ABSTRACT
Triazole antifungal compounds are the first treatment choice for invasive aspergillosis. However, in the last decade the rate of azole resistance among Aspergillus fumigatus strains has increased notoriously. The main resistance mechanisms are well defined and mostly related to point mutations of the azole target, 14-α sterol demethylase (cyp51A), with or without tandem repeat integrations in the cyp51A promoter. Furthermore, different combinations of five Cyp51A mutations (F46Y, M172V, N248T, D255E, and E427K) have been reported worldwide in about 10% of all A. fumigatus isolates tested. The azole susceptibility profile of these strains shows elevated azole MICs, although on the basis of the azole susceptibility breakpoints, these strains are not considered azole resistant. The purpose of the study was to determine whether these cyp51A polymorphisms (single nucleotide polymorphisms [SNPs]) are responsible for the azole susceptibility profile and whether they are reflected in a poorer azole treatment response in vivo that could compromise patient treatment and outcome. A mutant with a cyp51A deletion was generated and became fully susceptible to all azoles tested. Also, three cyp51A gene constructions with different combinations of SNPs were generated and reintroduced into an azole-susceptible wild-type (WT) strain (the ΔakuBKU80 strain). The alternative model host Galleria mellonella was used to compare the virulence and voriconazole response of G. mellonella larvae infected with A. fumigatus strains with WT cyp51A or cyp51A with SNPs. All strains were pathogenic in G. mellonella larvae, although they did not respond similarly to voriconazole therapeutic doses. Finally, the full genomes of these strains were sequenced and analyzed in comparison with those of A. fumigatus WT strains, revealing that they belong to different strain clusters or lineages.
KEYWORDS: Aspergillus fumigatus, Galleria mellonella, azole resistance, cyp51A polymorphisms, whole-genome sequencing
INTRODUCTION
Aspergillus fumigatus is a saprophytic fungus which produces volatile spores that are constantly inhaled by humans. It is the leading etiological agent of allergic and bronchopulmonary mycoses (1) and is largely responsible for the increased incidence of invasive aspergillosis (IA), which results in high mortality rates in some immunocompromised hosts (2). Azoles are the first-line drugs used to treat Aspergillus-caused diseases. However, the increasing rates of A. fumigatus azole resistance that have been reported are threatening the effectiveness of azole drugs (3). The main azole resistance mechanisms are mostly related to the azole antifungal target 14-α sterol demethylase, encoded by the cyp51A gene, in which several point mutations (G54, G138, M220, and G448) and/or tandem insertions at its promoter (a tandem repeat of 34 bp [TR34] plus L98H, TR46 plus Y121F and T289A, and TR53) have been described to be responsible for their azole-resistant phenotype (4). In addition, about 10% of A. fumigatus sensu stricto isolates can harbor a combination of eight polymorphisms (single nucleotide polymorphisms [SNPs]), which leads to five missense Cyp51A mutations (F46Y, M172V, N248T, D255E, and E427K) in two different SNP combinations and three silent mutations (G89G, L358L, and C454C) that are present in all strains (5–7). Basically, there are two possible combinations of amino acid substitutions: (i) the three missense substitutions F46Y, M172V, and D255E, here referred to as cyp51A-3SNPs, and (ii) the five missense substitutions consisting of the three previously mentioned substitutions (F46Y, M172V, and D255E) plus the N248T and E427K substitutions, here referred as cyp51A-5SNPs.
Strains with both SNP combinations have been described among clinical strains isolated from patients that have been undergoing azole treatment (6). In general, they have higher azole MICs than A. fumigatus strains with wild-type (WT) cyp51A. However, they show inconsistent azole susceptibility profiles and have been categorized as azole susceptible or resistant by different authors (5, 6, 8–16). On the basis of the azole susceptibility breakpoints, these strains with cyp51A SNPs cannot be considered azole resistant (by the use of either Clinical and Laboratory Standards Institute [CLSI] or European Committee on Antimicrobial Susceptibility Testing [EUCAST] criteria) (17–19).
The first strain with these mutations to be described was isolated in 2001 from a clinical sample in a Spanish hospital (5). These strains have now been reported in many European countries (6–10, 13, 20, 21), the United States (22), Canada (23), India (15), China (24), and Australia (16). Some authors have reported that these substitutions are not associated with azole resistance (5, 25). However, other authors have pointed out that the distribution of isolates harboring cyp51A SNPs could be influenced by azole preexposure (6).
The main aim of this study was to determine whether these cyp51A SNPs are responsible for the different azole susceptibility profiles as well as a poorer azole treatment response. On the basis of the Cyp51A protein homology model, the potential correlation of a mutation to azole resistance can be predicted (25). Two three-dimensional (3D) homology models based on the crystal structure of A. fumigatus Cyp51B in complex with voriconazole (VRC) were constructed in order to explore potential differences between the Cyp51A WT and Cyp51A-5SNP proteins. We generated strains that were deficient in cyp51A and that became fully susceptible to all azole drugs tested. Then, three independent gene copies with different combinations of cyp51A SNPs were constructed and reintroduced into an azole-susceptible WT strain (the ΔakuBKU80 strain). The alternative model host Galleria mellonella was used to compare the virulence of A. fumigatus WT or mutated strains and the VRC treatment response of G. mellonella larvae infected with these strains. Also, the whole-genome sequences (WGS) of both types of strains were analyzed.
RESULTS
Antifungal susceptibility testing.
Using the EUCAST methodology, the 15 A. fumigatus strains with cyp51A SNPs were found to be slightly less susceptible to azole drugs than the strains with WT cyp51A, with the VRC MICs being between 0.25 and 2 μg/ml (Table 1). Analyzing the results by strain group, the four strains which harbored cyp51A-5SNPs showed an average VRC geometric mean (GM) MIC value of 1 μg/ml, while the strains which harbored cyp51A-3SNPs had VRC GM MICs of 0.665 μg/ml. The remaining azole susceptibility values were also slightly higher for all strains with cyp51A SNPs; itraconazole (ITC) GM MICs were 0.557 μg/ml, and posaconazole (POS) GM MICs were 0.131 μg/ml (Table 1). There were no differences in amphotericin B (AMB) MICs between WT strains and strains with cyp51A SNPs (data not shown).
TABLE 1.
Cyp51A modifications and geometric mean MICs and MIC ranges obtained by EUCAST antifungal susceptibility testing for the A. fumigatus clinical strains
| Strain | Cyp51A amino acid change(s)a | MICb/MIC range (μg/ml) |
Isolation yr | ||
|---|---|---|---|---|---|
| ITC | VRC | POS | |||
| AF237 | WTc | 0.250/0.25 | 0.354/0.25–0.5 | 0.085/0.06–0.12 | |
| ΔakuBKU80 | WT | 0.175/0.06–0.25 | 0.297/0.25–0.5 | 0.036/0.03–0.06 | —d |
| CBS144.89/A1163 | WT | 0.210/0.125–0.25 | 0.500/0.5 | 0.036/0.015–0.06 | — |
| AF293 | F46Y, M172V, N248T, D255E, E427K | 0.354/0.25–0.5 | 1.000/1.0 | 0.085/0.06–0.12 | — |
| TP11 | F46Y, M172V, N248T, D255E, E427K | 0.630/0.25–2.0 | 1.000/0.5–2.0 | 0.139/0.06–0.5 | 2001 |
| TP12 | F46Y, M172V, N248T, D255E, E427K | 0.500/0.5 | 1.000/1.0 | 0.120/0.12 | 2001 |
| CM7632 | F46Y, M172V, N248T, D255E, E427K | 0.794/0.5–1.0 | 1.000/1.0 | 0.095/0.12 | 2015 |
| CM2495 | F46Y, M172V, E427K | 0.250/0.25 | 0.500/0.5 | 0.085/0.06–0.12 | 2002 |
| CM2730 | F46Y, M172V, E427K | 0.354/0.25–0.5 | 0.707/0.5–1.0 | 0.060/0.06 | 2004 |
| CM2733 | F46Y, M172V, E427K | 0.354/0.25–0.5 | 0.707/0.5–1.0 | 0.060/0.06 | 2004 |
| CM3249 | F46Y, M172V, E427K | 0.707/0.5–1.0 | 1.000/0.5–2.0 | 0.188/0.06–0.25 | 2005 |
| CM3262 | F46Y, M172V, E427K | 1.000/1.0 | 0.500/0.5 | 0.120/0.12 | 2005 |
| CM4602 | F46Y, M172V, E427K | 0.250/0.25 | 0.500/0.5 | 0.060/0.06 | 2006 |
| CM4946 | F46Y, M172V, E427K | 0.397/0.125–1.0 | 0.794/0.5–1.0 | 0.097/0.06–0.25 | 2007 |
| TP32 | F46Y, M172V, E427K | 0.630/0.25–1.0 | 0.397/0.25–1.0 | 0.095/0.06–0.12 | 2010 |
| CM7560 | F46Y, M172V, E427K | 0.707/0.5–1.0 | 0.707/0.5–1.0 | 0.122/0.06–0.25 | 2014 |
| CM7570 | F46Y, M172V, E427K | 0.630/0.5–1.0 | 0.500/0.5 | 0.397/0.25–0.5 | 2014 |
| CM8726 | F46Y, M172V, E427K | 1.000/1.0 | 1.000/1.0 | 0.250/0.25 | 2016 |
The Cyp51A protein with GenBank accession number AKK73659 was used as a reference.
MICs are the geometric mean MICs. ITC, itraconazole; VRC, voriconazole; POS, posaconazole. EUCAST susceptibility tests were repeated for each strain at least twice on different days.
WT, wild type.
Sequence analysis of the cyp51A and cyp51B genes.
The two cyp51-related genes were analyzed. The cyp51A full coding sequence and its promoter were sequenced. The above-described 6 or 8 synonymous and missense mutations were found in all strains when their cyp51A gene sequences were compared to the sequence of the WT cyp51A gene used as a reference (GenBank accession number AF338659). Also, two changes were found in the cyp51A promoter of all strains with cyp51A SNPs tested (T→C at c−335; C→T at c−70; GenBank accession number AF338659). In some strains, only three silent Cyp51B substitutions (S274S, P394P, I464I) in comparison to the sequence of the product of the cyp51B gene (GenBank accession number AF338660) were found (see Table S1 in the supplemental material).
A. fumigatus cyp51A gene expression.
The expression of cyp51A was analyzed to determine if the two changes at the promoter (at positions −335 and −70 bp) could be responsible for increased gene expression and therefore for the higher azole MICs. The cyp51A mRNA levels were quantified by reverse transcription-quantitative PCR (RT-qPCR) using β-tubulin as a reference for constitutive gene expression. Some strains were included as negative or positive expression controls. A mutant with a cyp51A deletion (the T1.15 mutant) was added as a negative control, and one azole-resistant strain (strain CM2627) with a tandem repeat of 34 bp (TR34) at the cyp51A promoter and the L98H substitution was added as a control for increased cyp51A expression (26). There were no differences in cyp51A gene expression between the strains with the cyp51A WT (strain AF237 and the ΔakuBKU80 strain), cyp51A-5SNPs (strains AF293 and TP11), and cyp51A-3SNPs (strain CM3249) (Table S2).
A. fumigatus Cyp51A protein 3D homology modeling.
In the absence of a crystal structure of A. fumigatus Cyp51A, automated homology modeling based on the crystal structure of A. fumigatus Cyp51B in complex with VRC was used (59% sequence identity).
Two 3D homology models were constructed in order to explore potential structural differences between A. fumigatus strains with the Cyp51A WT and Cyp51A-5SNPs and the relationship between these mutations and azole resistance. Both homology model structures were almost identical owing to their nearly matching amino acid sequences.
All the nonsynonymous mutations (M172V, N248T, D255E, and E427K) except one (F46Y) are located in nonconserved areas, mainly at the surface of the protein, and they were predicted neither to interact with azole compounds nor to affect structural integrity. However, our homology models show that a substitution at nucleotide T137A resulted in a nonconservative replacement of a neutral hydrophobic phenylalanine (F) with an uncharged but polar tyrosine (Y) residue (F46Y) located at the substrate channel entrance (Fig. 1).
FIG 1.
Overall view of the 3D model of the A. fumigatus Cyp51A protein in complex with VRC. Cyp51A-WT (A) and Cyp51A-F46Y (B) protein structures. (Panels 1 and 2) different protein views; (panels 3) structural formulas of the amino acid placed in position 46 showing the 2D structure (left) and the 3D conformer (right) of each amino acid. The location of the F46Y amino acid change is circled.
Generation of an A. fumigatus Δcyp51A strain and study of its phenotype.
In order to assess the role of the cyp51A polymorphism in azole drug susceptibility, the corresponding cyp51A-5SNPs gene was deleted from the TP11 strain and replaced by the resistance marker hygromycin (hyg) (Fig. 2A and B). The Δcyp51A mutant (T1.15) was morphologically indistinguishable from the parental strain regarding their macroscopic and microscopic morphologies and the radial growth of their colonies (results not shown). The Δcyp51A mutant showed an azole-hypersusceptible phenotype with an approximately 11-fold decrease of the VRC MIC, a 16-fold decrease of the ITC MIC, and a 7-fold decrease of the POS MIC compared with the MICs for parental strain TP11 and 3-fold, 4-fold, and 2-fold decreases in the MICs of the three azoles, respectively, compared with those for the WT ΔakuBKU80 strain (Table 2 and Fig. 2C).
FIG 2.
Construction of the A. fumigatus Δcyp51A-SNP fusion cassette, confirmation of the PCR mutant, and determination of VRC susceptibility by Etest. (A) Map of the parental strain with cyp51A-5SNPs (TP11) (map 1), map of the cyp51A WT strain (map 2), and design of the fusion vector for A. fumigatus cyp51A gene deletion (map 3). The deleted cyp51A coding fragment is indicated in light green. Primers (p) are indicated by arrows. 3′ T, 3′ cyp51A terminator. (B) PCR analysis of the TP11 parental strain and the T1.15 Δcyp51A mutant. PCR verification of integration of the fragment cassette at the 5′ end (p7 to p9) (lanes a) and the 3′ end (p10 to p8) (lanes b) and verification of the absence of the cyp51A fragment (p11 to p12) (lanes c). Expected fragment sizes are indicated at the bottom. Lanes M, 1-kb DNA molecular ladder. (C) VRC susceptibility of the ΔakuBKU80 strain (panel 1), strain TP11 (panel 2), and Δcyp51A-hypersusceptible mutant T1.15 (panel 3) by Etest.
TABLE 2.
Geometric mean MICs and MIC range obtained by EUCAST antifungal susceptibility testing for the A. fumigatus mutants generated and their control strains
| Strain | Cyp51A amino acid change(s)a | MICb/MIC range (μg/ml) |
|||
|---|---|---|---|---|---|
| AMB | ITC | VRC | POS | ||
| ΔakuBKU80 | WTc | 0.177/0.125–0.25 | 0.175/0.06–0.5 | 0.297/0.25–0.5 | 0.036/0.03–0.06 |
| TP11 | F46Y, M172V, N248T, D255E, E427K | 0.250/0.125–0.25 | 0.630/0.25–2.0 | 1.000/0.5–2.0 | 0.139/0.06–0.5 |
| CM3249 | F46Y, M172V, N248T | 0.250/0.125–0.25 | 0.707/0.5–1.0 | 1.000/0.5–2.0 | 0.188/0.06–0.5 |
| T1.15 | Δcyp51A (cyp51A knockout) | 0.250/0.25 | 0.038/0.03–0.06 | 0.087/0.06–0.125 | 0.019/0.015–0.03 |
| T1.5 | F46Y, M172V, E427K, D255E, E427K | 0.125/0.125 | 0.250/0.25 | 0.500/0.5 | 0.042/0.03–0.06 |
| T2.3 | F46Y, M172V, E427K | 0.125/0.125 | 0.250/0.25 | 0.500/0.5 | 0.030/0.03 |
| T3.1 | F46Y | 0.125/0.125 | 0.125/0.125 | 0.354/0.25–0.5 | 0.021/0.015–0.03 |
The Cyp51A protein with GenBank accession number AKK73659 was used as a reference.
MICs are the geometric mean MICs. AMB, amphotericin B; ITC, itraconazole; VRC, voriconazole; POS, posaconazole. Each assay was repeated in duplicate with RNA from two different extractions.
WT, wild type.
A. fumigatus cyp51A SNP heterologous gene expression in an A. fumigatus strain with the cyp51A WT.
In order to analyze the implication of each cyp51A SNP combination, each copy was expressed in an A. fumigatus WT strain (the ΔakuBKU80 strain), in which the original copy of cyp51A was replaced with a mutated copy of cyp51A (cyp51A-5SNPs, cyp51A-3SNPs, or cyp51A-F46Y) and the hyg cassette was included as a resistance marker, as explained in the appropriate section of Materials and Methods (Fig. 3A and B). The substitution F46Y was introduced into the ΔakuBKU80 strain in order to analyze whether the phenotype was caused only by this cyp51A mutation because it was expected to be the most determinative change according to the structural protein homology model.
FIG 3.
Construction of the fusion cassettes for heterologous expression of the A. fumigatus cyp51A SNPs in the ΔakuBKU80 strain with WT cyp51A and PCR confirmation of the mutants. (A) Design of the fusion cassettes for heterologous expression of cyp51A-5SNPs (map 1), cyp51A-3SNPs (map 2), and cyp51A F46Y (map 3). Primers are indicated by arrows. Blue, the homologous sequence outside cyp51A (5′ region, including the promoter, and 3′ region); dark green, the 3′ cyp51A terminator. (B) PCR analysis for verification of vector integration in the parental strain with WT cyp51A (the ΔakuBKU80 strain) and the strains with cyp51A-5SNPs (T1.5), cyp51A-3SNPs (T2.3), and cyp51A-F46Y (T3.1). Lanes: a, full vector (A to H2) cassette integration; b, 5′ fragment (A1 to p9) cassette integration; c, 3′ fragment (H1 to p10) cassette integration; and d, hyg fragment (HYGp1 to HYGp2) integration. Expected fragment sizes are indicated at the bottom. Lanes M, 1-kb DNA molecular ladder.
One mutant with each combination of mutations was selected: the T1.5 mutant had 5 SNPs in cyp51A, the T2.3 mutant had 3 SNPs in cyp51A, and the T3.1 mutant had the F46Y substitution.
Susceptibility testing was performed to investigate if cyp51A SNP gene replacements had an effect on the azole MICs of the newly generated expression mutants. All mutants of the ΔakuBKU80 strain with cyp51A gene replacement showed MICs lower than those obtained for the original strains, TP11 and CM3249 (Table 2); therefore, the expression mutants did not fully recover the parental phenotype (Table 2). Accordingly, there were no changes in the AMB susceptibility values for any mutant (0.125 to 0.25 μg/ml).
Virulence and voriconazole efficacy in an in vivo model of infection with A. fumigatus using G. mellonella. (i) Strain virulence.
To determine whether there were any differences in virulence, one strain with WT cyp51A (the ΔakuBKU80 strain), one strain each with each combination of cyp51A SNPs, and the generated mutants expressing their cyp51A SNP copies in the ΔakuBKU80 strain were tested in the alternative model of infection of G. mellonella larvae. Since the differences in virulence can be dependent on the strain mating type (Mat 1.1 or 1.2) (27), only strains with Mat 1.1 were used in these experiments. The selected strains with cyp51A SNPs were CM7632 (cyp51A-5SNPs) and CM3249 (cyp51A-3SNPs). Also, the expression mutants tested (mutants T1.5 and T2.3) were Mat 1.1, like their parental strain, the ΔakuBKU80 strain. The results showed that there were no differences in virulence among all strains tested (Fig. 4).
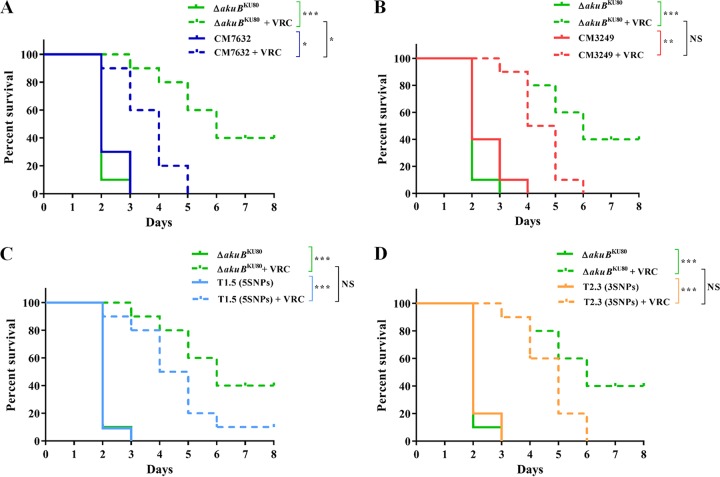
FIG 4.
Comparison of killing rates and VRC responses of G. mellonella larvae infected with A. fumigatus strains and their expression mutants. (A) Percent survival of G. mellonella larvae infected with the strain with the cyp51A WT (the ΔakuBKU80 strain) and the strain with cyp51A-5SNPs (CM7632) with or without VRC treatment. (B) Percent survival of G. mellonella larvae infected with the strain with the cyp51A WT (ΔakuBKU80) and the strain with cyp51A-3SNPs (CM3249) with or without VRC treatment. (C) Percent survival of G. mellonella larvae infected with the strain with the cyp51A WT (ΔakuBKU80) and the expression mutant with cyp51A-5SNP (T1.5) with or without VRC treatment. (D) Percent survival of G. mellonella larvae infected with the strain with the cyp51A WT (ΔakuBKU80) and the expression mutant with cyp51A-3SNPs (T2.3) with or without VRC treatment. NS, nonsignificant (P ≥ 0.01); *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
(ii) Voriconazole response.
Larvae infected with azole-susceptible A. fumigatus ΔakuBKU80 had significantly improved survival in relation to that of the untreated group after treatment with VRC at therapeutic doses (P < 0.0001), with a 40% larva survival rate after 8 days (Fig. 4A and B). In contrast, when G. mellonella larvae were infected with the A. fumigatus strain with cyp51A-3SNPs or the strain with cyp51A-5SNPs, there was a response to VRC treatment by both groups (P = 0.0001 and P = 0.001, respectively), but there was also a remarkable decrease in survival, showing 100% larva mortality 5 to 6 days after inoculation (P = 0.0174 and P = 0.0018, respectively) (Fig. 4A and B).
Similarly, VRC efficacy was tested in the A. fumigatus expression mutants (mutants T1.5 and T2.3). Results comparable to those for their cyp51A parental strains were obtained (Fig. 4C and D). There were no significant differences in the killing rate between A. fumigatus expression mutants T1.5 and T2.3 and the cyp51A parental strains (CM3249 or CM7632). The response to VRC therapy was significant (P < 0.0001 for both expression mutants), although as explained before, there was a remarkable decrease in survival compared to that of the ΔakuBKU80 strain (Fig. 4C and D).
WGS comparisons.
To gain insight into the full genotypic differences among the strains which harbored the two different combinations of Cyp51A mutations, a whole-genome sequence (WGS)-based comparison was conducted with 4 genomes of the strains with cyp51A-5SNPs and 11 genomes of the strains with cyp51A-3SNPs. Also, a set of 14 genomes from A. fumigatus strains with WT cyp51A were included to balance the number of cyp51A SNPs and WT genomes. Some of them were from control strains normally used in studies described in the literature, and others came from the Spanish National Center of Microbiology collection. Two whole genomes of A. fumigatus strains deposited in databases, strains AF293 and A1163 (CBS144.89), were included and sequenced in the Mycology Reference Laboratory, and comparisons against both published genomes were done.
The rates of identity of the mapped region of the genomes of all strains with the strain A1163 reference genome ranged from 70.94 to 97.91%, and the total coverage of the mapping ranged from 13.54 to 83.55 times. The rates of identity of the mapped region of the genomes of all strains compared to the strain AF293 reference genome ranged from 92.24 to 99.72%, and the total coverage of the mapping ranged from 16.97 to 85.08 times (Table S3).
The average number of single nucleotide variations (SNVs) calculated for the genome sequences of the strains from the group with cyp51A-5SNPs compared to the sequence of the strain A1163 reference genome was 51,133. However, when the genome sequences of strains of the group with cyp51A-5SNPs were compared to the sequence of the strain AF293 reference genome, the average number of SNVs was much lower at 14,893. On the other hand, when the genome sequences of strains from the group with cyp51A-3SNPs were compared to the sequences of both reference genomes, the number of SNVs was similar in both cases (131,391 SNVs in comparison with the AF293 sequence and 130,599 SNVs in comparison with the A1163 sequence). The average number of SNVs identified in A. fumigatus strains with the cyp51A WT was 34,237 when the sequences were compared with A1163 sequence and 55,523 when the sequences were compared with the AF293 sequence. Phylogenetic analysis identified a branched tree structure comprising three different clades. All isolates with cyp51A-3SNPs clustered together and separately from another branch which bifurcated into two subclades, one that grouped all the WT strains and the other that grouped the strains with cyp51A-5SNPs. These results were similar regardless of the reference genome (that of strain A1163 or AF293) that was used (Fig. 5A and B). Furthermore, two subclusters were observed among the WT strains.
FIG 5.
Phylogenetic analysis of A. fumigatus strains by whole-genome sequencing. Dendrograms were formed using the genome of strain A1163 (CBS144.89) (A) or AF293 (B) as a reference. Indigo dots, strains with the cyp51A WT; orange dots, strains with cyp51A-5SNPs; green dots, strains with cyp51A-3SNPs.
DISCUSSION
Azole antifungal drugs are currently the first-line treatment in the management of IA (28, 29). Azoles are the only orally administered compounds available with activity against Aspergillus spp.; therefore, their role is, at present, irreplaceable in long-term therapy (30). Current guidelines recommend VRC as initial therapy for almost all patients with suspected IA (28, 31). In the last decade, the population at risk has escalated, and therefore, the number of patients exposed to azole therapy has increased. Unfortunately, along with increased azole use, A. fumigatus azole resistance is emerging as a global health problem, threatening the effectiveness of azoles in the management of diseases caused by Aspergillus (32).
Azole antifungal drugs inhibit sterol 14-α demethylase, a member of the cytochrome P450 Cyp51 family (33, 34). In A. fumigatus, there are two isoenzymes, Cyp51A and Cyp51B, but most studies conclude that mutations in the cyp51A gene and/or its promoter (mainly, G54, G138, M220, G448, TR34 plus L98H, TR46 plus Y121F and T289A, and TR53) are responsible for the vast majority of the described azole resistance mechanisms (4).
Other single point mutations, such as N22D, F165L, F219I, D262Y, A284T, Y431C, G432S, G434C, T440A, N479D, and Y491H, have sporadically been reported to be associated with a reduced azole susceptibility profile but have not been demonstrated to have a role in azole resistance (5, 6, 9, 11, 35–38), while others, such as P216L and F219C, have been confirmed to be linked to azole resistance by gene replacement (39). In most cases, it is unknown whether these cyp51A SNPs have a role in A. fumigatus azole resistance or if they represent genotypic differences in local strains.
However, since 2001 (5) a combination of Cyp51A amino acid substitutions (F46Y, M172V, N248T, D255E, and E427K) has been increasingly reported worldwide (6–10, 13, 15, 16, 20–24). Strains with these mutations have been described to have different azole susceptibility profiles and to be azole susceptible or resistant, depending on the authors, but in all cases they have higher azole MICs than A. fumigatus strains with WT cyp51A (5, 6, 8–16). However, some authors have claimed that these substitutions are not associated with azole resistance (5, 26) because the azole MICs for strains with these substitutions normally remained below the accepted threshold defined for resistant isolates (19), while other authors concluded that VRC preexposure significantly influences the distribution of these strains, which are more frequently isolated from patients that have undergone azole treatment (6).
Cyp51A protein homology models can foresee the potential correlation of a mutation to azole resistance. In previous works, all of the nonsynonymous mutations were predicted not to be correlated with azole resistance (25). We had similar results with most of the nonsynonymous mutations (M172V, N248T, D255E, and E427K), which were mainly located at the surface of the protein in nonconserved areas. All of them were predicted neither to interact with azole compounds nor to affect structural integrity. However, a substitution at nucleotide T137A resulted in a nonconservative replacement of a neutral hydrophobic phenylalanine with an uncharged but polar tyrosine residue (F46Y). The corresponding phenylalanine (F) residue is located in the N terminus, downstream of the Cyp51A transmembrane domain, and constitutes part of the helix and loop which form the substrate access channel (40). The F46Y mutation could be partially or totally responsible for the slightly higher azole MICs that these strains show, due to a possible blockage at the entrance to the substrate access channel used by the inhibitor (Fig. 1). This residue (F46) is conserved among all analyzed filamentous fungi, such as other Aspergillus spp., Penicillium spp., and Fusarium spp. (data not shown). In yeasts, the amino acid conserved at this location is tryptophan (W). Interestingly, an A. fumigatus strain with this amino acid substitution (F46Y) has been isolated from a dog and showed azole resistance (41). However, the susceptibility profile of the A. fumigatus expression mutant with the cyp51A-F46Y mutation alone seemed to indicate that this mutation, on its own, could not be responsible for the higher MICs shown by these strains. Moreover, the azole susceptibility profile of the A. fumigatus expression mutants (T1.5 and T2.3) suggested that the synergic effect of these mutations in combination can be only partially related to the susceptibility profile of these mutants but cannot be completely responsible for their phenotype. Consequently, the remarkably decreased azole MICs obtained for the T1.15 Δcyp51A deletion mutant, similar to those obtained for other cyp51A deletion mutants of azole susceptible and resistant strains (42), suggests that the Cyp51A changes of these strains could somehow be implicated in their azole susceptibility profile. However, because the expression mutants did not fully recover the parental phenotype, it seems that their genetic background could also be involved and be of major importance.
On the other hand, in order to study whether the presence of these A. fumigatus SNPs has a role in the in vivo response to azoles, the alternative G. mellonella model was used. The results obtained in this model support the idea of a poorer response to VRC of strains with cyp51A-5SNPs or cyp51A-3 SNPs and higher azole MICs. These results are in agreement with those of previous works showing that VRC preexposure influences the distribution of A. fumigatus isolates carrying cyp51A polymorphisms and having higher ITC MICs (6). Moreover, there are some reports of patients harboring A. fumigatus strains with these cyp51A SNPs who did not respond to ITC treatment (43). Further well-designed studies are warranted to better assess the clinical outcomes of patients infected with these strains.
One of the easiest ways to compare genomes is to identify in an individual genome variations or nucleotide differences (SNVs) from the sequence of a reference genome at a given position, which is known as “variant calling.” Experimental investigations of A. fumigatus biology and virulence have historically used only a few strains, among which strains AF293 and A1163 are the most common. Our results show that even though both reference genomes were used, which resulted in different numbers of variants, the distribution of strains and how they clustered on the basis of whole-genome single nucleotide variations were not affected.
Our WGS analysis clearly shows that strains with these two sets of cyp51A SNP combinations were grouped into two different genetic clusters distinct from the WT cluster, in agreement with previous results obtained using typing analysis (6). Strikingly, the AF293 reference genome belongs to the cluster of strains with cyp51A-5SNPs, while the A1163 reference genome belongs to the cluster of WT strains. Taking this into account, the number of strains with these SNPs could be higher than suspected, as some of these strains could have been mistakenly assigned as a strain with WT cyp51A when AF293 was used as the reference genome. This is an important matter because the background heterogeneity among A. fumigatus isolates could potentially allow researchers and clinicians to be misled by the conclusions derived from these works (44). The existence of different A. fumigatus lineages could imply that these strains might have some important differences in biological features. In fact, recent research has shown that different WT strains of clinical origin elicit different immune responses (45). Similarly, other authors have shown that the AF293 and A1163 control strains displayed significant differences in virulence in a murine model of invasive pulmonary aspergillosis (46, 47). Therefore, the differences between strains which belong to different lineages and their singular biological behaviors should be explored further.
Conclusions.
A. fumigatus strains with a combination of three (F46Y, M172V, and D255E) or five (F46Y, M172V, N248T, D255E, and E427K) missense changes in the Cyp51A protein showed a tendency to have higher azole MICs than strains with WT cyp51A. In this work, we have demonstrated that both groups of strains represent different lineages of A. fumigatus isolates. The fact that these strains have been found in patients with azole preexposure is consistent with a better azole tolerance. It seems necessary to investigate whether the identification of A. fumigatus strains with cyp51A SNPs at the time of diagnosis could influence the treatment response and outcomes of patients.
MATERIALS AND METHODS
Aspergillus fumigatus strains.
In this study, we analyzed a total of 15 A. fumigatus strains obtained from clinical samples over a period of 16 years (2001 to 2016). The isolates harbored a combination of nonsynonymous and silent Cyp51A mutations (F46Y, G89G, M172V, D255E, L358L, and C454C or F46Y, G89G, M172V, N248T, D255E, L358L, E427K, and C454C). Most of the strains belong to the Spanish National Center of Microbiology strain collection, and one was received from the Netherlands (see Table S1 in the supplemental material). Identification to the species level was performed by PCR amplification and subsequent sequencing of a partial sequence of the β-tubulin gene (48). For the expression experiments, strain CM2726 was used as a positive control for increased cyp51A expression (27). For whole-genome sequencing experiments, several reference strains, including AF293, A1163 (also known as CBS144.89, CEA10, FGSC A1163, and AF10), and other control strains used frequently in studies described in the literature, such as strains AF237, ATCC 204305, ΔakuBKU80 (FGSC A1160), and ATCC 46645, were included. Also, nine A. fumigatus strains with the cyp51A WT (CM2141, CM2163, CM3248, CM5419, CM5757, CM6126, CM6458, CM7510, and CM7555) from the Spanish National Center of Microbiology collection were included to balance the number of strains with and without cyp51A SNPs.
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed using the broth microdilution method described by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (49, 50) and Etest (51). The antifungal agents tested were ITC (Janssen Pharmaceutical, Spain), VRC (Pfizer, Spain), POS (Schering-Plough, Spain), and AMB (Sigma, Spain). Aspergillus flavus ATCC 204304 and A. fumigatus ATCC 204305 were used as quality control strains. In vitro susceptibility and resistance were defined according to the clinical breakpoints published for A. fumigatus. Isolates with ITC and VRC MICs of ≤1 μg/ml were considered susceptible populations; for POS, this value was ≤0.125 μg/ml (52). For Etest susceptibility testing, an inoculum adjusted to 105 conidia per ml was used, and 200 μl of the inoculum was plated in RPMI 1640 (Sigma-Aldrich Quimica SA, Spain) agar base plates supplemented with 2% glucose, onto which gradient strips of VRC and POS were placed (bioMérieux, Spain). The MICs were read after 48 h at 35°C. Both susceptibility tests were repeated at least twice.
PCR amplification and sequence analysis of the cyp51A and cyp51B genes.
Conidia from each strain were cultured in 3 ml of GYEP broth (2% glucose, 0.3% yeast extract, 1% peptone) and grown overnight at 37°C, after which mycelium mats were harvested and DNA was extracted (53). The full coding sequences of the cyp51A and cyp51B genes, including the cyp51A promoter sequence, were amplified and sequenced using the PCR conditions described before (27). A DNA 1-kb molecular ladder (Promega, Spain) was used for all electrophoresis analyses. Each isolate was independently analyzed twice.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR).
An A. fumigatus inoculum reaching a total of 106 conidia was added to 10 ml of minimal medium broth and was grown for 18 h at 150 rpm and 37°C. Mycelial samples were harvested, blot dried, frozen with liquid nitrogen, and ground to a powder. RNA was isolated by using an RNeasy plant minikit (Qiagen, Spain) following the manufacturer's instructions. Reverse transcription was carried out in a 20-μl reaction volume which contained 0.5 μg of an oligo(dT)15 primer, 1 μl of reverse transcriptase from the ImProm-II Promega reverse transcription system (Promega, Spain), and 1 μg of total A. fumigatus RNA. The reaction conditions were a first step of 5 min at 25°C and then an hour at 42°C and 15 min at 70°C.
For transcription-level determination, a quantitative PCR (qPCR) assay was performed. The cyp51A expression level was quantified for each strain using the A. fumigatus β-tubulin gene (tub1, GenBank accession number AY048754) as a reference for gene expression. The primer set A6 (5′-ACCCCTTACATGATTCCTCCCC-3′) and A2 (5′-GGGGTCGTCAATGGACTA-3′) was used to amplify the cDNA from the cyp51A gene (54). The primer set Tub5 (5′-TGACCCAGCAGATGTT-3′) and Tub6 (5′-GTTGTTGGGAATCCACTC-3′) was used for amplification of a fragment of the A. fumigatus β-tubulin gene (27).
Bio-Rad qPCR mixtures were set up with SensiMix SYBR-Hi carboxy-X-rhodamine (Bioline, Spain). Each assay was repeated in duplicate with RNA from two different extractions. Each experiment included standard curves for the target gene (cyp51A) and the reference gene (tub1). The efficiencies of PCR amplification of β-tubulin and cyp51A cDNA were calculated from the slopes of the curves given by Bio-Rad CFX manager (version 2.0) software (Bio-Rad, USA), and the efficiency values were used to validate each experiment. Fold changes in expression were calculated using the 2−ΔΔCT threshold cycle (CT) method (55). The PCR conditions were 10 min at 95°C and 40 cycles of 10 s at 95°C, 5 s at 60°C, and 30 s at 72°C.
Comparative models of Cyp51A proteins.
Cyp51A 3D theoretical models of the Cyp51A WT and the Cyp51A-5SNPs from A. fumigatus strains were made by automated homology modeling techniques using SWISS-MODEL (56, 57). The crystal structure of the sterol 14-α demethylase (Cyp51B) from A. fumigatus in complex with VRC, deposited in the Protein Data Bank (PDB) under accession number 4UYM, was used as the template. The Cyp51A N terminus (residues 1 to 34) constitutes the membrane-spanning domain, which is not crystallized in the template protein.
Construction of deletion and heterologous expression cassettes.
For A. fumigatus cyp51A deletion and heterologous expression, different fusion cassettes were constructed by overlapping PCR (58). The final PCR product is a chimeric vector built with the previously amplified PCR fragments. The PCR conditions have been described elsewhere (59). The oligonucleotides used for the construction of all fusion cassettes are listed in Table S4. The primer locations and the fusion vector design are shown in Fig. 2A and 3A.
The constructed fusion knockout vector had about 2,000 bp of a sequence with homology to the 5′ upstream sequence of A. fumigatus TP11 (with cyp51A-5SNPs), including its promoter, followed by the hyg selectable marker cassette, ending with another 2,000 bp of sequence with homology to the 3′ downstream gene (Fig. 2A). The full cyp51A coding sequence is replaced by the hyg cassette in strain TP11.
For heterologous expression, three different cassettes were constructed. One copy had all five missense mutations, the second had three of them, and the third had only the F46Y substitution. All expression cassettes had (i) about 2,000 bp of homology to the 5′ upstream sequence of the WT cyp51A gene, including its promoter; (ii) one of the mutated copies of the cyp51A gene (cyp51A-5SNPs, cyp51A-3SNPs, or cyp51A-F46Y), including their 3′ terminators; (iii) the hyg selectable marker cassette; and (iv) another 2,000 bp of sequence with homology to the sequence downstream the WT cyp51A gene (Fig. 3A). Each heterologous expression cassette was used to replace the WT cyp51A copy in the ΔakuBKU80 strain.
A. fumigatus transformation and cassette verification by PCR amplification.
Transformation was achieved by the use of protoplasts (60). The resulting transformants were selected on minimal medium plates containing hygromycin B (Invitrogen, Spain) at 150 μg/ml for the deletion mutants and at 350 μg/ml for the expression mutants. The mutants were named with a letter T (transformant) and two numbers separated by a period (i.e., T1.15 and T2.7), in which the first number indicates the number of the transformation experiment and the second number indicates the number of the transformant.
The single integration and the replacement on the right locus were confirmed by PCR amplification (Fig. 2B and 3B) and sequencing (Table S4).
Computer analysis of the nucleotide sequences.
All the nucleotide sequences were analyzed using the MegAlign software package (DNAStar, Inc., Lasergene, USA). Also, the amino acid sequences of the putative 14-α demethylase genes cyp51A and cyp51B were deduced from the nucleotide sequences and analyzed using the same software.
Galleria mellonella survival assay and voriconazole response.
Wax moth larvae (TruLarv BioSystems Technology, UK) were infected with the A. fumigatus mutants and the ΔakuBKU80 strain as a control for the infection. Wax moth larva killing assays were performed as previously described (61). Basically, for each strain, 20 larvae (0.2 to 0.3 g) were inoculated with 105 conidia per larva. Treatment groups were injected with 5 μl (400 μg/ml) of a VRC solution (Pfizer, USA) (2 μg/larva, equivalent to a therapeutic dose of 10 mg/kg of body weight). Then, the larvae were incubated at 37°C for 10 days and mortality was recorded daily. Control groups, including untouched larvae and larvae inoculated only with VRC or phosphate-buffered saline, were omitted to simplify the figures. Each experiment was performed in duplicate at least 3 times, and the results are reported as mean values.
Statistical analyses were performed with the GraphPad Prism software package (version 11.0) (GraphPad Prism Software, San Diego, CA). Kaplan-Meier survival curves were analyzed by using a log-rank (Mantel-Cox) test for significance. A P value of <0.01 was considered significant.
Whole-genome sequencing library preparation methodology.
The preparation of fragmented genomic DNA libraries was performed using a Nextera XT library preparation kit (Illumina Inc., USA) according to the manufacturer's protocols. The mean length of the libraries ranged from 800 to 1,800 bp. Sequencing was conducted in a paired-end 2 × 150-bp mode on a NextSeq 500 system, according to the manufacturer's protocols (Illumina Inc., USA).
Whole-genome sequencing analysis.
The Illumina fastq data sets were trimmed using Trimmomatic (version 0.32) (62), removing adapters and sequences with low-quality scores at the 3′ ends (sequences in a 4-nt window with a Phred score of <20). Quality control analysis was performed with the FastQC (version 0.11.3) program (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) before and after the trimming process. Data sets were mapped against the sequences of two A. fumigatus reference genomes, the AF293 genome (AAHF00000000.1) and the A1163 genome (ABDB00000000.1), using the Bowtie 2 program (version 2.2.2.5) (63) with the very sensitive option in end-to-end mode. Duplicate reads were removed using the Picard (version 1.140) tool (http://broadinstitute.github.io/picard/). Realignment was performed using the GATK realignment (version 3.4) program (64). The BEDTools coverage (version 2.25) program (65) was used to perform further quality controls. The CFSAN SNP pipeline (66) was used for variant calling and SNV matrix generation. The VarScan2 program was used with the –min-var-freq 0.90 parameter. Consensus calling had to meet (i) a minimum coverage of 4 times in both strands, (ii) the presence of the consensus sequence in >90% of the base calls, (iii) the presence of a minimum fraction of 0.3 of the consensus-supporting reads in the forward and reverse sequences, and (iv) a minimum Phred quality score of 30 for a read. The remaining parameters were set at the default. The variant effect predictor script from ENSEMBL was used for variant annotation. Maximum likelihood phylogeny was carried out using RaxML software (67) with the GTRCAT model and 100 bootstrap replicates. A phylogenetic tree was visualized and annotated using the ggtree R package (68). SNP comparisons were performed using a custom R script.
Supplementary Material
ACKNOWLEDGMENTS
This work has been supported by the Fondo de Investigacion Sanitaria (FIS PI15_00019) and also by the Plan Nacional de I+D+i 2013–2016 and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/CIII/0004/0003), cofinanced by the European Development Regional Fund (ERDF; A way to achieve Europe), operative program Intelligent Growth 2014–2020.
We also thank the Clinical Microbiology and Infectious Diseases Department of the Hospital General Universitario Gregorio Marañón, Madrid, Spain.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00241-18.
REFERENCES
- 1.Latgé JP. 1999. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, Paiva JA, Blasco-Navalpotro M, De Laere E, Dimopoulos G, Rello J, Vogelaers D, Blot SI, AspICU Study Investigators. 2015. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagiwara D, Watanabe A, Kamei K, Goldman GH. 2016. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front Microbiol 7:1382. doi: 10.3389/fmicb.2016.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Rubio R, Cuenca-Estrella M, Mellado E. 2017. Triazole resistance in Aspergillus species: an emerging problem. Drugs 77:599–613. doi: 10.1007/s40265-017-0714-4. [DOI] [PubMed] [Google Scholar]
- 5.Escribano P, Recio S, Peláez T, Bouza E, Guinea J. 2011. Aspergillus fumigatus strains with mutations in the cyp51A gene do not always show phenotypic resistance to itraconazole, voriconazole, or posaconazole. Antimicrob Agents Chemother 55:2460–2462. doi: 10.1128/AAC.01358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alanio A, Cabaret O, Sitterlé E, Costa JM, Brisse S, Cordonnier C, Bretagne S. 2012. Azole preexposure affects the Aspergillus fumigatus population in patients. Antimicrob Agents Chemother 56:4948–4950. doi: 10.1128/AAC.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alstruey-Izquierdo A, Cuenca-Estrella M. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 52:2468–2472. doi: 10.1128/AAC.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 12.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Stensvold CR, Perlin DS, Arendrup MC. 2013. Azole resistance in Aspergillus fumigatus from bronchoalveolar lavage fluid samples of patients with chronic diseases. J Antimicrob Chemother 68:1497–1504. doi: 10.1093/jac/dkt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. 2015. Genomic context of azole resistance mutations in Aspergillus fumigatus determined by using whole-genome sequencing. mBio 6:e00536-15. doi: 10.1128/mBio.00536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 6:428. doi: 10.3389/fmicb.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 17.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 14:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ, Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol 47:3142–3146. doi: 10.1128/JCM.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist Updat 12:141–147. doi: 10.1016/j.drup.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Prigitano A, Venier V, Cogliati M, De Lorenzis G, Esposto MC, Tortorano AM. 2014. Azole-resistant Aspergillus fumigatus in the environment of northern Italy, May 2011 to June 2012. Euro Surveill 19(12):20747 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20747. [DOI] [PubMed] [Google Scholar]
- 21.Özmerdiven GE, Ak S, Ener B, Ağca H, Cilo BD, Tunca B, Akalın H. 2015. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother 21:581–586. doi: 10.1016/j.jiac.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Hurst SF, Berkow EL, Stevenson KL, Litvintseva AP, Lockhart SR. 2017. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J Antimicrob Chemother 72:2443–2446. doi: 10.1093/jac/dkx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shalhoub S, Luong ML, Howard SJ, Richardson S, Singer LG, Chaparro C, Keshavjee S, Akinlolu Y, Rotstein C, Mazzulli T, Husain S. 2015. Rate of cyp51A mutation in Aspergillus fumigatus among lung transplant recipients with targeted prophylaxis. Antimicrob Chemother 70:1064–1067. doi: 10.1093/jac/dkv085. [DOI] [PubMed] [Google Scholar]
- 24.Wang DY, Gricourt M, Arné P, Thierry S, Seguin D, Chermette R, Huang WY, Dannaoui E, Botterel F, Guillot J. 2014. Mutations in the Cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus isolated from avian farms in France and China. Poult Sci 93:12–15. doi: 10.3382/ps.2013-03541. [DOI] [PubMed] [Google Scholar]
- 25.Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob Agents Chemother 54:2425–2430. doi: 10.1128/AAC.01599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Melchers WJ, Verweij PE, Cuenca-Estrella M, Rodriguez-Tudela JL. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother 51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteiro MC, Garcia-Rubio R, Alcazar-Fuoli L, Peláez T, Mellado E. 2018. Could the determination of Aspergillus fumigatus mating type have prognostic value in invasive aspergillosis? Mycoses 61:172–178. doi: 10.1111/myc.12720. [DOI] [PubMed] [Google Scholar]
- 28.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF, Infectious Diseases Society of America. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 29.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 30.Lat A, Thompson GR III. 2011. Update on the optimal use of voriconazole for invasive fungal infections. Infect Drug Resist 4:43–53. doi: 10.2147/IDR.S12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Como JA, Dismukes WE. 1994. Oral azole drugs as systemic antifungal therapy. N Engl J Med 330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 34.Heimark L, Shipkova P, Greene J, Munayyer H, Yarosh-Tomaine T, DiDomenico B, Hare R, Pramani BN. 2002. Mechanism of azole antifungal activity as determined by liquid chromatographic/mass spectrometric monitoring of ergosterol biosynthesis. J Mass Spectrom 37:265–269. doi: 10.1002/jms.280. [DOI] [PubMed] [Google Scholar]
- 35.da Silva Ferreira ME, Capellaro JL, dos Reis Marques E, Malavazi I, Perlin D, Park S, Anderson JB, Colombo AL, Arthington-Skaggs BA, Goldman MH, Goldman GH. 2004. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob Agents Chemother 48:4405–4413. doi: 10.1128/AAC.48.11.4405-4413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albarrag AM, Anderson MJ, Howard SJ, Robson GD, Warn PA, Sanglard D, Denning DW. 2011. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51a promoter. Antimicrob Agents Chemother 55:5113–5121. doi: 10.1128/AAC.00517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol 49:2243–2251. doi: 10.1128/JCM.00213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bader O, Weig M, Reichard U, Lugert R, Kuhns M, Christner M, Held J, Peter S, Schumacher U, Buchheidt D, Tintelnot K, Groß U, MykoLabNet-D Partners . 2013. cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob Agents Chemother 57:3513–3517. doi: 10.1128/AAC.00167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 56:10–16. doi: 10.1128/AAC.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podust LM, Poulos TL, Waterman MR. 2001. Crystal structure of cytochrome P450 14α-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci U S A 98:3068–3073. doi: 10.1073/pnas.061562898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbot JJ, Kidd SE, Martin P, Beatty JA, Barrs VR. 2015. Azole resistance in canine and feline isolates of Aspergillus fumigatus. Comp Immunol Microbiol Infect Dis 42:37–41. doi: 10.1016/j.cimid.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Mellado E, Garcia-Effron G, Buitrago MJ, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2005. Targeted gene disruption of the 14-alpha sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob Agents Chemother 49:2536–2538. doi: 10.1128/AAC.49.6.2536-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seyedmousavi S, Mouton JW, Melchers WJ, Brüggemann RJ, Verweij PE. 2014. The role of azoles in the management of azole-resistant aspergillosis: from the bench to the bedside. Drug Resist Updat 17:37–50. doi: 10.1016/j.drup.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Keller NP. 2017. Heterogeneity confounds establishment of “a” model microbial strain. mBio 8:e00135-17. doi: 10.1128/mBio.00135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D. 2013. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PLoS One 8:e56651. doi: 10.1371/journal.pone.0056651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuller KK, Cramer RA, Zegans ME, Dunlap JC, Loros JJ. 2016. Aspergillus fumigatus photobiology illuminates the marked heterogeneity between isolates. mBio 7:e01517-16. doi: 10.1128/mBio.01517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowalski CH, Beattie SR, Fuller KK, McGurk EA, Tang Y-W, Hohl TM, Obar JJ, Cramer RA Jr. 2016. Heterogeneity among isolates reveals that fitness in low oxygen correlates with Aspergillus fumigatus virulence. mBio 7:e01515-16. doi: 10.1128/mBio.01515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob Agents Chemother 52:1244–1251. doi: 10.1128/AAC.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW, European Committee on Antimicrobial Susceptibility Testing Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST). 2012. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect 18:E248–E250. doi: 10.1111/j.1469-0691.2012.03890.x. [DOI] [PubMed] [Google Scholar]
- 50.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW. 2013. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat 16:81–95. doi: 10.1016/j.drup.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Pfaller JB, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. 2003. In vitro susceptibility testing of Aspergillus spp.: comparison of Etest and reference microdilution methods for determining voriconazole and itraconazole MICs. J Clin Microbiol 41:1126–1129. doi: 10.1128/JCM.41.3.1126-1129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.European Committee on Antimicrobial Susceptibility Testing. 2018. Antifungal agents. Breakpoint tables for interpretation of MICs. Version 9.0. www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_9.0_180212.pdf.
- 53.Tang CM, Cohen J, Holden DW. 1992. An Aspergillus fumigatus alkaline protease mutant constructed by gene disruption is deficient in extracellular elastase activity. Mol Microbiol 6:1663–1671. doi: 10.1111/j.1365-2958.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 54.Mellado E, Diaz-Guerra TM, Cuenca-Estrella M, Rodriguez-Tudela JL. 2001. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J Clin Microbiol 39:2431–2438. doi: 10.1128/JCM.39.7.2431-2438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1118. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 57.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino T, Bertoni M, Bordoli L, Schwede T. 2014. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:252–258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wurch T, Lestienne F, Pauwels PJ. 1998. A modified overlap extension PCR method to create chimeric genes in the absence of restriction enzymes. Biotechnol Tech 12:653–657. doi: 10.1023/A:1008848517221. [DOI] [Google Scholar]
- 59.Mellado E, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2011. Role of Aspergillus lentulus 14-α sterol demethylase (Cyp51A) in azole drug susceptibility. Antimicrob Agents Chemother 55:5459–5468. doi: 10.1128/AAC.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc 1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- 61.Alcazar-Fuoli L, Buitrago M, Gomez-Lopez A, Mellado E. 2015. An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp strains. Virulence 6:376–384. doi: 10.1080/21505594.2015.1025192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis S, Pettengill JB, Luo Y, Payne J, Shpuntoff A, Rand H, Strain E. 2015. CFSAN SNP Pipeline: an automated method for constructing SNP matrices from next-generation sequence data. Peer J Comput Sci 1:e20. doi: 10.7717/peerj-cs.20. [DOI] [Google Scholar]
- 67.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.