ABSTRACT
Aspergillus fumigatus can cause pulmonary aspergillosis in immunocompromised patients and is associated with a high mortality rate due to a lack of reliable treatment options. This opportunistic pathogen requires zinc in order to grow and cause disease. Novel compounds that interfere with fungal zinc metabolism may therefore be of therapeutic interest. We screened chemical libraries containing 59,223 small molecules using a resazurin assay that compared their effects on an A. fumigatus wild-type strain grown under zinc-limiting conditions and on a zinc transporter knockout strain grown under zinc-replete conditions to identify compounds affecting zinc metabolism. After a first screen, 116 molecules were selected whose inhibitory effects on fungal growth were further tested by using luminescence assays and hyphal length measurements to confirm their activity, as well as by toxicity assays on HeLa cells and mice. Six compounds were selected following a rescreening, of which two were pyrazolones, two were porphyrins, and two were polyaminocarboxylates. All three groups showed good in vitro activity, but only one of the polyaminocarboxylates was able to significantly improve the survival of immunosuppressed mice suffering from pulmonary aspergillosis. This two-tier screening approach led us to the identification of a novel small molecule with in vivo fungicidal effects and low murine toxicity that may lead to the development of new treatment options for fungal infections by administration of this compound either as a monotherapy or as part of a combination therapy.
KEYWORDS: Aspergillus fumigatus, animal models, antifungal agents, antifungal susceptibility testing, aspergillosis, bioluminescence, drug screening, fungal infection, immunosuppression, zinc metabolism
INTRODUCTION
Aspergillus fumigatus is a ubiquitous opportunistic fungal pathogen. It can cause invasive aspergillosis in immunocompromised individuals and is responsible for over 200,000 life-threatening infections per year (1). The preferred drug for treating this infection is voriconazole, which inhibits ergosterol synthesis, though amphotericin B, which binds to ergosterol, and echinocandins, which inhibit glucan synthesis, are alternatives (2). However, all these treatment options have limitations. Azole resistance is emerging across the world, which negatively impacts voriconazole-based treatments; also, amphotericin B is associated with significant toxicity, and echinocandins are only able to arrest the growth of the pathogen (3). Novel treatment options are thus urgently needed in order to combat invasive aspergillosis.
Zinc is the second most abundant transition metal after iron in humans and is essential for all organisms, as it is required for enzymes of all functional classes (4). Free zinc is tightly regulated within the human body and is only found at a concentration of 10 picomoles in order to prevent pathogens from acquiring it (5), a process termed nutritional immunity (6). In addition, infiltrated neutrophils in fungal abscesses release large amounts of calprotectin, a peptide heterodimeric protein that binds zinc and manganese with an extremely high affinity and limits their availability to pathogens (4). In order to obtain zinc, A. fumigatus utilizes three plasma membrane zinc transporters encoded by the zrfA, zrfB, and zrfC genes (7), which are regulated by the ZafA transcriptional activator (8). A loss of zrfC results in a partial loss of virulence, whereas the deletion of three genes results in a complete loss of virulence, which shows that they all function together to obtain zinc from the host (7).
Calprotectin, which has zinc-chelating activity, has been used to inhibit the growth of A. fumigatus in the corneas of immunocompetent mice (9). In addition, our group has used the zinc chelators N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN) and phenanthroline to successfully treat invasive pulmonary aspergillosis in mouse models (10). These findings support the suggestion that a reduction in the availability of zinc could have clinical applications for the treatment of aspergillosis (11). Following these promising findings, we carried out the screening of small-molecule libraries in order to find additional compounds that targeted the A. fumigatus zinc metabolism. We first performed a resazurin assay, as it is recommended for A. fumigatus screens (12) and has been successfully used to test the effects of antifungal drugs on A. fumigatus strains (13, 14), as well as to screen chemical libraries for novel antifungals (15, 16). This was followed by more exhaustive in vitro experiments utilizing luciferase and hyphal length measurements and then by in vivo tests on mouse models (10).
RESULTS
Screen and subsequent assays revealed six compounds of interest.
The primary resazurin screen comprised 59,223 compounds from commercial libraries (Chem-X-Infinity and Prestwick) and from the French academic library Chimiothèque Nationale (17). At an average concentration of 7.0 ± 3.5 μM, only 116 compounds were found to inhibit the growth of A. fumigatus wild type (AF14LUC) with no added zinc but permit the growth of the A. fumigatus triple zinc transporter knockout (AF721LUC) with 100 μM added zinc. Ninety-one percent of these compounds were validated using resazurin gradient concentration assays. These were followed up by luciferin gradient assays, where 15 compounds demonstrated at least a 2-fold difference in MIC50 between the wild type and triple knockout. Out of 15 compounds, 8 compounds were effective using hyphal length measurements. However, one compound was rejected due to high cytotoxicity on HeLa cells, another compound was rejected due to high toxicity on mice, and a third compound was omitted due to very limited availability. This left 6 compounds that were not toxic to HeLa cells (see Fig. S1 in the supplemental material) and that were used in further experiments. These compounds belonged to three different chemical series, with two pyrazolones, two porphyrins, and two polyaminocarboxylates.
Pyrazolone family.
Compounds in the pyrazolone family that were tested were Pyr05 and Pyr11 (Table 1 and Fig. S2). The addition of zinc fully restored the growth of A. fumigatus in the presence of both of these compounds (Fig. S3 and S4). Copper was also able to fully restore growth in the presence of both compounds, while iron and manganese were able to partially restore growth in the presence of Pyr05 (Fig. S3). This indicated that the compounds affected both copper and zinc metabolism. A little growth was observed upon incubating conidia for 8 h in medium containing either of the two compounds followed by 7 h of incubation in medium without the compounds, while incubating conidia for 8 h in medium free of the compounds followed by 7 h in medium containing the compounds resulted in almost complete inhibition (Fig. S5). This suggested that the two pyrazolones were acting at the early germination stage.
TABLE 1.
MICs of selected compoundsa
| Compound | MIC95 (μg/ml) | MIC50 (μg/ml) |
|---|---|---|
| Pyr05 | 13 | 1.3 |
| Pyr11 | 6.3 | 2.5 |
| Por06 | 14 | 1.4 |
| Por07 | 32 | 3.2 |
| Ami03 | 5 | 0.5 |
| Ami04 | 20 | 0.4 |
MIC95, ≥95% growth reduction; MIC50, ≥50% growth reduction.
Porphyrin family.
Compounds in the porphyrin family that were tested were Por06 and Por07 (Table 1 and Fig. S2). The addition of zinc restored growth in all but the highest tested concentrations for both compounds (Fig. S6 and S7), while copper and manganese were able to partially restore growth for both (Fig. S6). Incubating conidia for 8 h in medium containing either of the two compounds followed by 7 h of incubation in medium without the compounds or incubating conidia for 8 h in medium free of the compounds followed by 7 h in medium containing the compounds resulted in high levels of A. fumigatus inhibition (Fig. S8). This suggested that both compounds had a fast fungicidal effect. In vitro combination tests of Por06 resulted in indifferent results with all 3 antifungal drugs (Table 2). In addition, the administration of Por06 to infected mice resulted in no significant difference in survival compared to that in the control group. Thus, 5 out of 10 mice receiving 7.5 mg/kg of body weight/day (P = 0.4459) and 6 out of 10 mice receiving 11.25 mg/kg/day (P = 0.4004) of Por06 survived, compared to 12 out of 30 mice for the control group (Fig. S9). There was also no significant difference in luminescence between the groups (Fig. S9), and all three groups appeared to be similar (Fig. S10). The use of higher concentrations of Por06 to treat infected mice was not attempted, as 15 mg/kg/day resulted in 50% mortality when administered to uninfected mice.
TABLE 2.
Interactions between established antifungal drugs and library compounds using the FICIa
| Compound | FICI |
||
|---|---|---|---|
| Caspofungin | Voriconazole | Amphotericin B | |
| Por06 | 0.6 | 1.0 | 1.2 |
| Ami04 | 1.2 | 1.0 | 0.6 |
All results were between 0.5 and 4, indicating an indifferent result and no interactions between the compounds.
Polyaminocarboxylate family.
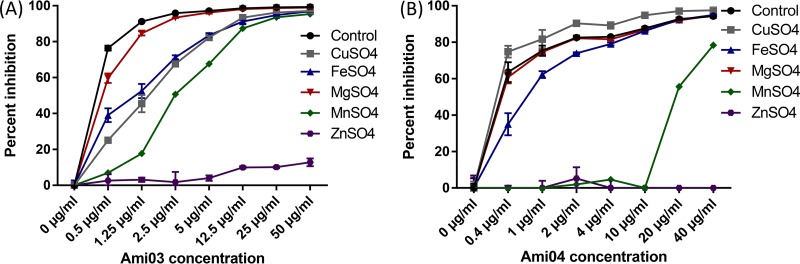
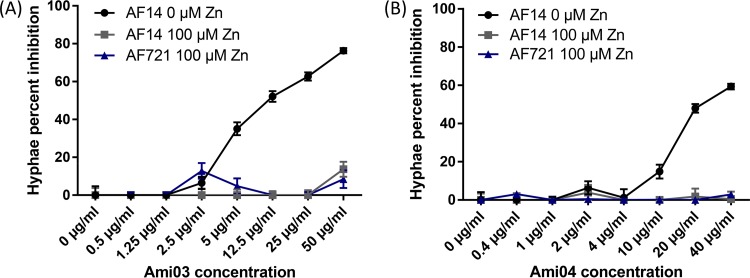
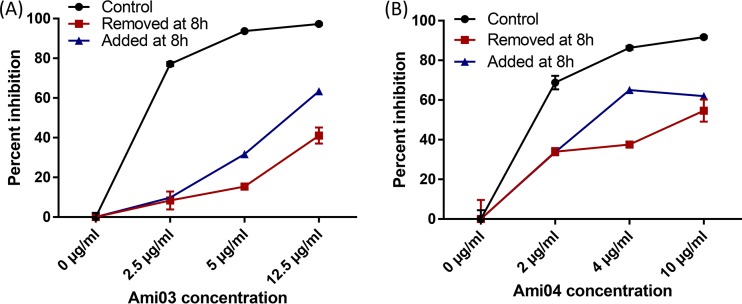
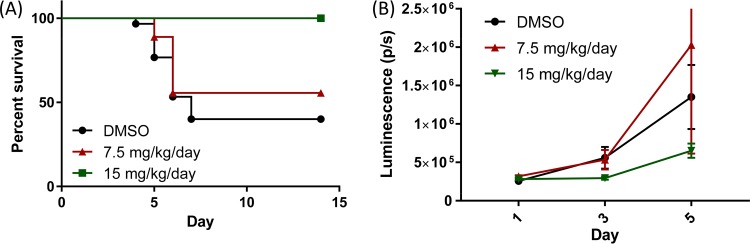
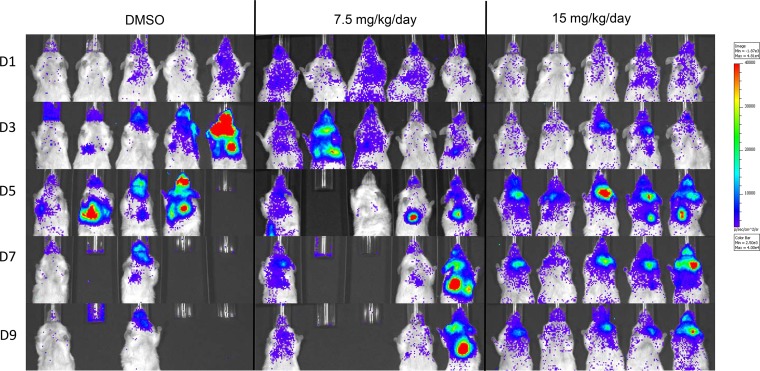
Compounds in the polyaminocarboxylate family that were tested were Ami03 and Ami04 (18) (Table 1 and Fig S2). The addition of zinc fully restored growth in the presence both these compounds, while manganese was able to partially restore growth in the presence of Ami04 (Fig. 1 and 2). The incubation of conidia for 8 h in medium containing the compounds followed by 7 h of incubation in medium without the compounds resulted in reduced growth inhibition of A. fumigatus conidia compared to having the compounds present throughout the incubation (Fig. 3). A reduction in inhibition was also observed when conidia were incubated for 8 h in the absence of the compounds followed by 7 h in their presence (Fig. 3). This suggested that these compounds were relatively slow acting and had a fungistatic effect on fungal growth. In vitro combination tests of Ami04 gave indifferent results with all 3 antifungal drugs (Table 2). Furthermore, in vivo experiments showed that a dose of 7.5 mg/kg/day of the polyaminocarboxylate compound Ami04 did not result in a significant difference, as only 5 out of 9 mice survived (P = 0.4264), and there was no significant difference in luminescence between the control group and the treated groups (Fig. 4 and 5). In contrast, a dose of Ami04 at 15 mg/kg/day was able to improve significantly the survival of immunosuppressed mice suffering from pulmonary aspergillosis (P = 0.0024), since 10 out of 10 infected mice recovered, compared to 12 out of 30 mice for the control group (Fig. 4). Though not statistically significant, there were 46% and a 52% reductions in luminescence on days 3 and 5, respectively, in the group receiving 15 mg/kg/day of Ami04 compared to the control.
FIG 1.
Percent inhibition based on luminescence measurements of A. fumigatus wild type (AF14) grown either with no added ions or with the addition of 100 μM CuSO4, FeSO4, MgSO4, MnSO4, or ZnSO4 for 15 h in the presence of the polyaminocarboxylate Ami03 (A) or Ami04 (B).
FIG 2.
Hyphal length percent inhibition of A. fumigatus wild type (AF14) or triple zinc transporter knockout (AF721) grown either with no added zinc or with 100 μM ZnSO4 for 10 h in the presence of the polyaminocarboxylate Ami03 (A) or Ami04 (B).
FIG 3.
Percent inhibition based on luminescence measurements of A. fumigatus wild type (AF14) grown in the presence of the polyaminocarboxylate Ami03 (A) or Ami04 (B). Removed at 8 h indicates that medium was replaced with fresh medium containing no tested compound after 8 h of incubation. Added at 8 h indicates that compounds were added to the medium after 8 h of incubation. The cultures were incubated for an additional 7 h, resulting in a total incubation time of 15 h.
FIG 4.
Percent survival (A) and luminescence (in photons per second) (B) of immunosuppressed mice that were intranasally infected with 7.5 × 104 A. fumigatus wild-type (AF14) conidia and treated with the polyaminocarboxylate Ami04. A dose of 15 mg/kg/day was able to significantly improve mouse survival (P = 0.0024) and resulted in a 46% reduction in luminescence on day 3 and 52% reduction on day 5 compared to the control group.
FIG 5.
Examples showing luminescence of mice treated with 7.5 or 15 mg/kg/day of the polyaminocarboxylate Ami04 and of a DMSO placebo group. Mice in all three groups developed aspergillosis; however, only the ones receiving 15 mg/kg/day showed 100% survival. Min, minimum; Max, maximum.
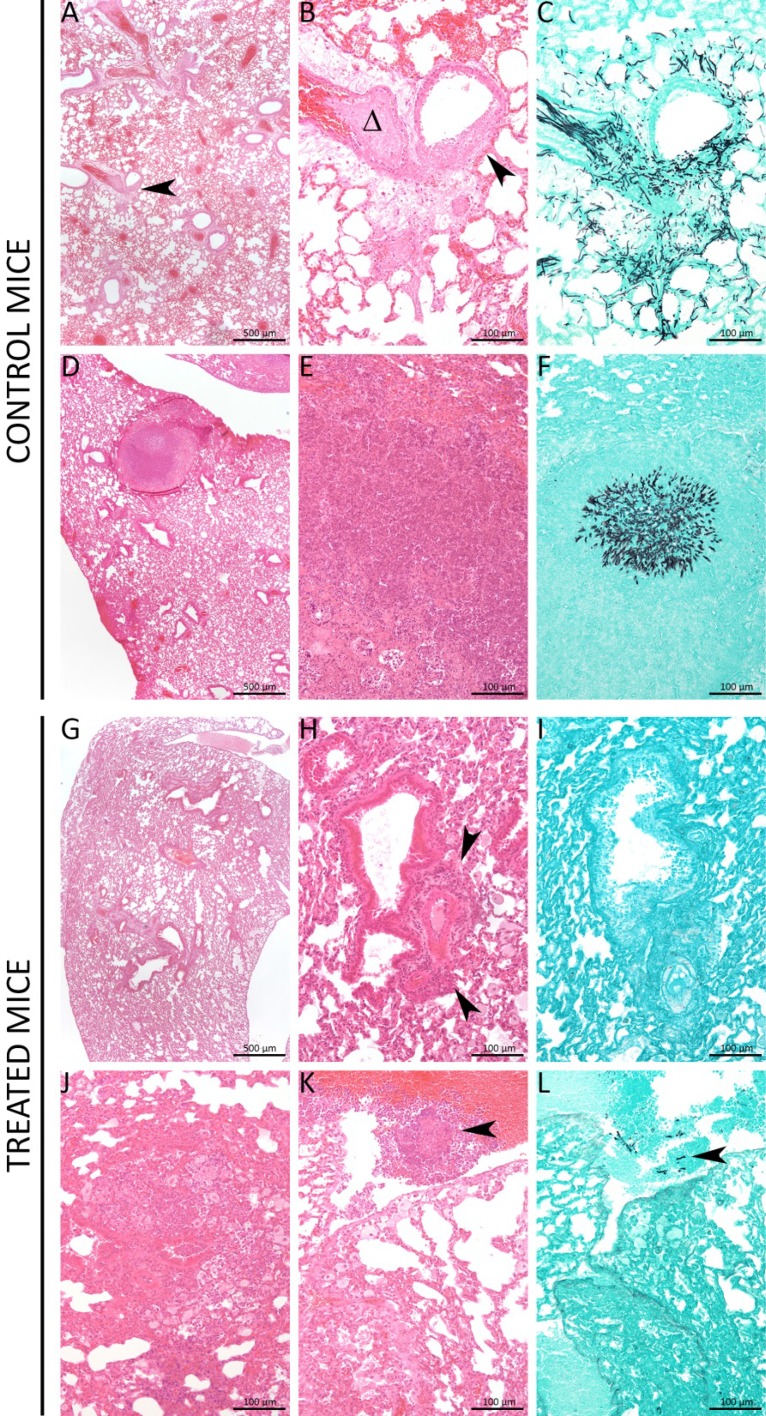
Lung sections from control mice displayed typical invasive aspergillosis lesions with small necrotic foci (Fig. 6A), destruction of bronchiole epithelium (Fig. 6B and C), blood vessel invasion by the fungus (Fig. 6D and E), and multifocal abscesses containing hyphae (Fig. 6F). In contrast, most treated mice displayed minimal to mild inflammatory lesions (Fig. 6G), characterized by perivascular lymphocyte and plasma cell infiltrates (Fig. 6H), with no fungi invading the parenchyma (Fig. 6I). A few mice displayed randomly distributed inflammatory (with neutrophils) or necrotic lesions (Fig. 6J and K), with few intralesional fungi (Fig. 6L).
FIG 6.
Treated mice displayed less severe lung invasion by the fungus. Control mice displayed very heterogeneous lesions from small necrotic foci (black arrowhead) (A), with destruction of bronchiole epithelium (black arrowhead) (B and C), and blood vessel invasion by the fungus (D and E), to randomly distributed multifocal abscesses containing hyphae (F). Most treated mice (6/10) displayed minimal to mild inflammatory lesions, characterized by perivascular lymphocyte and plasma cell infiltrates (black arrowheads) (G and H), with no fungi invading the parenchyma (I). Less frequently, mice (4/10) displayed randomly distributed inflammatory (with neutrophils) (J and K) or necrotic lesions (black arrowhead), with few intralesional fungi (black arrowhead) (L). Panels A, B, D, E, G, H, J, and K represent hematoxylin & eosin (HE) staining, and panels C, F, I, and L represent Grocott-Gomori's staining.
In summary, these results indicated that one polyaminocarboxylate compound (Ami04) significantly improved the survival of mice suffering from invasive pulmonary aspergillosis.
DISCUSSION
Zinc chelators have been shown to inhibit A. fumigatus growth (10). The 6 compounds identified by our protocol fell into 3 chemical families, and each family included two analogs. This supports the reliability of our approach, since it is very unlikely that structurally similar compounds would be selected by chance. Pyrazolones, porphyrins, and polyaminocarboxylates are all known to be metal ion chelators and include zinc chelators.
Pyrazolones have previously demonstrated antifungal activity against Aspergillus in vitro (19, 20), and there are pyrazolones which are known to bind to zinc to form complexes (21, 22). The two pyrazolones we identified proved effective in vitro; however, they were structurally similar to pyrazolones found to be metabolically unstable when tested in rat liver microsomes (23), which led us to focus on the other compounds we identified in our in vivo assays.
Certain porphyrins show strong selectivity toward zinc (24) and have been used as metal chelators (25, 26). The porphyrin Por06 (27) proved effective against fungi in vitro. Por06 had relatively low cytotoxicity against HeLa cells; however, a dose of 15 mg/kg/day proved toxic when administered to mice. The lower concentrations of 7.5 and 11.25 mg/kg/day did not improve the survival rates of infected mice compared to the ones receiving a placebo. Por06 fulfills only two of Lipinski's rules for determining if a compound is drug-like (28), as it has few hydrogen bond donors and acceptors but has a high molecular weight and logP value. The low solubility in water and the high molecular weight may therefore make it difficult for Por06 to reach the lungs from the peritoneal cavity. Another possible explanation for its lack of effect may be that it is rapidly degraded or cleared within the host body. The porphyrins identified by this study are therefore effective in vitro; however, the one we tested in vivo was not able to inhibit fungal growth when administered at concentrations that are not toxic to mice. However, it is possible that this porphyrin would be effective at that concentration if used in combination with other antifungal drugs.
Polyaminocarboxylates are commonly used in biological studies as metal chelators (29). One such compound is EDTA (30), which binds strongly to calcium, zinc, and magnesium and is able to inhibit metalloenzymes by rapidly capturing metal ions that spontaneously dissociate from them (18, 31). Polyaminocarboxylates have attracted interest as potential antimicrobial (32) or antitumor drugs (33, 34) due to their chelating activities. EDTA has proven effective in vitro as an antifungal agent either alone (35) or in combination with other compounds (36–38). EDTA has low toxicity, as mice can tolerate doses of 75 mg/kg/day (39), and it was able to reduce the mortality of rats suffering from pulmonary aspergillosis administered either alone or in combination with amphotericin B (40).
The polyaminocarboxylate Ami04 was able to significantly improve mouse survival in our model of invasive pulmonary aspergillosis at a dose of 15 mg/kg/day, as evidenced by the survival curve and the lung sections. This molecule proved more effective than EDTA, as EDTA required a dose of 30 mg/kg/day to improve survival in an invasive aspergillosis rat model (40). In addition, our molecule had greater specificity toward zinc than did EDTA, as it does not bind to magnesium. Moreover, the Ami04 compound did not show any toxicity toward HeLa cells or mice in the concentrations tested; Ami04 is less toxic than chelators that previously proved effective on infected mice, such as TPEN or phenanthroline, (10). Ami04 fulfills all but one of Lipinski's rules for determining if a compound is drug-like (28), as it has a low molecular weight, few hydrogen bond donors, and a low logP value.
The probable mode of action of this polyaminocarboxylate is that it sequesters free zinc outside the fungal cells and thus prevents them from acquiring the ions. It seems unlikely that it is able to enter the fungal cells, since other polyaminocarboxylates, such as EDTA or diethylenetriamine-N,N,N″,N″-tetraacetate (DTTA), are unable to cross cell membranes (41). This would explain why Ami03 and Ami04 primarily displayed a fungistatic effect. An inability to enter cells might also be the cause of the lower toxicity of polyaminocarboxylates than that of TPEN and phenanthroline in mammalian cells, so this could be advantageous, as it results in reduced host toxicity. When tested in combination with established antifungal drugs in vitro, Ami04, like Por06, had an indifferent effect, presumably because the mode of action of this compound is different from those of caspofungin, voriconazole, and amphotericin B. However, since there is no negative interaction between the drugs and our polyaminocarboxylate, it seems probable that they could be used in combination to produce an additive effect and to achieve higher survival rates in vivo.
In conclusion, our strategy was to select compounds that specifically interfere with zinc metabolism, and we were able to identify one compound that was effective in vivo. This polyaminocarboxylate did not show toxicity toward cell cultures or mice at the tested concentrations. Further investigation of this compound might lead to the development of novel antifungal treatment options either as monotherapy or in combination with existing drugs.
MATERIALS AND METHODS
Construction of strains used in this study.
The strains of Aspergillus fumigatus used in this study were AF14LUC (wild type [wt] [PgdpA→luccds]) and AF721LUC (ΔzrfA ΔzrfB ΔzrfC [PgdpA→luccds] mutant). Unlike their relative strains AF14 (wt) (42) and AF721 (ΔzrfA ΔzrfB ΔzrfC mutant) (7), the AF14LUC and AF721LUC strains were able to express constitutively at a high level a codon-optimized version of the firefly luciferase (luc) under the control of the glyceraldehyde-3-phosphate dehydrogenase promoter (PgdpA) from A. fumigatus.
To construct the AF14LUC and AF721LUC bioluminescent strains, we transformed both the CEA17 and AF2511 uridine-uracil-auxotrophic pyrG1 strains (43) with an EcoRI-SphI 4,777-bp DNA fragment excised from plasmid pLUC-pyrG-D (Fig. S11), which was generated by ligating a XbaI-XbaI DNA fragment (2,619 bp) obtained from plasmid PgpdAAf:LucOPTAf_ptrA (kindly provided by Matthias Brock) (44) into the only XbaI site of the pPYRGQ3 plasmid (43). Since the pPYRGQ3 plasmid had been designed previously to target specifically the introduction of any foreign DNA fragment between the AFUA_2G08360 (pyrG) and AFUA_2G08350 loci of any auxotrophic A. fumigatus strain (43), both strains harbored the [PgdpA→luc] construction inserted into the same locus, which allowed a comparison of the luminescence produced by these strains and, hence, a very accurate measurement of the fungal growth capacities of these strains.
Preparation of conidial suspensions.
Conidia were harvested from the AF14LUC and AF721LUC strains. Cultures were grown for 7 days on 2% malt agar slants and recovered by vortexing with 0.01% aqueous Tween 20 (VWR International) solution. Homogenous conidial suspensions were collected following filtration through a 40-μm-pore-size filter (Falcon) (10).
Chemical library screening.
The medium for our resazurin assay consisted of 70% (vol/vol) RPMI 1640 (1×) without phenol red (Thermo Fisher Scientific), 30% (vol/vol) sterile water, 0.07% (vol/vol) Tween 20 (VWR International), 0.00002% (wt/vol) resazurin sodium salt, 10 μM FeSO4 (Merck Millipore), 2 μM CuSO4 (Merck Millipore), 2 μM MnSO4 (Merck Millipore). This medium was inoculated with either 8 × 104 conidia/ml of AF14 with no additional ZnSO4 or with 8 × 104 conidia/ml of AF721 with an additional 100 μM ZnSO4 (Merck Millipore). This ZnSO4 concentration was sufficiently high to allow the amount of zinc necessary for normal growth to diffuse through the cell membrane without the need for transporters. The salt solutions were made using sterile water. Using a Tecan Freedom EVO 200 platform, 130 μl of these mixtures was added to 96-well plates (F-bottom, clear, barcoded tissue culture plates; Greiner Bio-One); each well was previously spiked with 1 μl of compound in dimethyl sulfoxide (DMSO), except columns 1 and 12, which were dedicated to controls. Amphotericin B dissolved in DMSO was used as a negative control to kill all cells, while DMSO was used as a positive control to define 100% growth. The plates were incubated for 38 to 40 h in a 5% CO2 incubator at 37°C. Then, a dual-wavelength measurement was performed (measurement wavelength, 570 nm; reference wavelength, 604 nm) using a Tecan Infinite M1000 Pro microplate reader (13). For each plate, the Z′-factor (45) was calculated based on positive and negative controls, and all values that were above the threshold were considered an excellent assay (average Z′-factor, 0.793 ± 0.120 for AF14 and 0.929 ± 0.029 for AF721). The data were normalized as the percentage of viability relative to positive and negative controls using the following formula: % viability = 100 × (sample value − average value of negative controls)/(average value of positive controls − average value of negative controls). Compounds that caused less than 70% viability of AF14 with no additional zinc but more than 95% viability of AF721 in the presence of zinc were considered hits and selected for further experiments.
Resazurin dilution series assay.
The medium and strains (8 × 104 conidia/ml) in these assays were the same as those used in the library screen, except that this assay used 130 μl of medium per well (13). The compound concentrations used in the dilution series were 100, 50, 25, 12.5, 6.25, 3.1, 1.6, 0.8, 0.4, and 0.2 μM compound in addition to positive controls containing 1 μl of DMSO and negative controls containing 1 μl of DMSO with 2 μg of amphotericin B. The plates were incubated in a 5% CO2 incubator at 37°C for 42 h for the resazurin assay. Each concentration was tested in duplicate, as were the plates, resulting in four total replicates. The measurements on the resazurin plates were performed as previously described.
Luciferin dilution series assays.
The medium and strains (8 × 104 conidia/ml) in these assays were the same as those used in the library screen, except that this assay used 65 μl of medium with no resazurin (10). The compound concentrations used in the dilution series were 100, 50, 25, 12.5, 6.25, 3.1, 1.6, 0.8, 0.4, and 0.2 μM compound, in addition to positive controls containing 1 μl of DMSO and negative controls containing 1 μl of DMSO with 2 μg of amphotericin B. The plates were incubated in a 5% CO2 incubator at 37°C for 15 h. Each concentration was tested in duplicate, as were the plates, resulting in four total replicates. The plates had 5 μl phosphate-buffered saline (PBS) containing 4.3 μg of d-luciferin added to each well, and the plates were incubated for 10 min prior to luminescence acquisition on an IVIS 100 system (PerkinElmer, Boston, MA). Bioluminescence images were analyzed and the light emission (total photon flux per second) from a region of interest (ROI) quantified with Living Image software (version 3.1; PerkinElmer) (10). The percent growth at each concentration was calculated using the equation (ample well/positive control average) × 100.
Hyphal measurement and luciferin assay.
To obtain more precise results, the compounds demonstrating an effect in luciferin dilution series assays were tested on AF14 and AF721 as in the luciferase dilution series assay, except that this assay used 24-well plates with 500 μl of medium per well seeded with 5 × 104 conidia. The plates were incubated for 10 h at 37°C, at which point photographs were taken using an Evos Core microscope (Thermo Fisher Scientific, Waltham, MA) at a magnification of ×20. The ImageJ software was used to measure the lengths of 100 hyphae for each sample, using the freehand line tool to trace the hyphae from the conidium to the tip of the longest hypha (10).
The plates were then incubated for an additional 5 h at 37°C, and luminescence measurements were taken as described in the previous section, except that each well received 5 μl of phosphate-buffered saline (PBS) containing 0.16 mg of d-luciferin. The experiments were repeated twice for each concentration, and cultures were made in triplicate (44). The MIC95 was defined as the lowest concentration of a compound tested that was sufficient to cause at least 95% reduction in A. fumigatus bioluminescence compared to the positive control that received no treatment, while the MIC50 was the minimum concentration tested that was able to cause at least 50% reduction.
The assays for measuring the effects of other ions on the compounds were identical to the zinc assay, with the exception that the 100 μM ZnSO4 was replaced with 100 μM CuSO4, FeSO4, MgSO4, or MnSO4.
Fungal growth phase luciferin assay.
The effects of a short early conidial exposure to the compounds were determined by adding the compounds at the start of the incubation, removing them after 8 h by centrifuging the plate to pellet the conidia, washing the plates twice before adding fresh medium, and continuing the incubation for 7 h, followed by luminescence measurements. The effects of the compounds at the later conidial growth stages were examined by adding the compounds after 8 h of incubation, at which point the conidia start to germinate, and then continuing the incubation for 7 h, followed by luminescence measurements (10).
In vitro combination treatment assay.
This procedure used the same medium and incubation conditions as the luciferase assay. It was performed on the Por06, Por07, Ami03, and Ami04 compounds. The interactions between established antifungal drugs and library compounds were measured using the fractional inhibitory concentration index (FICI) via a checkerboard method (46). Caspofungin, amphotericin B, and voriconazole were selected because they are representatives of different classes of established antifungal drugs, and their mode of action does not involve zinc metabolism. The dilution series for the selected molecules was 24, 18, 12, 6, 2.4, and 0 μM, while that for the antifungal drugs was 0.1, 0.75, 0.50, 0.25, 0.1, and 0 μg/ml. Fifty percent inhibition was employed as an endpoint for assays involving caspofungin, as it is cytostatic rather than cytotoxic and cannot achieve high levels of inhibition (47), and 90% inhibition was employed for assays involving amphotericin B and voriconazole. The FICI was defined as (Ac/Aa) + (Bc/Ba), where Ac and Bc are the endpoint values of the library compound and antifungal drug in combination, respectively, Aa is the endpoint value of the library compound alone, and Ba is the endpoint value of the antifungal alone. Interactions were classified as synergistic (FICI, ≤0.5), indifferent (FICI, >0.5 and ≤4), or antagonistic (FICI, >4) (10).
HeLa cell cytotoxicity assay.
Analysis of toxicity to human cells was performed as previously described (16) using the Cytotoxicity detection kit (lactate dehydrogenase [LDH]) (Roche), according to the manufacturer's instructions. This assay measures the activity of lactate dehydrogenase in a culture's supernatant to estimate percent cytotoxicity. It was performed on the compounds selected by the luciferin dilution series assay. Briefly, 100 μl of a 5 × 105 cells/ml suspension in PAA Quantum 286 complete epithelial medium (Brunschwig Chemie) with l-glutamine, penicillin, and streptomycin, but no serum, was placed in the wells of a 96-well plate and left to grow overnight at 37°C with 10% CO2. The supernatant was replaced with 200 μl of fresh medium containing 10 μM or 100 μM library compound in the sample wells, 1% Triton X-100 in the positive-control wells, and nothing in the negative-control wells, while the background control consisted solely of 200 μl of medium. All samples and controls were in triplicate. The cells were left to grow for 24 h at 37°C with 5% CO2. One hundred microliters of culture supernatant from each well was transferred to a new 96-well plate, to which 100 μl of reaction mixture containing the dye iodonitrotetrazolium was added, and the plate was incubated for 0.5 h. Absorbance at 492 nm and 604 nm was measured using a Dynex enzyme-linked immunosorbent assay (ELISA) processor (Magellan Biosciences), and the percent cytotoxicity was calculated using the equation 100 × (mean of sample triplicates − negative control)/(positive control − negative control).
Murine toxicity assays and infection.
In this procedure, we used our model of invasive pulmonary aspergillosis (10) with male BALB/cJ mice (23 to 28 g, 8 weeks old) supplied by the R. Janvier breeding center (Le Genest-Saint-Isle, France). Mice were cared for in accordance with Institut Pasteur guidelines, in compliance with European animal welfare regulations. This study was approved by the ethics committee for animal experimentation (Comité d'Éthique en Experimentation Animale [CETEA], project license number 2013-0020). At 4 days and 1 day before the start of a toxicity assay or of infection, each mouse received an immunosuppressive regimen by intraperitoneal (i.p.) injection of 200 μl cyclophosphamide (4 mg/ml). The mice remained immunosuppressed for around 7 days, which was sufficient for them to succumb to infection if left untreated. Mice used for toxicity assays received 100-μl i.p. injections of 20% DMSO in saline solution containing the compounds on a daily basis for 10 days.
Mice to be infected were inoculated intranasally with a dose of 7.5 × 104 conidia in 25 μl of PBS containing 0.01% Tween. Following infection, the compounds or placebo were administered by i.p. injection at the concentrations indicated in Fig. 4 and 5 in a final volume of 100 μl. The placebo consisted of 20% DMSO in saline solution. Bioluminescence imaging was started 24 h after infection and was continued every other day. Images were acquired using an IVIS 100 system, as previously described (48). The experiments extended to 14 days postinfection, including 10 days of daily treatment. Infected mouse experiments were only performed on the Por06 and the Ami04 small molecules, as they each represented one of the two most promising chemical families we identified and because they were the compounds for which we had sufficient amounts to perform the mouse experiments.
Histological analysis of lung sections.
Mice were euthanized at day 3 postinoculation. Lungs were immediately fixed in 4% neutral-buffered formalin and embedded in paraffin. Five-micrometer sections were cut and stained with Grocott-Gomori's methenamine silver staining (GMS) for the detection of fungi (44).
Statistical analyses.
For the in vitro tests, the luminescence values of the different cultures in the presence of chelators and/or metal ions were compared to those of the control cultures using unpaired Student t tests with Welch's correction. Levels of significance for hyphal lengths were calculated using the Mann-Whitney test. For the in vivo tests, survival rates were determined by creating Kaplan-Meier plots and then performing log rank tests. Comparisons of luminescence between different mouse groups were done using an unpaired Student t test with Welch's correction. All results are expressed as means ± standard errors of the mean (SEM), and comparisons for survival studies were considered significant if the P value was <0.05. All tests were performed using the GraphPad Prism 7 software. All MIC50 and MIC95 values reported were statistically significant, and the P values indicate the level of significance compared to the positive controls (10).
Supplementary Material
ACKNOWLEDGMENTS
O.I.-G., H.M.-L., and J.-P.L. were supported by the PTR468 funding program. P.L. received funding from PTR468 and CARNOT MS for a postdoctoral fellowship. J.A.C. was supported by the Spanish Ministry of Economy and Competitiveness through grant SAF2013-48382-R.
We thank Yves Janin for the synthesis of the pyrazolones Pyr05 and Pyr11, Constance Bochot and Pierrette Battioni of UMR8601 for the supply of Por06 and Por07 compounds, and the National Library of National Chemistry for providing access to the complete database for screening. We thank Hervé Waxin from the Institut Pasteur education department and Marie-Anne Nicolas from the Institut Pasteur Photonic BioImaging (UTechS PBI) for their assistance in live imaging.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02510-17.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Cadena J, Thompson GR III, Patterson TF. 2016. Invasive aspergillosis: current strategies for diagnosis and management. Infect Dis Clin North Am 30:125–142. doi: 10.1016/j.idc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Jensen RH, Cuenca-Estrella M. 2015. In vitro activity of ASP2397 against Aspergillus isolates with or without acquired azole resistance mechanisms. Antimicrob Agents Chemother 60:532–536. doi: 10.1128/AAC.02336-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford A, Wilson D. 2015. Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res 15:fov071. doi: 10.1093/femsyr/fov071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watly J, Potocki S, Rowinska-Zyrek M. 2016. Zinc homeostasis at the bacteria/host interface-from coordination chemistry to nutritional immunity. Chemistry 22:15992–16010. doi: 10.1002/chem.201602376. [DOI] [PubMed] [Google Scholar]
- 6.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amich J, Vicentefranqueira R, Mellado E, Ruiz-Carmuega A, Leal F, Calera JA. 2014. The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol 16:548–564. doi: 10.1111/cmi.12238. [DOI] [PubMed] [Google Scholar]
- 8.Moreno MA, Ibrahim-Granet O, Vicentefranqueira R, Amich J, Ave P, Leal F, Latge JP, Calera JA. 2007. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol 64:1182–1197. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 9.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. 2016. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol 196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laskaris P, Atrouni A, Calera JA, d'Enfert C, Munier-Lehmann H, Cavaillon JM, Latge JP, Ibrahim-Granet O. 2016. Administration of zinc chelators improves survival of mice infected with Aspergillus fumigatus both in monotherapy and in combination with caspofungin. Antimicrob Agents Chemother 60:5631–5639. doi: 10.1128/AAC.00324-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicentefranqueira R, Amich J, Laskaris P, Ibrahim-Granet O, Latge JP, Toledo H, Leal F, Calera JA. 2015. Targeting zinc homeostasis to combat Aspergillus fumigatus infections. Front Microbiol 6:160. doi: 10.3389/fmicb.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TM, Richie DL, Tao J. 2016. A fluorescence-based high-throughput screening assay to identify growth inhibitors of the pathogenic fungus Aspergillus fumigatus. Methods Mol Biol 1439:171–179. doi: 10.1007/978-1-4939-3673-1_11. [DOI] [PubMed] [Google Scholar]
- 13.Clavaud C, Beauvais A, Barbin L, Munier-Lehmann H, Latge JP. 2012. The composition of the culture medium influences the beta-1,3-glucan metabolism of Aspergillus fumigatus and the antifungal activity of inhibitors of beta-1,3-glucan synthesis. Antimicrob Agents Chemother 56:3428–3431. doi: 10.1128/AAC.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi H, Uchida K, Nagino K, Matsunaga T. 2002. Usefulness of a colorimetric method for testing antifungal drug susceptibilities of Aspergillus species to voriconazole. J Infect Chemother 8:374–377. doi: 10.1007/s10156-002-0201-Y. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro MC, de la Cruz M, Cantizani J, Moreno C, Tormo JR, Mellado E, De Lucas JR, Asensio F, Valiante V, Brakhage AA, Latge JP, Genilloud O, Vicente F. 2012. A new approach to drug discovery: high-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J Biomol Screen 17:542–549. doi: 10.1177/1087057111433459. [DOI] [PubMed] [Google Scholar]
- 16.Raj S, Krishnan K, Askew DS, Helynck O, Suzanne P, Lesnard A, Rault S, Zeidler U, d'Enfert C, Latge JP, Munier-Lehmann H, Saveanu C. 2015. The toxicity of a novel antifungal compound is modulated by endoplasmic reticulum-associated protein degradation components. Antimicrob Agents Chemother 60:1438–1449. doi: 10.1128/AAC.02239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibert MF. 2009. French/European academic compound library initiative. Drug Discov Today 14:723–725. doi: 10.1016/j.drudis.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet CS, Laine S, Buron F, Tircso G, Pallier A, Helm L, Suzenet F, Toth E. 2015. A pyridine-based ligand with two hydrazine functions for lanthanide chelation: remarkable kinetic inertness for a linear, bishydrated complex. Inorg Chem 54:5991–6003. doi: 10.1021/acs.inorgchem.5b00804. [DOI] [PubMed] [Google Scholar]
- 19.Krishnasamy SK, Namasivayam V, Mathew S, Eakambaram RS, Ibrahim IA, Natarajan A, Palaniappan S. 2016. Design, synthesis, and characterization of some hybridized pyrazolone pharmacophore analogs against Mycobacterium tuberculosis. Arch Pharm (Weinheim) 349:383–397. doi: 10.1002/ardp.201600019. [DOI] [PubMed] [Google Scholar]
- 20.Padmavathi V, Reddy SN, Mahesh K. 2009. Synthesis, antimicrobial and antioxidant activities of sulfone linked bis heterocycles-pyrazolyl oxadiazoles and pyrazolyl thiadiazole. Chem Pharm Bull (Tokyo) 57:1376–1380. doi: 10.1248/cpb.57.1376. [DOI] [PubMed] [Google Scholar]
- 21.Liguori PF, Valentini A, Palma M, Bellusci A, Bernardini S, Ghedini M, Panno ML, Pettinari C, Marchetti F, Crispini A, Pucci D. 2010. Non-classical anticancer agents: synthesis and biological evaluation of zinc(II) heteroleptic complexes. Dalton Trans 39:4205–4212. doi: 10.1039/b922101h. [DOI] [PubMed] [Google Scholar]
- 22.Vamja AC, Surati KR. 2017. Photoluminescent properties of novel design heteroleptic Zn(II) complexes. Luminescence 32:1197–1202. doi: 10.1002/bio.3311. [DOI] [PubMed] [Google Scholar]
- 23.Cadieux JA, Zhang ZH, Mattice M, Brownlie-Cutts A, Fu JM, Ratkay LG, Kwan R, Thompson J, Sanghara J, Zhong J, Goldberg YP. 2012. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1). Bioorg Med Chem Lett 22:90–95. doi: 10.1016/j.bmcl.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 24.Li CY, Zhang XB, Dong YY, Ma Q J, Han ZX, Zhao Y, Shen GL, Yu RQ. 2008. A porphyrin derivative containing 2-(oxymethyl)pyridine units showing unexpected ratiometric fluorescent recognition of Zn2+ with high selectivity. Anal Chim Acta 616:214–221. doi: 10.1016/j.aca.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Halime Z, Lachkar M, Toupet L, Coutsolelos AG, Boitrel B. 2007. Coordination and structural studies of crowned-porphyrins. Dalton Trans 33:3684–3689. [DOI] [PubMed] [Google Scholar]
- 26.Vlascici D, Popa I, Chiriac VA, Fagadar-Cosma G, Popovici H, Fagadar-Cosma E. 2013. Potentiometric detection and removal of copper using porphyrins. Chem Central J 7:111. doi: 10.1186/1752-153X-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochot C, Bartoli JF, Frapart Y, Dansette PM, Mansuy D, Battioni P. 2007. Synthesis and spectroscopic, electrochemical, and catalytic properties of a new manganese porphyrin bearing four positive charges close to the metal. J Mol Catal A: Chem 263:200–205. doi: 10.1016/j.molcata.2006.08.032. [DOI] [Google Scholar]
- 28.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 29.Cabelli DE, Bielski BHJ. 1990. Use of polyaminocarboxylates as metal chelators. Methods Enzymol 186:116–120. doi: 10.1016/0076-6879(90)86098-G. [DOI] [PubMed] [Google Scholar]
- 30.Nowack B. 2002. Environmental chemistry of aminopolycarboxylate chelating agents. Environ Sci Technol 36:4009–4016. doi: 10.1021/es025683s. [DOI] [PubMed] [Google Scholar]
- 31.Auld DS. 1995. Removal and replacement of metal ions in metallopeptidases. Methods Enzymol 248:228–242. doi: 10.1016/0076-6879(95)48016-1. [DOI] [PubMed] [Google Scholar]
- 32.Finnegan S, Percival SL. 2015. EDTA: an antimicrobial and antibiofilm agent for use in wound care. Adv Wound Care (New Rochelle) 4:415–421. doi: 10.1089/wound.2014.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chon HS, Ma X, Lee H, Bui P, Song HA, Birch N. 2008. Synthesis and evaluation of novel polyaminocarboxylate-based antitumor agents. J Med Chem 51:2208–2215. doi: 10.1021/jm701307j. [DOI] [PubMed] [Google Scholar]
- 34.Kang CS, Ren S, Sun X, Chong HS. 2016. Theranostic polyaminocarboxylate-cyanine-transferrin conjugate for anticancer therapy and near-infrared optical imaging. ChemMedChem 11:2188–2193. doi: 10.1002/cmdc.201600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramage G, Wickes BL, Lopez-Ribot JL. 2007. Inhibition on Candida albicans biofilm formation using divalent cation chelators (EDTA). Mycopathologia 164:301–306. doi: 10.1007/s11046-007-9068-x. [DOI] [PubMed] [Google Scholar]
- 36.Al-Bakri AG, Othman G, Bustanji Y. 2009. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin-EDTA combination and their effectiveness as antibiofilm agents. J Appl Microbiol 107:280–286. doi: 10.1111/j.1365-2672.2009.04205.x. [DOI] [PubMed] [Google Scholar]
- 37.Raad II, Hachem RY, Hanna HA, Fang X, Jiang Y, Dvorak T, Sherertz RJ, Kontoyiannis DP. 2008. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int J Antimicrob Agents 32:515–518. doi: 10.1016/j.ijantimicag.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Raad I, Chatzinikolaou I, Chaiban G, Hanna H, Hachem R, Dvorak T, Cook G, Costerton W. 2003. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob Agents Chemother 47:3580–3585. doi: 10.1128/AAC.47.11.3580-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosayebi G, Haghmorad D, Namaki S, Ghazavi A, Ekhtiari P, Mirshafiey A. 2010. Therapeutic effect of EDTA in experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol 32:321–326. doi: 10.3109/08923970903338367. [DOI] [PubMed] [Google Scholar]
- 40.Hachem R, Bahna P, Hanna H, Stephens LC, Raad I. 2006. EDTA as an adjunct antifungal agent for invasive pulmonary aspergillosis in a rodent model. Antimicrob Agents Chemother 50:1823–1827. doi: 10.1128/AAC.50.5.1823-1827.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kicic A, Chua AC, Baker E. 2001. Effect of iron chelators on proliferation and iron uptake in hepatoma cells. Cancer 92:3093–3110. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Vicentefranqueira R, Moreno MA, Leal F, Calera JA. 2005. The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot Cell 4:837–848. doi: 10.1128/EC.4.5.837-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amich J, Vicentefranqueira R, Leal F, Calera JA. 2009. Aspergillus fumigatus survival in alkaline and extreme zinc-limiting environments relies on the induction of a zinc homeostasis system encoded by the zrfC and aspf2 genes. Eukaryot Cell 9:424–437. doi: 10.1128/EC.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galiger C, Brock M, Jouvion G, Savers A, Parlato M, Ibrahim-Granet O. 2013. Assessment of efficacy of antifungals against Aspergillus fumigatus: value of real-time bioluminescence imaging. Antimicrob Agents Chemother 57:3046–3059. doi: 10.1128/AAC.01660-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Su X, Sun WK, Chen F, Xu XY, Shi Y. 2014. Efficacy of the combination of voriconazole and caspofungin in experimental pulmonary aspergillosis by different Aspergillus species. Mycopathologia 177:11–18. doi: 10.1007/s11046-013-9719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayr A, Aigner M, Lass-Florl C. 2012. Caspofungin: when and how? The microbiologist's view. Mycoses 55:27–35. doi: 10.1111/j.1439-0507.2011.02039.x. [DOI] [PubMed] [Google Scholar]
- 48.Brock M, Jouvion G, Droin-Bergere S, Dussurget O, Nicola MA, Ibrahim-Granet O. 2008. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl Environ Microbiol 74:7023–7035. doi: 10.1128/AEM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.