Abstract

The gut microbiome has an enormous impact on the life of the host, and the diet plays a fundamental role in shaping microbiome composition and function. The way food is processed is a key factor determining the amount and type of material reaching the gut bacteria and influencing their growth and the production of microbiota metabolites. In this perspective, the current possibilities to address food design toward a better feeding of gut microbiota are highlighted, together with a summary of the most interesting microbial metabolites that can be made from dietary precursors.
Keywords: food ingredients, phytochemicals, metabolism, food processing, Mediterranean diet, melanoidins
Introduction
Advances in food technology combined with the preference of the consumer resulted in the wide adoption of ultraprocessed foods having high calorie density.1 This is considered one of the causes underpinning the current obesity epidemic. Food reformulation strategies are currently based on sugar and fat reduction, mainly targeting food calorie reduction. Unfortunately, this strategy has limited efficacy because it works only for health-conscious, restrained consumers, and more specifically, it does not take into account the needs of the gut microbiota. It is now firmly established that the gut microbiome has enormous functional potential for the host. The diet plays a fundamental role in shaping the composition of gut microbiota and, thus, determines the inter-relationship between the gut microbiome and the host.2 Microbial activities can influence the host, and compelling evidence shows that microbiome composition and functions are responsible for human metabolism and regulate the balance between health and disease. In this vein, food design should take into account the needs of our commensal bacteria together with those of the human body. The simple concept is that microbes in the gut thrive on what is not bioavailable for the human body. In other words, unavailable food components are the actual food for commensal bacteria in the gut. This concept is exemplified in the sketch of Figure 1. In the context of an excess of macronutrient intake and high-calorie-dense foods, a design that reduces the bioaccessibility is possibly beneficial for the host. The reduced bioavailability could raise some concerns for infants and malnourished people because, in these subjects, the priority is still host feeding.
Figure 1.
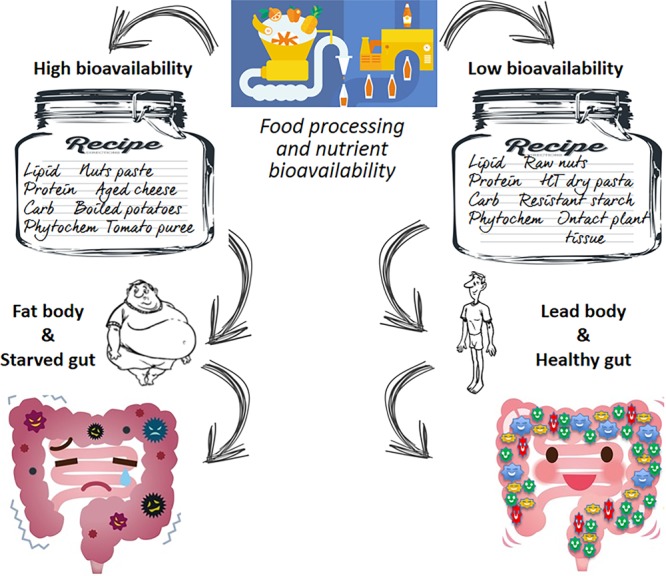
Food design has a pivotal role in defining the quantity and type of material reaching the gut microbiota. More abundant and diverse feeding material favor its health status.
In the framework of microbiome-related nutrition, the attention has been mainly devoted to the dietary fiber, which is surely a key dietary component for the microbiome. However, the material reaching the gut is not limited to dietary fiber. Between 45 and 85 g of solid matter containing around 20–40% of proteins can partially escape the absorption in the small intestine and reach the gut every day.3 Beside proteins, a significant amount of resistant starch, fats, and phytochemicals is also part of this material. Multiple studies showed the triglyceride bioavailability can be modulated by the physical structure of the food.4 Macronutrients stored inside the intact plant cell wall are not accessible for the human digestive enzymes. In the same way, oil and protein bodies that are not damaged during food preparation can be degraded only to a limited extent by digestive lipase and proteases, respectively.
Different considerations must be drawn for most of the plant phytochemicals, such as all poyphenols. It is well-known that they have a limited bioavailability (usually only 1–5% is absorbed), while a large moiety is delivered to the gut. Recent evidence showed how they play multiple functions in the gut, shaping the microbiome and triggering various biochemical pathways, influencing inflammatory and immune statuses.5 Phytochemicals are extensively metabolized by the microbiota and converted in simple phenolic acids, which are absorbed in the body, contributing to the communication between the gut and the human body.
It is worth noticing that virtually all of the undigested material can be a substrate for the community of the gut microbes: the higher the variability of the substrates available, the higher the diversity of the microbiome. Mounting evidence showed that this microbiome diversity is associated with a low inflammatory status and lean phenotype. This is not a random association: the expression of genes in the microbiome triggers biochemical pathways, ensuring proper intestinal permeability and immunomodulation.6
The diet/microbiome interplay is the current basis for implementation of personalized nutrition,7,8 and microbiota composition is a key factor affecting responsiveness to nutritional interventions2 that will soon take into account initial stratification of individuals on the basis of the microbiota.
In this framework, it is timely to start restyling the concept of food and to add the role of feeding the gut microbiota by providing precursors of microbial metabolites that are responsible for the microbiome/host relationship.
Delivery of Nutrients to the Gut Microbiota
The traditional nutritional recommendation to feed human microbiota is to formulate food or prescribe a diet with plenty of dietary fiber. Unfortunately, fiber-rich foods are often not palatable and not liked by many consumers. Thus, it is pragmatic to develop food design strategies able to properly feed the microbiota while keeping the sensory characteristics that make Western foods attractive and rewarding.1
Besides the intrinsic nature of the food component, the type and amount of food material reaching the lower gut depend upon how the whole food is designed: by changing the food matrix, it is possible to modulate the nutrient digestibility. Mounting evidence indicates that nutrient bioavailability is not an absolute value defined for each nutrient but is influenced by the food matrix, as the digestion and absorption process is a time-dependent function. Most of the food components can only be absorbed after conversion into their basic units: proteins, triglycerides, and polysaccharides into amino acids, free fatty acids, and glucose, respectively. If a food has a strong and entangled matrix, this conversion into the basic unit becomes very slow and the nutrients become de facto less bioavailable. In this case, they will become potential substrates for the gut microbiota. As illustrated in Figure 1, designing food with a limited bioaccessibility could result in a low bioavailability of proteins, carbohydrates, lipids, and phytochemicals, resulting in higher levels of nutrient delivery to the microbiota and less calorie absorption for the host. This is a win–win situation, especially for subjects living in an obesogenic environment and having no macro- or micronutrient deficiencies.
Also looking at phytochemicals and micronutrients, the proper balance between bioavailability and microbiota should be taken into account: the natural low bioavailability of food phytochemicals favored the evolutionary capability of several microbes to metabolize them into signaling molecules. There is evidence showing how the microbiome variability is responsible for the formation of metabolites that can be measured in human urine and plasma and correlated with health status.5
From this viewpoint, it is clear that the way a food is designed can deeply influence the distribution of nutrients between the human body (i.e., the bioaccessible fraction) and the microbiota. Delaying the digestion kinetics can be an effective strategy to deliver the gut nutrients and micronutrients, which could be, in theory, 100% bioavailable to humans. In this vein, food design can be a powerful tool to modulate the microbiota. This targeted delivery can be achieved in many different ways both at the industrial level and during domestic preparation of food. Some examples are provided in the following paragraphs, illustrating strategies that can be useful for both macro- and micronutrients.
Food Particle Size and Macronutrient Bioavailability: The Bigger, the Lower
An extensive mechanical processing is often performed to make foods highly palatable. Conversion of raw materials into “flours and juices” is the easiest starting point to design any type of food. In fact, from the perspective of food designers, to work with ultraprocessed, homogeneous, and flexible ingredients is a great advantage. The extensive processing makes all nutrients fully accessible to the digestive enzymes accelerating their degradation and absorption, while limiting mechanical processing can reduce their bioavailability. Despite the common belief that digestion starts in the mouth, the human masticatory apparatus is not able to perform an extensive particle size reduction. The formation of the bolus is mainly designed to avoid choking and not to facilitate the digestion process. Mounting evidence showed that the dimension of the food particle size is inversely related to the absorption by the human body. Studies with ileostomy subjects showed that 17% of starch from barley was not absorbed when 3 mm flaked particles rather than flour was provided. Similarly, soybean protein digestibility was improved processing the seeds into a fine flour. Therefore, it is not surprising that a significant reduction of the overall calorie intake can be achieved just by avoiding fine grinding.4 In this framework, it has been reported that the anti-inflammatory effects of wheat bran are dependent upon the particle size (the bigger, the better), and this could be related to the changes in cecal Enterobacteriaceae.9
Thermal Treatment Increases Nutrient Bioavailability
Industrial and domestic cooking induce severe modifications in food, resulting in a generalized increase of macro- and micronutrient bioavailability. The most relevant effect is starch gelatinization: thermal treatment induces physical modifications of starch granules, which are not well digestible to humans. Gelatinized starch becomes a good substrate for human amylases, and starch is rapidly converted to glucose. Also, heat-induced protein denaturation usually facilitates their digestion, especially for plant proteins; however, in some cases, thermal protein aggregation or thermally induced protein cross-linking can reduce or even revert this effect. Thermal treatment causes the swelling of the plant food matrix, which is a prerequisite for small molecule bioaccessibility. Solid evidence has been provided on the positive effect of domestic cooking on phytochemical bioaccessibility, especially using steaming and a microwave.10 In this respect, the effect of thermal treatment on carotenoid bioavailability can be used as the gold standard: processed tomatoes, especially in the presence of oil, provide 3 times more bioavailable lycopene than the corresponding raw tomato.11 All together, the amount of phytochemicals reaching the microbiota in a cooked food diet is lower than the corresponding raw food diet. Moreover, the opening of the vegetable food matrix makes the gut biotransformation of all food components more pronounced.12
Intactness of Plant Food Tissue: Plant Cell Walls Can Reduce Nutrient Bioavailability
Intact plant cells are not a good substrate for the human digestion system: amylase, lipase, and proteases cannot trespass the intact cell wall barrier and reach their substrates present in the cytoplasm.4 A large moiety of all macronutrients can reach the microbiota if the plant cell structure have not been destroyed by mechanical and thermal processes. This effect is well-known in raw almonds: up to 30% of lipids travel through the gastrointestinal tract entrapped within intact cells, limiting the extent of lipid digestion.13 On one hand, fine milling and an extrusion cooking process combining heating, pressure, and mechanical sheering to produce plasticized, expanded, and cooked products disrupt all of the barriers and organelles of the original plant material, maximizing starch and lipid digestibility. On the other hand, it is possible to design a food process to keep the cell wall intact and deliver more material to the gut microbiota, as recently shown in bean cotyledon cells by Rovalino and co-workers.14
Designing a Stronger Food Matrix To Reduce Macronutrient Bioavailability
There are different ways to design a strong food matrix favoring the delivery of nutrients into the gut. A network of highly cross-linked proteins is poorly bioavailable, and it can delay the digestibility of the starch, as observed in pasta. A network of proteins can delay the accessibility of lipid droplets to lipase: in cheese, it was found that the structure of the casein network and the size of the fat globules, which can be both modulated during cheese processing, are the main factors determining the lipid degradation kinetics into fatty acids.4 Also, strong non-covalent interactions formed in the gastrointestinal environment can result in massive aggregation, limiting the bioaccessibility of proteins, lipids, and starch. Protein gels obtained by cross-linking or ionic gelation have different structural properties and different digestibilities.15 Finally, the Maillard reaction (MR) is a potent tool to strengthen the food matrix and reduce the protein digestibility, as it happens in pasta dried at a high temperature. MR promotes the formation of high-molecular-weight protein aggregates called melanoidins, whose formation can well explain the decrease of protein digestibility observed in several roasted products. Also, in this case, the processing conditions of products, such as bread, bakery coffee, cocoa, and roasted nuts, can be designed to promote the formation of melanoidins and bring them to the gut microbiota.16 A limitation of this approach is that often the formation of melanoidins parallels that of potentially toxic compounds, such as acrylamide. However, in these cases, it is possible to implement strategies able to disentangle the formation of melanoidins from those of hazardous products.17.
Micronutrients and Phytochemicals: Naturally Delivered to the Gut
The low bioavailability of many micronutrients and phytochemicals was considered for many years to be a barrier to exploit their potential benefit on human health. This is actually a true concern for some vitamins, such as vitamin A, and some minerals, such as iron, zinc, and calcium. However, for most of the phytochemicals, the low bioavailability implies that they are actually well-available for the commensal bacteria, which can metabolize them and also produce new metabolites that are beneficial for human health. As highlighted in the following paragraph, the ability of human microbiota to convert specific phytochemicals into metabolites that can be absorbed in the body is highly variable. It is dependent upon the presence of specific bacteria functions, which are, in turn, dependent upon the dietary exposure as well as the interaction with the host.5 The ultimate goal of food design should be to favor the delivery of phytochemicals to the gut by preserving their degradation during food processing and their excessive exposure during the passage in the upper part of the gastrointestinal tract, where they can be oxidized by the radicals formed during the digestion process.
Nutrients for a Profitable Feeding of the Gut Microbiota
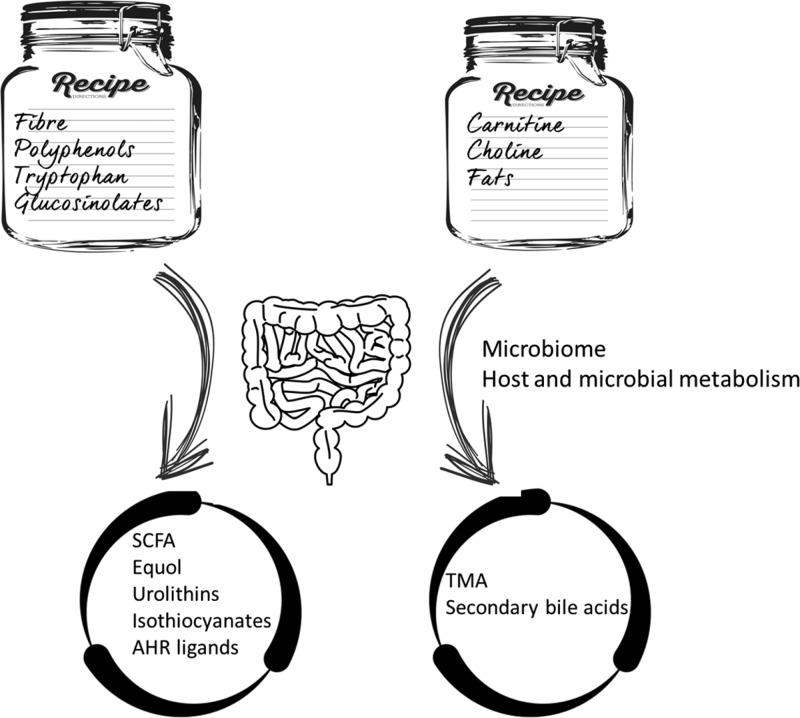
The gut microbiota is characterized by huge microbial diversity, and its members can have different feeding requirements. It is known that an abundant supply of diverse foods promotes the biodiversity in the microbiota and also the variety of the microbial genes expressed that can be triggers of health status. The next step is to obtain details on the specific conversion pathways of the various species: different precursors supplied through the diet can be converted to beneficial or detrimental metabolites by members of the gut microbiota, as schematized in Figure 2.
Figure 2.
Dietary precursors and possible beneficial and/or detrimental metabolites produced by the gut microbiota.
The main food component to impact gut microbiota composition and activity is certainly fiber. Ancient dietary regimes in agrarian populations could reach 100 g intake per day, while urban Western populations eat only 15 g per day of fiber, with the recommended intake being above 25 g per day. By definition, the dietary fiber goes through the small intestine, reaches the colon, and can be used by fiber-degrading members of the microbiota. The main results of such a metabolism are short-chain fatty acids (SCFAs), namely, acetate, propionate, and butyrate, which have recognized health-promoting activities, such as anti-inflammatory, anticarcinogenic, and immune-regulatory functions.18 The production of such beneficial molecules depends upon the composition of the gut microbiota but also the quantity of consumed dietary fiber. Accordingly, increased levels of SCFAs were found in vegan, vegetarian, and also omnivore subjects with high-level adherence to the Mediterranean diet, having a remarkable daily intake of plant-based foods, such as fruit vegetables and legumes.19 In addition, dietary fiber supplementation has been recently demonstrated to select specific groups of fiber-degrading bacteria and to increase SCFA levels with beneficial effects on type 2 diabetes patients.20 It is well-known that the equilibrium of the gut bacteria is modulated by the dietary components reaching the gut, and the metabolic degradation pathways of several specific classes of macro- and micronutrients have been elucidated. However, the interindividual variability is very high, and in many cases, prolonged dietary exposure is the key factor underpinning the microbiota metabolic capacity. Some examples of both positive and negative consequences of microbiota action are provided in the following paragraphs.
Phytoestrogens are plant-derived polyphenols with a chemical structure similar to human estrogens that are associated with multiple health benefits. They occur at high levels in soy, seeds, fruits, vegetables, and cereals, as well as also in coffee, tea, and chocolate. Ingested polyphenols are poorly absorbed in the small intestine, and remarkable quantities may be available in the colon. The gut microbiota can convert isoflavones, ellagitannins, and lignans to equol, urolithins, and enterolignans, respectively, which are recognized to have anti-inflammatory effects and induce antiproliferative activities. These compounds are more bioavailable and display a higher level of estrogenic/anti-estrogenic and antioxidant activities compared to their precursors.21 The polyphenol-converting bacteria belong to the dominant phyla of the human intestine,22 although the knowledge on the capability to metabolize polyphenols by gut bacteria is currently far from exhaustive. The individual gut microbiota seems to play an important role on the potential for colon activation of phytoestrogens. Equol is formed from isoflavones present in soy-based foods, particularly daidzein. The introduction of soy-based foods in a diet does not necessarily determine equol production,23 suggesting an important role of the individual composition of the gut microbiota and its functional capability on equol production. Interestingly, more than 60% of Asian residents were found as equol producers from soy isoflavones, while only 30% of the Western populations display the same functionality.24 Indeed, Westernization could be the cause of the loss of the equol-producing ability, as a consequence of different gut microbiome structures and different dietary patterns consumed. Accordingly, Wu and co-worker found that less than 50% of recruited Western vegans produced equol.25
The aryl hydrocarbon receptor (AhR) is an important factor in intestinal homeostasis. AhR is not only able to respond to exogenous stimuli but also to endogenous ligands that are generated from host–cell interactions, diet, and microbiota metabolism. Therefore, AhR is considered as a sensor that connects the gut lumen environment with cellular processes with consequences for immune functioning.26 Microbe-mediated metabolism of polyphenols, glucosinolates, and tryptophan can generate ligands for AhR, thus contributing to homeostasis. Glucosinolates are supplied by vegetable foods and can also be converted by gut microbes to isothiocyanates, which are able to activate cytoprotective, antioxidant responses.27
Interestingly, also compounds only present in processed foods, such as the MR products, can be metabolized by specific members of the microbiota, as demonstrated for a strain of Intestinimonas that was proven able to convert fructoselysine into butyrate.28 Moreover, the capability of human microbiota to use melanoidins as a preferential substrate for Bifidobacteria is documented, especially for those present in coffee and bread crust.16
Some other microbial metabolisms of dietary components lead to the production of detrimental molecules. Choline and carnitine are particularly abundant in foods of animal origin, such as meat, poultry, and eggs. They are precursors of trimethylamine (TMA) that is produced from choline and carnitine by some members of the gut microbiota. Once absorbed, TMA is oxidized to trimethylamine oxide (TMAO) in the liver, and TMAO has been associated with cardiovascular risk. The most recent studies are targeting the discovery of yet not well-known members of the gut microbiota that are able to degrade TMA and, thus, contrast the activity of TMA producers.29 In addition, a fat-rich diet determines higher levels of bile in the colon, where members of the microbiota may turn bile acids into secondary bile acids, mainly deoxycholic and lithocholic acids. These can be involved in processes linked to colorectal carcinogenesis, such as apoptosis, cell proliferation, and DNA damage induction.30
In summary, mounting evidence indicates that the way a food is designed will ultimately define the diversity of microbial metabolites released in the gut. This is relevant for not only the microbiota wellness but also the host health through a number of physical connections and biochemical signaling, indicated as the gut–organ axis.
Gut–Organ Axis
The connection of microbiota wellness with the functioning of the liver is obvious: microbiota metabolites are carried to the liver through the portal vein, and microbial dysbiosis is often the cause of liver inflammatory status. Similarly, for all of the parameters connected to the circulatory system: mounting evidence indicates that the signal triggered by the gut microbes and their metabolites is directly responsible for the low-density lipoprotein (LDL) and endothelium functionality as well as many factors connected to the glucose-managing capability. Finally, current trends in gut–brain axis science recognize the role of the gut microbiome interacting with the brain. Psychobiotics have been recently defined as “any substance that exerts a microbiome-mediated psychological effect” and are thus not limited to probiotics and prebiotics.30 Psychobiotics exert anxiolytic and antidepressant effects characterized by changes in emotional, cognitive, systemic, and neural indices.30 For example, bacteria crucially affect the metabolism of tryptophan into serotonin, whose effect on mood is recognized. In addition, the potential anti-inflammatory activity of gut microbes upon fiber degradation and SCFA production can stimulate positive responses at the systemic level and be involved in emotional responses. In light of this, it is tantalizing to imagine food designed to act as a psychobiotic, which may be enriched with prebiotic fiber or probiotics with a recognized effect on human behavior. Indeed, in first evidence on humans, supplying Bimuno-galactooligosaccharide (B-GOS) determined a significant reduction of waking-cortisol response, with a possible consequent decrease in emotional disturbances.
In summary, the recipe to design the perfect food is not available. However, the message that we can take from the available knowledge is that, beyond having the desired nutritional, technological, sensory, and health properties, the design of novel foods should take care of the availability of nutrients for the host and its microbes. Ingredient formulation and processing technology tailoring the bioavailability of nutrients on the specific need of each individual and especially our powerful symbionts will be the starting point to enter the new era of personalized nutrition.
The authors declare no competing financial interest.
References
- Pellegrini N.; Fogliano V. Cooking, industrial processing and caloric density of foods. Curr. Opin Food Sci. 2017, 14, 98–102. 10.1016/j.cofs.2017.02.006. [DOI] [Google Scholar]
- Shanahan F.; van Sinderen D.; O’Toole P. W.; Stanton C. Feeding the microbiota: Transducer of nutrient signals for the host. Gut 2017, 66, 1709–1717. 10.1136/gutjnl-2017-313872. [DOI] [PubMed] [Google Scholar]
- Silvester K. R.; Englyst H. N.; Cummings J. H. Ileal recovery of starch from whole diets containing resistant starch measured in vitro and fermentation of ileal effluent. Am. J. Clin. Nutr. 1995, 62, 403–411. 10.1093/ajcn/62.2.403. [DOI] [PubMed] [Google Scholar]
- Capuano E.; Oliviero T.; Fogliano V.; Pellegrini N. Digestion and the true energy content of foods. Nutr. Rev. 2018, 76, 274–289. 10.1093/nutrit/nux072. [DOI] [PubMed] [Google Scholar]
- Tomas-Barberan F.; Gonzalez-Sarrias A.; Garcia-Villalba R.; et al. Urolithins, the rescue of ″old″ metabolites to understand a ″new″ concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- Bischoff S. C.; Barbara G.; Buurman W.; Ockhuizen T.; Schulzke J.-D.; Serino M.; Tilg H.; Watson A.; Wells J. M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M.; Veiga P. Rethinking diet to aid human-microbe symbiosis. Trends Microbiol. 2017, 25, 100–112. 10.1016/j.tim.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Kashyap P. C.; Chia N.; Nelson H.; Segal E.; Elinav E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin. Proc. 2017, 92, 1855–1864. 10.1016/j.mayocp.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriano F.; Neyrinck A. M.; Verspreet J.; Olivares M.; Leclercq S.; Van de Wiele T.; Courtin C. M.; Cani P. D.; Bindels L. B.; Delzenne N. M. Particle size determines the anti-inflammatory effect of wheat bran in a model of fructose over-consumption: Implication of the gut microbiota. J. Funct. Foods 2018, 41, 155–162. 10.1016/j.jff.2017.12.035. [DOI] [Google Scholar]
- Palermo M. A.; Pellegrini N.; Fogliano V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. 10.1002/jsfa.6478. [DOI] [PubMed] [Google Scholar]
- Rao A. V. Processed tomato products as a source of dietary lycopene: Bioavailability and antioxidant properties. Can. J. Diet Pract Res. 2004, 65, 161–165. 10.3148/65.4.2004.161. [DOI] [PubMed] [Google Scholar]
- Juániz I.; Ludwig I. A.; Bresciani L.; Dall’Asta M.; Mena P.; Del Rio D.; Cid C.; de Peña M.-P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Foods 2017, 32, 195–207. 10.1016/j.jff.2017.02.033. [DOI] [Google Scholar]
- Grassby T.; Mandalari G.; Grundy L.; et al. In vitro and in vivo modeling of lipid bioaccessibility and digestion from almond muffins: The importance of the cell-wall barrier mechanism. J. Funct. Foods 2017, 37, 263–271. 10.1016/j.jff.2017.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovalino-Córdova A. M.; Fogliano V.; Capuano E. A closer look to the cell structural barriers affecting starch digestibility in beans. Carbohydr. Polym. 2018, 181, 994–1002. 10.1016/j.carbpol.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Rui X.; Fu Y.; Zhang Q.; et al. A comparison study of bioaccessibility of soy protein gel induced by magnesiumchloride, glucono-δ-lactone and microbial transglutaminase. LWT—Food Sci. Technol. 2016, 71, 234–242. 10.1016/j.lwt.2016.03.032. [DOI] [Google Scholar]
- Delgado-Andrade C.; Fogliano V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of Their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. 10.1146/annurev-food-030117-012441. [DOI] [PubMed] [Google Scholar]
- Palermo M.; Gökmen V.; De Meulenaer B.; Ciesarová Z.; Zhang Y.; Pedreschi F.; Fogliano V. Acrylamide mitigation strategies: Critical appraisal of the FoodDrinkEurope toolbox. Food Funct. 2016, 7, 2516–2525. 10.1039/C5FO00655D. [DOI] [PubMed] [Google Scholar]
- O’Keefe S. J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F.; Pellegrini N.; Vannini L.; Jeffery I. B.; La Storia A.; Laghi L.; Serrazanetti D. I.; Di Cagno R.; Ferrocino I.; Lazzi C.; Turroni S.; Cocolin L.; Brigidi P.; Neviani E.; Gobbetti M.; O’Toole P. W.; Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Zhang F.; Ding X.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- Gaya P.; Medina M.; Sánchez-Jiménez A.; Landete J. M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. 10.3390/molecules21081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braune A.; Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. 10.1080/19490976.2016.1158395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson C.; Frankenfeld C. L.; Lampe J. W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–70. 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- Magee P. J. Is equol production beneficial to health?. Proc. Nutr. Soc. 2011, 70, 10–18. 10.1017/S0029665110003940. [DOI] [PubMed] [Google Scholar]
- Wu G. D.; Compher C.; Chen E. Z.; Smith S. A.; Shah R. D.; Bittinger K.; Chehoud C.; Albenberg L. G.; Nessel L.; Gilroy E.; Star J.; Weljie A. M.; Flint H. J.; Metz D. C.; Bennett M. J.; Li H.; Bushman F. D.; Lewis J. D. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. 10.1136/gutjnl-2014-308209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T.; Iannitti R. G.; Cunha C.; De Luca A.; Giovannini G.; Pieraccini G.; Zecchi R.; D’Angelo C.; Massi-Benedetti C.; Fallarino F.; Carvalho A.; Puccetti P.; Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova T.; Kostov R. V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Bui T. P.; Ritari J.; Boeren S.; de Waard P.; Plugge C. M.; de Vos W. M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. 10.1038/ncomms10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrel G.; McCann A.; Deane J.; Neto M. C.; Lynch D. B.; Brugère J.-F.; O’Toole P. W. Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome. ISME J. 2017, 11, 2059–2074. 10.1038/ismej.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A.; Lehto S. M.; Harty S.; Dinan T. G.; Cryan J. F.; Burnet P. W. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]