Abstract
Objectives:
To estimate prevalence of physical activity and its associations with various psychiatric disorders and the use of psychotropic medications.
Methods:
A cross-sectional observational study was carried out between July 2012 and June 2014. Patients were enrolled from a number of hospitals located in 5 regions of the Kingdom of Saudi Arabia.
Results:
A total of 1185 patients were included in current analysis: 796 were outpatients, and 389 were inpatients. Out of 1,185 patients, 153 (12.9%) were physically active. Much higher rates of physical activity were reported among males than females (15.9% versus 9.6%, p<0.001). According to the univariate analysis, higher rates of physical activity were positively correlated with primary bipolar disorders, the use of antianxiety medications and, to a lesser extent, use of antipsychotic medications, but they were negatively correlated with primary anxiety disorders, use of antidepressant medications, and use of multiple psychotropic medications. The associations between physical activity and primary bipolar disorders (odds ratio [OR]=2.47, p=0.002), use of antianxiety medications (OR=3.58, p=0.003), and use of multiple psychotropic medications (OR=0.33, p<0.001) remained significant after adjusting for demographic and clinical characteristics.
Conclusion:
We report a variable but generally low prevalence of physical activity among a large, mixed sample of psychiatric patients in Saudi Arabia. These findings may highlight the importance of assessing physical activity status of psychiatric patients and the critical need for physical activity promotion programs among this group of disadvantaged patients.
Physical activity has been shown to considerably reduce the burden of several non-communicable disorders, such as heart disease, stroke, diabetes, and breast and colon cancers.1 Additionally, physical activity is associated with a 20-35% reduction in cardiovascular and all-cause mortality, particularly in women, even after adjusting for other relevant risk factors.2,3 Despite the clear benefits of physical activity, the rates of insufficient physical activity remain high in several parts of the world. For example, the World Health Organization (WHO) estimated that 23% of adults worldwide (>18 years) displayed insufficient physical activity (20% of men and 27% of women).4 Comparable rates in Saudi Arabia are worse. For example, the Saudi Ministry of Health estimated that 80% of Saudi men and 88% of Saudi women do not engage in physical activity related to work or recreation.5 Moreover, only one-third of Saudi adults engage in moderate to vigorous types of physical activity.5,6 Patients with psychiatric illnesses are a vulnerable group of patients who exhibit multiple poor health-associated behaviors and use medications that can induce physical side effects, such as metabolic syndrome.7-9 Additionally, these patients have a higher risk of mortality than the general population, mainly due to cardiovascular disease, stroke and respiratory tumors.10,11 Therefore, the beneficial impact of physical activity on psychiatric patients is even more advantageous. For example, physical activity and exercise have been used to reduce depressive and anxiety symptoms in psychiatric patients, results that may be mediated by the positive metabolic responses, neuroprotective effects, and improved quality of life.12-14 Moreover, based on high-quality evidence, physical activity reduces depression and anxiety in non-clinical populations, and even low levels of physical activity (for example, walking <150 minutes/week) prevent future depression.15,16 Although Saudi Arabia has experienced major socioeconomic changes over the last few decades that have fostered reduced levels of physical activity, the country has also experienced increased awareness and better management of psychiatric disorders.6,17-19 Nevertheless, data assessing the prevalence of physical activity in patients with psychiatric illnesses in Saudi Arabia are lacking. Therefore, we sought to estimate the prevalence of physical activity among a mixed group of patients with psychiatric illnesses in Saudi Arabia. Additionally, we sought to evaluate the associations between physical activity, patients with different psychiatric diagnoses and the use of psychotropic medications
Methods
Setting
The sample for the current study comprised patients seeking psychiatric advice at major hospitals in Saudi Arabia. We recruited patients from a number of hospitals located in the central, eastern, western, northern, and southern regions of Saudi Arabia. The hospitals included were King Khalid University Hospital in Riyadh, Zulfi General Hospital (central region), the Jeddah Mental Health Hospital (western region), the Al-Amal Complex for Mental Health - Dammam (eastern region), Aljouf Mental Health Hospital (northern region), and Abha Mental Health Hospital (southern region). Except for King Khalid University Hospital, which is a university-affiliated government hospital, all other hospitals are government-funded hospitals under the authority of the Ministry of Health. All included hospitals deliver free psychiatric inpatient and outpatient healthcare services.
Study design
A cross-sectional observational study was carried out between July 2012 and June 2014. We attained required ethical approvals from the Institutional Review Board of the Faculty of Medicine at King Saud University in Riyadh and administrative approval from the corresponding hospitals.
Population
We invited all consecutive male and female patients seeking psychiatric help at the included hospitals during the study period to join the study. Patients who provided written informed consent were included, irrespective of the type of psychiatric disorder, disease duration, and use of psychotropic medications.
Data collection
A mini-interview form was developed by the authors of this study. It comprised socio-demographic characteristics, medical history, current psychiatric disorders, and recent use of psychotropic medications. The information was mainly acquired by reviewing the patients’ charts. The diagnoses of psychiatric disorders including both Axis I and Axis II disorders in this study were established on routine clinical interviews. The psychiatric consultants in charge at each study site diagnosed the psychiatric illnesses of their patients using the criteria in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR). The psychiatric diagnoses were confirmed by the treating teams following a longitudinal and follow-up assessment in the psychiatric clinic. No semi-structured diagnostic interview or validated assessment scales were used. Trained psychiatric residents/staff were in charge for reviewing the chart and conducting the mini-interviews with the patients and/or their families. These mini-interviews aim to collect any unclear or missing information except for the major clinical data like psychiatric diagnoses and treatments which are certainly documented in the patients’ charts. Patients were asked to define their physical activity status as active or not active. A physically active status was defined as engaging in aerobic activity, such as walking, jogging, swimming, or garden/yard work, for a minimum of 20 minutes twice weekly.
Classification of psychiatric disorders
The psychiatric disorders of the particpants who were diagnosed by their primary psychiatrists using the DSM-IV-TR criteria were categorized into 8 groups to analyze the data.20,21 Primary psychotic disorders included schizophrenia, schizoaffective disorder, delusional disorder and brief psychotic disorder. Primary bipolar disorders included bipolar disorders types I and II. Primary depressive disorders included major depressive disorder and dysthymic disorder. Primary anxiety disorders included generalized anxiety disorder, obsessive-compulsive disorder, social anxiety disorder, specific phobia, panic disorder, post-traumatic stress disorder, and acute stress disorder. Personality disorders included personality disorder not otherwise specified (mixed personality disorder), paranoid personality disorder, antisocial personality disorder, and borderline personality disorder. Secondary psychiatric disorders included psychotic disorders caused by another medical condition, depression caused by another medical condition, dementia, substance abuse disorder, and substance-induced depressive disorder. Other disorders included undifferentiated somatoform disorder, conversion disorder, mental retardation, attention deficit hyperactivity disorder, dissociative disorder, primary insomnia, adjustment disorder, enuresis disorder, trichotillomania, and anorexia nervosa. Multiple disorders included the presence of 2 or more psychiatric disorders from the previously mentioned classes.
Classification of psychotropic medications
Both the individual psychotropic medications and the pharmacological groups were used in the analysis. These medications included antipsychotics: low-potency first-generation (Chlorpromazine, Thioridazine, Sulpiride), high-potency first-generation (Haloperidol, Trifluoperazine, Fluphenazine, Flupentixol, Zuclopenthixol) and second-generation; antidepressants: selective serotonin reuptake inhibitors (SSRIs), tricyclics, and others; mood stabilizers; and antianxiety drugs.
Statistical analysis
Data are presented as frequencies and percentages for categorical data and as means and standard deviations (SDs) for continuous data. Significant differences in the demographics, clinical characteristics, comorbidity, and medications between patients who were physically active and patients who were not physically active were analyzed using the chi-square test or Fisher’s exact test, as appropriate, for categorical data and Student’s t-test or the Mann-Whitney U test, as appropriate, for continuous data. Independent associations between physical activity, different psychiatric disorders and psychotropic medications were evaluated using multivariate logistic regression models with stepwise backward elimination, after adjusting for relevant demographic and clinical characteristics (p<0.10 in a univariate analysis). Characteristics with prevalence rates <5% were excluded from the model to avoid instability. All p-values were 2-tailed. A p-value <0.05 was considered significant. The Statistical Package for the Social Science (SPSS) software version 23.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
A total of 1,185 patients (647 males and 538 females) were included in the current analysis (Figure 1); 796 (67.2%) were outpatients and 389 (32.8%) were inpatients. Out of the 1,185 patients, 153 (12.9%) patients were physically active. As shown in Figure 2, the prevalence of physical activity was significantly higher among males than females (15.9% and 9.6%, p<0.001), and this gender difference in physical activity was maintained across all age groups (p<0.001).
Figure 1.
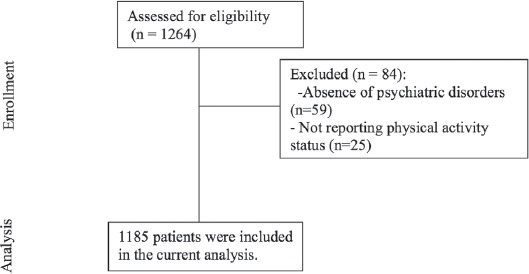
Flowchart of patients included in the analysis of the study.
Figure 2.
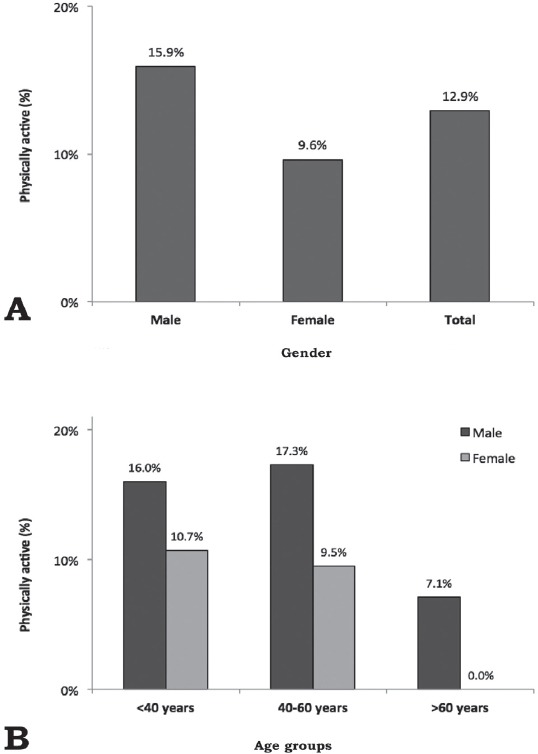
Prevalence of physical activity among psychiatric patients (N=1185) stratified according to A) gender and B) age.
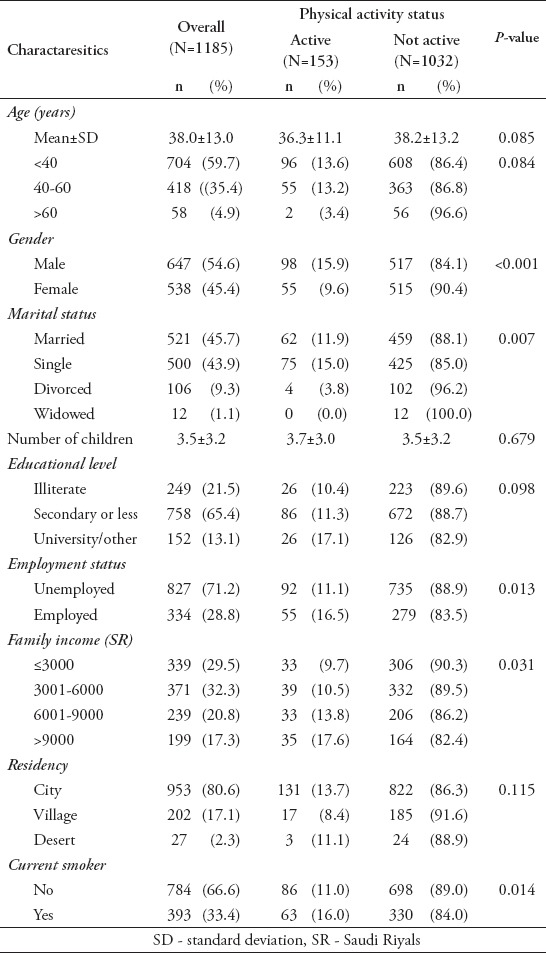
Demographic characteristics are presented in Table 1. The average age was 38.0±13.0 years, and 54.3% of the patients were unmarried. The majority of patients had attained secondary education or less; 86.9% were illiterate, and 71.2% were unemployed. Additionally, 61.8% had a family income of 6,000 Saudi Riyals (SR) or less per month, 80.6% were living in urban communities, and 66.6% of patients were non-smokers. Significantly higher rates of physical activity were observed for males versus females (p<0.001), single versus married patients (p=0.007), patients with higher family incomes versus lower family incomes (p=0.031), and smokers versus non-smokers (p=0.014). A trend (p<0.10) toward higher rates of physical activity was also observed among younger patients (p=0.084) and patients with higher education (p=0.098).
Table 1.
Demographic characteristics of psychiatric patients stratified according to physical activity status (N=1185)
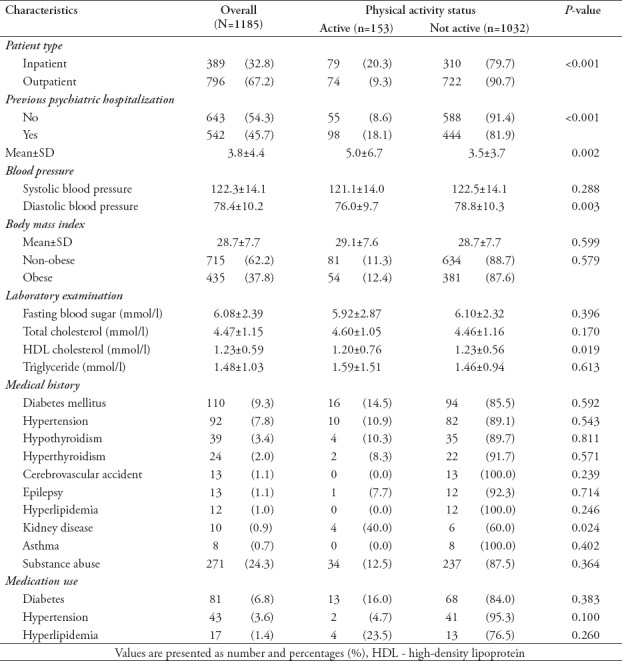
The clinical characteristics of the examined patients are presented in Table 2. The average systolic blood pressure was 122.3±14.1 mmHg, and the average diastolic blood pressure was 78.4±10.2 mmHg. The average body mass index (BMI) was 28.7±7.7, and 37.8% of the patients were obese. Blood examinations showed fasting blood glucose levels of 6.08±2.39 mmol/l, total cholesterol levels of 4.47±1.15 mmol/l, high-density lipoprotein (HDL) cholesterol levels of 1.23±0.59 mmol/l, and triglycerides levels of 1.48±1.03 mmol/l. Common comorbidities included diabetes, hypertension, and thyroid dysfunction. Approximately one-quarter (24.3%) of the patients presented with substance abuse disorders. Commonly treated non-psychotropic conditions included diabetes, hypertension, and hyperlipidemia. Approximately half (45.7%) of the patients had an average of 3.8±4.4 previous psychiatric hospitalizations. Higher rates of physical activity were significantly associated with a slightly lower diastolic blood pressure, slightly lower HDL cholesterol level, a history of kidney disease, and previous psychiatric hospitalizations.
Table 2.
Clinical characteristics of psychiatric patients stratified according to physical activity status (N=1185).
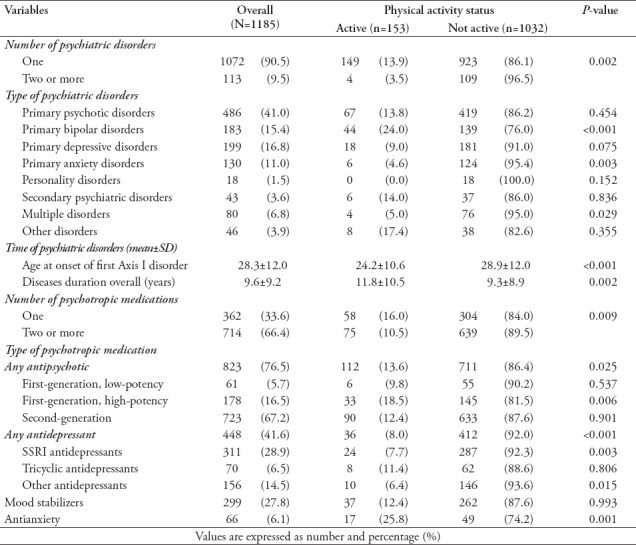
The correlations between physical activity with different psychiatric disorders and the use of different psychotropic medications are displayed in Table 3. The majority of patients were diagnosed with a single psychiatric disorder (90.5%), with 41% diagnosed with primary psychotic disorders, 16.8% with primary depressive disorders, and 15.4% with primary bipolar disorders. The average age of onset of psychiatric disorders was 28.3±12.0 years, with an average disease duration of 9.6±9.2 years. The majority of patients were using 2 or more psychotropic medications (66.4%), mainly including antipsychotics (76.5%), antidepressants (41.6%), and mood stabilizers (27.8%). Higher rates of physical activity were positively correlated with primary bipolar disorders, the use of antianxiety medications and, to a lesser extent, the use of antipsychotic medications (particularly high-potency first-generation antipsychotics) but were negatively correlated with primary anxiety disorders, multiple disorders, and the use of antidepressant medications.
Table 3.
Psychiatric disorders and use of psychotropic medications among psychiatric patients stratified according to physical activity status (N=1185).
As shown in Figure 3, the prevalence of physical activity was higher in patients using antipsychotic medications or those who did not use any psychotropic medications and was lower in patients using antidepressant medications alone or in combination with antipsychotic medications. Among the commonly used (>5% of the patients) psychotropic medications, a higher prevalence of physical activity was observed in patients who used quetiapine or haloperidol, and a lower prevalence was observed in patients who used mirtazapine, aripiprazole, olanzapine, escitalopram, or fluoxetine. Among the less commonly used (<5%) psychotropic medications, a higher prevalence of physical activity was observed (>40%) in patients who used clonazepam or fluphenazine.
Figure 3.
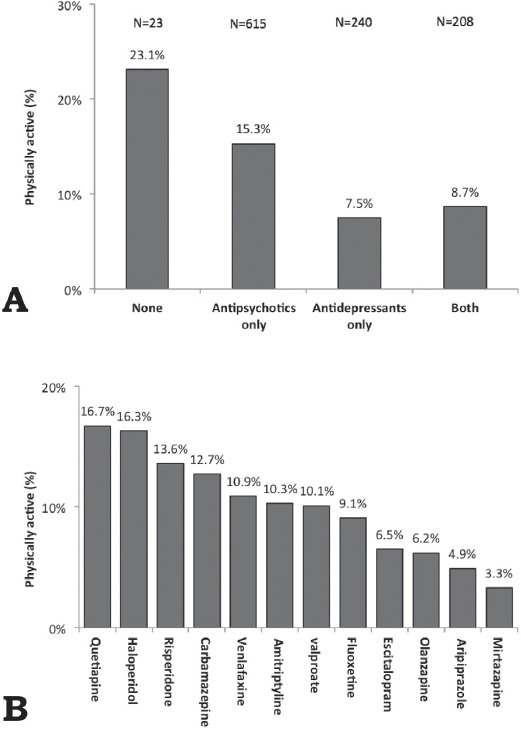
Prevalence of physical activity among psychiatric patients (N=1185) stratified according to the A) psychotropic medication group and B) the use of individual psychotropic medications.
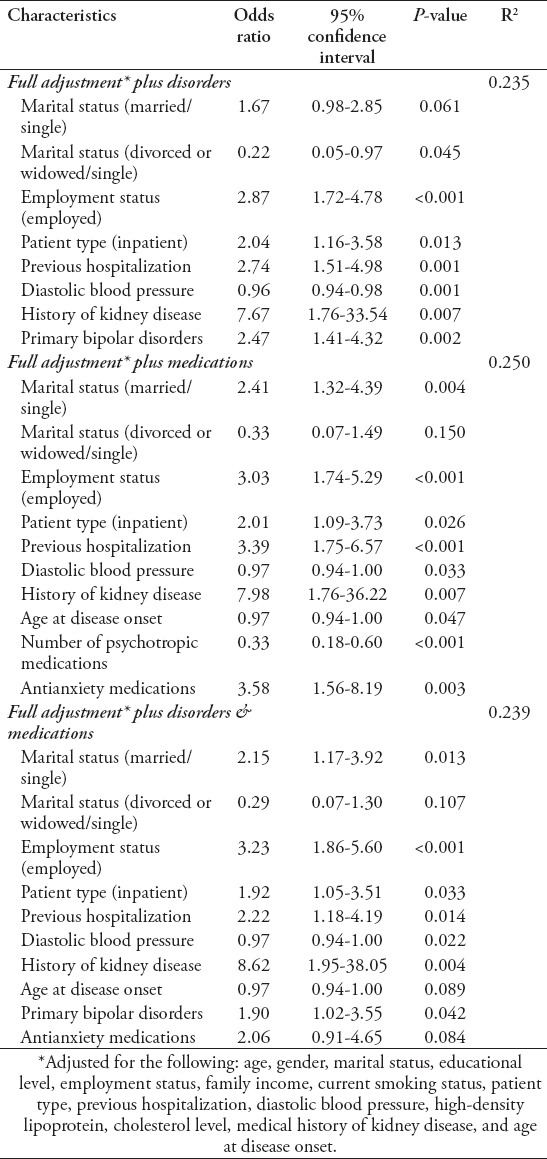
Table 4 presents the multivariate logistic regression analysis of the associations with physical activity. The models tested the association between physical activity and the different psychiatric disorders and the use of different psychotropic medications, after adjusting for demographic and clinical characteristics (Tables 1 & 2). Significant associations or significant trends for age, gender, marital status, educational level, employment status, family income, current smoking status, patient type, previous psychiatric hospitalization, diastolic blood pressure, HDL cholesterol level, a medical history of kidney disease, and age at disease onset were observed in the univariate analysis. In models adjusted for psychiatric disorders, either alone (odds ratio (OR)=2.47, p=0.002) or with psychotropic medications (OR=1.90, p=0.042), physical activity was independently and positively associated with primary bipolar disorders. In models adjusted for psychotropic medications but not psychiatric disorders, physical activity was positively associated with the use of antianxiety medications (OR=3.58, p=0.003) but negatively associated with the number of psychotropic medications used (OR=0.33, p<0.001). Additionally, in almost all models, physical activity was positively associated with marriage, employment, inpatient setting, previous psychiatric hospitalizations, and a history of kidney disease but negatively associated with diastolic blood pressure and age at disease onset.
Table 4.
Multivariate logistic regression analysis of associations of physical activity with different characteristics of psychiatric patients (N=1185).
Discussion
We report a low prevalence of physical activity in a large, mixed sample of patients with psychiatric illnesses in both inpatient and outpatient settings in Saudi Arabia. For example, the level of physical activity observed in the current study was much lower than comparable rates reported by the Saudi Ministry of Health for the general population (13% versus 32%).5 This low community level of physical activity remained the same, even a decade after the Saudi Ministry of Health report, and this level is known to be much lower than the global average.4,6 Hot weather, the lack of appropriate nearby sports facilities, and a lack of time have been reported as barriers to leisure-related physical activities among both genders in Saudi Arabia.22,23 Unfortunately, the lack of physical activity data for patients with psychiatric illnesses in Saudi Arabia has limit our ability to compare the current findings with the general population. However, a number of international studies consistently reported lower physical activity levels among patients with psychiatric illnesses than in the general population.24-26 For example, the amount of physical activity was estimated at 32.6 metabolic equivalents per day in patients with psychiatric illnesses compared with 43.3 metabolic equivalents per day in healthy controls in Denmark.24 Similarly, a negative correlation between physical activity and the burden/risk of psychiatric disorders has been reported among different populations in Brazil and Norway.27,28
This finding may be explained by the observation that patients with psychiatric illnesses frequently exhibit low resilience and low self-efficacy for practicing exercise and by the likely lack of physicians who provide counseling regarding exercise.29-31 Additionally, several barriers to physical activity have been described among patients with psychiatric illnesses in the US and UK, including symptoms of mental illness, medication-associated sedation, weight gain, fear of unsafe conditions, limited social support, and fear of discrimination.30,32 Lifestyle changes that focus on the cumulative performance of at least 150 minutes of moderate-intensity aerobic physical activity throughout the week may be the most appropriate strategy in real world clinical settings.33,34 However, supervised exercise interventions, including structured individual and group programs delivered by qualified professionals such as physiotherapists, may enhance adherence to physical activity among patients with serious mental illnesses, such as schizophrenia and depression.35,36
Interestingly, in both the current study and in the national Saudi general population data, males exhibited significantly higher levels of physical activity than females.5,6,17 For example, 15.9% of males and 9.6% of females in the current study and 39% of males and 26% of females in the general Saudi population engaged in moderate to vigorous types of physical activity.5 The same findings were reported in several non-Saudi studies.37 This lower physical activity rate among females in the general Saudi population was attributed to fewer work-related physical activities.23,38,39 Additionally, the conservative culture of Saudi Arabia restricts females from performing outdoor activities, which may be another barrier to leisure-related physical activities among females in Saudi Arabia.23 Interestingly, the correlation between gender and physical activity observed in the univariate analysis in the current study disappeared after adjusting for demographic characteristics (data not provided). Thus, gender differences in physical activity observed in the univariate analysis may be partially explained by gender-specific differences in marital status and employment status, both of which were independently associated with physical activity. For example, females in the current study were more likely to be married (55.5% versus 36.4%) or divorced/widowed (13.6% versus 7.2%) and less likely to be employed (13.9% versus 42.4%) than males.
Unlike the consistently reported lower physical activity levels among patients with psychiatric illnesses compared with the general population, the variability of physical activity among patients with different psychiatric diagnoses has received little attention, and conflicting findings have been reported.24-26,28 Patients with primary bipolar disorders in the current study displayed higher levels of physical activity than patients with other psychiatric illnesses in both the univariate and multivariate analyses. Patients with bipolar disorders exhibit more active profiles during the manic stage than during the depressive stage.40 Additionally, patients with bipolar disorders tend to overestimate the actual physical activity practiced, probably as a result of the concomitant neurocognitive impairment.41 Finally, patients with bipolar disorder in the current study were primarily treated with antipsychotic medications (95.3%) and to a lesser extent with antianxiety medications (16.4%). Both treatments were associated with greater levels of physical activity in the current study, probably due to reductions in the disease symptoms and in the negative impact of the disease on physical activity. However, patients with primary anxiety disorders exhibited lower levels of physical activity than patients with other psychiatric illnesses in the univariate analysis in the present study but not in the multivariate analysis. Supporting the current findings, anxiety complicated with or without depression is negatively associated with physical activity levels, particularly leisure activities.25,28,42 Additionally, patients with anxiety disorders in the current study were primarily treated with antidepressant medications (93.7%), which was associated with lower levels of physical activity among our patients.
Information regarding the impact of psychotropic medications on physical activity is generally lacking. However, the sedative effects of many psychotropic medications and the tendency of some medications to cause weight gain and metabolic syndrome are among the reasons for lower physical activity among patients with psychiatric illnesses compared with the general population.32,43 Accordingly, in the current study, physical activity exhibited varying correlations with psychotropic medications. For example, antianxiety and antipsychotic medications were positively correlated with physical activity, whereas antidepressant medications were negatively correlated with physical activity. However, the antianxiety medications that were mainly used to treat patients with primary bipolar disorders were the only medications that retained the positive correlation with physical activity after adjusting for demographic and clinical characteristics.44 Additionally, the antidepressants used in the current study, particularly tricyclic antidepressants, are associated with metabolic syndrome, which is traditionally associated with a lower physical activity profile.45,46 However, the current findings should be interpreted with caution for several reasons. For example, a differentiation between the actual effect of psychotropic medication use and the effects of disease symptoms is difficult, even after statistical adjustment.25 In addition, more than 90% of the patients in the present study were taking psychotropic mediations, and two-thirds were using multiple psychotropic medications. Finally, the degree of patient compliance with the prescribed medications and the difference between transient and long-term use of psychotropic medications was not assessed in the current study.
The current study has several strengths. It is the first local study to estimate the prevalence of physical activity among patients with psychiatric illnesses and to assess variability within a population. The mixed patient population and the relatively large sample size enabled us to examine the correlations between physical activity and a wide range of psychiatric diagnoses and psychotropic medications. Nevertheless, we acknowledge a number of limitations.
Study limitations
First, the use of convenience sampling may limit the generalizability of our findings to patients with psychiatric illnesses in Saudi Arabia. The cross-sectional design did not allow us to ascertain causality between physical activity and psychiatric disorders or medications. Furthermore, as the presence of physical activity was self-reported data, the possibility of recall bias cannot be excluded. Finally, our definition of physical activity was broad and much lower than the recommended 150 minutes of moderate to vigorous activity per week. However, given the low percentage of individuals who achieve the recommended level of activity in the general Saudi population, we chose to use a lower cut-off.47 Moreover, even a low dose of moderate-to-vigorous physical activity has been found to be beneficial compared to inactivity.48-50 Notably, some of the studies that used the 150-minute cut-off for patients with psychiatric illnesses did not identify any participants who were able to achieve the recommended activity level.51 Accordingly, the lower activity level (13%) reported in the current study is probably overestimated.
In conclusion, we report a low prevalence of physical activity among a large, mixed sample of patients with psychiatric illnesses in Saudi Arabia. Additionally, the physical activity level varies by the psychiatric disease type and the medication(s) used, even after adjusting for sociodemographic and clinical characteristics. The current findings highlight the importance of assessing the physical activity status in patients with psychiatric illnesses and the critical need for physical activity promotion programs among this group of disadvantaged patients.
Acknowledgment
The authors extend their sincere appreciation for the support they received from the College of Medicine Research Center, Deanship of Scientific Research, King Saud University. The authors also express their gratitude to Ms. Fatima Jama and Dr. Aiman El-Saed for their assistance with data entry and analysis, respectively
Footnotes
Ethical Consent.
All manuscripts reporting the results of experimental investigations involving human subjects should include a statement confirming that informed consent was obtained from each subject or subject’s guardian, after receiving approval of the experimental protocol by a local human ethics committee, or institutional review board. When reporting experiments on animals, authors should indicate whether the institutional and national guide for the care and use of laboratory animals was followed
References
- 1.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 3.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Prevalence of insufficient physical activity. Geneva: WHO; [[cited 2015]]. Available from URL: http://www.who.int/gho/ncd/risk_factors/physical_activity_text/en/ [Google Scholar]
- 5.Saudi Ministry of Health. WHO STEPwise Approach to NCD Surveillance. Country Specific Standard Report. Saudi Arabia 2005. WHO (KSA): Ministry of Health; 2005. [Google Scholar]
- 6.Al-Zalabani AH, Al-Hamdan NA, Saeed AA. The prevalence of physical activity and its socioeconomic correlates in Kingdom of Saudi Arabia: A cross-sectional population-based national survey. J Taibah Univ Med Sci. 2015;10:208–215. [Google Scholar]
- 7.Chuang HT, Mansell C, Patten SB. Lifestyle characteristics of psychiatric outpatients. Can J Psychiatry. 2008;53:260–266. doi: 10.1177/070674370805300407. [DOI] [PubMed] [Google Scholar]
- 8.Chwastiak LA, Rosenheck RA, Kazis LE. Association of Psychiatric Illness and Obesity, Physical Inactivity, and Smoking among a National Sample of Veterans. Psychosomatics. 2011;52:230–236. doi: 10.1016/j.psym.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centorrino F, Masters GA, Talamo A, Baldessarini RJ, Öngür D. Metabolic syndrome in psychiatrically hospitalized patients treated with antipsychotics and other psychotropics. Hum Psychopharmacol. 2012;27:521–526. doi: 10.1002/hup.2257. [DOI] [PubMed] [Google Scholar]
- 10.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications. JAMA Psychiatry. 2015;72:334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn DPJ, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Research Database. Arch Gen Psychiatry. 2007;64:242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 12.Strohle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- 13.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 14.Knöchel C, Oertel-Knöchel V, O'Dwyer L, Prvulovic D, Alves G, Kollmann B, et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol. 2012;96:46–68. doi: 10.1016/j.pneurobio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9:366–378. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- 16.Mammen G, Faulkner G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am J Prev Med. 2013;45:649–657. doi: 10.1016/j.amepre.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Al-Nozha MM, Al-Hazzaa HM, Arafah MR, Al-Khadra A, Al-Mazrou YY, Al-Maatouq MA, et al. Prevalence of physical activity and inactivity among Saudis aged 30-70 years. A population-based cross-sectional study. Saudi Med J. 2007;28:559–568. [PubMed] [Google Scholar]
- 18.Mabry R, Koohsari MJ, Bull F, Owen N. A systematic review of physical activity and sedentary behaviour research in the oil-producing countries of the Arabian Peninsula. BMC Public Health. 2016;16:1003. doi: 10.1186/s12889-016-3642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig HG, Al Zaben F, Sehlo MG, Khalifa DA, Al Ahwal MS. Current state of psychiatry in Saudi Arabia. Int J Psychiatry Med. 2013;46:223–242. doi: 10.2190/PM.46.3.a. [DOI] [PubMed] [Google Scholar]
- 20.Hales RE. The American Psychiatric Publishing Textbook of Psychiatry. 5th ed. The American Journal of Psychiatry. 2008;165:1220. doi: 10.1176/appi.ajp.2008.08040493. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- 22.Alsubaie ASR, Omer EOM. Physical activity behavior predictors, reasons and barriers among male adolescents in Riyadh Saudi Arabia: evidence for obesogenic environment. Int J Health Sci (Qassim) 2015;9:400–408. [PMC free article] [PubMed] [Google Scholar]
- 23.Amin TT, Suleman W, Ali A, Gamal A, Al Wehedy A. Pattern, prevalence, and perceived personal barriers toward physical activity among adult Saudis in Al-Hassa, KSA. J Phys Act Health. 2011;8:775–784. doi: 10.1123/jpah.8.6.775. [DOI] [PubMed] [Google Scholar]
- 24.Nyboe L, Lund H. Low levels of physical activity in patients with severe mental illness. Nord J Psychiatry. 2013;67:43–46. doi: 10.3109/08039488.2012.675588. [DOI] [PubMed] [Google Scholar]
- 25.de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BWJH. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress Anxiety. 2010;27:1057–1065. doi: 10.1002/da.20738. [DOI] [PubMed] [Google Scholar]
- 26.Mangerud W, Bjerkeset O, Lydersen S, Indredavik M. Physical activity in adolescents with psychiatric disorders and in the general population. Child Adolesc Psychiatry Ment Health. 2014;8:2. doi: 10.1186/1753-2000-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha SV, de Araújo TM, de Almeida MMG, Virtuoso JS., Júnior Practice of physical activity during leisure time and common mental disorders among residents of a municipality of Northeast Brazil. Rev Bras Epidemiol. 2012;15:871–883. doi: 10.1590/s1415-790x2012000400017. [DOI] [PubMed] [Google Scholar]
- 28.Harvey SB, Hotopf M, Overland S, Mykletun A. Physical activity and common mental disorders. Br J Psychiatry. 2010;197:357–364. doi: 10.1192/bjp.bp.109.075176. [DOI] [PubMed] [Google Scholar]
- 29.Min JA, Jung YE, Kim DJ, Yim HW, Kim JJ, Kim TS, et al. Characteristics associated with low resilience in patients with depression and/or anxiety disorders. Qual Life Res. 2013;22:231–241. doi: 10.1007/s11136-012-0153-3. [DOI] [PubMed] [Google Scholar]
- 30.Ussher M, Stanbury L, Cheeseman V, Faulkner G. Physical activity preferences and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr Serv. 2007;58:405–408. doi: 10.1176/ps.2007.58.3.405. [DOI] [PubMed] [Google Scholar]
- 31.Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA. 1999;282:1583–1588. doi: 10.1001/jama.282.16.1583. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt J, Snyder M, Miller A, Wilbur J. Perceptions of barriers and benefits to physical activity among outpatients in psychiatric rehabilitation. J Nurs Scholarsh. 2006;38:50–55. doi: 10.1111/j.1547-5069.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 33.Richardson CR, Faulkner G, McDevitt J, Skrinar GS, Hutchinson DS, Piette JD. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatr Serv. 2005;56:324–331. doi: 10.1176/appi.ps.56.3.324. [DOI] [PubMed] [Google Scholar]
- 34.Word Health Organization. Physical Activity and Adults. Geneva: World Health Organization; [[cited 2017 September 22]]. Available from: http://www.who.int/dietphysicalactivity/factsheet_adults/en/ [Google Scholar]
- 35.Vancampfort D, Rosenbaum S, Schuch FB, Ward PB, Probst M, Stubbs B. Prevalence and predictors of treatment dropout from physical activity interventions in schizophrenia: a meta-analysis. Gen Hosp Psychiatry. 2016;39:15–23. doi: 10.1016/j.genhosppsych.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A, et al. Dropout from exercise randomized controlled trials among people with depression: A meta-analysis and meta regression. J Affect Disord. 2016;190:457–466. doi: 10.1016/j.jad.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Zechner MR, Gill KJ. Predictors of physical activity in persons with mental illness: Testing a social cognitive model. Psychiatr Rehabil J. 2016;39:321–327. doi: 10.1037/prj0000191. [DOI] [PubMed] [Google Scholar]
- 38.Al-Hazzaa HM, Abahussain NA, Al-Sobayel HI, Qahwaji DM, Musaiger AO. Physical activity, sedentary behaviors and dietary habits among Saudi adolescents relative to age, gender and region. Int J Behav Nutr Phys Act. 2011;8:140. doi: 10.1186/1479-5868-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Nuaim AA, Al-Nakeeb Y, Lyons M, Al-Hazzaa HM, Nevill A, Collins P, et al. The Prevalence of Physical Activity and Sedentary Behaviours Relative to Obesity among Adolescents from Al-Ahsa, Saudi Arabia: Rural versus Urban Variations. J Nutr Metab. 2012;2012:417589. doi: 10.1155/2012/417589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sylvia LG, Friedman ES, Kocsis JH, Bernstein EE, Brody BD, Kinrys G, et al. Association of exercise with quality of life and mood symptoms in a comparative effectiveness study of bipolar disorder. J Affect Disord. 2013;151:722–727. doi: 10.1016/j.jad.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Vancampfort D, Firth J, Schuch F, Rosenbaum S, De Hert M, Mugisha J, et al. Physical activity and sedentary behavior in people with bipolar disorder: A systematic review and meta-analysis. J Affect Disord. 2016;201:145–152. doi: 10.1016/j.jad.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Pasco JA, Williams LJ, Jacka FN, Henry MJ, Coulson CE, Brennan SL, et al. Habitual physical activity and the risk for depressive and anxiety disorders among older men and women. Int Psychogeriatrics. 2011;23:292–298. doi: 10.1017/S1041610210001833. [DOI] [PubMed] [Google Scholar]
- 43.Choong E, Bondolfi G, Etter M, Jermann F, Aubry J-M, Bartolomei J, et al. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46:540–548. doi: 10.1016/j.jpsychires.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Alosaimi FD, Alhabbad A, Abalhassan MF, Fallata EO, Alzain NM, Alassiry MZ, et al. Patterns of psychotropic medication use in inpatient and outpatient psychiatric settings in Saudi Arabia. Neuropsychiatr Dis Treat. 2016;12:897–907. doi: 10.2147/NDT.S100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alosaimi FD, Abalhassan M, Alhaddad B, Alzain N, Fallata E, Alhabbad A, et al. Prevalence of metabolic syndrome and its components among patients with various psychiatric diagnoses and treatments: A cross-sectional study. Gen Hosp Psychiatry. 2017;45:62–69. doi: 10.1016/j.genhosppsych.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Vancampfort D, Probst M, Scheewe T, De Herdt A, Sweers K, Knapen J, et al. Relationships between physical fitness, physical activity, smoking and metabolic and mental health parameters in people with schizophrenia. Psychiatry Res. 2013;207:25–32. doi: 10.1016/j.psychres.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Amin TT, Al Khoudair AS, Al Harbi MA, y Ali AR. Leisure time physical activity in Saudi Arabia: prevalence, pattern and determining factors. Asian Pac J Cancer Prev. 2012;13:351–360. doi: 10.7314/apjcp.2012.13.1.351. [DOI] [PubMed] [Google Scholar]
- 48.Eijsvogels TMH, Thompson PD. Exercise is medicine: At any dose? JAMA. 2015;314:1915–1916. doi: 10.1001/jama.2015.10858. [DOI] [PubMed] [Google Scholar]
- 49.Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults. Ann Intern Med. 2015;162:325. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- 50.Hupin D, Roche F, Gremeaux V, Chatard J-C, Oriol M, Gaspoz J-M, et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1262–1267. doi: 10.1136/bjsports-2014-094306. [DOI] [PubMed] [Google Scholar]
- 51.Janney CA, Fagiolini A, Swartz HA, Jakicic JM, Holleman RG, Richardson CR. Are adults with bipolar disorder active?Objectively measured physical activity and sedentary behavior using accelerometry. J Affect Disord. 2014;152-154:498–504. doi: 10.1016/j.jad.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


