Summary
Alongside the development of sexual characteristics and reproductive competence, adolescents undergo marked cognitive, social, and emotional development [1]. A fundamental question is whether these changes are triggered by activation of the hypothalamic-pituitary-gonadal (HPG) axis at puberty (puberty-dependent), or whether they occur independently of HPG activation (puberty-independent). Disentangling puberty-dependent from puberty-independent mechanisms is difficult because puberty and adolescence typically proceed concurrently. Here, we test a new approach that leverages natural adaptations of a seasonally breeding species to dissociate pubertal status from chronological age. Siberian hamsters (Phodopus sungorus) reared in a long, summer-like day length (LD) exhibit rapid pubertal development, whereas those reared in a short, winter-like day length (SD) delay puberty by several months to synchronize breeding with the following spring [2,3]. We tested whether the SD-induced delay in puberty delays the peri-adolescent decline in juvenile social play and the rise in aggression that characterizes adolescent social development in many species [4–6], and compared the results to those obtained after prepubertal gonadectomy. Neither SD-rearing nor prepubertal gonadectomy altered the age at which hamsters transitioned from play to aggression; SD-reared hamsters completed this transition prior to puberty. SD-rearing and prepubertal gonadectomy, however, increased levels of play in male and female juveniles, implicating a previously unknown role for prepubertal gonadal hormones in juvenile social behavior. Levels of aggression were also impacted (decreased) in SD-reared and gonadectomized males. These data demonstrate that puberty-independent mechanisms regulate the timing of adolescent social development, while prepubertal and adult gonadal hormones modulate levels of age-appropriate social behaviors.
Keywords: social play, aggression, gonadal hormones, Siberian hamster
eTOC Blurb
Paul et al. leverage natural adaptations of seasonal-species to investigate the role of pubertal hormones in adolescent social development. The authors find that puberty-independent mechanisms regulate the timing of adolescent social development, whereas prepubertal and adult gonadal hormones modulate levels of age-appropriate social behaviors.

Results
Play Behavior in Siberian hamsters
Play behavior of Siberian hamsters (Phodopus sungorus) has not previously been described. We found that Siberian hamsters exhibited similar social play behaviors to those of laboratory rats (Rattus norvegicus), Syrian hamsters (Mesocricetus auratus), and Djungarian hamsters (Phodopus campbelli) [7–9]. These include pounces (lunges toward the playmates face or nape), pins (one animal on top, holding its playmate in the supine position), and boxing (both animals standing on their hind legs pushing or batting each other with their forepaws). Offensive aggressive behaviors in Siberian hamsters were typified by rapid strikes/bites typically directed toward the rump and genitals and vigorous chases that usually ended with the subordinate animal in the supine defensive posture [as in 10,11]. Unlike during playful pins, the dominant aggressor did not sustain contact during this supine posture. Playful and aggressive interactions were easily discriminated in Siberian hamsters by considering the target of attacks (face/head versus rump/genitals), the vigor of attacks (aggressive attacks are much more rapid and raucous), the duration of contact during pins, and the presence or absence of accompanying vocalizations (playful interactions are typically silent, whereas aggression is accompanied by long, audible calls).
SD-rearing dissociates puberty and adolescent social development
To test whether a SD-induced delay in puberty delays the peri-adolescent transition from juvenile social play to adult aggression, male and female Siberian hamsters were reared under LD (14h light/day; 14L) or SD (10L) conditions, and play, aggression, and reproductive measures (estimated testis volume [ETV] or vaginal opening [VO]) were assessed every 10 days from P20 to either P60 (LD groups) or P120 (SD groups). Measures of LD-reared hamsters were not taken past P60 because the transition from play to aggression was expected to (and did) occur by this age in LD groups.
Figure 1 depicts the reproductive and social development of LD- and SD-reared male and female Siberian hamsters. As in previous studies [2,3], SD-rearing delayed reproductive development of Siberian hamsters by 2–3 months (in males) or more (in females) compared to LD-reared hamsters (Figure 1A,B). The mean onset of puberty occurred at P30 for LD-reared males, P80 for SD-reared males, P52 for LD-reared females, and later than P120 for SD-reared females (only a single SD-reared female underwent vaginal opening by P120). Despite these markedly different reproductive profiles, play declined and aggression emerged at similar ages between LD- and SD-reared hamsters (Figure 1C,D). For SD-reared hamsters, the decline in play was completed by P40 (for SD males) or P60 (for SD females) (P>0.10, vs. final time point, Fisher’s PLSD), and aggression emerged at P40 (for SD males) or P50 (for SD females; Figure 1D), well before the onset of puberty (P80 for SD males and >P120 for SD females). Hence, this behavioral transition cannot be explained by pubertal factors indicating puberty-independent regulation.
Figure 1.
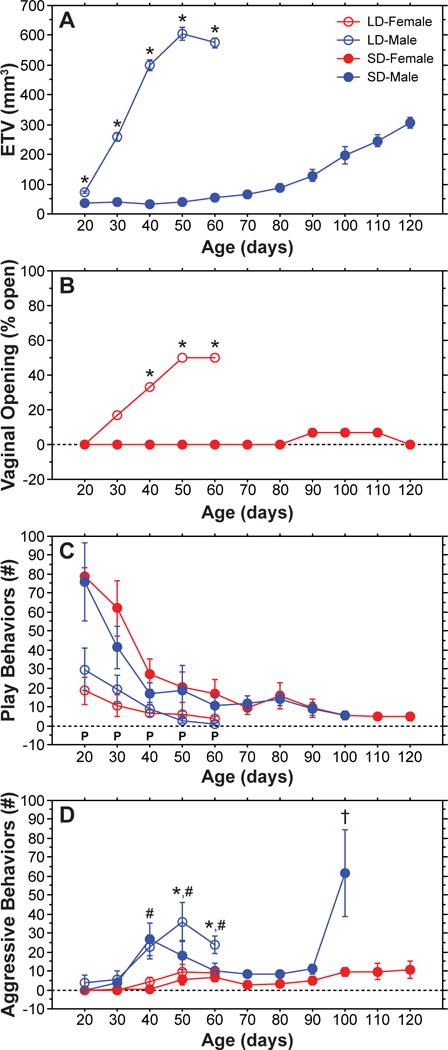
SD-rearing dissociates reproductive and social development in Siberian hamsters. Developmental profiles of (A) mean (±s.e.) estimated testis volume (ETV), (B) percent females exhibiting vaginal opening, (C) mean (±s.e.) number of play behaviors, and (D) mean (±s.e.) number of aggressive behaviors (D) of LD- and SD-reared male and female Siberian hamsters. ‘P’ indicates a main effect of Photoperiod within each time point (P<0.02, ANOVA); * indicates a significant difference between LD-reared males and SD-reared males (P<0.02, Fisher’s PLSD); # indicates a significant sex difference between LD-reared males and LD-reared females (P<0.003, Fisher’s PLSD); † indicates a significant increase in SD-male aggression at P100 versus all other ages (P<0.005, Fishers PLSD). Social behaviors were analyzed at the level of the pair and sample sizes were: LD-Female = 13; LD-Male = 10; SD-Female = 8; SD-Male = 7. Reproductive measures were analyzed at the level of the individual, hence samples sizes were double those of behavioral measures.
Assessment of dyadic playful and aggressive interactions further supports this conclusion. Juvenile social play is reciprocal with both participants engaging in the behavior [5,12]. To assess the reciprocal/asymmetric nature of play and aggression in Siberian hamsters, we calculated an asymmetry score for which complete symmetry equals 0 (both animals exhibit the same number of behaviors) and complete asymmetry equals 1 (one animal accounts for all behaviors; see methods for equation). As noted for other species, asymmetry was low for play in juvenile hamsters (P20 and P30 in Figure 2A). In contrast, aggression asymmetry equaled or approached 1 (Figure 2B). Play asymmetry increased from P20 to P60 as LD-reared hamsters underwent pubertal development (P<0.02, nonparametric sign test), which is reminiscent of the increase in dominance-associated play postures in male rats across adolescence [13,14]. Notably, SD-reared hamsters exhibited the same developmental increase in play asymmetry from P20 to P60, even though they had not yet undergone puberty (P<0.007, nonparametric sign test). Play asymmetry did not differ between LD- and SD-reared hamsters at any age (P>0.28 at each age from P20 to P60, ANOVA), indicating that photoperiod does not alter the reciprocal nature of play.
Figure 2.
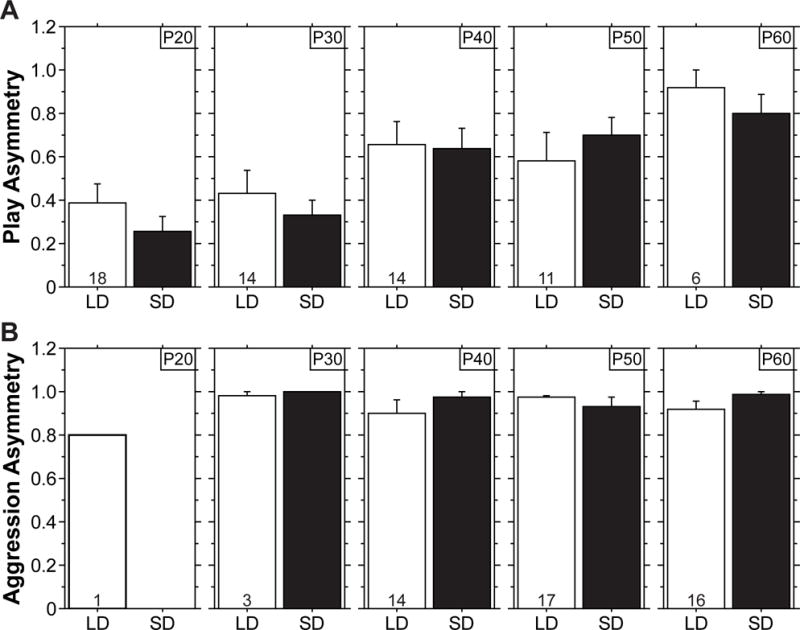
SD-rearing does not alter play asymmetry or its developmental increase across adolescence. Mean (+s.e.) asymmetry score for (A) playful and (B) aggressive interactions of LD- and SD-reared hamsters at postnatal day (P)20, P30, P40, P50, and P60. Play and aggression asymmetry of males and females did not differ; therefore data were collapsed across sex. Play and aggression asymmetry did not differ between LD and SD-reared hamsters at any age (P>0.28 for play at each age from P20 to P60, P>0.13 for aggression at each age from P30 to P60, ANOVA). Play asymmetry increased from P20 to P60 in both LD- and SD-reared hamsters (P<0.02, nonparametric sign test). Only a single pair exhibited aggression at P20. Sample sizes (pairs per group) are indicated within bars.
SD-rearing modulates levels of juvenile social play and adult male aggression
Although SD-rearing did not alter the temporal profiles of play or aggression, it did impact absolute levels of these behaviors (Figure 1C,D; P<0.04, main effect of Photoperiod for both behavioral measures, repeated measures ANOVA). SD-reared hamsters displayed higher total number of play behaviors than their LD-reared counterparts at all ages (P<0.02, main effect of Photoperiod at each age from P20 to P60, ANOVA). Once aggression emerged, the total number of aggressive behaviors increased to higher levels in LD-reared males compared to SD-reared males (P<0.02 at P50 and P60, Fisher’s PLSD). Notably, SD-reared males displayed a second increase in aggression at P100 (P100 vs. all other ages, P<0.005, Fisher’s PLSD), around the time of increased testicular development (see Figure 1A). Additionally, 3 of 7 SD-reared male pairs needed to be separated between P100 and P110 due to fighting in their home cages. Photoperiod modulation of aggression was not detected in females, for which aggression was much lower than for males (P<0.003, LD-Males versus LD-Females at each age from P40 to P60, Fisher’s PLSD) and did not differ between LD- and SD-reared hamsters (P>0.52, LD-Females versus SD-Females at each age from P30 to P60, Fisher’s PLSD). These data suggest that increased secretion of pubertal hormones promote high levels of aggression in males. Because photoperiod modulated play behavior at P20 and P30, a pubertal mechanism for altered levels of play is doubtful. In addition to delaying puberty, SD-rearing suppresses gonadal function as early as P20 [3,15], raising the possibility of a prepubertal gonadal mechanism.
Prepubertal gonadectomy impacts levels, but not developmental timing of social behaviors
To test possible contributions of the gonads to the developmental timing and levels of play and aggression, LD-reared male and female Siberian hamsters were gonadectomized (GNX) or sham-operated (Sham) on P15, and play and aggressive behaviors were assessed every 10 days from P20 to P60. GNX and Sham groups were conducted concurrently with the photoperiod manipulations described above. Therefore, LD-reared hamsters from the photoperiod experiment were used as non-surgical controls (Intact) for this experiment. We first assessed whether Sham and Intact groups could be combined into a single control group (Control). Sham surgery at P15 markedly increased play behavior at P20 in both males and females (Figure 3A,B; Sham vs. Intact, P≤0.05, main effect of surgery, ANOVA), which prevented the combining of these groups at this age. This surgical effect on play was no longer evident from P30 onwards and did not manifest for aggression at any age (P>0.13, Sham vs. Intact, P30–P60 for play and P20–P60 for aggression, Fisher’s PLSD). Therefore, Sham and Intact groups were combined into a single Control group at P30–P60 for play and at P20–P60 for aggression.
Figure 3.
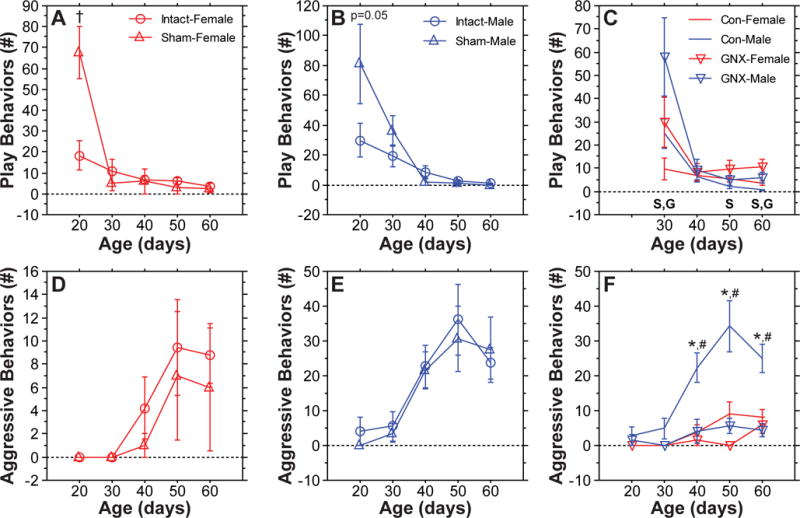
Prepubertal gonadectomy increases juvenile social play and decreases adult male aggression. Panels A, B, D, and E illustrate the mean (±s.e.) number of play and aggressive behaviors in sham-operated (Sham) and unoperated (Intact) LD-reared male and female hamsters; † indicates a significant difference between Sham and Intact female hamsters at P20 (P<0.008, Fisher’s PLSD). Because this surgical effect did not manifest at other ages for play or at any age for aggression, Sham and Intact groups were combined into a single control group (Con) at P30-P60 for play and at P20-P60 for aggression for comparisons with gonadectomized (GNX) hamsters in Panels C and F; ‘G’ indicates a significant main effect of GNX (P<0.004, ANOVA); ‘S’ indicates a significant main effect of Sex (P<0.02, ANOVA); * indicates a significant difference between GNX-Males and Con-Males (P<0.004, Fisher’s PLSD); # indicates significant a sex difference between Con-Males and Con-Females (P<0.001, Fisher’s PLSD). Sample sizes (pairs per group) were: Intact-Female = 13; Intact-Male = 10; Sham-Female = 3; Sham-Male = 5; Con-Female = 16; Con-Male = 15; GNX-Female = 7; GNX-Male = 6.
As with SD-rearing, GNX did not delay the developmental decline in play (Figure 3C) or block the increase in asymmetric play interactions from P20 to P60 (P<0.02 for GNX hamsters, nonparametric sign test; Figure 4), further demonstrating puberty-independent and gonadal-independent regulation of the timing of this developmental transition. These data agree with those in rats, for which prepubertal GNX also does not alter the decline in play [16,17]. Unlike in rats [18,19], however, prepubertal GNX increased levels of play in Siberian hamsters at P30 (P<0.004, main effect of GNX, ANOVA). These data, in conjunction with the play-promoting effects of SD-rearing, implicate a role for the prepubertal gonads in juvenile social behavior; one in which the prepubertal gonads suppress juvenile social play, and removal of this suppression, either by GNX or SD-rearing, results in elevated play.
Figure 4.
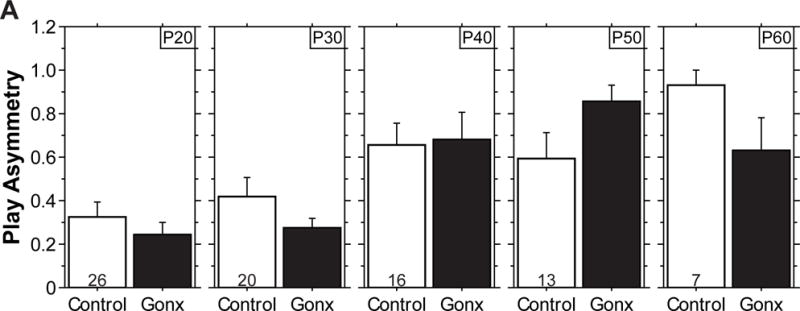
Gonadectomy does not alter play asymmetry or its developmental increase across adolescence. Mean (+s.e.) asymmetry score for playful interactions of LD-reared gonadectomized (GNX) and control hamsters at P20, P30, P40, P50, and P60. Play asymmetry of males and females did not differ; therefore data were collapsed across sex. Play and aggression asymmetry did not differ between GNX and Control hamsters at any age (P>0.09 at each age from P20 to P60, ANOVA). Play asymmetry increased from P20 to P60 in GNX and Control hamsters (P<0.02, nonparametric sign test). Sample sizes (pairs per group) are indicated within bars.
Prepubertal GNX decreased aggression in male hamsters (P<0.004, GNX-Males vs. Control-Males at each age from P40 to P60, Fisher’s PLSD) and eliminated the sex difference in this behavior (P>0.59, GNX-Males vs. GNX-Females, at each age from P40 to P60, Fisher’s PLSD). Unlike SD-rearing, GNX prevented the developmental rise in male aggression (P>0.34, GNX-Males, main effect of Age, repeated measures ANOVA; Figure 3F).
Discussion
The present study demonstrates that adolescent social development is regulated by both puberty-dependent and puberty-independent mechanisms. Delaying puberty by rearing hamsters in a SD failed to delay the developmental transition from play to aggression. This same manipulation, however, increased play and reduced male aggression. Similarly, gonadectomy increased play and reduced male aggression without shifting the peri-adolescent decline in play. Based on these results, we propose that: 1) Puberty-independent mechanisms initiate the transition from juvenile social play to adult aggression, thereby determining the timing of this developmental transition. 2) Pubertal increases in testicular hormones synergize with this puberty-independent shift toward aggression to further increase levels of aggression in males. 3) Prepubertal gonadal hormones suppress levels of juvenile play behavior. Taken together, these data indicate that adolescent social development requires coordinated interactions between puberty-dependent and puberty-independent mechanisms.
The use of seasonal species to study such interactions has several unique advantages over gonadectomy and hormone replacement [20]. SD-rearing circumvents potential confounds that can accompany gonadectomy, including early-life surgical stress [21,22] and compensatory neuroendocrine changes after removal of gonadal steroid negative feedback [23]. Indeed, confounds from early-life surgery were evident in the present study, where sham surgery substantially, albeit transiently, increased play behavior, which prevented assessment of gonadal influences at P20. A second advantage is that unlike gonadectomy and hormone replacement, delaying puberty by SD-rearing maintains endogenous hormone rhythms [28]. Finally, SD-rearing more accurately mimics the prepubertal state by altering the development of the entire reproductive axis rather than just the gonads. Data in adult hamsters suggests that SD-rearing likely extends the prepubertal period of increased sensitivity to gonadal steroid negative feedback on gonadotropin secretion [23–25], thereby delaying the pubertal switch to decreased steroid-dependent gonadotropin restraint [26]. This expands the question of puberty-dependence/independence to include non-gonadal levels of the HPG axis (e.g., pubertal changes in hypothalamic and pituitary hormones). Nevertheless, caveats must be considered when using the seasonal-species approach. It is not known whether neural circuits that regulate behavior undergo similar changes in sensitivity to gonadal steroids during adolescence, and if so, whether photoperiod alters this process. SD-housing of adult Syrian hamsters increases the steroid sensitivity of neural circuits regulating territorial aggression of females, but decreases the steroid sensitivity of neural circuits regulating sex behavior of males and females [27,28]. Hence, SD-rearing might increase or decrease steroid sensitivity of neural circuits regulating play and/or aggression of juvenile and/or adolescent Siberian hamsters. If this occurs, behavioral development seen in the seasonal-species approach could be regulated by the combination of changing pubertal hormones, changes in the sensitivity to these hormones, as well as puberty-independent changes in neural circuits. Photoperiod also regulates many seasonal traits in addition to puberty (e.g., body mass, immune function, thermoregulation) [29,30], and effects of SD-rearing could be due to photoperiod influences on these non-reproductive systems. This is less of an issue when photoperiod does not alter the adolescent trait, as seen in the present study for the timing of the transition from play to aggression. However, experiments that demonstrate effects of SD-rearing, as seen in the present experiment for levels of play and aggression, should be followed up by neuroendocrine manipulations designed to test whether these effects are due to pubertal or non-pubertal influences of photoperiod.
In the present study, results from the seasonal-species and gonadectomy approaches were convergent, but not identical. For example, the developmental rise in aggression was evident in SD-reared, but not GNX male hamsters. This highlights an important difference between the two approaches: gonadectomy tests the role of the gonads, whereas the seasonal-species approach tests the role of changes in pubertal hormones. Because gonadectomy removes all gonadal hormones, whereas SD-rearing maintains low levels of gonadal hormones [3], the present findings suggest that the expression of male aggression, and consequently its developmental rise, require at least some level of gonadal hormone. However, the SD findings demonstrate that the emergence of aggression does not require changes in pubertal hormones. In males, the onset of puberty was accompanied by a further increase in aggression. Hence, the full developmental rise in aggression is brought about by synergistic actions between puberty-dependent and puberty-independent mechanisms. Prepubertal castration decreases adult aggression in some species, but not others [31]. To our knowledge, the present findings are the first to demonstrate coordinated actions between puberty-dependent and puberty-independent mechanisms within a single species.
Although gonadal steroids are known to promote aggression in numerous species [31], experiments have repeatedly shown that SD-housing increases, and gonadectomy has little to no effect on, aggression in adult male and female Siberian hamsters when tested in the resident-intruder paradigm [32–36]. Instead, adrenal hormones have been proposed to regulate aggression in SD-housed Siberian hamsters [37–39] and several other seasonal species [40]. It is likely that the conflicting findings in the present study, which indicated that testicular hormones increase aggression, are due to the type of aggression examined. The present experiments tested familiar animals in a neutral setting, which assesses aggression related to social dominance, whereas previous experiments used the resident-intruder test, which assesses territorial aggression toward a stranger. Extrapolating from findings across studies suggests that gonadal hormones play a larger role in regulating social dominance than territorial aggression in Siberian hamsters.
Unexpectedly, removing prepubertal gonadal inhibition, either by SD-rearing or gonadectomy, increased juvenile social play. This finding adds to the growing body of literature that challenges the widely held belief that the gonads, particularly the ovaries, are functionally quiescent during the juvenile period. The prepubertal gonads secrete measurable levels of hormones [41–49], and some evidence suggests that prepubertal ovarian hormones program sexually dimorphic adult behaviors [50–54]. The present findings demonstrate that the prepubertal ovary and testis also impact juvenile social behaviors. In rats, neither prepubertal castration nor prepubertal ovariectomy alters levels of juvenile social play [14,17,55,56, but see 57]. Discrepant findings between Siberian hamsters and rats likely reflect a species difference, and highlight the importance of using multiple, diverse animal models in biological research [58]. Further comparative studies are needed to determine whether findings in Siberian hamsters or those in laboratory rats more accurately reflect the role of prepubertal gonadal hormones in juvenile social development across mammals, including humans.
The underlying substrates that mediate puberty-independent regulation of adolescent social development are not known. The hypothalamic-pituitary-adrenal (HPA) axis, serotonin, and vasopressin have each been proposed to regulate the transition from juvenile social play to adult aggression in Syrian hamsters [6]. HPA axis stress reactivity undergoes developmental changes during adolescence [59], and manipulations that activate type II corticosteroid receptors accelerate the transition from play to aggression in male Syrian hamsters [60,61]. One such manipulation is social subjugation, which, in addition to increasing cortisol and adult aggressive behaviors in juvenile male Syrian hamsters [62,63], increases serotonin innervation and decreases vasopressin content in the anterior hypothalamus [64], an area known to regulate both play and aggression in this species [65–68]. Vasopressin has long been known to regulate aggression in adult animals [69], and has recently been implicated in play [67,70–72]. Brattleboro rats, which contain a mutation in the vasopressin gene, exhibit lower levels of play than wild type littermates while maintaining the same temporal profile of play development [73], and gonadectomy markedly reduces vasopressin mRNA and/or peptide in the bed nucleus of the stria terminalis, medial amygdala, and their projection areas [74,75]. These findings suggest a gonadal-dependent rather than puberty-independent role for vasopressin. Nevertheless, other vasopressin projections are not significantly altered by gonadal manipulations [e.g., 76,77], and intracranial vasopressin manipulations can have opposite effects on play depending on the brain area injected [71], raising the possibility that vasopressin has multiple roles in social development depending on its site of action.
The present study demonstrates how natural adaptations of seasonal species can be used to investigate puberty-dependent and puberty-independent regulation of adolescent social development. Using both photoperiod and gonadal manipulations, we found that puberty-independent mechanisms regulate the timing of adolescent social development, whereas prepubertal and pubertal/adult gonadal hormones modulate levels of age-appropriate social behaviors. At present we can only speculate on the functional significance of this type of synergistic regulation for seasonal species. Perhaps decoupling of puberty (and photoperiod) from the timing of social behavior transitions allows certain developmental milestones (e.g., dispersal) to occur at a given chronological age, regardless of whether the hamster was born at the beginning or end of the breeding season. Conversely, maintaining the link between pubertal hormones and levels of social behaviors would maintain the ability to adjust or fine-tune levels of behaviors to match the seasonal environment. Moving forward, a combination of approaches will be required to elucidate the functional significance and underlying mechanisms of puberty-dependent and puberty-independent regulation. The seasonal-species approach will provide a valuable tool to untangle the many neural, endocrine, and behavioral changes that occur during adolescence. Such experiments will begin to map puberty-dependent and puberty-independent neuroendocrine circuits and specify how they interact to regulate adolescent development.
STAR Methods
Contact For Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Matthew Paul (mjpaul@buffalo.edu).
Experimental Model and Subject Details
Siberian hamsters (Phodopus sungorus) were obtained from the breeding colony of Dr. Eric Bittman at the University of Massachusetts, Amherst, which was originally derived from breeding stock supplied by Dr. Klaus Hoffmann (Munster, Germany). Male and female subjects for this experiment were gestated and reared in either a long day length (LD; 14 h light/day) or short day length (SD; 10 h light/day; lights off at 4pm EST for both photoperiods) and weaned at 19 days of age. At weaning, hamsters were fitted with ear tags for individual identification. Unless otherwise noted, hamsters were housed in same-sex, same-treatment pairs in polypropylene cages (28 × 17 × 12 cm) with Carefresh bedding. Ambient temperature was maintained at 22 ± 4°C. Tap water and Purina Rodent Chow (no. 5008) was available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at UMass Amherst and conducted in accordance to the NIH Guidelines for the Care and Use of Laboratory Animals.
Method Details
Experimental Timeline and Groups
At 15 days of age, LD-reared hamsters underwent gonadectomy (GNX; 14 females, 12 males), sham-surgery (Sham; 6 females, 10 males), or no surgical (Intact; 26 females, 20 males) procedures. SD-reared hamsters did not undergo surgical procedures (Intact; 16 females, 18 males). Social play, aggressive behaviors, and reproductive measures were assessed every 10 (±1) days from postnatal day (P)20 to P60 (LD-reared hamsters) or P120 (SD-reared hamsters). Measures of LD-reared hamsters were not taken past P60 because puberty and the transition from play to aggression were expected to be complete (and were) by this age in LD groups. Behavioral testing and reproductive measures for all groups were conducted concurrently. Data from LD- and SD-Intact males and females were used to test the influence of photoperiod on social development (Experiment 1a), and data from all LD-reared groups (Intact, Sham, and GNX) were used to test the influence of the gonads on social development (Experiment 1b); note that the same LD-Intact hamsters were used in both experiments.
Pair-housed hamsters were checked daily for signs of fighting. Those exhibiting fighting wounds were separated into single-housing conditions and removed from further behavioral testing. This resulted in the removal of 2 male LD-Intact and 1 male LD-Sham pairs at P60 as well as 3 male SD-Intact pairs at P110; data prior to removal were included in all analyses. In a small percentage of hamsters, SD-housing fails to suppress reproductive function [78]. In the present study, this occurred in two pairs of male SD-reared hamsters, therefore, data from these hamsters were not included in analyses. Notably, these SD-reared hamsters underwent the transition from play to aggression around P40-P50 (as was true for all animals regardless of photoperiod) and exhibited high levels of aggression coincident with increased testis size (P50 for one pair and P60 for the other).
Surgeries
Gonadectomies were conducted under isoflurane anesthesia. For castrations, the right and left testes and epididymes were externalized sequentially through a single incision in the abdominal wall. The testicular veins were ligated with sterile sutures, and the testes and epididymes were removed. The abdominal wall and skin were closed, and the wound was treated with antibiotic ointment. Ovariectomies were carried out in a similar manner except that two incisions were made, one on each of the animal’s flanks for the left and right gonad. Hamsters receiving sham surgeries underwent identical procedures except that the testicular/ovarian veins were not ligated and the gonads were not removed. Both sham-operated and gonadectomized animals received a subcutaneous injection of buprenorphine (0.05 mg/kg) as a post-operative analgesic.
Social Interaction Tests
Twenty-five-minute social interaction tests were conducted between 0.5 – 2.5 h after lights off under dim red light. Same-sex, same-treatment cagemate pairs were separated and subsequently reunited the following day in a fresh cage, at which point behaviors were recorded using a Sony Handycam video camera (DCR-SR85). This separation paradigm is commonly used to stimulate play behavior at the time of testing [70,73,79,80]. Play and aggressive behaviors were scored from video by an observer unaware of experimental group assignments using JWatcher software (http:/jwatcher.ucla.edu/) or Observer XT12 (Noldus Information Technology Inc.). Play behavior was scored as the number of play attacks, boxing events, and pins. Aggression was scored as the number of offensive aggressive attacks. See text for description of behaviors, including the distinction between play and aggression. Hamsters within each testing pair were given a unique shave pattern on their dorsal skin to enable tracking of individuals during behavioral scoring. This allowed for the quantification of the proportion of behaviors exhibited by each individual, e.g. play and aggression asymmetry scores. Hamsters were shaved under light isoflurane anesthesia the day before behavior testing.
Reproductive measures
Reproductive development was tracked using estimated testis volume (ETV) or vaginal opening (VO). ETV and VO were recorded at the time of dorsal fur shaving (day before behavioral testing) while hamsters were under light isoflurane anesthesia. For ETV, the skin around the left testis is shaved and wet with 70% ethanol. The length and width of the left testis is then measured externally through the skin using calipers. ETV is quantified as the length × width2, which is highly correlated with testis weight, circulating testosterone, and spermatogenesis [81–83]. VO can be used as a marker of reproductive development [84,85], and photoperiod suppression of VO is associated with low uterine and ovarian weights [86].
Quantification and Statistical Analysis
Play, aggression, and ETV were analyzed by repeated measures ANOVA, with Photoperiod and Sex as independent variables for Experiment 1a and Surgery and Sex as independent variables for Experiment 1b. Photoperiod’s influence on the percentage of hamsters exhibiting VO was assessed using the Chi-Square test at each age. The onset of puberty was defined as the first significant increase in mean ETV from baseline (for male groups) or mean age of vaginal opening (for female groups). Play and aggression were analyzed at the level of the pair, meaning that sample sizes for these measures equaled the number of pairs in each group (Female LD-Intact = 13; Male LD-Intact = 10; Female SD-Intact = 8; Male SD-Intact = 7; Female LD-Sham = 3; Male LD-Sham = 5; Female LD-GNX = 7; Male LD-GNX = 6). Given the low number of Sham pairs, behavioral data from Sham and Intact hamsters were combined into a single Control group for Experiment 1b where differences were not detected between these groups (see Text and Figure 3). There were recording/camera focus issues that prevented behavioral scoring in 6 of the 334 behavior tests (2 Female LD-Intact tests, 1 Male LD-Sham test, 1 Female LD-GNX test, 1 Female SD-Intact test, and 1 Male SD-Intact test). These 6 missing data points along with the 3 data points that could not be tested due to fighting between LD-reared male cagemates (see Experimental Timeline and Groups section above) were replaced with the mean of the experimental group for that time point (total of 9 data points out of 334). Mean replacement was not used for the missing P110 and P120 data points of the 3 SD-reared male cagemate pairs that were removed for fighting because this constituted 43% of Male SD-Intact pairs (3 of 7 pairs). Instead, P110 and P120 time points were not included in SD-reared male behavioral analyses.
Play and aggression asymmetry scores were calculated as: (# play/aggressive behaviors of the more active hamster - # play/aggressive behaviors of the less active hamster)/Total number of play/aggressive behaviors of the pair. If no play/aggressive behaviors were exhibited during a given test, the asymmetry score was undefined and the pair was removed from the analysis for that age. Hence, not all pairs contributed to each time point. Therefore, group differences in play and aggression asymmetry data were analyzed at each age by ANOVA, and differences between P20–30 versus P50–60 were assessed using the nonparametric sign test. Post hoc comparisons were conducted using Fisher’s PLSD when the overall ANOVA yielded significant main effects or interactions. Statistical tests were conducted using Statview 5.0.1 (SAS Institute, Cary, NC), and significance was assumed when P < 0.05.
Supplementary Material
Highlights.
Timing of the adolescent transition from play to aggression is puberty-independent.
Puberty-dependent mechanisms regulate levels of aggression in males.
Prepubertal gonadal hormones suppress levels of juvenile play behavior.
Acknowledgments
We thank Dr. Eric Bittman for providing Siberian hamsters, Dr. Jeffrey Blaustein for help overseeing the animal colony, Christina Rainey for technical assistance, and Dr. Ann-Marie Torregrossa for assistance with statistical analyses. The research was supported by NIMH RO1 MH47538 (to Geert J. de Vries).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.J.P. designed experiments. G.J.D. supervised the project and provided funding. M.J.P. and C.K.P. conducted experiments. M.J.P. and L.M.B. conducted analyses. M.J.P, G.J.D., L.M.B., and C.K.P. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus) J Reprod Fertil. 1978;54:29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- 3.Yellon SM, Goldman BD. Photoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus) Endocrinology. 1984;114:664–70. doi: 10.1210/endo-114-2-664. [DOI] [PubMed] [Google Scholar]
- 4.Goldman L, Swanson HH. Developmental changes in pre-adult behavior in confined colonies of golden hamsters. Dev Psychobiol. 1975;8:137–150. doi: 10.1002/dev.420080206. [DOI] [PubMed] [Google Scholar]
- 5.Fagen R. Animal play behavior. Oxford University Press; 1981. [Google Scholar]
- 6.Wommack JC, Delville Y. Stress, aggression, and puberty: neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain Behav Evol. 2007;70:267–273. doi: 10.1159/000105490. [DOI] [PubMed] [Google Scholar]
- 7.Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes MC, Taravosh-Lahn K, Wommack JC, Delville Y. Characterization of offensive responses during the maturation of play-fighting into aggression in male golden hamsters. Dev Psychobiol. 2007;49:87–97. doi: 10.1002/dev.20183. [DOI] [PubMed] [Google Scholar]
- 9.Pellis SM, Pellis VC. Targets of attack and defense in play-fighting of the Djungarian hamster Phodopus campbelli: Links to fighting and sex. Aggr Behav. 1989;15:217–234. [Google Scholar]
- 10.Castro WL, Matt KS. The importance of social condition in the hormonal and behavioral responses to an acute social stressor in the male Siberian dwarf hamster (Phodopus sungorus) Horm Behav. 1997;32:209–216. doi: 10.1006/hbeh.1997.1423. [DOI] [PubMed] [Google Scholar]
- 11.Scotti MAL, Rendon NM, Greives TJ, Romeo RD, Demas GE. Short-day aggression is independent of changes in cortisol or glucocorticoid receptors in male Siberian hamsters (Phodopus sungorus) J Exp Zool A Ecol Genet Physiol. 2015;323:331–342. doi: 10.1002/jez.1922. [DOI] [PubMed] [Google Scholar]
- 12.Bekoff M, Byers JA. Animal Play: Evolutionary, Comparative and Ecological Perspectives. Cambridge University Press; 1998. [Google Scholar]
- 13.Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 14.Smith LK, Forgie ML, Pellis SM. Mechanisms underlying the absence of the pubertal shift in the playful defense of female rats. Dev Psychobiol. 1998;33:147–156. [PubMed] [Google Scholar]
- 15.Park SU, Bernstein AN, Place NJ. Complementary histological and genomic analyses reveal marked differences in the developmental trajectories of ovaries in Siberian hamsters raised in long- and short-day lengths. Mol Reprod Dev. 2014;81:248–256. doi: 10.1002/mrd.22292. [DOI] [PubMed] [Google Scholar]
- 16.Thor DH, Holloway WR. Play soliciting behavior in prepubertal and postpubertal male rats. Animal Learning & Behavior. 1985;13:327–330. [Google Scholar]
- 17.Smith LK, Field EF, Forgie ML, Pellis SM. Dominance and age-related changes in the play fighting of intact and post-weaning castrated male rats (Rattus norvegicus) Aggr Behav. 1996;22:215–226. [Google Scholar]
- 18.Meaney MJ. The sexual differentiation of social play. Trends Neurosci. 1988;11:54–58. doi: 10.1016/0166-2236(88)90164-6. [DOI] [PubMed] [Google Scholar]
- 19.Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- 20.Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ. Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J Neurosci. 2017;37:10855–10866. doi: 10.1523/JNEUROSCI.1834-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012;227:224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittman EL, Jetton AE, Villalba C, De Vries GJ. Effects of photoperiod and androgen on pituitary function and neuropeptide staining in Siberian hamsters. Am J Physiol. 1996;271:R64–72. doi: 10.1152/ajpregu.1996.271.1.R64. [DOI] [PubMed] [Google Scholar]
- 24.Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the Syrian hamster. Endocrinology. 1976;99:1528–1533. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- 25.Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101:1210–5. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- 26.Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reprodution. New York: Raven Press; 1994. pp. 363–409. [Google Scholar]
- 27.Campbell CS, Finkelstein JS, Turek FW. The interaction of photoperiod and testosterone on the development of copulatory behavior in castrated male hamsters. Physiol Behav. 1978;21:409–15. doi: 10.1016/0031-9384(78)90101-4. [DOI] [PubMed] [Google Scholar]
- 28.Elliott AS, Nunez AA. Photoperiod modulates the effects of steroids on sociosexual behaviors of hamsters. Physiol Behav. 1992;51:1189–93. doi: 10.1016/0031-9384(92)90307-n. [DOI] [PubMed] [Google Scholar]
- 29.Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–61. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson TJ, Prendergast BJ, Nelson RJ. 1.13 - Mammalian Seasonal Rhythms: Behavior and Neuroendocrine Substrates. In: Pfaff DW, Joëls M, editors. Hormones, Brain and Behavior. Third. Oxford: Academic Press; 2017. pp. 371–398. Available at: http://www.sciencedirect.com/science/article/pii/B9780128035924000134 [Accessed August 17, 2017] [Google Scholar]
- 31.Nelson RJ, Kriegsfeld LJ. An Introduction to Behavioral Endocrinology. 5. Sunderland, Massachusetts: Sinauer Associates is an imprint of Oxford University Press; 2016. [Google Scholar]
- 32.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–10. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- 33.Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Scotti MAL, Belén J, Jackson JE, Demas GE. The role of androgens in the mediation of seasonal territorial aggression in male Siberian hamsters (Phodopus sungorus) Physiol Behav. 2008;95:633–640. doi: 10.1016/j.physbeh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Bedrosian TA, Fonken LK, Demas GE, Nelson RJ. Photoperiod-dependent effects of neuronal nitric oxide synthase inhibition on aggression in Siberian hamsters. Horm Behav. 2012;61:176–180. doi: 10.1016/j.yhbeh.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Rendon NM, Amez AC, Proffitt MR, Bauserman ER, Demas GE. Aggressive behaviours track transitions in seasonal phenotypes of female Siberian hamsters. Funct Ecol. 2017;31:1071–1081. doi: 10.1111/1365-2435.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demas GE, Polacek KM, Durazzo A, Jasnow AM. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2004;46:582–91. doi: 10.1016/j.yhbeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Rendon NM, Rudolph LM, Sengelaub DR, Demas GE. The agonistic adrenal: melatonin elicits female aggression via regulation of adrenal androgens. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2015.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rendon NM, Demas GE. Bi-directional actions of dehydroepiandrosterone and aggression in female Siberian hamsters. J Exp Zool A Ecol Genet Physiol. 2016;325:116–121. doi: 10.1002/jez.2001. [DOI] [PubMed] [Google Scholar]
- 40.Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: insights from comparative studies of aggression. J Steroid Biochem Mol Biol. 2015;145:261–272. doi: 10.1016/j.jsbmb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 42.Van den Hurk R, Dijkstra G, De Jong FH. Enhanced serum oestrogen levels and highly steroidogenic, luteinized atretic follicles in the ovaries of the Djungarian hamster (Phodopus sungorus) kept under a short photoperiod from birth. Eur J Endocrinol. 2002;147:701–710. doi: 10.1530/eje.0.1470701. [DOI] [PubMed] [Google Scholar]
- 43.Kabithe EW, Place NJ. Photoperiod-dependent modulation of anti-Müllerian hormone in female Siberian hamsters, Phodopus sungorus. Reproduction. 2008;135:335–342. doi: 10.1530/REP-07-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phalen AN, Wexler R, Cruickshank J, Park SU, Place NJ. Photoperiod-induced differences in uterine growth in Phodopus sungorus are evident at an early age when serum estradiol and uterine estrogen receptor levels are not different. Comp Biochem Physiol A Mol Integr Physiol. 2010;155:115–121. doi: 10.1016/j.cbpa.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannan MA, O’Shaughnessy PJ. Steroidogenesis during postnatal development in the mouse ovary. J Endocrinol. 1991;130:101–106. doi: 10.1677/joe.0.1300101. [DOI] [PubMed] [Google Scholar]
- 46.Bidlingmaier F, Versmold H, Knorr D. Sexual endocrinology of the perinatal period. Paris: Institut National de la Santé et de la Recherche Médicale; 1974. Plasma estrogens in newborns and infants; pp. 299–314. [Google Scholar]
- 47.Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, Klein KO. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: a 10 year experience. J Pediatr Endocrinol Metab. 2006;19:901–909. doi: 10.1515/jpem.2006.19.7.901. [DOI] [PubMed] [Google Scholar]
- 48.Dionyssiou-Asteriou A, Zachari A. Serum sex hormone binding globulin in prepubertal girls and adult women. Clin Physiol Biochem. 1992;9:127–131. [PubMed] [Google Scholar]
- 49.Winter JS, Ellsworth L, Fuller G, Hobson WC, Reyes FI, Faiman C. The role of gonadal steroids in feedback regulation of gonadotropin secretion at different stages of primate development. Acta Endocrinol. 1987;114:257–268. doi: 10.1530/acta.0.1140257. [DOI] [PubMed] [Google Scholar]
- 50.Gerall AA, Dunlap JL, Hendricks SE. Effect of ovarian secretions on female behavioral potentiality in the rat. J Comp Physiol Psychol. 1973;82:449–465. doi: 10.1037/h0034113. [DOI] [PubMed] [Google Scholar]
- 51.Hendricks SE. Role of estrogens and progestins in the development of female sexual behavior potential. In: Gerall AA, Moltz H, Ward IL, editors. Sexual Differentiation Handbooks of Behavioral Neurobiology. Springer; US: 1992. pp. 129–155. [Google Scholar]
- 52.Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21:311–327. doi: 10.1017/s0140525x98001216. Peer Commentary 327-352. [DOI] [PubMed] [Google Scholar]
- 53.Bakker J, Brock O. Early oestrogens in shaping reproductive networks: evidence for a potential organisational role of oestradiol in female brain development. J Neuroendocrinol. 2010;22:728–735. doi: 10.1111/j.1365-2826.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- 54.Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beatty WW, Dodge AM, Traylor KL, Meaney MJ. Temporal boundary of the sensitive period for hormonal organization of social play in juvenile rats. Physiol Behav. 1981;26:241–243. doi: 10.1016/0031-9384(81)90017-2. [DOI] [PubMed] [Google Scholar]
- 56.Meaney MJ, Stewart J. Neonatal androgens influence the social play of prepubescent rats. Hormones and Behavior. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- 57.Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Devel Neurobio. 2009;69:141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yartsev MM. The emperor’s new wardrobe: Rebalancing diversity of animal models in neuroscience research. Science. 2017;358:466–469. doi: 10.1126/science.aan8865. [DOI] [PubMed] [Google Scholar]
- 59.Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wommack JC, Salinas A, Delville Y. Glucocorticoids and the development of agonistic behaviour during puberty in male golden hamsters. J Neuroendocrinol. 2005;17:781–787. doi: 10.1111/j.1365-2826.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 61.Wommack JC, Delville Y. Cortisol controls the pubertal development of agonistic behavior in male golden hamsters via type II corticosteroid receptors. Horm Behav. 2007;51:306–312. doi: 10.1016/j.yhbeh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Wommack JC, Taravosh-Lahn K, David JT, Delville Y. Repeated exposure to social stress alters the development of agonistic behavior in male golden hamsters. Horm Behav. 2003;43:229–236. doi: 10.1016/s0018-506x(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 63.Wommack JC, Delville Y. Repeated social stress and the development of agonistic behavior: individual differences in coping responses in male golden hamsters. Physiol Behav. 2003;80:303–308. doi: 10.1016/j.physbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Delville Y, Melloni RH, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J Neurosci. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- 66.Ferris CF, M RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/Serotonin Interactions in the Anterior Hypothalamus Control Aggressive Behavior in Golden Hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng SY, Delville Y. Vasopressin facilitates play fighting in juvenile golden hamsters. Physiol Behav. 2009;98:242–246. doi: 10.1016/j.physbeh.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 68.Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31:1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- 69.Caldwell HK, Albers HE. Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr Top Behav Neurosci. 2016;27:51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- 70.Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, De Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. Front Behav Neurosci. 2014;8:58. doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38:2554–2561. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bredewold R, Smith CJW, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, de Vries GJ. Atypical social development in vasopressin-deficient Brattleboro rats. eNeuro. 2016;3 doi: 10.1523/ENEURO.0150-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- 75.Miller MA, De Vries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 77.Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521:2321–2358. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- 78.Freeman DA, Goldman BD. Evidence that the circadian system mediates photoperiodic nonresponsiveness in Siberian hamsters: the effect of running wheel access on photoperiodic responsiveness. J Biol Rhythms. 1997;12:100–109. doi: 10.1177/074873049701200202. [DOI] [PubMed] [Google Scholar]
- 79.Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 80.Panksepp J, Beatty WW. Social deprivation and play in rats. Behav Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- 81.Gorman MR. Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol Reprod. 1995;53:110–5. doi: 10.1095/biolreprod53.1.110. [DOI] [PubMed] [Google Scholar]
- 82.Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol Reprod. 1995;53:1169–77. doi: 10.1095/biolreprod53.5.1169. [DOI] [PubMed] [Google Scholar]
- 83.Paul MJ, Park JH, Horton TH, Alvarez MI, Burke MK, Place NJ, Zucker I. Photoperiodic regulation of compensatory testicular hypertrophy in hamsters. Biol Reprod. 2006;75:261–269. doi: 10.1095/biolreprod.106.050781. [DOI] [PubMed] [Google Scholar]
- 84.Haigh GR, Cushing BS, Bronson FH. A novel postcopulatory block of reproduction in white-footed mice. Biol Reprod. 1988;38:623–6. doi: 10.1095/biolreprod38.3.623. [DOI] [PubMed] [Google Scholar]
- 85.Paul MJ, Galang J, Schwartz WJ, Prendergast BJ. Intermediate-duration day lengths unmask reproductive responses to nonphotic environmental cues. Am J Physiol. 2009;296:R1613–1619. doi: 10.1152/ajpregu.91047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Place NJ, Tuthill CR, Schoomer EE, Tramontin AD, Zucker I. Short day lengths delay reproductive aging. Biol Reprod. 2004;71:987–92. doi: 10.1095/biolreprod.104.029900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


