Abstract
Purpose
To report two rare cases of filamentous fungal keratitis.
Methods
Two non-consecutive patients presented with suspicious fungal keratitis. After performing the smear and culture, medical therapy was started for them. They underwent slit photography and in vivo confocal microscopy (IVCM) in their follow-up visits.
Results
The patients were 33-year-old and 56-year-old farmer men. They both mentioned a history of ocular trauma by plants. During their follow-up visits, corneal infiltration density and fungal hyphae density decreased in slit-lamp biomicroscopy and IVCM, respectively. The corresponding organisms were Pseudallescheria boydii (P. boydii) and Colletotrichum coccodes.
Conclusions
It is important to be aware of these rare organisms and their antibiotic susceptibility. There was not any specific confocal feature for the presented fungal keratitis that was different from other filamentous fungal hyphae; however, confocal scan is a good choice to follow the response to the treatment.
Keywords: Fungal keratitis, Pseudallescheria boydii, Colletotrichum coccodes
Introduction
Microbial keratitis is one of the major causes of ocular morbidity and a potentially leading cause of blindness. It is reported that in warm, tropical climates, 30–62% of all microbial keratitis cases are caused by fungi,1, 2, 3 but they are rare in regions with temperate climates.4 Trauma with plants is one of the main predisposing factors in fungal keratitis.1, 2, 3, 4 Common causes include Candida spp., Fusarium spp., and Aspergillus spp.5
The standard diagnostic methods for infectious keratitis, including fungal keratitis, are corneal smear and culture. However, it may take several days to obtain the result of a corneal culture from a patient with suspicious fungal keratitis. On the other hand, the result could be negative in cases with deep infiltrates.6 It is obvious that the sooner diagnosis of causative agent, the better the prognosis.
In clinical examination, they are usually determined by gray-white infiltrates and satellite lesions with feathery borders, but it is not a perennial finding.
Considering long time to obtain culture results and variable clinical pictures, it is wise to use other diagnostic methods such as in vivo confocal microscopy (IVCM) with the resolution of 1 μm.7, 8 This resolution is enough to show fungal hyphae that are larger than a micrometer. IVCM is useful in diagnosis, management, and follow-up of patients with fungal keratitis.9
In this report, we report two patients with fungal keratitis caused by rare molds.
Case reports
Case 1
A 33-year-old male farmer was referred to our emergency department with the impression of microbial keratitis. He mentioned a history of ocular trauma with herbal material 5 days earlier.
On clinical examination, his best corrected visual acuity without any glasses was counting fingers from 50 cm. On slit-lamp biomicroscopy, he had a superficial corneal infiltration with epithelial defect and feathery border. Its diameter was 5 mm, and there was a deeper infiltration with 6.5 mm diameters of infiltration (Fig. 1). Corneal scraping was done with a surgical blade, and mycelium was seen. Samples were taken on sabouraud agar, blood agar, and chocolate agar media and were sent to laboratory for culture and defining the organism.
Fig. 1.
Clinical picture of the case 1. A, B: clinical picture of the patient at presentation. C, D: the patient developed hypopyon 20 days after initiating medication, but the density of infiltration was reducing. E, F: the infiltration is almost cleared with a central thinning.
Based on clinical picture and the result of corneal scraping fortified topical voriconazole, topical levofloxacin and oral itraconazole 100 mg were started hourly, then every 4 hours and every 12 hours for the patient, respectively. Clinical signs and symptoms of the patients improved with medical therapy. Considering clinical improvement, the anti-microbial therapy was tapered down, and non-preserved lubricating agents were added.
20 days after initiating medication, the patient developed a 1.5 mm hypopyon, but the corneal infiltration was reducing. Despite formation of the hypopyon, medical regimen was not change. The patient underwent IVCM (Heidelberg engineering, Heidelberg, Germany) weekly. He had hyper-reflective linear, branching and interlocking structures typical of fungal hyphae in IVCM that reduced in density with therapy (Fig. 2). When the patient developed hypopyon, the density of hyphae in IVCM was just reducing. Seven days after corneal sampling, the organism was defined by culture, and it was Pseudallescheria boydii (P. boydii) The topical voriconazole was tapered from hourly in the first week to every six hours in the fifth week, when it was discontinued because of resolving the infiltration and scar formation. Systemic itraconazole was discontinue after two weeks.
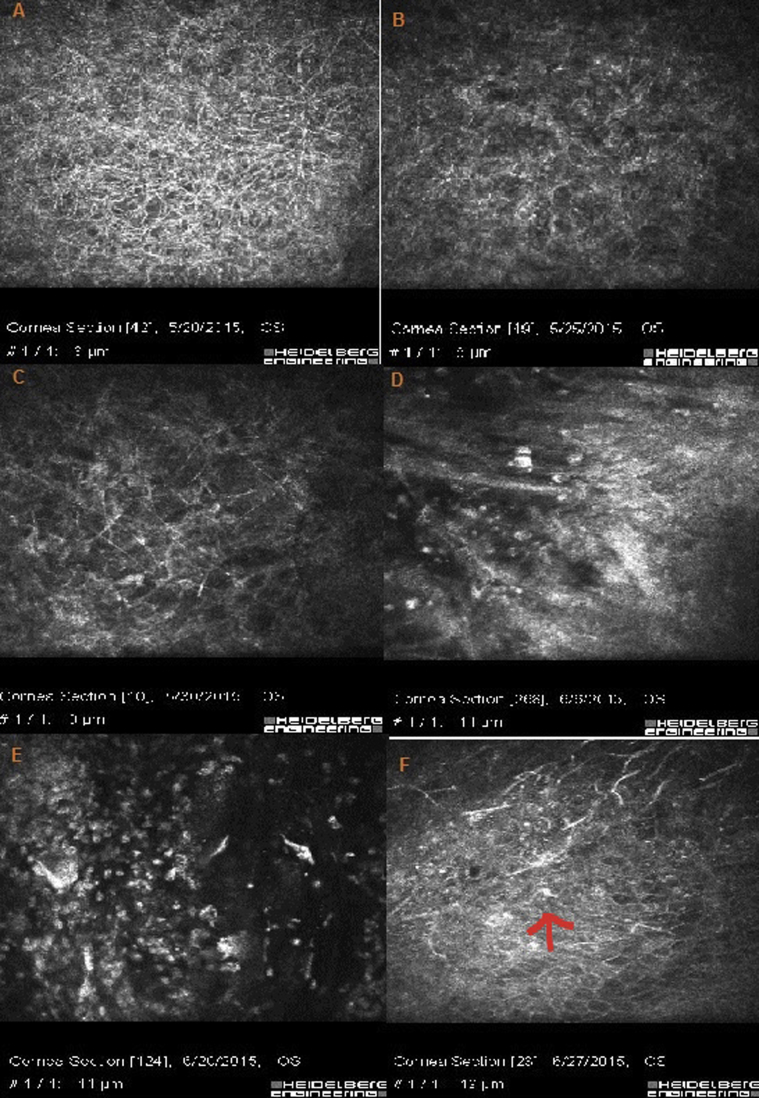
Fig. 2.
In vivo confocal microscopy (IVCM) of the patient. A: diffuse, hyper-reflective, branching and interlocking structures typical of fungal hyphae. B, C: The density of hyphae decreased after 5 days. D: The cornea is infiltrated by inflammatory cells. This image is synchronous with development of hypopyon. E, F: presence of inflammatory cells in the cornea (arrow).
Case 2
A 56-year-old male farmer came to our emergency department with chief complaint of ocular pain and burning. He had minimal ocular trauma with rice plant one week earlier. He was not a contact lens user.
His best corrected visual acuity was 20/40 without glasses. On slit-lamp biomicroscopy he had conjunctival injection, and there was an area of corneal infiltration in infranasal quadrant with gritty appearance and feathery border. The diameter of infiltration was 3.5*2 mm (Fig. 3). In corneal scraping samples that were obtained with surgical blade, mycelium was seen. Topical natamycin every 1 hour and topical levofloxacin every 4 hours were started for the patient with the impression of fungal keratitis. Clinical signs and symptoms were improved after starting medications, and anti-microbial agents were tapered while signs and symptoms were improving. Besides this regimen, lubricating agents were also prescribed. IVCM was done for the patient weekly. Multiple hyper-reflective, branching, and interlocking structures characteristics of fungal hyphae were detected in IVCM (including interlocking and branching structures with septate appearance) (Fig. 4). These fungal hyphae decreased in density with treatment. Five days after culturing on sabouraud agar, blood agar, and chocolate agar, the organism was detected as Colletotrichum coccodes. Topical natamycin was tapered weekly until the sixth week when the patient developed a scar. Systemic anti-fungal was discontinued two weeks after startup of the treatment.
Fig. 3.
Clinical picture of the case 2. A: Slit photograph of the patient at presentation. B, C: reduced infiltration after 1 week and 3 weeks, respectively. D: slit photograph 2 months after presentation. Infiltration has turned into scar tissue with central thinning.
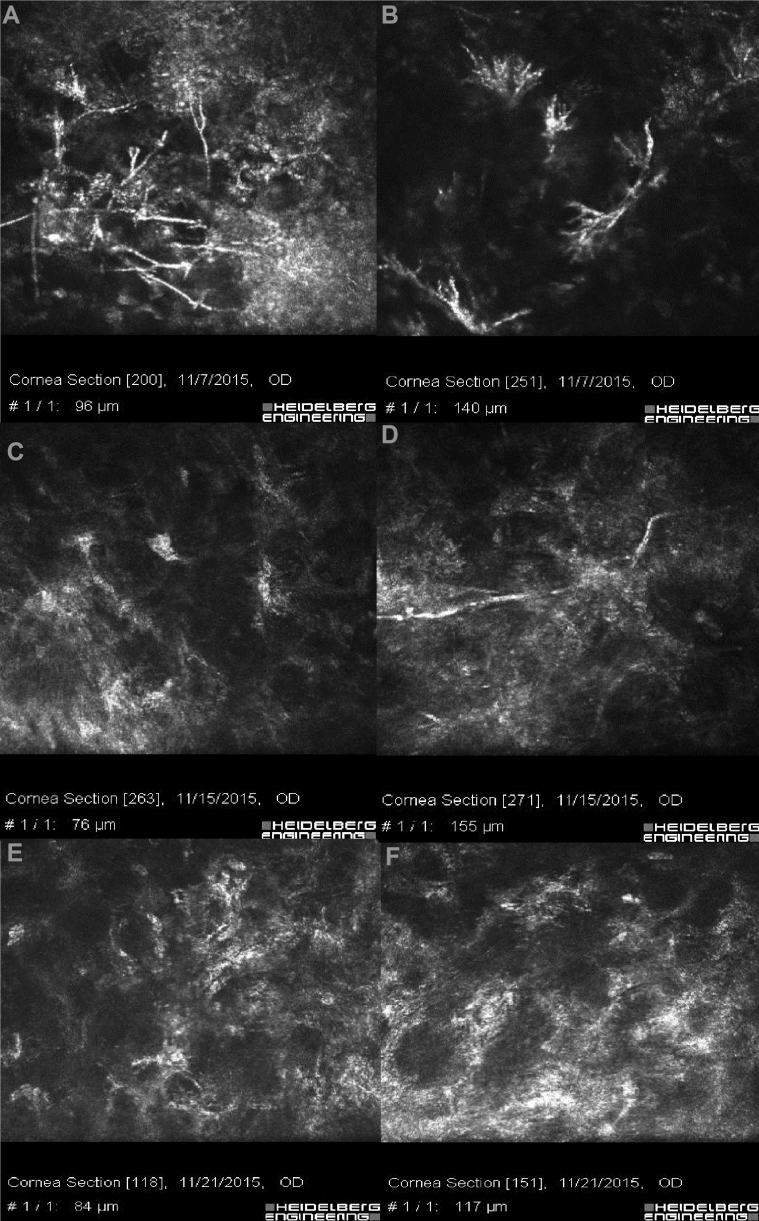
Fig. 4.
In vivo confocal microscopy (IVCM) of the patient. A, B: hyper-reflective, branching, and interlocking structures typical of fungal hyphae. C, D: The reduction of fungal hyphae density can be seen in IVCM after 8 days. E, F: The IVCM is free of hyphae 2 weeks after starting the medication.
Discussion
P. boydii is a recognized cause of mycetoma, a chronic fungal disease that usually affects the extremities.10 It is a ubiquitous filamentous fungi found in soil, water, and sewage.10, 11 First described in 1955, the organism can cause fungal keratitis that is usually unresponsive to medical treatment. Wong et al presented the first case of P. boydii keratitis that was successfully treated with topical natamycin. Wu et al reported a rate of enucleation or evisceration about 45% in these patients. They related this failure rate to misrecognition of this pathogen and initial bacterial treatment. There are other reports of successful treatment using topical voriconazole, miconazole, nystatin, and amphotericin B.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 This organism has septate, thin-walled, branching hyphae and an angioinvasive tendency.14 There is not any report of confocal scanning of this type of fungal keratitis in the literature. However, its clinical picture is almost the same as other keratitis caused by fungal organisms with white-gray infiltration and feathery border. The diagnosis is made by studying the smear and culture of corneal scraping samples. When the organism is stained with hematoxylin and eosin, it is indistinguishable from Aspergillus spp. morphologically.20 P. boydii is generally sensitive to azoles, and there are some reports of its resistance to polyenes.20 In cases that suffer from keratitis caused by this organism, fortified topical voriconazole can be prescribed. We added oral itraconazole as an adjuvant medication.
Colletotrichum spp. are anamorphic stages of fungi of the family Glomerellaceae.21, 22, 23, 24, 25 Colletotrichum spp. exist in hot and moderate climates.22, 23, 24, 26 There are rare reports of keratitis caused by Colletotrichum spp.27, 28, 29, 30 On KOH smear, septate hyaline hyphae can be seen. Fusarium spp. and Colletotrichum spp. both have curved conidia, so morphological discrimination is difficult. However, the presence of appressoria, non-septate conidia, and (in the later stages) acervuli with setae in Colletotrichum help in the discrimination.23 Povidone iodine is fungicidal to aerial mycelium of Colletotrichum dematium, Colletotrichum gloeosporioides, and Colletotrichum acutatum, and it is fungistatic to C. coccodes.31 Amphotericin B is the first choice for this organism.31 It is also sensitive to clotrimazole and miconazole.31 There is a high minimum inhibitory concentration (MIC) by in vitro susceptibility testing for natamycin, but it has good clinical response27 the same as our case.
In conclusion, these cases bold the importance of determining causative organism of fungal keratitis and their antibiotic susceptibility. There was not any specific confocal feature for the presented fungal keratitis that was different from other filamentous fungal hyphae; however, confocal scan is a good choice to follow the response to the treatment, as in the first case. Despite increasing hypopyon, medical treatment was continued because of decreasing hypha density in confocal scanning.
Footnotes
Conflicts of interest and source of funding: None.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Chang H.Y., Chodosh J. Diagnostic and therapeutic considerations in fungal keratitis. Int Ophthalmol Clin. 2011;51(4):33–42. doi: 10.1097/IIO.0b013e31822d64dc. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P.A. Fungal infections of the cornea. Eye (London) 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen E., Heegaard S., Prause J.U., Ivarsen A., Mortensen K.L., Hjortdal J. Fungal keratitis – improving diagnostics by confocal microscopy. Case Rep Ophthalmol. 2013;4(3):303–310. doi: 10.1159/000357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S.C., Lin Y.Y., Kuo C.N., Lai L.J. Cladosporium keratitis – a case report and literature review. BMC Ophthalmol. 2015;15:106. doi: 10.1186/s12886-015-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takezawa Y., Shiraishi A., Noda E. Effectiveness of in vivo confocal microscopy in detecting filamentous fungi during clinical course of fungal keratitis. Cornea. 2010;29(12):1346–1352. doi: 10.1097/ICO.0b013e3181cd3c84. [DOI] [PubMed] [Google Scholar]
- 7.Das S., Samant M., Garg P., Vaddavalli P.K., Vemuganti G.K. Role of confocal microscopy in deep fungal keratitis. Cornea. 2009;28(1):11–13. doi: 10.1097/ICO.0b013e318181cff7. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R.L., Cruzat A., Hamrah P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin Ophthalmol. 2010;25(5-6):166–170. doi: 10.3109/08820538.2010.518516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman S.C., Musch D.C., Belin M.W. Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111(2):396–406. doi: 10.1016/j.ophtha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Travis L.B., Roberts G.D., Wilson W.R. Clinical significance of Pseudallescheria boydii: a review of 10 years' experience. Myco Clin Proc. 1985;60(8):531–537. doi: 10.1016/s0025-6196(12)60571-0. [DOI] [PubMed] [Google Scholar]
- 11.Wong A.C., Mak S.T., Luk S., Tse R.K. Successful treatment of Pseudallescheria boydii keratitis with topical natamycin as monotherapy. J Ocul Pharmacol Ther. 2010;26(5):519–521. doi: 10.1089/jop.2010.0069. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z., Ying H., Yiu S., Irvine J., Smith R. Fungal keratitis caused by Scedosporium apiospermum: report of two cases and review of treatment. Cornea. 2002;21(5):519–523. doi: 10.1097/00003226-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Guber I., Bergin C., Majo F. Repeated intrastromal injections of voriconazole in combination with corneal debridement for recalcitrant fungal keratitis – a case series. Klin Monbl Augenheilkd. 2016;233(4):369–372. doi: 10.1055/s-0041-111814. [DOI] [PubMed] [Google Scholar]
- 14.Bradley J.C., Hirsch B.A., Kimbrough R.C., 3rd, McCartney D.L. Pseudallescheria boydii keratitis. Scand J Infect Dis. 2006;38(11-12):1101–1103. doi: 10.1080/00365540600643211. [DOI] [PubMed] [Google Scholar]
- 15.Pautler E.E., Roberts R.W., Beamer P.R. Mycotic infection of the eye. Monosporium apiospermum associated with corneal ulcer. Arch Ophthalmol. 1955;53(3):385–389. doi: 10.1001/archopht.1955.00930010387009. [DOI] [PubMed] [Google Scholar]
- 16.Ozkurt Y., Oral Y., Kulekci Z., Benzonana N., Ustaoglu R., Dogan O.K. Pseudallescheria boydii keratitis. J Pediatr Ophthalmol Strabismus. 2006;43(2):114–115. doi: 10.3928/0191-3913-20060301-14. [DOI] [PubMed] [Google Scholar]
- 17.Mancini N., Ossi C.M., Perotti M. Direct sequencing of Scedosporium apiospermum DNA in the diagnosis of a case of keratitis. J Med Microbiol. 2005;54(Pt 9):897–900. doi: 10.1099/jmm.0.46029-0. [DOI] [PubMed] [Google Scholar]
- 18.Sridhar M.S., Garg P., Bansal A.K., Sharma S. Fungal keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(4):613–615. doi: 10.1016/s0886-3350(99)00459-9. [DOI] [PubMed] [Google Scholar]
- 19.D'Hondt K., Parys-Van Ginderdeuren R., Foets B. Fungal keratitis caused by Pseudallescheria boydii (Scedosporium apiospermum) Bull Soc Belge Ophtalmol. 2000;277:53–56. [PubMed] [Google Scholar]
- 20.Saracli M.A., Erdem U., Gonlum A., Yildiran S.T. Scedosporium apiospermum keratitis treated with itraconazole. Med Mycol. 2003;41(2):111–114. doi: 10.1080/mmy.41.2.111.114. [DOI] [PubMed] [Google Scholar]
- 21.Joseph J., Fernandez M., Sharma S. Colletotrichum dematium keratitis. J Postgard Med. 2004;50(4):309–310. [PubMed] [Google Scholar]
- 22.Mendiratta D.K., Thamke D., Shukla A.K., Narang P. Keratitis due to Colletotrichum dematium – a case report. Indian J Med Microbiol. 2005;23(1):56–58. doi: 10.4103/0255-0857.13876. [DOI] [PubMed] [Google Scholar]
- 23.Kirk P.M., Cannon P.F., Minter D.W., Stalpers J.A. 10th ed. CAB International; CABI Europe–UK, Wallingford: 2008. Ainsworth and Bisby's Dictionary of the Fungi; p. 771. [Google Scholar]
- 24.Machowicz-Stefaniak Z. Occurrence and characterization of Colletotrichum dematium (Fr.) Grove. Pol J Microbiol. 2010;59(3):191–200. [PubMed] [Google Scholar]
- 25.Kotwal A., Biswas D., Kakati B., Bahadur H., Gupta N. Non traumatic keratitis due to Colletotrichum coccodes: a case report. J Clin Diagn Res. 2015;9(2) doi: 10.7860/JCDR/2015/10843.5529. DD01-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivaprakash M.R., Appannanavar S.B., Dhaliwal M. Colletotrichum truncatum: an unusual pathogen causing mycotic keratitis and endophthalmitis. J Clin Microbiol. 2011;49(8):2894–2898. doi: 10.1128/JCM.00151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liesegang T.J., Forster R.K. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan S.V., Rekha N.S., Sharda R.D., Mahalingam N. Colletotrichum keratitis: a rare but definite clinical entity. J Clin Diagn Res. 2013;7(7):1430–1433. doi: 10.7860/JCDR/2013/5513.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machowicz-Matejko E., Zalewska E.D. Pharmacological substances in vitro in limiting growth and development of fungi colletotrichum genera. J Ocul Pharma Ther. 2015;31(5):303–309. doi: 10.1089/jop.2014.0128. [DOI] [PubMed] [Google Scholar]
- 30.De Hoog G.S., Guarro J., Gene J., Figueras M.J. 2nd ed. Utrecht; The Netherlands: 1995. Atlas of Clinical Fungi: Central Bureau Voor Schimmelcultures. [Google Scholar]
- 31.Shukla P., Kumar M., Keshava G. Mycotic keratitis: an overview of diagnosis and therapy. Mycoses. 2008;51(3):183–199. doi: 10.1111/j.1439-0507.2007.01480.x. [DOI] [PubMed] [Google Scholar]