk1 activation could be associated with a mechanism to down regulate the initial chemotactic signal. The mechanism by which Erk2 function regulates the phosphorylation of Erk1 is unclear but it requires the activation of MekA and possibly intercellular signaling, as suggested by a previous study [17]. Interestingly, the timing of Erk1 phosphorylation in response to chemotactic signals in Dictyostelium is similar to that of mammalian Erk1/Erk2 phosphorylation in mammalian neutrophils after chemotactic stimulation with fMLP, in that the phosphorylated form persists for over 5 minutes [60–62]. If Dictyostelium and mammalian MAPK orthologs play analogous roles in chemotaxis then it is possible that mammalian Erk1/Erk2 could be involved with an adaptive secondary response to chemoattractants and the mammalian MAPK15 might have a role in mediating the initial chemotactic signaling. Studies of the mammalian MAPK15 regulation have often focused on relatively slow or long term responses (10 minutes to hours after stimulation) rather than rapid responses (within a couple minutes), like the rapid phosphorylation of Erk2 in Dictyostelium, and so possible chemotactic regulation of MAPK15 activity might have been overlooked [63–65]. Dictyostelium and mammals share many similarities in chemotactic responses including G protein-mediated signaling and a rapid rise in cAMP suggesting similarities could possibly extend to MAPK function and regulation [45, 66, 67].
The sequence similarity of the Dictyostelium Erk2 with the mammalian MAPK15 and inability of these MAPKs to be activated by typical MAP2Ks suggest that these MAPKs might share related functions and regulation [5, 10, 11]. Thus far no gene disruptions have been created in the animal orthologs but recently mutations within the kinase domain of a nematode (Caenorhabditis elegans) ortholog have been shown to interfere with the formation of motile cilium formation [68]. RNA interference and kinase inhibitor analyses suggest that orthologs in trypanosomes (Trypanosoma brucei) are important for proliferation [69, 70]. The human MAPK15 has been found widespread in tissue distribution and throughout development and studies using RNA interference suggest this MAPK can regulate proliferation in a variety of cell types [11, 71–73]. The corresponding ortholog in flies, Erk7, regulates insulin-like peptide secretion and perhaps this production of a secreted hormone has some analogy with the intercellular signaling associated with Erk1 activation in Dictyostelium [74]. Understanding the activation kinetics of mammalian MAPK15 and other orthologs has been hampered due to the limited characterization of possible endogenous extracellular signals that activate these pathways [64, 65]. Therefore defining the regulation and function of the Dictyostelium Erk2 MAPK in response to known endogenous signals is likely to provide a useful model for characterizing the regulation and function of this group of atypical MAPKs.
5. Conclusion
The Dictyostelium Erk2 MAPK is required for chemotactic movement to both folate and cAMP suggesting Erk2 plays a critical role in the general cellular response to chemoattractants. While not necessary for early chemoattractant detection, the signaling pathway mediated by Erk2 function is responsible for the secondary response of Erk1 phosphorylation. Both Erk2 and Erk1 have overlapping roles in both cell growth and cell movement. In contrast to Erk1, Erk2 is an ortholog of atypical MAPKs and has an activation mechanism independent of conventional MAP2Ks. Therefore, Erk2 represents a good model for the study of atypical MAPKs.
Supplementary Material
Highlights.
Dictyostelium Erk2 is essential for chemotaxis and the secondary activation of Erk1.
Erk2 and Erk1 have overlapping roles in cell movement and growth
Erk2 is an ortholog of atypical MAP kinases
Acknowledgments
This work was supported by grants NIGMS R15 GM097717-01 and OCAST HR13-36 to JAH. MP and TJ are supported by intramural funding of the NIAID, NIH. The authors thank Joshua Moore for technical assistance and the laboratory of P. Devreotes for reporter plasmids.
Funding
This work was supported by grants National Institute of General Medical Sciences R15 GM097717-01 and Oklahoma Center for the Advancement of Science and Technology HR13-36 to JAH. MP and TJ are supported by Division of Intramural Research, National Institute of Allergies and Infectious Diseases, National Institutes of Health.
Abbreviations
- MAPK
mitogen activated protein kinase
- MAP2K
MAPK kinase
- ERK
extracellular-signal regulate kinase
- JNK
c-Jun N-terminal kinase
- PHCRAC
pleckstrin homology domian
- RBD
ras binding domain
- cAMP
cyclic adenosine monophosphate
- fMLP
formylmethionyl-leucyl-phenylalanine
- MCCC1
mitochondrial 3-methylcrotonyl-CoA carboxylase α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
DJS, NA, NAK, and JAH created the strains, analyzed chemotaxis and growth phenotypes, and examined MAPK activation. MP and TJ analyzed phagocytosis and early chemotactic signaling. JAH wrote the manuscript and all authors contributed comments.
References
- 1.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 2.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 3.Hadwiger JA, Nguyen HN. MAPKs in development: insights from Dictyostelium signaling pathways. Biomol Concepts. 2011;2(1–2):39–46. doi: 10.1515/BMC.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochimica et biophysica acta. 2007;1773(8):1311–40. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and molecular biology reviews: MMBR. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogoyevitch MA, Court NW. Counting on mitogen-activated protein kinases–ERKs 3, 4, 5, 6, 7 and 8. Cellular signalling. 2004;16(12):1345–54. doi: 10.1016/j.cellsig.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Fremin C, Saba-El-Leil MK, Levesque K, Ang SL, Meloche S. Functional Redundancy of ERK1 and ERK2 MAP Kinases during Development. Cell Rep. 2015;12(6):913–21. doi: 10.1016/j.celrep.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Breitkreutz A, Tyers M. MAPK signaling specificity: it takes two to tango. Trends Cell Biol. 2002;12(6):254–7. doi: 10.1016/s0962-8924(02)02284-5. [DOI] [PubMed] [Google Scholar]
- 9.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochimica et biophysica acta. 2007;1773(8):1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochimica et biophysica acta. 2007;1773(8):1376–87. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Abe MK, Saelzler MP, Espinosa R, 3rd, Kahle KT, Hershenson MB, Le Beau MM, Rosner MR. ERK8, a new member of the mitogen-activated protein kinase family. The Journal of biological chemistry. 2002;277(19):16733–43. doi: 10.1074/jbc.M112483200. [DOI] [PubMed] [Google Scholar]
- 12.Doczi R, Okresz L, Romero AE, Paccanaro A, Bogre L. Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci. 2012;17(9):518–25. doi: 10.1016/j.tplants.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Escalante R, Vicente JJ. Dictyostelium discoideum: a model system for differentiation and patterning. Int J Dev Biol. 2000;44(8):819–35. [PubMed] [Google Scholar]
- 15.Loomis WF. Genetic control of morphogenesis in Dictyostelium. Dev Biol. 2015;402(2):146–61. doi: 10.1016/j.ydbio.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H, Gamper M, Parent C, Firtel RA. The Dictyostelium MAP kinase kinase DdMEK1 regulates chemotaxis and is essential for chemoattractant-mediated activation of guanylyl cyclase. The EMBO journal. 1997;16(14):4317–32. doi: 10.1093/emboj/16.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwebs DJ, Hadwiger JA. The Dictyostelium MAPK ERK1 is phosphorylated in a secondary response to early developmental signaling. Cellular signalling. 2015;27(1):147–55. doi: 10.1016/j.cellsig.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobko A, Ma H, Firtel RA. Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev Cell. 2002;2(6):745–56. doi: 10.1016/s1534-5807(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 19.Gaskins C, Clark AM, Aubry L, Segall JE, Firtel RA. The Dictyostelium MAP kinase ERK2 regulates multiple, independent developmental pathways. Genes Dev. 1996;10(1):118–28. doi: 10.1101/gad.10.1.118. [DOI] [PubMed] [Google Scholar]
- 20.Maeda M, Lu S, Shaulsky G, Miyazaki Y, Kuwayama H, Tanaka Y, Kuspa A, Loomis WF. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304(5672):875–8. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HN, Hadwiger JA. The Galpha4 G protein subunit interacts with the MAP kinase ERK2 using a D-motif that regulates developmental morphogenesis in Dictyostelium. Dev Biol. 2009;335(2):385–95. doi: 10.1016/j.ydbio.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen HN, Raisley B, Hadwiger JA. MAP kinases have different functions in Dictyostelium G protein-mediated signaling. Cellular signalling. 2010;22(5):836–47. doi: 10.1016/j.cellsig.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segall JE, Kuspa A, Shaulsky G, Ecke M, Maeda M, Gaskins C, Firtel RA, Loomis WF. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. The Journal of cell biology. 1995;128(3):405–13. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Liu J, Segall JE. MAP kinase function in amoeboid chemotaxis. J Cell Sci. 1998;111(Pt 3):373–83. doi: 10.1242/jcs.111.3.373. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka C, Pears CJ. Chemoattractants induce tyrosine phosphorylation of ERK2 in Dictyostelium discoideum by diverse signalling pathways. Biochem J. 1997;324(Pt 1):347–52. doi: 10.1042/bj3240347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan M, Xu X, Chen Y, Jin T. Identification of a Chemoattractant G-Protein-Coupled Receptor for Folic Acid that Controls Both Chemotaxis and Phagocytosis. Dev Cell. 2016;36(4):428–39. doi: 10.1016/j.devcel.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda M, Firtel RA. Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. The Journal of biological chemistry. 1997;272(38):23690–5. doi: 10.1074/jbc.272.38.23690. [DOI] [PubMed] [Google Scholar]
- 28.Brzostowski JA, Kimmel AR. Nonadaptive regulation of ERK2 in Dictyostelium: implications for mechanisms of cAMP relay. Mol Biol Cell. 2006;17(10):4220–7. doi: 10.1091/mbc.E06-05-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aubry L, Maeda M, Insall R, Devreotes PN, Firtel RA. The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by Ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. The Journal of biological chemistry. 1997;272(7):3883–6. doi: 10.1074/jbc.272.7.3883. [DOI] [PubMed] [Google Scholar]
- 30.Maeda M, Aubry L, Insall R, Gaskins C, Devreotes PN, Firtel RA. Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium. Role of heterotrimeric G proteins. The Journal of biological chemistry. 1996;271(7):3351–4. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- 31.Bloomfield G, Traynor D, Sander SP, Veltman DM, Pachebat JA, Kay RR. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife. 2015;4 doi: 10.7554/eLife.04940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veltman DM, Williams TD, Bloomfield G, Chen BC, Betzig E, Insall RH, Kay RR. A plasma membrane template for macropinocytic cups. eLife. 2016;5 doi: 10.7554/eLife.20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadwiger JA, Firtel RA. Analysis of G alpha 4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992;6(1):38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- 34.Watts DJ, Ashworth JM. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970;119(2):171–4. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadwiger JA. Developmental morphology and chemotactic responses are dependent on G alpha subunit specificity in Dictyostelium. Dev Biol. 2007;312(1):1–12. doi: 10.1016/j.ydbio.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levi S, Polyakov M, Egelhoff TT. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44(3):231–8. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HN, Hadwiger JA. The Galpha4 G protein subunit interacts with the MAP kinase ERK2 using a D-motif that regulates developmental morphogenesis in Dictyostelium. Developmental Biology. 2009;335(2):385–95. doi: 10.1016/j.ydbio.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson AJ, King JS, Insall RH. The use of streptavidin conjugates as immunoblot loading controls and mitochondrial markers for use with Dictyostelium discoideum. Biotechniques. 2013;55(1):39–41. doi: 10.2144/000114054. [DOI] [PubMed] [Google Scholar]
- 39.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8(3):275–82. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016 doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–65. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- 42.Kuburich NA, Adhikari N, Hadwiger JA. Acanthamoeba and Dictyostelium Use Different Foraging Strategies. Protist. 2016;167(6):511–525. doi: 10.1016/j.protis.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meena NP, Kimmel AR. Chemotactic network responses to live bacteria show independence of phagocytosis from chemoreceptor sensing. eLife. 2017;6 doi: 10.7554/eLife.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devreotes P, Horwitz AR. Signaling networks that regulate cell migration. Cold Spring Harb Perspect Biol. 2015;7(8):a005959. doi: 10.1101/cshperspect.a005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44(1):1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95(1):81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 47.Skoge M, Adler M, Groisman A, Levine H, Loomis WF, Rappel WJ. Gradient sensing in defined chemotactic fields. Integr Biol (Camb) 2010;2(11–12):659–68. doi: 10.1039/c0ib00033g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider N, Weber I, Faix J, Prassler J, Muller-Taubenberger A, Kohler J, Burghardt E, Gerisch G, Marriott G. A Lim protein involved in the progression of cytokinesis and regulation of the mitotic spindle. Cell Motil Cytoskeleton. 2003;56(2):130–9. doi: 10.1002/cm.10139. [DOI] [PubMed] [Google Scholar]
- 49.Lim CJ, Spiegelman GB, Weeks G. RasC is required for optimal activation of adenylyl cyclase and Akt/PKB during aggregation. The EMBO journal. 2001;20(16):4490–9. doi: 10.1093/emboj/20.16.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim CJ, Zawadzki KA, Khosla M, Secko DM, Spiegelman GB, Weeks G. Loss of the Dictyostelium RasC protein alters vegetative cell size, motility and endocytosis. Exp Cell Res. 2005;306(1):47–55. doi: 10.1016/j.yexcr.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Segall JE. EppA, a putative substrate of DdERK2, regulates cyclic AMP relay and chemotaxis in Dictyostelium discoideum. Eukaryot Cell. 2006;5(7):1136–46. doi: 10.1128/EC.00383-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bessard A, Fremin C, Ezan F, Fautrel A, Gailhouste L, Baffet G. RNAi-mediated ERK2 knockdown inhibits growth of tumor cells in vitro and in vivo. Oncogene. 2008;27(40):5315–25. doi: 10.1038/onc.2008.163. [DOI] [PubMed] [Google Scholar]
- 53.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 54.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 55.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochimica et biophysica acta. 2011;1813(9):1619–33. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31(2):151–74. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 57.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–35. [PubMed] [Google Scholar]
- 58.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–4. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 60.Grinstein S, Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. The Journal of biological chemistry. 1992;267(25):18122–5. [PubMed] [Google Scholar]
- 61.Nick JA, Avdi NJ, Young SK, Knall C, Gerwins P, Johnson GL, Worthen GS. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. The Journal of clinical investigation. 1997;99(5):975–86. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rane MJ, Carrithers SL, Arthur JM, Klein JB, McLeish KR. Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase. Journal of immunology. 1997;159(10):5070–8. [PubMed] [Google Scholar]
- 63.Iavarone C, Acunzo M, Carlomagno F, Catania A, Melillo RM, Carlomagno SM, Santoro M, Chiariello M. Activation of the Erk8 mitogen-activated protein (MAP) kinase by RET/PTC3, a constitutively active form of the RET proto-oncogene. The Journal of biological chemistry. 2006;281(15):10567–76. doi: 10.1074/jbc.M513397200. [DOI] [PubMed] [Google Scholar]
- 64.Klevernic IV, Martin NM, Cohen P. Regulation of the activity and expression of ERK8 by DNA damage. FEBS letters. 2009;583(4):680–4. doi: 10.1016/j.febslet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Klevernic IV, Stafford MJ, Morrice N, Peggie M, Morton S, Cohen P. Characterization of the reversible phosphorylation and activation of ERK8. Biochem J. 2006;394(Pt 1):365–73. doi: 10.1042/BJ20051288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahadeo DC, Janka-Junttila M, Smoot RL, Roselova P, Parent CA. A chemoattractant-mediated Gi-coupled pathway activates adenylyl cyclase in human neutrophils. Mol Biol Cell. 2007;18(2):512–22. doi: 10.1091/mbc.E06-05-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Artemenko Y, Lampert TJ, Devreotes PN. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell Mol Life Sci. 2014;71(19):3711–47. doi: 10.1007/s00018-014-1638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazatskaya A, Kuhns S, Lambacher NJ, Kennedy JE, Brear AG, McManus GJ, Sengupta P, Blacque OE. Primary Cilium Formation and Ciliary Protein Trafficking Is Regulated by the Atypical MAP Kinase MAPK15 in Caenorhabditis elegans and Human Cells. Genetics. 2017 doi: 10.1534/genetics.117.300383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackey ZB, Koupparis K, Nishino M, McKerrow JH. High-throughput analysis of an RNAi library identifies novel kinase targets in Trypanosoma brucei. Chemical biology & drug design. 2011;78(3):454–63. doi: 10.1111/j.1747-0285.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valenciano AL, Ramsey AC, Santos WL, Mackey ZB. Discovery and antiparasitic activity of AZ960 as a Trypanosoma brucei ERK8 inhibitor. Bioorg Med Chem. 2016;24(19):4647–4651. doi: 10.1016/j.bmc.2016.07.069. [DOI] [PubMed] [Google Scholar]
- 71.Colecchia D, Strambi A, Sanzone S, Iavarone C, Rossi M, Dall’Armi C, Piccioni F, Verrotti di Pianella A, Chiariello M. MAPK15/ERK8 stimulates autophagy by interacting with LC3 and GABARAP proteins. Autophagy. 2012;8(12):1724–40. doi: 10.4161/auto.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu YM, Zhu F, Cho YY, Carper A, Peng C, Zheng D, Yao K, Lau AT, Zykova TA, Kim HG, Bode AM, Dong Z. Extracellular signal-regulated kinase 8-mediated c-Jun phosphorylation increases tumorigenesis of human colon cancer. Cancer research. 2010;70(8):3218–27. doi: 10.1158/0008-5472.CAN-09-4306. [DOI] [PubMed] [Google Scholar]
- 73.Jin DH, Lee J, Kim KM, Kim S, Kim DH, Park J. Overexpression of MAPK15 in gastric cancer is associated with copy number gain and contributes to the stability of c-Jun. Oncotarget. 2015;6(24):20190–203. doi: 10.18632/oncotarget.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasygar K, Hietakangas V. p53- and ERK7-dependent ribosome surveillance response regulates Drosophila insulin-like peptide secretion. PLoS genetics. 2014;10(11):e1004764. doi: 10.1371/journal.pgen.1004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


