Abstract
Purpose of review
Although viral infections of the central nervous system (CNS) are known to acutely cause pathology in the form of cytokine-mediated neural tissue damage and inflammation, the pathophysiology of neurologic sequelae after viral clearance is incompletely understood.
Recent findings
Alterations in microglial and glial biology in response to initial infiltration of immune cells that persist within the CNS have recently been shown to promote neuronal dysfunction and cognitive deficits in animal models of viral encephalitis.
Summary
The current review summarizes the current knowledge on the possible role of innate immune signaling during acute infections as triggers of neurologic sequelae that persist, and may even worsen, after clearance of viral infections within the CNS.
Keywords: encephalomyelitis, Guillain-Barré syndrome, HIV-associated neurocognitive disorder, microcephaly
INTRODUCTION
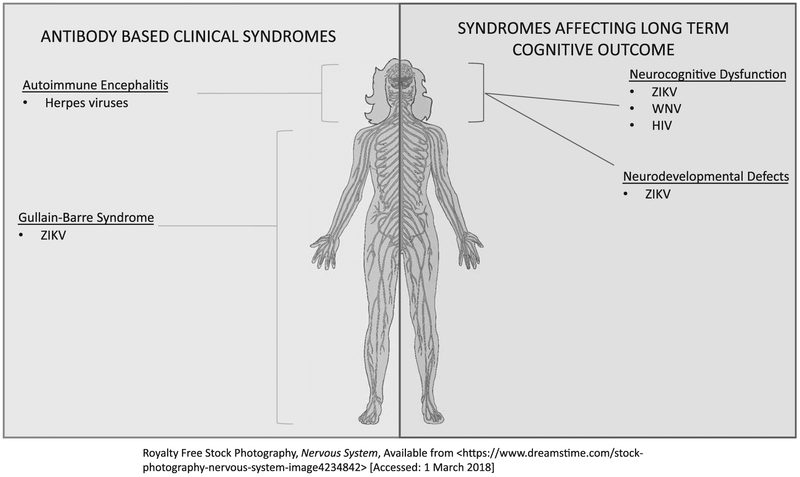
Emerging virus epidemics and their neurologic sequelae of the past few years have demonstrated that viruses cause central nervous system (CNS) pathology via multiple mechanisms. The most common way in which viruses can affect the nervous system is by encephalomyelitis in the acute phase of infection. However, subacute and chronic sequelae have now been demonstrated for many viral infections, including long-term cognitive impairment, acute immune neuropathies such as Gullain–Barre syndrome and complications of congenital infection including microcephaly and cognitive impairment. These subacute and chronic complications can persist long after viral clearance and can be related to viral tropism for specific brain regions, the immune responses triggered by viral infection or persistent presence of viral RNA rather than the virus itself. In this review, we highlight some of the major advances in the understanding of mechanisms by which viral infections cause CNS pathology beyond the acute phase of infection. Figure 1 illustrates the localization of virus-related neurologic syndromes we review in this article. We examine the effect of Zika virus (ZIKV) infection on neural progenitor cells in both congenital and adult mouse models. We also review the recent increase in acute immune-mediated neuropathy associated with ZIKV outbreaks and mechanisms discovered in a mouse model study evaluating how ZIKV might cause acute immune neuropathy. In addition, we highlight a mouse model for cognitive impairment in West Nile virus (WNV) that closely mimics the incidence and spatial learning deficits seen in human infections along with the mechanisms proposed to cause such impairment. We review recent data examining the controversial link between herpes simplex virus (HSV) and autoimmune encephalitis. Finally, we review a clinical imaging study as well as a mouse model for HIV-associated neuro-cognitive disorder (HAND).
FIGURE 1.
Localization of virus-related neurologic syndromes beyond encephalomyelitis. WNV, West Nile virus; ZIKV, Zika virus.
NEURODEVELOPMENTAL DEFECTS AND ZIKA VIRUS
Infection with ZIKV, a single-stranded RNA flavivirus with an evolving epidemic worldwide, continues to demonstrate a variety of long-lasting effects on the CNS in some patients. Through December 2016, the WHO reported 69 countries or territories having reported mosquito-borne ZIKV transmission since January 2015, 29 countries or territories had reported ZIKV congenital syndrome and 20 countries or territories have reported at least one case of Guillain–Barré syndrome (GBS) associated with ZIKV infection [1].
The mechanisms for tropism of ZIKV for the developing nervous system remain unclear. However, Chavali et al. [2■] reported that the protein Musashi 1 (MSI-1) is highly expressed in neural progenitor cells, which have been shown to be targeted by ZIKV [3]. In various cell lines with defective MSI-1, ZIKV replication was decreased. Of interest, exome sequencing of two cases of isolated familial microcephaly revealed that MSI-1 mutations, including those that limit ZIKV replication, were also implicated as pathogenic mutations, further supporting a possible role for MSI-1 in the ZIKV-associated tropism for neural progenitor cells and in the microcephaly phenotype.
NEUROLOGIC SYNDROMES AFTER CENTRAL NERVOUS SYSTEM VIRAL INFECTIONS IN ADULTS
West Nile virus
WNV is another Flavivirus that has demonstrated sequelae of neuroinvasive disease beyond acute encephalomyelitis. Over 50% of patients who survive neuroinvasive disease will have chronic cognitive deficits that may worsen over time [4]. Recently, a murine model of human WNV neuroinvasive disease (WNND) was developed with similar rates of survival as well as neurocognitive deficits. Vasek et al. [5■■] demonstrated that WNV-recovered mice that exhibit poor learning show increased expression of genes within the hippocampus that drive microglial activation and phagocytosis of synapses, including members of the classical complement cascade. For example, C1qa was upregulated in microglia, and infected neurons, whereas C3d protein was localized to VGlut1-positive presynaptic terminals, which were eliminated in recovered animals. The validity of this model is supported by the fact that in both murine and human postmortem pathology, there is significant loss of presynaptic terminals within the CA3 region of the hippocampus [5■■]. Of interest, hippocampal and total brain volumes and total numbers of neurons after recovery from infection do not differ between mock-infected and WNV-NS5-E218A-infected animals, suggesting more subtle defects in synapse physiology underlie cognitive deficits. These data suggest that complement-mediated loss of synapses may promote memory dysfunction during recovery from WNND.
In a another recent study, Garber et al. [6■] showed that WNND-recovered mice exhibit decreased neurogenesis in favor of increased astrogenesis that persisted up to 30 days postinfection. These animals also showed lack of hippocampal synapse recovery detected in Vasek et al. [5■■]. Transcriptional profiling in WNND-recovered mice detected increased expression of antineurogenic cytokines, including IL-1. In IL-lRl-deficient, WNND-recovered mice, normal neurogenesis, recovery of presynaptic termini and resistance to spatial learning defects were demonstrated. Improved spatial learning was also demonstrated in infected mice treated with an IL-1R1 antagonist during acute WNND. Cytokine production in ex-vivo isolated microglia and astrocytes revealed astrocytes to be the predominant source of IL-1. The authors suggest that preferential generation of proinflammatory astrocytes impairs neuronal progenitor cell homeostasis via expression of IL-1 and is associated with the long-term cognitive consequences of WNND. This study also demonstrates a therapeutic target in prevention of long-term cognitive deficits in WNND.
Zika virus
Infection with ZIKV has also been associated with rare cognitive dysfunction in adult humans [7,8]. Murine models of ZIKV encephalitis in adult animals have demonstrated that neural progenitor cells in the adult mouse can be infected by ZIKV and may have impaired proliferation after ZIKV infection. In a study by Li et al. [3], they showed that in an interferon regulatory factor triple knockout mouse model infected with FSS13025, a ZIKV 2010 Cambodian isolate, via retro-orbital injection, there were high viral titers in brain regions with high densities of proliferative cells, including the subventricular zone (SVZ) and subgranular zone (SGZ), the two regions in which adult neurogenesis occurs. In addition, ZIKV staining colocalized with caspase-3 activated cells in neural progenitor cells in the SVZ and SGZ. The authors concluded from this finding that ZIKV infection may induce apoptotic cell death in adult in neural progenitor cells in these regions. This study also examined cell proliferation post-ZIKV infection by examining immunostaining using ethynyl deoxyuridine (EdU), a thymidine analog, as well as the cell cycle markers, Ki-67 and phospho-Histone H3. ZIKV-infected mice had decreased numbers of cells positive for EdU, Ki-67 and phosphor-histone H3 compared with mock infected mice, despite the fact that total brain volumes and sizes of the SGZ and SVZ were not different. Given the importance of the SGZ in the hippocampus in memory and learning, this study demonstrates that there may be long-term cognitive consequences for adults with neuroinvasive ZIKV infection, and longer term clinical studies will be needed to see if this animal model will reflect human complications of neuroinvasive ZIKV infections in adults.
HIV-associated neurocognitive disorder
Combined antiretroviral therapy has dramatically improved the lifespan and quality of life for HIV-positive patients. As patients are living longer with HIV, HAND, which is characterized by memory impairment and cognitive dysfunction, has been recognized in patients even with well controlled disease, and the mechanisms for this are unknown. This year, in a brain imaging study by Sanford et al. [9■], patients who were HIV-positive, yet aviremic and on combination antiretroviral therapy (cART), had decreased cortical thickness and lower subcortical brain volumes than matched HIV-negative controls. However, when followed over a 2-year period, they had similar brain atrophy velocities, indicating that although there may be factors related to initial infection that lead to an early degenerative process, cART may still slow the process of neurodegeneration over longer periods of time.
In another study aiming to look at the mechanism for neurodegeneration in HIV-positive patients, Dickens et al. [10■] examined the expression of Tat, a nonstructural protein encoded by the HIV genome and its possible role in neurodegeneration. They examined Tetracycline-inducible glial fibrillary acidic protein-driven HIV-1 Tat transgenic mice (rtTA-Tat) from two age groups (3–5 and 11–12 months) and aged matched control mice expressing only the Tetracycline-inducible transgene (rtTA) promoter. Using in-vivo MRI, they found that in 11–12-month-old doxycycline-na’ive rtTA-Tat mice, total ventricular volume was increased, primary motor cortex thickness was reduced, and dentate gyrus thickness was increased. Ventricular volume and primary motor cortex findings were attributed to neurodegeneration, whereas dentate gyrus enlargement was hypothesized to the attributed to edema secondary to reactive gliosis. Ki-67 expression was not increased in doxycycline-na’ive rtTA-Tat mice, further supporting that neurogenesis was not increased in this group but perhaps enlargement was due to edema. Doxycycline-naive rtTA-Tat mice had reductions in cortical levels of the neuronal-specific cytoskeletal protein βIII-Tubulin and the presynaptic vesicle protein p38 (Synaptophysin), and the postsynaptic density 95 protein compared with rtTA mice, suggesting a decrease in cortical synaptic density in concert with the decrease in thickness of the primary motor cortex. Thus far, this has only been studied in this model of Tat protein expression; however, this study demonstrates an exciting avenue of research for understanding the long-term neurologic consequences of HIV infection in the face of absent viremia.
ANTIBODY-BASED CLINICAL SYNDROMES
Herpesviridae
Herpesviridae, double-stranded DNA viruses including HSV and varicella zoster virus (VZV), have long been known to cause infectious encephalitis and cranial neuropathies. Recent literature has emerged reporting their association with the generation of anti-N-methyl-D-aspartate receptor antibodies, causing postviral encephalitis and epilepsy in both adults and children [11–13]. In all cases, immunotherapies have been tried, all with some benefit. However, Poulheim et al. [14] evaluated 113 patients with epilepsy and suspected autoimmune encephalitis to see if there was an under-recognition of postinfectious autoimmunity. Cerebrospinal spinal fluid from these patients was evaluated by real-time PCR for HSV 1/2, cytomegalovirus, epstein barr virus, VZV, human herpesvirus (HHV)-6A, HHV-6B, HHV-7 and HHV-8 as well as autoantibodies by immunofluorescence. They found antineuronal antibodies in 48 patients and no autoantibodies in 65 patients. Real-time PCR analysis revealed positive results for HSV in three patients who were autoantibody-negative patients; these patients had no evidence for further virus DNA. The authors concluded that this argues against herpes infection as a trigger for autoimmune-mediated encephalitis but also raise the possibility of appearance of autoantibodies after a short period of active virus infection. Given the exact mechanism of this postviral immunemediated disease is unknown, additional investigation is warranted to identify an optimal treatment.
Zika virus
Zika infection also continues to be implicated in the autoimmune-mediated disease, GBS, in which patients exhibit motor with or without sensory deficits. Surges of GBS cases were temporally associated with ZIKV epidemics over the past 3 years [15–17]. Jurado et al. [18] showed in a murine model of ZIKV infection (Infar—/— with Zika Cambodia strain infection) that depletion of CD8+ T cells, but not CD4+ T cells, led to higher viral titers and hindlimb paralysis, a phenotype thought to be akin to human GBS. This may suggest that ZIKV-associated GBS is due to an immune response that is more complex that the classical antibody-mediated GBS.
Limitations and future directions
In this review, we highlight some of the clinical and animal model studies that contribute to our understanding of the effects of viral infection on the central nervous system. Overwhelmingly, the trend is toward understanding immune responses to viral infections and how the immune mechanisms triggered to assist in viral clearance may cause long-lasting consequences for the CNS after neuroinvasive infection has resolved. Although insights provided by these studies are promising, some caveats must be noted. For example, in mouse models of ZIKV infection, interferon-signaling is altered to produce neuroinvasive disease. Interferon has been suggested in other studies to be related to neurodegeneration [19], so modification of interferon signaling could impact neuronal survival even in the absence of viral infections.
CONCLUSION
Viral infections can have varied neurologic consequences that may last beyond the acute phase of infection. This can be influenced both by tropism for certain brain regions as well the immune response to the virus and any pathologic sequelae of the immune responses intended to eradicate the infection. Animal models continue to be an important vehicle for understanding the complex system in which viruses invade the central nervous system, cause damage and trigger a cascade of immune responses that are both immediate and long-lasting. In addition, CNS cytokine milieus, immune cell regulation of neurotransmitter systems and synaptic plasticity, and newly discovered antimicrobial roles for biomarkers of neurodegenerative diseases [20] suggest that the immune and nervous systems may have arisen and continue to evolve as a single system. Examples from the evolutionary literature comparing immune traits in humans versus chimpanzees may support this notion. Many differences between humans and nonhuman primates occur from changes in gene regulation, including 1356 human-specific conserved noncoding sequences (CNC), which comprise 0.3% of the human genome. Included among these CNCs is a single amino acid substitution at position 2524 in the nuclear factor of activated T cells binding elements of the promoter of IL-4 [21], a protein recently shown to be critical for performance of cognitive tasks [22], This polymorphism leads to three-fold greater expression of this cytokine in humans versus the great apes, suggesting it may contribute to improved cognition in humans over chimpanzees. Future studies focused on innate immune mechanisms of brain function in health and disease will likely uncover new mechanisms and targets to improve recovery from CNS infections and promote cognitive function in human populations.
KEY POINTS.
Activation of the innate immune system during acute viral infection may play a key role in the neurologic sequelae that persist even after viral clearance.
Study of viral tropism for specific brain regions will lead to better understanding of the mechanisms by which viruses alter brain structure and function after viral infection.
The possible association between viral infection and autoimmune encephalitides continues to be a controversial and poorly understood entity, requiring continued study of the immune system response during and after acute viral infection.
Acknowledgements
We would like to thank the members of the Klein Lab for their intellectual discussions of the topics herein.
Financial support and sponsorship
R.S.K. acknowledges the following funding: NIH/NINDS R01NS052632, R21NS096363, and P01 NS059560; NIH/NIAID U19 AI083019 and R01AI101400.
Footnotes
Conflicts of interest
S.C.A. is a coinventor and has previously received royalties on patent US 9235887 B2: Classification of biological tissue by multimode data registration, segmentation and characterization.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.WHO. WHO’s response to Zika virus and associated oomplioations - report to donors. Feb-Dec 2016; who.int. [Google Scholar]
- 2.■.Chavali PL, Stojic L, Meredith LW, et al. Neurodevelopmental protein Musashi 1 interacts with the Zika genome and promotes viral replication. Science 2017; 357:83–88.Discusses role of Musashi 1 protein in Zika virus associated tropism for neural progenitor cells and in microcephaly.
- 3.Li C, Xu D, Ye Q, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 2016; 19:120–126. [DOI] [PubMed] [Google Scholar]
- 4.Samaan Z, McDermid Vaz S, Bawor M, et al. Neuropsychological impact of West Nile virus infection: an extensive neuropsychiatric assessment of 49 oases in Canada. PLoS One 2016; 11:e0158364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.■■.Vasek MJ, Garber C, Dorsey D, et al. A complement–microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016; 534:538.Presents a novel mouse model of West Nile neuroinvasive disease (WNND) and its associated cognitive deficits; also demonstrates the model’s use in understanding complement-mediated synapse loss in WNND.
- 6.■.Garber C, Vasek MJ, Vollmer LL, et al. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol 2018; 19:151–161.Study suggests preferential generation of proinflammatory astrocytes impairs neural progenitor cell homeostasis via IL-1 expression and is associated with long-term cognitive dysfunction in WNND.
- 7.Zucker J, Neu N, Chiriboga CA, et al. Zika virus-associated cognitive impairment in adolescent, 2016. Emerg Infect Dis 2017; 23:1047–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuele N, Castilletti C, Balestra P, et al. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis 2016; 22:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.■.Sanford R, Fellows LK, Ances BM, Collins DL. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol 2018; 75:72–79.Demonstrates that combined antiretroviral therapy may slow neurodegeneration.
- 10.■.Dickens AM, Yoo SW, Chin AC, et al. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep 2017; 7:7748.Study of the nonstructural HIV-assooiated protein, Tat, and its possible role in HIV-associated neurodegeneration.
- 11.Popkirov S, Ismail FS, Grönheit W, et al. Progressive hippocampal sclerosis after viral encephalitis: potential role of NMDA receptor antibodies. Seizure 2017; 51:6–8. [DOI] [PubMed] [Google Scholar]
- 12.Strippel C, Mönig C, Golombeck KS, et al. Treating refractory postherpetic anti-N-methyl-d-aspartate receptor encephalitis with rituximab. Oxf Med Case Rep 2017; 2017:omx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothur K, Gill D, Wong M, et al. Cerebrospinal fluid cyto-/chemokine profile during acute herpes simplex virus induced anti-N-methyl-d-aspartate receptor encephalitis and in chronic neurological sequelae. Dev Med Child Neurol 2017; 59:806–814. [DOI] [PubMed] [Google Scholar]
- 14.Poulheim F, Esposito L, Eiger CE, et al. Large-scale analysis of herpesviridae in epilepsy-patients with signs of autoimmune encephalitis. Seizure 2017; 53:100–102. [DOI] [PubMed] [Google Scholar]
- 15.Parra B, Lizarazo J, Jiménez-Arango JA, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med 2016; 375:1513–1523. [DOI] [PubMed] [Google Scholar]
- 16.Sebastián UU, Ricardo AVA, Alvarez BC. Zika virus-induced neurological critical illness in Latin America: severe Guillain–Barre syndrome and encephalitis. J Crit Care 2017; 42:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Styczynski AR, Malta JMAS, Krow-Lucal ER, et al. Increased rates of Guillain–Barré syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trap Dis 2017; 11:e0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurado KA, Yockey LJ, Wong PW, et al. Antiviral CD8 T cells induce Zika-virus-assooiated paralysis in mice. Nat Microbiol 2018; 3:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ejlerskov P, Hultberg JG, Wang J, et al. Lack of neuronal IFN-β-IFNAR causes Lewy Body- and Parkinson’s disease-like dementia. Cell 2015; 163:324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar DKV, Choi SH, Washicosky KJ, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 2016; 8:340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bird CP, Stranger BE, Liu M, et al. Fast-evolving noncoding sequences in the human genome. Genome Biol 2007; 8:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol 2012; 189:4213. [DOI] [PMC free article] [PubMed] [Google Scholar]