Abstract
Methionine aminopeptidase (MetAP) is a dinuclear metallo-protease responsible for the cleavage of methionine initiator residues from nascent proteins. MetAP activity is necessary for bacterial proliferation and is therefore a projected novel antibacterial target. A compound library consisting of 294 members containing metal-binding functional groups was screened against Rickettsia prowazekii MetAP to determine potential inhibitory motifs. The compounds were first screened against the target at a concentration of 10 μM and potential hits were determined to be those exhibiting greater than 50% inhibition of enzymatic activity. These hit compounds were then rescreened against the target in 8-point dose-response curves and 11 compounds were found to inhibit enzymatic activity with IC50 values of less than 10 μM. Finally, compounds (1 – 5) were docked against RpMetAP with AutoDock to determine potential binding mechanisms and the results were compared with crystal structures deposited within the PDB.
Graphical Abstract

The discovery of chemical therapies for the treatment of bacterial infection continues to be the focus of numerous research programs around the world. Epidemic typhus, a disease often neglected by research programs, is characterized by a characteristic rash that is often accompanied by sustained fever, muscle pain, chills, and delirium. The causative agent of epidemic typhus in humans is Rickettsia prowazekii,1,2 and transmission of R. prowazekii generally occurs in crowded populations with compromised sanitation and hygienic practices, as the parasite is transmitted between hosts via the human body louse.3,4 Because R. prowazekii is an obligate intracellular parasite, there are limited clinically effective antibiotic treatments to treat rickettsioses. Additionally, R. prowazekii strains resistant to both tetracycline and chloramphenicol antibiotics have been reported,5 and the identification of novel targets for the development of anti-rickettsial therapeutics is necessary.
To discover effective inhibitory compounds encompassing novel chemical space and complexity while also possessing the necessary and desirable antibiotic activity, research programs should target pathways responsible for necessary functions of bacterial life and proliferation.6,7 This will lead to two outcomes: first, the optimized antibacterial compounds may exhibit broad spectrum activity against a wide number of distinct bacterial species in the event a universal pathway is successfully regulated, and second, the targeted bacterial species will not have had the opportunity on the evolutionary time scale to develop resistance mechanisms to these compounds.6 Additionally, if a suitably potent inhibitory scaffold is discovered, derivatization could afford potent compounds tailored to target various infective agents. Recently, methionine aminopeptidase (MetAP), a ubiquitous enzyme responsible for the cleavage of methionine initiatory residues from nascent proteins, has been suggested as a potential broad spectrum antibacterial target.8 MetAP is a dinuclear metalloprotease, with demonstrated in vitro activity when Co, Mn, Fe, Zn, and Ni divalent cofactors are utilized.9–11 Additionally, current inhibitory motifs demonstrate a significant correlation with cofactor identity and are generally only potent against enzymes binding specific metals.12,13 Regarding MetAP inhibition resulting in antibacterial outcomes, inactivation of the gene encoding MetAP in Escherichia coli14 and Salmonella typhimurium15 has demonstrated MetAP production and function is necessary for bacterial proliferation. Thus, the inhibition of MetAP is a proposed mechanism of antibacterial activity, and compounds demonstrated to potently bind MetAP have bactericidal activity against species in whole cell in vitro assays.16–19 However, MetAP is present in all eukaryotic life forms, and selective inhibition of bacterial MetAPs is formidable. Indeed, human and bacterial isoforms have significant conservation, with Homo sapiens and E. coli isoforms of MetAP type 1 demonstrating 47% sequence identity.20 Additionally, many of the residues composing the substrate binding pocket are conserved between human and bacterial MetAPs, resulting in difficulties associated with isoform selective binding of inhibitors (Figure 1).20
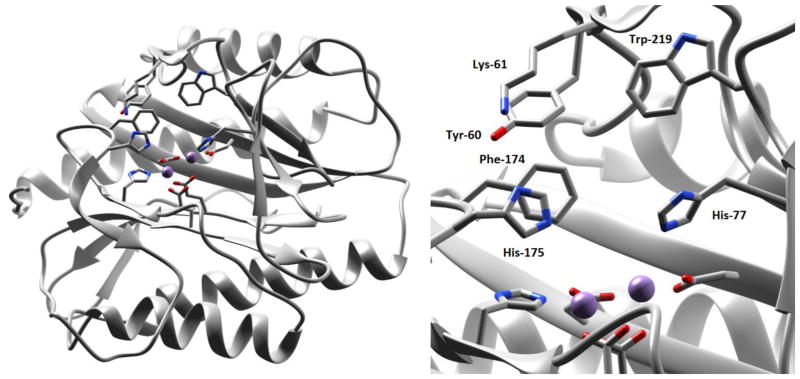
Figure 1.
Left: RpMetAP with active site residues displayed; Mn(II) cofactors are shown as purple spheres. Right: Zoom-in of the RpMetAP active site with bound Mn(II) cofactors (PDB 3MX621).
We therefore set out to determine inhibitory motifs capable of regulating the activity MetAP from R. prowazekii. Because R. prowazekii is an obligate intracellular pathogen, the parasite cannot survive for extended periods outside of a host. Consequently, screening campaigns targeting R. prowazekii must therefore be performed within host cells,21 affording the bacteria an additional resistance mechanism; the host cells must first absorb the compounds, which must then be absorbed by the bacteria. For this reason, R. prowazekii is resistant to a wide number of commercially available antibiotics and few antibiotics are approved to treat this infection.19 Thus, RpMetAP was chosen as the enzymatic target and was expressed and purified according to our previously published methods with slight modifications21–25 (see Supplementary Content). Additionally, the choice of the metal cofactor to be utilized in the enzymatic activity assay is important, as inhibitory values are dependent upon cofactor identity. Here, Mn(II) was used for our enzyme assays as it is the suggested native cofactor in human isoforms 11 and is the cofactor present in available crystal structures of RpMetAP.21
With the enzyme in hand, enzymatic activity was monitored via a fluorescence-based activity assay where methionine-amino-methylcoumarin (Met-AMC) was utilized as the substrate.18,21 Enzymatic activity cleaves the peptide bond of the Met-AMC substrate, resulting in free AMC, which is fluorescent (excitation: 360 nm; emission: 460 nm) (Scheme 1). Because only the product of enzymatic activity is fluorescent, an increase in fluorescence over time is directly related to enzyme activity. The addition of inhibitory compounds and the resulting decrease in fluorescence is a measure of inhibitory activity, where activity is calculated as the slope of a fluorescence versus time plot as compared to an uninhibited control.26
Scheme 1.
The members of the compound library screened against the RpMetAP enzyme were chosen based upon published inhibitory scaffolds and compounds possessing functional groups capable of binding to the metal cofactors.27 Typically, MetAP inhibitors contain functional groups capable of potently coordinating the metal cofactors found within all species of MetAP.20 These functional groups generally include carboxylic acid, 2,2′-bipyridyl, 1,2,4-triazolo, thiazolo, or oxamide derivatives. A commercially available library was therefore assembled from Otava Chemicals catalog to include compounds possessing these (or derivatives of these) functional groups. Additionally, reactive functional groups (alkyl halides, hydrides, oxidizing agents, etc.) were intentionally excluded from the screening library to minimize the likelihood of false positive results.
Initially composed of 294 individual chemical species, the inhibition activities were assessed using a single-point screening (10 μM compound). Compounds found to exhibit inhibition greater than 50% were considered initial hits and were rescreened in 8-point dose-response curves to determine IC50 values. The final determinant for hit motifs was IC50 values <10 μM. A total of 11 compounds met this cut-off (Table 1). The compounds all possess metal binding functional groups (carboxylic acids, nitrogenous heterocycles) and are thus similar to hit motifs already discussed in the literature20 (ex: furoic acid and 1,2,4-triazoles), although none have been previously described as inhibitors of MetAP. For example, 5-phenyl-2-furoic acid-based inhibitors have been extensively described as inhibitors of bacterial MetAPs,13,16–19,28–35 but the corresponding 5-sulfonamidefuroic acids (1) and 5-phenylisoxazol-3-carboxylic acids (2) have not yet been reported as possessing MetAP inhibitory activity. Additionally, aryl carboxylic acids (3 – 5) have not been discussed in any literature reports to date regarding MetAP activity. Biaryl chelating inhibitors similar in structure to (6) have been discovered and often are included in screening libraries,36–42 although compound (6) has not been explicitly reported. Finally, numerous 1,2,4-triazole based inhibitors have been discovered,18,19,30,35,43 although essentially all are derivatives of 5-(benzylthio)-4H-1,2,4-triazol-3-amines. It is therefore interesting that a few phenethanone derivatives (7 – 9) were found to possess inhibitory activity against RpMetAP.
Table 1.
Experimental and predicted binding affinities of compounds identified as hits against RpMetAP
| Cmpd | Structure | IC50a | Predicted Kib |
|---|---|---|---|
| (1) |

|
0.27 ± 0.01 | 0.42c |
| (2) |

|
1.4 ± 0.1 | 0.04c |
| (3) |

|
3.7 ± 0.2 | 0.07c |
| (4) |

|
3.1 ± 0.3 | 0.21c |
| (5) |

|
8.3 ± 0.9 | 0.03c |
| (6) |

|
1.1 ± 0.1 | 21.8d |
| (7) |

|
0.40 ± 0.07 | 0.37d |
| (8) |

|
1.2 ± 0.1 | 0.06d |
| (9) |

|
10.0 ± 1.5 | 4.35d |
| (10) |
|
9.4 ± 0.7 | 3.98d |
| (11) |

|
5.0 ± 0.6 | 1.24d |
IC50 values are expressed in units of μM; Mn(II) cofactors, measured in triplicate
Ki values represent those predicted by docking software (AutoDock)44 and are expressed in units of μM
Ki value for deprotonated carboxylic acid; protonated species were predicted to bind in the same manner with only a slight reduction in predicted binding affinity
No output docking poses correlated well with published crystal structures including inhibitors of similar structure. The Ki values therefore represent those for the predicted lowest energy docking pose.
Of the compounds screened, the most potent was sulfonamide (1) with an inhibitory value (IC50) of 0.27 μM. It is noteworthy that this compound is based upon the 5-aryl-2-furoic acid scaffold that has been extensively demonstrated to exhibit inhibition of MetAP isoforms possessing Mn(II) cofactors. Similar aryl carboxylic acid-based inhibitors demonstrated slightly weaker activity (2 – 5, IC50 = 1.4 – 8.3 μM), although none of these inhibitors have been previously reported to inhibit any MetAP isoform. We have previously identified similar 5-aryl-2-furoic acids possessing inhibitory activity against RpMetAP, notably 5-(2-chlorophenyl)-2-furoic acid (IC50 = 0.6 μM).21 Interestingly, 2-(pyridin-2-yl)thiazole (6) also demonstrated moderate activity (IC50 = 1.1 μM), although similar aryl chelating MetAP inhibitors only have demonstrated activity against MetAP isoforms utilizing Co(II) cofactors.20 Finally, 1,2,4-triazole based inhibitors made up half of the hit compounds identified through this screening campaign. Of these, the most potent inhibitor was found to be derivative (7), (IC50 = 0.40 μM), and the other identified inhibitors (8 – 11) exhibited moderate activity (IC50 = 1.2 – 10 μM). These inhibitory activities are noteworthy, as compounds of this general scaffold typically exhibit the most potent activity against bacterial MetAP isoforms utilizing Co(II) cofactors, although these specific compounds have never been identified as MetAP inhibitors.20
To gain additional insights into the binding mechanisms and corresponding activities of the hit compounds, each was docked into the active site of RpMetAP using the open source docking software AutoDock.44 Co-crystal structures of bacterial MetAPs containing bound inhibitors based upon similar motifs (PDB: 1XNZ,29 1YVM,45 and 3IU919) were first examined to determine the expected binding mechanism of compounds (1 – 11). Briefly, furoic acid based inhibitors are expected to chelate one of the metal cofactors through the acid O atoms, 1,2,4-triazole based inhibitors are expected to coordinate to both metal cofactors through the triazole 1 and 2 N atoms, and biaryl chelating inhibitors (such as (6)) are expected to coordinate to a third metal present in the active site of all crystal structures containing compounds of this class, even for H. sapiens MetAP isoforms (see PDB: 1YVM,45 2G6P,46 4IU6,41 4HXX,42 4IKR,39 4IKS,39 and 4IKT39).
With this information, the compounds were docked against the RpMetAP target (PDB: 3MX621) to determine whether the predicted binding modes mirrored those observed experimentally. For compounds similar in structure to furoic acid based inhibitors (1 – 5), all docked structures predicted the inhibitors bind to the metal cofactors through the acid O atoms (Figure 2). This result was not unexpected, as published crystal structures (PDB: 1XNZ,29 2EVM,28 2Q92,47 2Q93,47 2Q94,47 2Q95,47 2Q9647 and 3IU719) demonstrate this binding mechanism. It is noteworthy, however, that sulfonamide (1) was predicted to coordinate the metal cofactors through the carboxylate, as this compound could conceivably bind via the sulfonamide functionality. Additionally, no specific interactions between the inhibitor and the protein target were predicted to suggest a drastic difference in activity compared to the other compounds of this series (2 – 5). This may explain why (1) was predicted to possess the least potent activity against RpMetAP as compared to the other derivatives (2 – 5), although the compound was the most potent at inhibiting enzymatic activity. Sulfonamide (1) is a good starting point for further exploration, as both the acid and sulfonamide functionalities can be easily modified to determine the effect of both on the inhibitory activity against RpMetAP. Additionally, 5-sulfonamide-2-furoic acids are novel inhibitors of MetAP and appear to exhibit superior activity to the corresponding 5-aryl-2-furoic acid based inhibitors, which are generally the most potent inhibitors of bacterial MetAPs. Unfortunately, the docking method was unable to predict docking poses for compounds (6 – 11) that closely mirror crystal structures from MetAP species containing similar inhibitors.
Figure 2.
Comparison of the known binding mechanism of furoic acid-based inhibitors against EcMetAP (left, PDB: 1XNZ29) and the predicted docking pose of sulfonamide (1) against RpMetAP (right). Compound (1) was predicted to bind via chelation to the Mn(II) cofactors through the acid functionality which mirrors the experimentally determined binding mechanism depicted for EcMetAP containing 5-(2-chlorophenyl)-2-furoic acid.
Through the testing of a screening library composed of a limited number of compounds (294 members), we have identified 11 compounds found to inhibit MetAP expressed from R. prowazekii with IC50 values less than 10 μM. The compounds fit into three distinct classes of inhibitors for bacterial MetAP species, and none of the specific compounds identified herein have been previously reported as inhibitors of this enzyme class. To discern the potential binding interactions that lead to potent inhibition of MetAP activity, the compounds were docked with the currently available crystal structure of RpMetAP (PDB: 3MX621). The docking output for some compounds (1 – 5) suggested similar binding modes to those of currently available crystal structures containing bound inhibitors of similar composition; however, the remaining compounds (6 – 11) were predicted to bind in orientations not currently revealed by crystal structures of MetAP species containing similar compounds. With the continual emergence of bacterial species resistant to currently available therapeutics, the discovery of a class of antibiotics targeting new pathways is paramount. The regulation of MetAP is therefore attractive as the enzymatic activity has been demonstrated as essential for bacterial proliferation and remains relatively unexplored for this purpose. As novel inhibitory compounds are identified, MetAP regulation may prove to be a viable method for the mitigation of bacterial infection, and future efforts should focus on both the discovery of additional inhibitory motifs as well as the exploration of the motifs reported herein.
Supplementary Material
Acknowledgments
This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN272200700057C and HHSN272201200025C. Molecular graphics were generated with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bechah Y, Capo C, Raoult D, Mege J-L. Infection of endothelial cells with virulent Rickettsia prowazekii increases the transmigration of leukocytes. J Infect Dis. 2010;197:142–147. doi: 10.1086/523649. [DOI] [PubMed] [Google Scholar]
- 2.Audia JP. Rickettsial physiology and metabolism in the face of reductive evolution. In: Palmer GH, Azad AF, editors. Intracellular Pathogens II: Rickettsiales. Washington, DC: ASM Press; 2012. pp. 221–242. [Google Scholar]
- 3.Andersson JO, Andersson SG. A century of typhus, lice and Rickettsia. Res Microbiol. 2000;151:143–150. doi: 10.1016/s0923-2508(00)00116-9. [DOI] [PubMed] [Google Scholar]
- 4.Raoult D, Woodward T, Dumler JS. The history of epidemic typhus. Infect Dis Clin North Am. 2004;18:127–140. doi: 10.1016/S0891-5520(03)00093-X. [DOI] [PubMed] [Google Scholar]
- 5.Weiss E, Dressler HR. Increased resistance to chloramphenicol in Rickettsia prowazekii with a note on failure to demonstrate genetic interaction among strains. J Bacteriol. 1962;83:409–414. doi: 10.1128/jb.83.2.409-414.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J., Jr The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 8.Rose JA, Lahiri SD, McKinney DC, Albert R, Morningstar ML, Shapiro AB, Fisher SL, Fleming PR. Novel broad-spectrum inhibitors of bacterial methionine aminopeptidase. Bioorg Med Chem Lett. 2015;25:3301–3306. doi: 10.1016/j.bmcl.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 9.D’souza VM, Holz RC. The methionyl aminopeptidase from Escherichia coli can function as an iron(II) enzyme. Biochemistry. 1999;38:11079–11085. doi: 10.1021/bi990872h. [DOI] [PubMed] [Google Scholar]
- 10.Walker KW, Bradshaw RA. Yeast methionine aminopeptidase I can utilize either Zn2+ or Co2+ as a cofactor: A case of mistaken identity? Protein Sci. 1998;7:2684–2687. doi: 10.1002/pro.5560071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Sheppard GS, Lou P, Kawai M, Park C, Egan DA, Schneider A, Bouska J, Lesniewski R, Henkin J. Physiologically relevant metal cofactor for methionine aminopeptidase-2 is manganese. Biochemistry. 2003;42:5035–5042. doi: 10.1021/bi020670c. [DOI] [PubMed] [Google Scholar]
- 12.Chai SC, Wang W-L, Ye Q-Z. Fe(II) is the native cofactor for Escherichia coli methioinine aminopeptidase. J Biol Chem. 2008;283:26879–26885. doi: 10.1074/jbc.M804345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q, Huang M, Nan F, Ye Q. Metalloform-selective inhibition: Synthesis and structure–activity analysis of Mn(II)-form-selective inhibitors of Escherichia coli methionine aminopeptidase. Bioorg Med Chem Lett. 2005;15:5386–5391. doi: 10.1016/j.bmcl.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Chang S-YP, McGary EC, Chang S. Methionine aminopeptidase gene of Escherichia coli is essential for cell growth. J Bacteriol. 1989;171:4071–4072. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CG, Kukral AM, Miller JL, Movva NR. pepM is an essential gene in Salmonella typhimurium. J Bacteriol. 1989;171:5215–5217. doi: 10.1128/jb.171.9.5215-5217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W-L, Chai SC, Ye Q-Z. Synthesis and biological evaluation of salicylate-based compounds as a novel class of methionine aminopeptidase inhibitors. Bioorg Med Chem Lett. 2011;21:7151–7154. doi: 10.1016/j.bmcl.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huguet F, Melet A, Alves de Sousa R, Lieutaud A, Chevalier J, Maigre L, Deschamps P, Tomas A, Leulliot N, Pages JM, Artaud I. Hydroxamic acids as potent inhibitors of Fe(II) and Mn(II) E. coli methionine aminopeptidase: Biological activities and X-ray structure of oxazole hydroxamate-EcMetAP-Mn complexes. Chem Med Chem. 2012;7:1020–1030. doi: 10.1002/cmdc.201200076. [DOI] [PubMed] [Google Scholar]
- 18.Wangtrakuldee P, Byrd MS, Campos CG, Henderson MW, Zhang Z, Clare M, Masoudi A, Myler PJ, Horn JR, Cotter PA, Hagen TJ. Discovery of inhibitors of Burkholderia pseudomallei methionine aminopeptidase with antibacterial activity. ACS Med Chem Lett. 2013;4:699–703. doi: 10.1021/ml400034m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Chai SC, Ye Q. Catalysis and inhibition of Mycobacterium tuberculosis methionine aminopeptidase. J Med Chem. 2010;53:1329–1337. doi: 10.1021/jm901624n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helgren TR, Wangtrakuldee P, Staker BL, Hagen TJ. Advances in bacterial methionine aminopeptidase inhibition. Curr Top Med Chem. 2016;16:397–414. doi: 10.2174/1568026615666150813145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helgren TR, Chen C, Wangtrakuldee P, Edwards TE, Staker BL, Abendroth J, Sankaran B, Housley NA, Myler PJ, Audia JP, Horn JR, Hagen TJ. Rickettsia prowazekii methionine aminopeptidase as a promising target for the development of antibacterial agents. Bioorg Med Chem. 2017;25:813–824. doi: 10.1016/j.bmc.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myler PJ, Stacy R, Stewart L, Staker BL, Van Voorhis WC, Varani G, Buchko GW. The Seattle Structural Genomics Center for Infectious Disease (SSGCID) Infect Dis Drug Targets. 2009;9:493–506. doi: 10.2174/187152609789105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serbzhinskiy DA, et al. Structure of an ADP-ribosylation factor, ARF1, from Entamoeba histolytica bound to Mg+2-GDP. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2015;71(Pt 5):594–599. doi: 10.1107/S2053230X15004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacy R, Begley DW, Phan I, Staker BL, Van Voorhis WC, Varani G, Buchko GW, Stewart LJ, Myler PJ. Structural genomics of infectious disease drug targets: the SSGCID. Acta Crystallogr Sect F Struct Biol Commun. 2011;67:979–984. doi: 10.1107/S1744309111029204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan CM, Bhandari J, Napuli AJ, Leibly DJ, Choi R, Kelley A, Van Voorhis WC, Edwards TE, Stewart LJ. High-throughput protein production and purification at the Seattle Structural Genomics Center for Infectious Disease. Acta Crystallogr Sect F Struct Biol Commun. 2011;67:1010–1014. doi: 10.1107/S1744309111018367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant SK, Skylar JG, Cummings RT. Development of novel assays for proteolytic enzymes using rhodamine-based fluorogenic substrates. J Biomol Screen. 2002;7:531–540. doi: 10.1177/1087057102238627. [DOI] [PubMed] [Google Scholar]
- 27.Martin DP, Puerta DT, Cohen SM. Metalloprotein inhibitors. In: Storr T, editor. Ligand Design in Medicinal Inorganic Chemistry. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2014. pp. 375–403. [Google Scholar]
- 28.Xie S-X, Huang W-J, Ma Z-Q, Huang M, Hanzlik RP, Ye Q-Z. Structural analysis of metalloform-selective inhibition of methionine aminopeptidase. Acta Crystallogr Sect D Struct Biol. 2006;62:425–432. doi: 10.1107/S0907444906003878. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q-Z, Xie S-X, Huang M, Huang W-J, Lu J-P, Ma Z-Q. Metalloform-selective inhibitors of Escherichia coli methionine aminopeptidase and X-ray structure of a Mn(II)-form enzyme complexed with an inhibitor. J Am Chem Soc. 2004;126:13940–13941. doi: 10.1021/ja045864p. [DOI] [PubMed] [Google Scholar]
- 30.Yuan H, Chai SC, Lam CK, Xu HH, Ye Q-Z. Two methionine aminopeptidases from Acinetobacter baumannii are functional enzymes. Bioorg Med Chem Lett. 2011;21:3395–3398. doi: 10.1016/j.bmcl.2011.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vedantham P, Zhang M, Gor PJ, Huang M, Georg GI, Lushington GH, Mitscher LA, Ye Q-Z, Hanson PR. Studies towards the synthesis of methionine aminopeptidase inhibitors: Diversification utilizing a ROMP-derived coupling reagent. J Comb Chem. 2008;10:195–203. doi: 10.1021/cc7000869. [DOI] [PubMed] [Google Scholar]
- 32.Chai SC, Ye Q-Z. A cell-based assay that targets methionine aminopeptidase in a physiologically relevant environment. Bioorg Med Chem Lett. 2010;20:2129–2132. doi: 10.1016/j.bmcl.2010.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vedantham P, Guerra JM, Schoenen F, Huang M, Gor PJ, Georg GI, Wang JL, Neuenswander B, Lushington GH, Mitscher LA, Ye Q-Z, Hanson PR. Ionic immobilization, diversification, and release: Application to the generation of a library of methionine aminopeptidase inhibitors. J Comb Chem. 2008;10:185–194. doi: 10.1021/cc700085c. [DOI] [PubMed] [Google Scholar]
- 34.Huang M, Xie S-X, Huang Q-Q, Nan F-J, Ye Q-Z. Inhibition of monometalated methioinine aminopeptidase: Inhibitor discovery and crystallographic analysis. J Med Chem. 2007;50:5735–5742. doi: 10.1021/jm700930k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J-P, Ye Q-Z. Expresssion and characterization of Mycobacterium tuberculosis methionine aminopeptidase type 1a. Bioorg Med Chem Lett. 2010;20:2776–2779. doi: 10.1016/j.bmcl.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishor C, Gumpena R, Reddi R, Addlagatta A. Structural studies of Enterococcus faecalis methionine aminopeptidase and design of microbe specific 2,2′-bipyridine based inhibitors. Med Chem Comm. 2012;3:1406–1412. [Google Scholar]
- 37.Altmeyer MA, Marschner A, Schiffmann R, Klein CD. Subtype-selectivity of metal-dependent methioinine aminopeptidase inhibitors. Bioorg Med Chem Lett. 2010;20:4038–4044. doi: 10.1016/j.bmcl.2010.05.093. [DOI] [PubMed] [Google Scholar]
- 38.Musonda CC, Whitlock GA, Witty MJ, Brun R, Kaiser M. Synthesis and evaluation of 2-pyridyl pyrimidines with invitro antiplasmodial and antileishmanial activity. Bioorg Med Chem Lett. 2009;19:401–405. doi: 10.1016/j.bmcl.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 39.Kishor C, Arya T, Ravikumar R, Chen X, Saddanapu V, Marapaka AK, Gumpena R, Ma D, Liu JO, Addlagatta A. Identification, biochemical and structural evaluation of species-specific inhibitors against type I methionine aminopeptidases. J Med Chem. 2013;56:5295–5305. doi: 10.1021/jm400395p. [DOI] [PubMed] [Google Scholar]
- 40.Schiffmann R, Neugebauer A, Klein CD. Metal-mediated inhibition of Escherichia coli methionine aminopeptidase: Structure - activity relationships and development of a novel scoring function for metal - ligand interactions. J Med Chem. 2006;49:511–522. doi: 10.1021/jm050476z. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Bhat S, Gabelli SB, Chen X, Miller MS, Nacev BA, Cheng YL, Meyers DJ, Tenney K, Shim JS, Crews P, Amzel LM, Ma D, Liu JO. Pyridinylquinazolines selectively inhibit human methionine aminopeptidase-1 in cells. J Med Chem. 2013;56:3996–4016. doi: 10.1021/jm400227z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P, Yang X, Zhang F, Gabelli SB, Wang R, Zhang Y, Bhat S, Chen X, Furlani M, Amzel LM, Liu JO, Ma D. Pyridinylpyrimidines selectively inhibit human methionine aminopeptidase-1. Bioorg Med Chem. 2013;21:2600–2617. doi: 10.1016/j.bmc.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oefner C, Douangamath A, D’Arcy A, Häfeli S, Mareque D, Mac Sweeney A, Padilla J, Pierau S, Schulz H, Thormann M, Wadman S, Dale GE. The 1.15 Å crystal structure of the Staphylococcus aureus methionyl-aminopeptidase and complexes with triazole based inhibitors. J Mol Biol. 2003;332:13–21. doi: 10.1016/s0022-2836(03)00862-3. [DOI] [PubMed] [Google Scholar]
- 44.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffmann R, Heine A, Klebe G, Klein CDP. Metal ions as cofactors for the binding of inhibitors to methionine aminopeptidase: A critical view of the relevance of in vitro metalloenzyme assays. Angew Chemie Int Ed. 2005;44:3620–3623. doi: 10.1002/anie.200500592. [DOI] [PubMed] [Google Scholar]
- 46.Hu X, Addlagatta A, Matthews BW, Liu JO. Identification of pyridinylpyrimidines as inhibitors of human methionine aminopeptidases. Angew Chemie Int Ed. 2006;45:3772–3775. doi: 10.1002/anie.200600757. [DOI] [PubMed] [Google Scholar]
- 47.Ma Z-Q, Xie S-X, Huang Q-Q, Nan F-J, Hurley TD, Ye Q-Z. Structural analysis of inhibition of E. coli methionine aminopeptidase: implication of loop adaptability in selective inhibition of bacterial enzymes. BMC Struct Biol. 2007;7:84. doi: 10.1186/1472-6807-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.