Abstract
Objective
This study aimed to assess the diagnostic value of serum neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C for renal dysfunction in older patients with coronary disease.
Methods
A total of 84 older patients with coronary artery disease were included in this study. Serum NGAL and cystatin C levels were analysed using commercially available kits. Medical data of all patients were recorded and analysed.
Results
NGAL and cystatin C levels were significantly positively correlated with N-terminal prohormone of brain natriuretic peptide levels and negatively correlated with the estimated glomerular filtration rate. The areas under the receiver operating characteristic curves of serum NGAL and cystatin C levels for diagnosing early renal dysfunction were 0.884 and 0.744, respectively.
Conclusion
Serum NGAL and cystatin C are potential early and sensitive markers of renal dysfunction in older patients with coronary artery disease.
Keywords: Neutrophil gelatinase-associated lipocalin (NGAL), cystatin C, chronic heart failure, coronary artery, renal function, older patient
Introduction
Chronic heart failure (CHF) is the end stage of various cardiovascular diseases and one of its common causes is coronary artery disease (CAD). Because of the increase in the ageing population and improved treatment options for CAD, including acute myocardial infarction, the incidence of CHF has increased yearly.1–3 Renal impairment is common among patients with heart failure. Approximately 35%–70% of patients with heart failure have concurrent chronic kidney dysfunction (CKD).4–8 CHF and CKD have many common risk factors and pathophysiological pathways, such as inflammatory and direct cellular immune-mediated responses, as well as renin–angiotensin–aldosterone system activation.9–13 Poor outcomes regarding CKD in patients with heart failure have been shown in some trials.5,14 CKD can increase the risk of re-hospitalization because of worsening of heart failure and cardiac death in patients with heart failure.15 Early diagnosis of renal impairment for patients with CHF is important. Serum creatinine (Scr) and blood urea nitrogen (BUN) levels have been the most widely used parameters for clinical evaluation of renal function. While Scr levels are affected by many factors, such as age, sex, weight, height, muscle mass, and dietary structure, BUN levels are easily affected by body conditions and can be reabsorbed by the renal system. Only severe renal damage can be detected by increased levels of Scr and BUN. In recent years, there has been increasing interest and effort in developing new biomarkers for assessing early renal damage in patients with CAD, including neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid-binding protein, kidney injury molecule-1, cystatin C, and C-reactive protein.16 Among them, NGAL and cystatin C are the two most promising potential biomarkers. Therefore, this study aimed to evaluate the potential of serum NGAL and cystatin C levels in diagnosing early renal impairment in older patients with CAD.
Subjects and Methods
Subjects
This prospective study enrolled 84 older patients who were diagnosed with CAD at the Xuanwu Hospital of Capital Medical University between October 2012 and September 2013. CAD was diagnosed by a history of CAD, computed tomography angiographic or digital subtraction angiographic evidence of coronary artery stenosis >50%, typical angina pectoris, or myocardial ischaemic changes detected by an electrocardiogram.
Based on the Framingham diagnostic criteria, CHF was considered when patients had at least two major criteria or one major criterion with two minor criteria.17 The patients were divided into three groups according to the New York Heart Association (NYHA) cardiac function classification as follows: NYHA class I group (n = 30), NYHA class II group (n = 28), and NYHA class III–IV group (n = 26). A total of 31 healthy age-matched subjects were selected for the control group. Exclusion criteria were as follows: acute exacerbations of CHF, acute coronary syndrome, unstable control of blood pressure, diabetes, cancer, haematological diseases, chronic obstructive pulmonary disease, autoimmune disease, severe hepatic insufficiency, and end-stage renal failure. All subjects had normal Scr levels (<133 µmol/L in men and <108 µmol/L in women).17 All subjects signed informed consent forms prior to the study, which was approved by the Ethics Committee of Xuanwu Hospital at the Capital Medical University in China.
Clinical data
Weight, height, and systolic and diastolic blood pressure were recorded at admission. Body mass index was calculated. Venous blood was collected after 8 to 10 hours of fasting. Scr, BUN, serum albumin (ALB), and urinary microalbumin (UmALB) levels were measured using a Hitachi 7600 automatic analyser (Hitachi Medical Corporation, Tokyo, Japan) in the laboratory at Xuanwu Hospital. The estimated glomerular filtration rate (eGFR) was determined using the simplified modification of diet in renal disease (MDRD) formula: 186.3 × Scr−1.154 × age−0.203 (× 0.742 if female and × 1.212 if Black). Renal dysfunction was defined by an eGFR < 60 mL/min/1.73 m2. Serum N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were detected using the PATHFAST system analyser (Mitsubishi, Tokyo, Japan).
Measurement of Serum NGAL and cystatin C levels
Serum NGAL levels were analysed using an ELISA kit from AntibodyShop (Gentofte, Denmark). Cystatin C levels were measured using a particle-enhancing immunonephelometric assay (Dade Behring, Marburg, Germany).
Echocardiography
Echocardiographic parameters, including the left ventricular ejection fraction and left ventricular end-diastolic dimension, were assessed in every patient using a Philips iE33 ultrasound system (Philips Healthcare Systems, Eindhoven, The Netherlands).
Statistical analysis
Statistical analyses were conducted using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). The data are shown as the mean ± standard deviation (SD). Continuous variables among the four groups were compared by using one-way analysis of variance. The χ2 test was used for comparisons of enumeration data. To obtain the optimal threshold value, the sensitivity and specificity of serum NGAL and cystatin C in diagnosing early renal impairment were determined using receiver operating characteristic (ROC) curves. Correlations between serum NGAL or cystatin C levels and NT-proBNP levels or the eGFR were evaluated by Pearson’s or Spearman’s test. A P value <0.05 indicates statistical significance.
Results
The baseline clinical and biochemical characteristics of all subjects are shown in Table 1. Serum NGAL levels of CAD patients from the NYHA class I, NYHA class II, and NYHA class III–IV groups were significantly higher than those of the control group (P = 0.009, P < 0.001, P < 0.001, respectively). Serum cystatin C levels of the NYHA class II and NYHA class III–IV groups were significantly higher than those of the control group (both P < 0.001). There was no significant difference in serum cystatin C levels between the NYHA class I group and the control group. Scr, BUN, and UmALB levels were significantly higher in patients of the NYHA class III–IV group compared with those in the other groups (all P < 0.001).
Table 1.
Baseline clinical and biochemical characteristics of the subjects
| Characteristic | Control group n = 31 |
NYHA class I n = 30 |
NYHA class II n = 28 |
NYHA class III–IV n =26 |
F or χ 2 | P value |
|---|---|---|---|---|---|---|
| Age, years | 79.00 ± 9.32 | 76.33 ± 4.82 | 78.00 ± 12.32 | 80.67 ± 8.86 | 1.06 | NS |
| Sex, male% | 54.83 | 56.66 | 57.14 | 53.84 | 0.80 | NS |
| Systolic BP, mm Hg | 136.00 ± 19.82 | 128.17 ± 19.31 | 126.20 ± 28.32 | 126.43 ± 22.49 | 1.24 | NS |
| Diastolic BP, mm Hg | 78.33 ± 7.48 | 78.28 ± 7.19 | 74.80 ± 13.60 | 79.00 ± 11.26 | 0.98 | NS |
| BMI, kg/m2 | 22.05 ± 3.44 | 23.57 ± 2.65 | 23.25 ± 2.63 | 22.43 ± 2.99 | 1.69 | NS |
| LVEDD, mm | 42.58 ± 11.48 | 44.97 ± 12.64 | 51.87 ± 16.45○ | 58.88 ± 14.25•▲▽ | 8.01 | <0.001 |
| LVEF, % | 61.56 ± 12.15 | 55.17 ± 17.63 | 48.76 ± 16.58○ | 44.55 ± 11.51• | 7.30 | <0.001 |
| NT-proBNP, ng/L | 453.29 ± 286.50 | 1553.40 ± 900.77• | 2073.10 ± 1299.49• | 3658.92 ± 2486.51•▲▼ | 24.47 | <0.001 |
| Scr, µmol/L | 85.20 ± 18.13 | 90.5 ± 23.62 | 98.11 ± 22.31 | 110.54 ± 17.27•▲▽ | 7.97 | <0.001 |
| BUN, mmol/L | 12.67 ± 6.98 | 13.38 ± 8.12 | 15.22 ± 7.14 | 23.15 ± 8.25•▲▼ | 10.86 | <0.001 |
| eGFR, mL/min | 79.26 ± 18.72 | 75.40 ± 31.70 | 65.21 ± 34.17 | 59.87 ± 32.17 | 2.55 | NS |
| ALB, g/L | 40.00 ± 1.29 | 39.36 ± 3.41 | 38.08 ± 4.08 | 35.02 ± 6.27•▲▼ | 8.21 | <0.001 |
| UmALB, µg/min | 12.865 ± 11.31 | 19.88 ± 19.69 | 29.67 ± 22.26 | 98.89 ± 36.27•▲▼ | 77.06 | <0.001 |
| NGAL, µg/L | 36.96 ± 21.23 | 87.80 ± 61.40• | 141.21 ± 92.96•▲ | 198.15 ± 98.46•▲▼ | 24.43 | <0.001 |
| Cystatin C, mg/L | 0.75 ± 0.64 | 1.40 ± 1.88 | 2.33 ± 2.03•△ | 3.45 ± 1.81•▲▽ | 13.95 | <0.001 |
| Medical history | ||||||
| Myocardial infarction, % | NA | 13.33 | 21.42 | 30.76 | 2.51 | NS |
| Ischaemic stroke or TIA,% | NA | 36.66 | 39.28 | 46.15 | 0.54 | NS |
| Hypertension, % | NA | 40.00 | 46.42 | 53.84 | 1.07 | NS |
| Atrial fibrillation, % | NA | 16.66 | 21.42 | 30.76 | 1.61 | NS |
| Medication | NS | |||||
| ACE inhibitor or ARB, % | NA | 76.66 | 78.57 | 69.23 | 0.69 | NS |
| BB, % | NA | 56.66 | 53.57 | 53.84 | 0.31 | NS |
| CCB, % | NA | 26.08 | 25.00 | 23.07 | 0.33 | NS |
| Diuretics, % | NA | 20.00 | 71.42▲ | 84.61▲ | 27.24 | <0.001 |
| Aspirin or clopidogrel, % | NA | 83.33 | 75.00 | 73.07 | 0.97 | NS |
| Warfarin or NOAC, % | NA | 16.66 | 17.85 | 26.92 | 1.05 | NS |
Values are mean ± SD or %. BMI, body mass index; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; Scr, serum creatinine; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; ALB, albumin; UmALB, urinary micro-albumin; NGAL, neutrophil gelatinase-associated lipocalin; TIA, transient ischaemic attack; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, β-receptor blocker; CCB, calcium channel blockers; NOAC, new oral anticoagulant.
Comparisons of continuous variables among the four groups were conducted by ANOVA followed by the Student–Newman–Keuls test. The χ2 test was used for comparisons of enumeration data. NS, not significant (P > 0.05, ANOVA). •P < 0.01 vs. the control group; △P < 0.05, ▲P < 0.01 vs. the NYHA class I group, ▽P < 0.05, ▼P < 0.01 vs. the NYHA class II group.
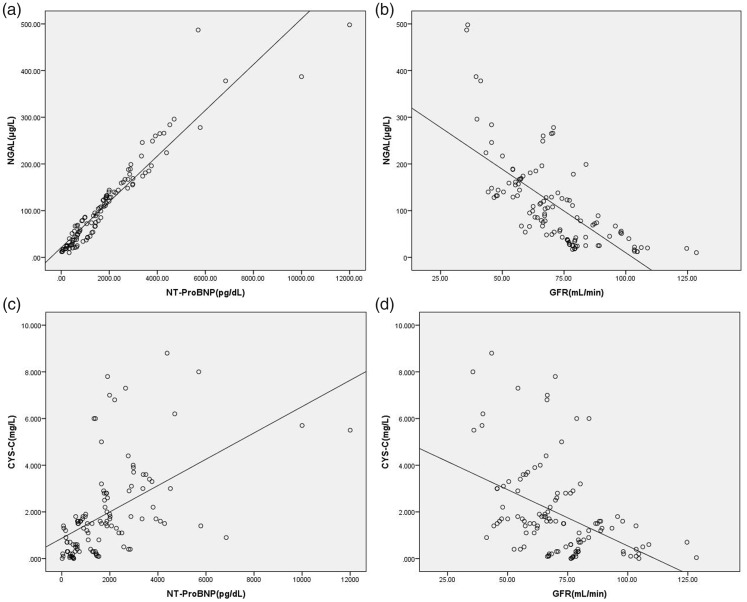
Serum NGAL and cystatin C levels were positively correlated with NT-proBNP levels (r = 0.842 and r = 0.718, respectively; P < 0.001) and were negatively correlated with the eGFR (r = −0.689 and r = −0.448, respectively; P < 0.001). (Figure 1).
Figure 1.
Scatter plots showing correlations of serum NGAL or cystatin C with NT-proBNP or the eGFR
(a) Correlation of serum NGAL with NTproBNP levels; (b) correlation of serum NGAL levels with the eGFR; (c) correlation of serum cystatin C with NT-proBNP levels; and (d) correlation of serum cystatin C levels with the eGFR. NGAL, neutrophil gelatinase-associated lipocalin; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; eGFR, estimated glomerular filtration rate.
ROC curve analysis showed that the areas under the curves of serum NGAL and cystatin C levels were 0.884 and 0.744, respectively (P < 0.001, Figure 2). A serum NGAL level of 127 g/L showed the highest sensitivity and specificity in diagnosing renal dysfunction at 93.5% and 84.5%, respectively. Moreover, a serum cystatin C level of 1.35 mg/L showed the highest sensitivity and specificity in diagnosing renal dysfunction at 83.9% and 54.8%, respectively.
Figure 2.

ROC curves of serum NGAL and cystatin C levels for diagnosing renal dysfunction
ROC, receiver operating characteristic; NGAL, neutrophil gelatinase-associated lipocalin.
Discussion
In this study, we found that serum NGAL and cystatin C levels increased with increased NYHA class classification. We also observed significant correlations between serum NGAL or cystatin C levels and the eGFR or NT-proBNP levels. Our results are consistent with previous data on the relationship between NGAL and brain natriuretic peptide with the GFR.18 Additionally, serum NGAL and cystatin C levels were highly sensitive and specific in diagnosing early renal dysfunction, as determined by ROC curves, and the sensitivity and specificity of serum NGAL levels were higher than those of serum cystatin C levels.
NGAL is a 25-kD protein that is present in activated neutrophils. NGAL is mainly expressed in the proximal tubules of the mature kidney. This protein is also expressed in the trachea, lung, stomach, small intestine, pancreas, prostate, and thymus.19 NGAL can be absorbed by the early primitive renal tubular epithelial cells and promotes maturation of primary tubular epithelial cells by mediating the transport of iron.20 NGAL is a marker of renal tubular injury and is elevated in the serum and urine of patients prior to an increase of Scr levels in the early stage of acute kidney injury.21–26 Several studies have also indicated a potential value for NGAL in heart failure after acute myocardial infarction. In the left ventricle of a rat myocardial infarction model, Yndestad et al.27 observed NGAL expression in the non-ischaemic area from 2 days to 2 months after myocardial infarction. Patients with CHF also had elevated serum NGAL levels. Cystatin C is a small protein consisting of 122 amino acids and is produced in vivo by nucleated cells at a constant rate. Cystatin C is almost completely filtrated by the glomerulus in the proximal tubule, and after degradation by renal tubular epithelial cells, it is completely reabsorbed into the blood. The concentration of serum cystatin C is entirely dependent on the GFR and is rarely affected by other factors. Therefore, serum cystatin C levels are considered to be one of the ideal endogenous markers of the GFR. Additionally, cystatin C is involved in the pathogenesis of heart failure, such as tissue remodelling, atherosclerosis, and left ventricular hypertrophy.28–30
In this study we chose to use the eGFR instead of measurement of the creatinine clearance rate for evaluating renal dysfunction because creatinine-based measurement may not be suitable for all populations. Creatinine-based estimation of renal function is only useful when renal function is stable and Scr values that are obtained during a change in renal function cannot provide an accurate estimate of renal function. For measuring the eGFR, we used the MDRD formula because it has been validated extensively in Caucasian and African American populations with renal dysfunction and has shown good performance for patients with all common causes of kidney disease.31
In our study, there was no significant difference in the eGFR, as calculated by the MDRD equation, among the study groups. We found that serum NGAL and cystatin C levels increased with the NYHA class and were the highest in the NYHA class III–IV group. Serum NGAL levels in the NYHA class I group were significantly higher than those of the control group. Serum cystatin C levels in the NYHA class II group were significantly higher than those of the control group and the NYHA class I group. There was no significant difference in serum cystatin C levels between the NYHA class I group and the control group. Levels of Scr, BUN, and UmALB were higher in patients of the NYHA class III–IV group compared with those in the other groups. These data indicated that renal impairment may have already occurred in patients in the early stage of CHF. Moreover, serum NGAL levels were increased earlier than cystatin C levels. Therefore, even if biochemical indicators of patients with CHF suggest that Scr, BUN, and UmALB levels are normal, doctors should still pay close attention to their renal function.
This study also showed that serum NGAL and cystatin C levels were negatively correlated with the eGFR. Furthermore, serum NGAL levels were more closely correlated with the eGFR than serum cystatin C levels. Therefore, both serum NGAL and cystatin C levels could reflect the degree of renal dysfunction. However, serum NGAL levels were a better indicator than serum cystatin C levels. This study also used ROC curves to study the threshold, sensitivity, and specificity of serum NGAL and cystatin C in diagnosing renal dysfunction. The area under the curve of serum NGAL levels was greater than that of cystatin C. The sensitivity and specificity of serum NGAL in diagnosing renal dysfunction were also higher than those of serum cystatin C. These results suggest that serum NGAL levels can more accurately diagnose renal dysfunction than cystatin C among older patients with CAD.
Even mild renal impairment in patients with CHF leads to a higher mortality rate compared with those with normal renal function.32–34 CHF and CKD appear to interact in a vicious cycle, as one situation worsens the other. Patients with CHF and normal Scr levels are usually undertreated. Changes in renal function, even small changes in Scr levels, may worsen the prognosis of patients with CHF. Therefore, detection of early kidney dysfunction can aid implementation of early treatment to prevent or delay progression of renal impairment and effectively improve the patient’s prognosis.
In conclusion, serum NGAL and cystatin C are potential early and sensitive markers of kidney impairment among older patients with CAD. Detection of serum NGAL and cystatin C levels may contribute to early diagnosis of renal injury, and improve the treatment and prognosis of older patients with CAD.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the Beijing Municipal Bureau of Health Healthcare Committee Office (Beijing No.11-13).
References
- 1.Hoes AW, Mosterd A, Grobbee DE. An epidemic of heart failure? Recent evidence from Europe. Eur Heart J 1998; 19(Suppl L): L2–L9. [PubMed] [Google Scholar]
- 2.Rich MW. Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol A Biol Sci Med Sci 2001; 56: M88–M96. [DOI] [PubMed] [Google Scholar]
- 3.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezekowitz J1, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol 2004; 44: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 5.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 6.McAlister FA, Ezekowitz J, Tonelli M, et al. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 7.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol 2004; 15: 1912–1919. [DOI] [PubMed] [Google Scholar]
- 8.van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014; 16: 103–111. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Cruz DN, Aspromonte N, et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25: 1406–1416. [DOI] [PubMed] [Google Scholar]
- 10.House AA, Anand I, Bellomo R, et al. Definition and classification of Cardio-Renal Syndromes: workgroup statements from the 7thADQI Consensus Conference. Nephrol Dial Transplant 2010; 25: 1416–1420. [DOI] [PubMed] [Google Scholar]
- 11.Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int 2014; 2014: 937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016; 13: 28–35. [DOI] [PubMed] [Google Scholar]
- 13.Filippatos G, Farmakis D, Parissis J. Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J 2014; 35: 416–418. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Scott C, Chen HH. The renal-cardiac connection in subjects with preserved ejection fraction: a population based study. ESC Heart Fail 2017; 4: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiba N, Shimokawa H. Chronic kidney disease and heart failure–Bidirectional close link and common therapeutic goal. J Cardiol 2011; 57: 8–17. [DOI] [PubMed] [Google Scholar]
- 16.Paapstel K, Zilmer M, Eha J, et al. Early Biomarkers of Renal Damage in Relation to Arterial Stiffness and Inflammation in Male Coronary Artery Disease Patients. Kidney Blood Press Res 2016; 41: 488–497. [DOI] [PubMed] [Google Scholar]
- 17.McKee PA, Castell WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 18.Donadio C. Effect of glomerular filtration rate impairment on diagnostic performance of neutrophil gelatinase-associated lipocalin and B-type natriuretic peptide as markers of acute cardiac and renal failure in chronic kidney disease patients. Crit Care 2014; 18: R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2007; 18: 407–413. [DOI] [PubMed] [Google Scholar]
- 20.Patsaoura A, Tatsi E, Margeli A, et al. Plasma neutrophil gelatinase-associated lipocalin levels are markedly increased in patients with non-transfusion-dependent thalassemia: Lack of association with markers of erythropoiesis, iron metabolism and renal function. Clin Biochem 2014; 47: 1060–1064. [DOI] [PubMed] [Google Scholar]
- 21.Hekmat R, Mohebi M. Comparison of serum creatinine, cystatin C, and neutrophil gelatinase-associated lipocalin for acute kidney injury occurrence according to risk, injury, failure, loss, and end-stage criteria classification system in early after living kidney donation. Saudi J Kidney Dis Transpl 2016; 27: 659–664. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007; 22: 2089–2095. [DOI] [PubMed] [Google Scholar]
- 24.Ling W, Zhaohui N, Ben H, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 2008; 108: c176–c181. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008; 36: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh C, Jani A, Mishra J, et al. Urine NGAL and IL‐18 are Predictive Biomarkers for Delayed Graft Function Following Kidney Transplantation. Am J Transplant 2006; 6: 1639–1645. [DOI] [PubMed] [Google Scholar]
- 27.Yndestad A, Landrø L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009; 30: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 28.Patel PC, Ayers CR, Murphy SA, et al. Association of cystatin C with left ventricular structure and function: The Dallas Heart Study. Circ Heart Fail 2009; 2: 98–104. [DOI] [PubMed] [Google Scholar]
- 29.McMurray MD, Trivax JE, McCullough PA. Serum cystatin C, renal filtration function, and left ventricular remodeling. Circ Heart Fail 2009; 2: 86–89. [DOI] [PubMed] [Google Scholar]
- 30.Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann NY Acad Sci 1998; 857: 110–118. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masson S, Latini R, Milani V, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail 2010; 3: 65–72. [DOI] [PubMed] [Google Scholar]
- 33.Braam B, Joles JA, Danishwar AH, et al. Cardiorenal syndrome-current understanding and future perspectives. Nat Rev Nephrol 2014; 10: 48–55. [DOI] [PubMed] [Google Scholar]
- 34.Scrutinio D, Passantino A, Lagioia R, et al. Detection and prognostic impact of renal dysfunction in patients with chronic heart failure and normal serum creatinine. Int J Cardiol 2011; 147: 228–233. [DOI] [PubMed] [Google Scholar]