Abstract
Compression apparel is popular in both medical and sport performance settings. Perceived benefits are suggested to include changes in sensory feedback transmission caused by activation of mechanoreceptors. However, little is known about effects of compression apparel on sensorimotor control. Our purpose was to mechanistically examine whether compression apparel modulates sensory feedback transmission and reaching accuracy in the upper limb. Two experiments were completed under CONTROL and COMPRESSION (sleeve applied across the elbow joint) conditions. M-waves and H-reflexes were elicited by stimulating the median nerve and were recorded via surface electromyography (EMG). In experiment 1, H-reflexes and M-H recruitment curves were assessed at REST, during wrist flexion (10% EMGmax), and during a cutaneous conditioning of the superficial radial (SR) or distal median (MED) nerve. Cutaneous reflexes were elicited during 10% wrist flexion via stimulation of SR or MED. In experiment 2, unconditioned H-reflex measures were assessed at rest, during arm cycling, and during a discrete reaching task. Results indicate that compression apparel modulates spinal cord excitability across multiple sensory pathways and movement tasks. Interestingly, there was a significant improvement in reaching accuracy while wearing the compression sleeve. Taken together, the compression sleeve appears to increase precision and sensitivity around the joint where the sleeve is applied. Compression apparel may function as a “filter” of irrelevant mechanoreceptor information allowing for optimal task-related sensory information to enhance proprioception.
NEW & NOTEWORTHY Wearing a customized compression sleeve was shown to alter the excitability of multiple pathways within the central nervous system regardless of conditioning input or movement task and was accompanied by improved accuracy of reaching movements and determination of movement end point. Compression apparel may assist as a type of “filter function” of tonic and nonspecific mechanoreceptor information leading to increased precision and movement sensitivity around the joint where compression is applied.
Keywords: afferent feedback, compression, conditioning, cutaneous, electromyography, H-reflex, proprioception
INTRODUCTION
Compression garments have traditionally been thought to provide mechanical pressure to the body surface to stabilize or support the underlying tissue. Several attempts have been made to investigate the effects (if any) of wearing compression apparel with varying outcomes. Two review papers and a meta-analysis concluded that compression apparel promotes numerous physiological processes capable of assisting athletic performance and subsequent recovery (Born et al. 2013; Hill et al. 2014; MacRae et al. 2011). The majority of clinical work has focused on increasing venous return or other cardiovascular parameters in cases such as lymphedema, pulmonary embolism, or deep vein thrombosis (Gandhi et al. 1984; Kraemer et al. 2000; MacRae et al. 2011, 2012). Currently, little evidence of possible mechanisms or loci of adaptation has been identified for many of the perceived benefits.
While some improvements with compression are likely due to mechanical or cardiovascular effects (Berry and McMurray 1987; MacRae et al. 2012; Perrey 2009), benefits such as improved power output, joint position sense (Birmingham et al. 1998; Kraemer et al. 1998) and one-leg balance (Michael et al. 2014) may be related to adaptation in the nervous system. A major limitation of the current literature is the lack of information on how compression apparel interacts with the nervous system and may impact sensorimotor control. It remains entirely possible that many perceived benefits of compression apparel may be due to alterations in sensory feedback transmission caused by activation of mechanoreceptors beneath and around the site of compression.
One review discussed the idea that compression apparel could improve proprioception, allowing for improved information on the direction, acceleration, and speed of the limbs during movement (Born et al. 2013). Previous research on proprioception has mainly focused on the primary and secondary endings of the muscle spindle, which are heavily involved in kinesthetic feedback (Proske and Gandevia 2009). However, two separate lines of research using microneurography to record afferent discharge (Edin 1992, 2004) and skin stretch to isolate the afferent input to cutaneous mechanoreceptors (Collins and Prochazka 1996; Collins et al. 2000, 2005) indicate that cutaneous feedback from the skin also provides accurate perceptual information about joint position and movement (Proske and Gandevia 2012). This information from the skin is then integrated with feedback from muscle spindles to provide judgments of position and movement at joints throughout the body.
It has been established that both cutaneous and muscle afferent feedback is “tuned” by the nervous system depending on the phase of the locomotor cycle and the type of task performed (Zehr and Kido 2001; Zehr et al. 2003) and is required for smooth coordinated limb movements (Zehr and Stein 1999). Although these contributions go largely unnoticed due to their relatively fast acting effects on motor output, they are integrated at multiple levels of the nervous system including the spinal cord and brain (Aimonetti et al. 1999; Birmingham et al. 1998; Iles 1996; Zehr and Stein 1999).
An interesting proxy for compression apparel is the use of tape applied under tension across joints or muscles. Previously, the H-reflex was employed to assess changes in excitability with different taping techniques in the trapezius muscle (Alexander et al. 2003). Results showed a 22% reduction in H-reflex amplitude when rigid tape was applied compared with no change when the tape was removed or only under tape was applied. A follow-up study found a 19% reduction in H-reflex amplitude when rigid sports tape was applied in parallel to the medial gastrocnemius compared with no change when the tape was applied across the muscle fibers (Alexander et al. 2008). Compression apparel is likely to activate both cutaneous and muscle mechanoreceptors at rest and during movement, which could modulate sensory feedback transmission and ultimately proprioception (McNair and Heine 1999; Simoneau et al. 1997). Effects of sensorimotor integration could account for a significant portion of the perceived benefit of this technology, especially if performance of a motor task can be improved.
Thus the overall objective of this experiment was to examine whether compression apparel produces measurable modulation of sensory feedback and motor task performance in the arm. Using both Hoffmann (H-) and cutaneous reflexes as probes, the purpose of experiment 1 was to explore the effects of compression apparel on sensory feedback transmission in a stationary limb. Based on the limited available literature, it was hypothesized that compression apparel would inhibit H-reflex amplitude in a similar fashion as tape applied under tension. The twofold purpose of experiment 2 was to assess 1) whether a compression sleeve differentially modulated the transmission of sensory information based on the task (static vs. locomotor vs. reaching) or phase of movement; and 2) any differences in performance of a discrete reaching task.
METHODS
Participants
Two separate experiments were performed with a total of 25 neurologically intact participants free from metabolic disorders (e.g., diabetes) that could affect neurotransmission: 13 participants (5 male; 8 female, 22.9 ± 3.0 yr, 170.8 ± 9.0 cm, 69.8 ± 13.1 kg) performed experiment 1 while 12 participants (5 male; 7 female, 25.2 ± 2.3 yr, 171.6 ± 8.9 cm, 68.6 ± 11.2 kg) completed experiment 2. Participants were informed of all experimental procedures and signed a written consent form. Protocols used in the experiments were approved by the University of Victoria Human Research Ethics Committee and performed according to the Declaration of Helsinki (1964).
Participants were fitted with a custom-made, non-medical-grade compression sleeve containing silicone at the proximal and distal ends to improve friction between the compression garment and the skin surface to provide skin stretch. Arm circumference was measured in each participant and the closest fit according to the circumference of both the forearm and upper arm was then assigned. This fit was sized to maintain a similar amount of compression (between 10 and 20 mmHg) between participants. Table 1 provides details of the six sleeve sizes, the number of participants for each size, the average forearm and upper arm circumferences and the calculated compression applied to each site in experiment 1 of the study. Overall, the compression applied was similar to previous investigations (Beliard et al. 2015). Interestingly, the review by Beliard et al. (2015) concluded the mechanical characteristics of a compression garment (value or spatial patterns of the pressure applied) have not shown to impact its effects on recovery and soreness.
Table 1.
Compression sleeve sizing with corresponding compression
| Sizing (n = 13) | Forearm Circumference, cm | Forearm Compression, mmHG | Upper Arm Circumference, cm | Upper Arm Compression, mmHG |
|---|---|---|---|---|
| Male | ||||
| Large (n = 1) | 28 | 17 | 36.5 | 12 |
| Medium (n = 2) | 27.8 | 14 | 32.8 | 13 |
| Small (n = 2) | 26 | 16 | 31.5 | 13 |
| Female | ||||
| Large (n = 1) | 25 | 16 | 32.5 | 17 |
| Medium (n = 4) | 22.9 | 15 | 29.1 | 17 |
| Small (n = 3) | 21.5 | 15 | 25.3 | 17 |
Experiment 1: Effects of Compression on Afferent Transmission During a Static Task.
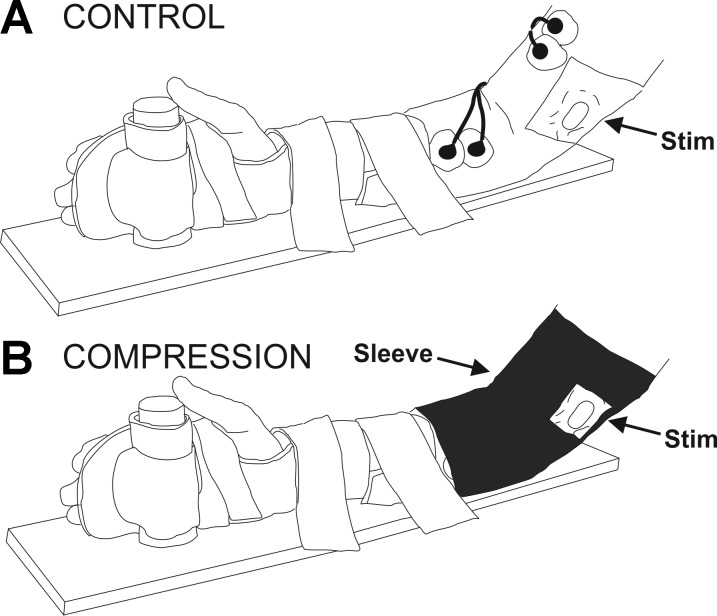
To establish whether wearing a compression sleeve produced measurable changes in sensory feedback transmission, reflex measures were initially assessed in a static position. The right forearm, wrist, and hand were fixed in a customized brace to restrict movement and maintain joint angles throughout the experiment (Fig. 1). All measures were assessed without (CONTROL) or with a customized compression sleeve (COMPRESSION). H-reflex recruitment curves were collected during REST (No contraction), VOL (10% voluntary contraction of the flexor carpi radialis), SR (Contraction + superficial radial nerve conditioning), and MED (Contraction + distal median nerve conditioning). H-reflexes were also collected at the intensity required to match direct motor response (M-wave) amplitude between all conditions. Cutaneous reflexes were elicited via stimulation of the SR or MED nerve. For all conditions except REST, participants were asked to maintain a consistent low-level contraction (~10% of maximal voluntary contraction) of the right flexor carpi radialis (FCR) using visual feedback of the rectified and filtered EMG signal displayed on a computer screen.
Fig. 1.
A: experimental setup of experiment 1 without compression sleeve. B: experimental setup of experiment 1 with compression sleeve. Stimulation electrode (Stim) placement on the median nerve proximal to the elbow to elicit H-reflexes in the flexor carpi radialis is depicted in both setups. FCR, flexor carpi radialis.
Electrical nerve stimulation.
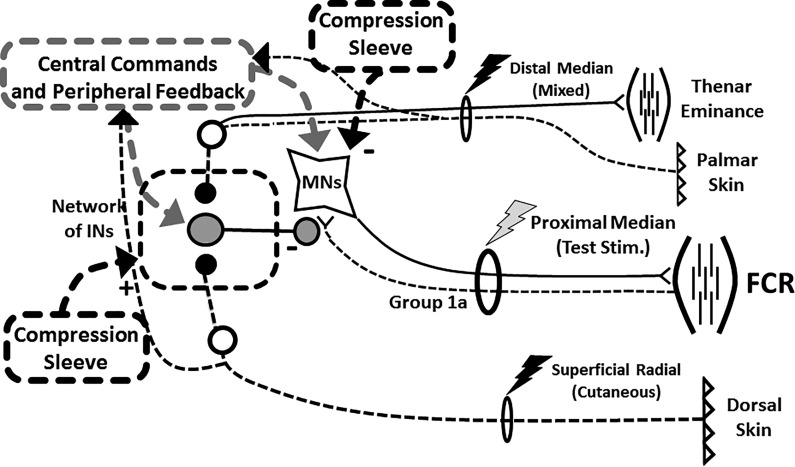
Electrical stimulation of the median nerve (Fig. 2; gray bolt) evoked M-wave and H-reflex responses in the FCR were collected using bipolar surface electrodes placed just proximal to the medial epicondyle, as in previous studies (Nakajima et al. 2013; Vasudevan and Zehr 2011; Zehr and Hundza 2005; Zehr et al. 2007). Square wave pulses (1 ms) were delivered with a constant current stimulator (Digitimer model DS7A) pseudo-randomly every 2–5 s. Cutaneous nerve stimulation was used to condition H-reflexes and for generating cutaneous reflexes in trains of 5 × 1.0 ms pulses at 300 Hz using a Grass S88 stimulator with SIU5 stimulus isolation and a CCU1 constant current unit (Grass Instruments, Austin, TX) (Nakajima et al. 2013) (Fig. 2). Cutaneous trains were delivered at three times the radiating threshold (RT). Perceptual threshold (PT) and RT were determined as the point at which nerve stimulation produces a perceivable stimulation and the point at which a stimulation produced radiating paresthesia in the cutaneous receptive field of that nerve, respectively. Stimulation of the SR nerve was delivered at a conditioning-test (C-T) interval of 37 ms as suggested for the FCR by previous researchers (Nakajima et al. 2013). Cutaneous stimulation was applied to either the SR or MED nerves just proximal to the wrist (Fig. 2; black bolts). As shown in Fig. 2, the SR nerve carries primarily cutaneous afferent information while MED is a mixed peripheral nerve that carries both motor and sensory information. All reflex measures were normalized to the corresponding maximally evoked motor response (Mmax).
Fig. 2.
Schematic of H-reflex and conditioning pathways. Schematic diagram outlining possible neural pathways for integration of inputs on Ia afferents arising from a compression sleeve placed around the elbow joint. FCR, flexor carpi radialis; INs, interneurons; MNs, motoneurons.
Electromyography.
EMG was recorded unilaterally from the FCR, extensor carpi radialis (ECR), biceps, and triceps brachii. Once the skin was cleaned with alcohol wipes, surface electrodes were placed in a bipolar configuration on the skin, oriented longitudinally along the predicted fiber direction in accordance with SENIAM procedures (see Hermens et al. 2000). EMG was preamplified (GRASS P511, AstroMed) and band-pass filtered 30–1,000 Hz (FCR) or 30–300 Hz (ECR, BB, TB). The output was sent to the analog-to-digital interface (National Instruments) where it was converted to a digital signal and sampled at 2,000 Hz using custom-built continuous acquisition software (LabVIEW, National Instruments). The level of muscle activation during the different tasks and conditions was determined via background EMG amplitudes from the reflex trials. During each trial, prestimulus EMG was rectified and averaged to provide a mean level of muscle activation during reflex sampling.
H-reflex.
H-reflex amplitude was evaluated while M-waves were kept constant across all conditions. Initial M-wave amplitude was determined by finding an intensity that produced an H-reflex amplitude of ~70% maximally evoked H-reflex (Hmax) on the ascending limb of the recruitment curve while producing a small but measurable motor response to minimize antidromic effects. M-waves were monitored across all conditions, and stimulation intensity was adjusted as needed to maintain consistent amplitude. Twenty stimuli were averaged to directly compare the amplitudes of the H-reflex during CONTROL and COMPRESSION. FCR H-reflex and M-wave amplitudes were averaged for each condition and analyzed off line using MATLAB (MathWorks, Natick, MA).
M-H recruitment curves were collected by applying 40 stimuli over a range of intensities while stimulation current was concurrently measured (mA-2000 Noncontact Milliammeter, Bell Technologies, Orlando, FL). Peak-to-peak amplitudes of M- and H-waves were calculated offline from the single unrectified sweeps of EMG with custom-written software (MATLAB). Average Hmax and Mmax were calculated from the three largest H-reflexes and M-waves, respectively.
Cutaneous reflex stimulation.
Cutaneous reflexes were determined by analyzing averages of 20 sweeps of rectified EMG. The stimulus artifact was removed from the reflex trace and data were then low-pass filtered at 30 Hz using a dual-pass, fourth order Butterworth filter. The peak long-latency response (110–140 ms poststimulus) was evaluated to assess whether compression resulted in altered integration of sensory information at supraspinal levels.
Experiment 2: Effects of Compression on Afferent Transmission and Performance During Movement
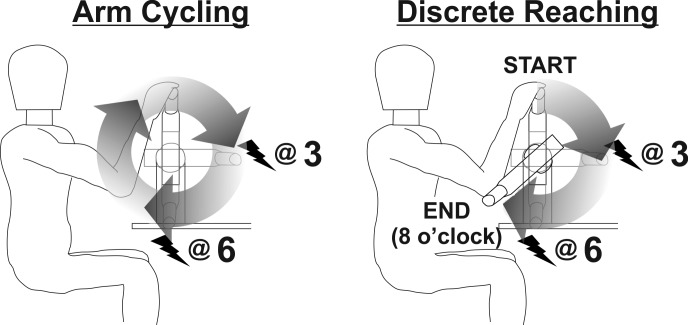
To assess whether wearing a compression sleeve differentially modulates the transmission of sensory information based on the task (STATIC vs. CYCLING vs. REACHING) or phase of a movement, 12 participants completed experiment 2. Once again, each participant completed all experimental protocols during both CONTROL and COMPRESSION. H-reflexes were collected at the intensity required to match M-wave amplitude between all conditions with M-H recruitment curves being elicited in a similar manner to experiment 1 by stimulating the median nerve at two different phases of movement (elbow flexion and extension) during three different tasks (STATIC, CYCLING, and REACHING). The stimulation was delivered pseudo-randomly once every 1–3 s, 1–3 cycles, or 1–2 reaches for the static, cycling and reaching tasks, respectively. All three tasks were performed on a custom-made hydraulic arm cycle ergometer and remained in the seated position throughout the experiment (Fig. 3). The movement cycle was divided into 12 equal phases of movement (bins), which correspond to a clock face when viewing the right arm from a lateral view. Details of each task are listed below.
Fig. 3.
Movement tasks during experiment 2. Arm cycling was performed at a cadence of 1 Hz (60 rpm). The discrete reaching task was initiated at the 12 o’clock position and ended at 8 o’clock. Stimulation for both tasks was provided at the 3 (extension) and 6 o’clock (flexion) positions during separate trials.
Static grip.
To provide a position-matched control for the cycling and reaching tasks and a reference point to experiment 1, participants were asked to grip the ergometer handle and maintain a 10% voluntary contraction in the FCR muscle of the right forearm using visual feedback of the rectified and filtered EMG signal displayed on a computer screen (3 o’clock: CONTROL 10.1% EMGmax vs. COMPRESSION 10.0% EMGmax). Participants held the ergometer handle at the 3 (extension) and 6 o’clock (flexion) positions during separate trials while H-reflexes were evoked (Fig. 3). At 3 o’clock background muscle activation in the FCR was 10.1 and 10.0% EMGmax for CONTROL and COMPRESSION, respectively. At 6 o’clock background muscle activation was 10.1 and 9.9% EMGmax for CONTROL and COMPRESSION, respectively.
Upper limb cycling.
Unloaded rhythmic arm cycling (no resistance) was performed using both limbs on a custom-made hydraulic arm ergometer at a constant frequency of 1 Hz (~60 rpm) (Zehr et al. 2003). H-reflexes were evoked during separate trials at two positions corresponding to 3 (extension) and 6 o’clock (flexion) (Fig. 3). At 3 o’clock background muscle activation in the FCR was 8.7 and 8.0% EMGmax for CONTROL and COMPRESSION, respectively. At 6 o’clock background muscle activation was 10.5 and 10.3% EMGmax for CONTROL and COMPRESSION, respectively.
Reaching task.
A discrete reaching task was performed with the right arm while the left arm remained in the participant’s lap. While holding the ergometer handgrip, the participants initiated a movement from the 12 o’clock position to an end point at the 8 o’clock position (Fig. 3). H-reflexes were evoked during separate trials at the same 3 (extension) and 6 o’clock (flexion) positions as during cycling. Five minutes of practice were provided to all participants to account for learning effects of the task itself. Participants were instructed to stop exactly at the 8 o’clock position, which was detected by optical-encoded position sensors in the ergometer and visually represented on a monitor in front of them. Ten trials were performed under each condition with deviation from the intended target being used as a measure of reaching accuracy with and without compression apparel. Differences in reaching accuracy were assessed to provide data on whether a compression garment can alter motor task performance. The difference between the intended target and actual end position was calculated as a voltage from the optical encoders and converted to degrees of difference. The cadence of reaching matched their previous self-generated cycling frequency of 1 Hz (60 rpm). Trials were collected after a familiarization period in a randomized order. A similar protocol has been used previously (Hundza and Zehr 2007). At 3 o’clock background muscle activation in the FCR was 9.5 and 8.2% EMGmax for CONTROL and COMPRESSION, respectively. At 6 o’clock background muscle activation was 14.1 and 13.1% EMGmax for CONTROL and COMPRESSION, respectively.
Statistical Analysis
Experiment 1.
To assess effects on sensory transmission in the upper limb, dependent measures of background muscle activity, Mmax, Hmax, M-wave at constant M-value, H-wave at constant M-value, and long-latency cutaneous reflex amplitude were assessed using SPSS Statistics 20 (Chicago, IL). Factorial ANOVAs contained within factors of condition (CONTROL vs. COMPRESSION) and task (REST, VOL, SR, MED).
Experiment 2.
To assess effects on sensory transmission during movement, dependent measures of background muscle activity, Mmax, Hmax, M-wave at constant M-value, H-wave at constant M-value, and reaching accuracy were assessed. Within factors of condition (CONTROL vs. COMPRESSION) and task (STATIC, CYCLING, REACHING) were compared using ANOVA, while each position (3 O’CLOCK, 6 O’CLOCK) was treated separately.
All values are expressed as mean ± standard deviation except in the figures where standard error was used for clarity of display. If significant main effects or interactions were detected in experiment 1 or 2, simple main effects analysis and pairwise comparisons were used. Significance was accepted at P < 0.05.
RESULTS
Experiment 1: Effects of Compression on Afferent Transmission During a Static Task
Effect of somatosensory conditioning on M-Hmax.
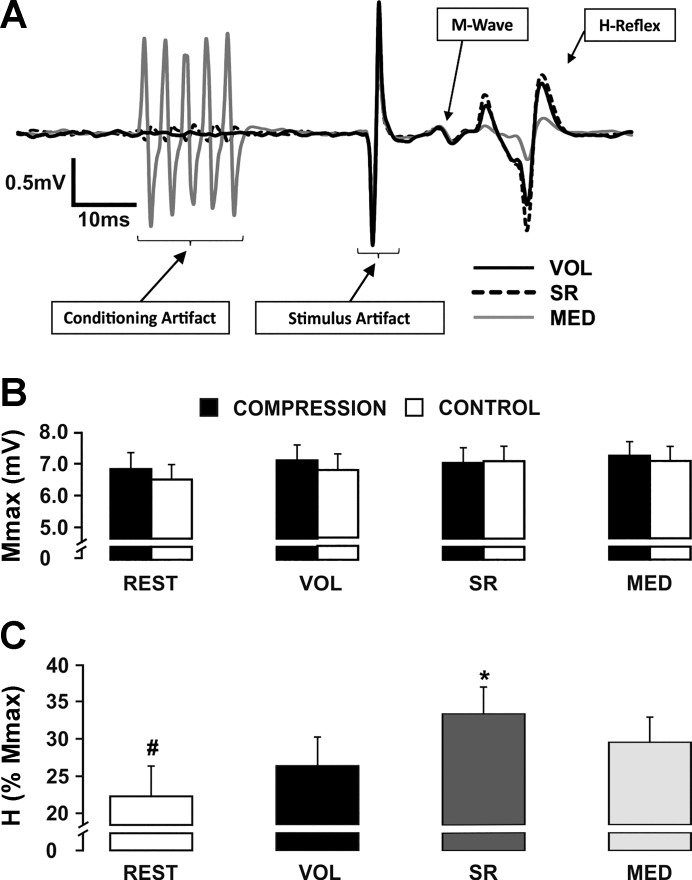
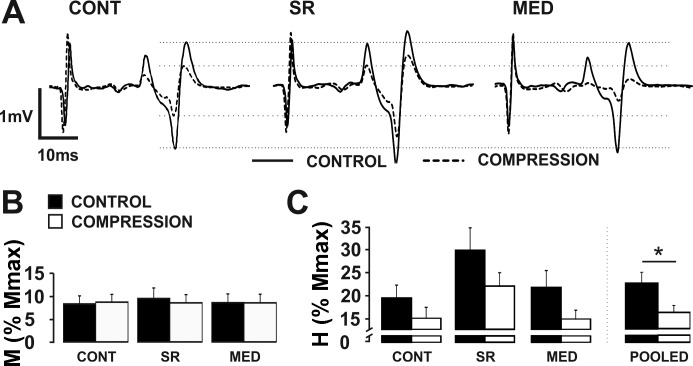
Somatosensory conditioning significantly altered Hmax but not Mmax amplitudes. Figure 4A demonstrates the trend of the group means with single subject average H-reflex traces. Greater H-reflex amplitude is evident during the SR condition compared with both VOL and MED conditions. Maximal M-wave amplitudes evoked in experiment 1 (ranged from 6.6 to 7.2 mV) were not affected by somatosensory conditioning or compression (P > 0.05). Figure 4B shows the Mmax amplitudes for each task and condition. For Hmax amplitude, a 2 (condition) × 4 (task) factorial ANOVA revealed a significant main effect for task (F1.924,23.094 = 10.570, P = 0.001). Pairwise comparisons showed that REST (22.3 ± 13.12%) was significantly lower than VOL (26.4 ± 13.31%, P = 0.026), SR (33.3 ± 12.56%, P = 0.001) and MED (29.5 ± 11.68%, P = 0.014). Furthermore, the SR Hmax amplitude was significantly higher than both the VOL (P = 0.001) and MED (P = 0.023) Hmax amplitudes (Fig. 4C).
Fig. 4.
Effects of conditioning paradigm. A: individual subject traces that show the effect of conditioning on H-reflex amplitude while M-wave is maintained constant. B: group average of maximally evoked M-wave (Mmax) amplitude across the four conditions, with and without compression. C: group average of maximally evoked H-reflex (Hmax) amplitude pooled between CONTROL and COMPRESSION across all participants. #Significantly lower Hmax than all other conditions. *Significantly higher Hmax than all other conditions. Values are means ± SE (P < 0.05). MED, distal median; SR, superficial radial; VOL, voluntary contraction.
Effect of the compression sleeve on maximally evoked M-waves and H-reflexes.
During experiments 1 and 2, background muscle activity was not significantly different within a task between CONTROL or COMPRESSION for any muscle group (P > 0.05). Wearing the compression sleeve did not alter Mmax or Hmax values. There were no significant differences in Mmax between CONTROL and COMPRESSION at REST (6.7 ± 1.8 vs. 6.6 ± 1.8 mV), VOL (7.1 ± 1.7 vs. 7.0 ± 1.8 mV), SR (7.0 ± 1.6 vs. 7.1 ± 1.8 mV), or MED (7.2 ± 1.6 vs. 7.1 ± 1.7 mV) (P > 0.05). This indicates the ability to maximally recruit motor axons was not measurably affected by a compression sleeve (Fig. 4B). There were no significant differences in Hmax between CONTROL and COMPRESSION at REST (23.7 ± 15.6 vs. 20.8 ± 15.0%), VOL (26.6 ± 12.8 vs. 26.1 ± 16.1%), SR (35.3 ± 13.3 vs. 31.3 ± 13.7%), or MED (31.0 ± 12.9 vs. 28.1 ± 13.4%) (P > 0.05). This indicates the ability to maximally recruit afferent axons was not measurably affected by a compression sleeve.
Effect of the compression sleeve on H-reflex amplitude evoked with a constant M-wave.
Wearing the compression sleeve caused a general suppression of the H-reflex amplitude elicited with constant M-wave amplitude, regardless of somatosensory conditioning. Figure 5A shows single subject average traces representing the group trends of a reduction in H-reflex amplitude. Evoked M-wave amplitudes were ~8–9% of Mmax and were not significantly different in amplitude across conditions (P > 0.05), as seen in Fig. 5B. For H-reflex amplitude, the 2 (condition) × 3 (task) factorial ANOVA revealed a significant main effect for condition (F1,12 = 5.142, P = 0.043) (Fig. 5C).
Fig. 5.
Effects of compression sleeve during experiment 1. A: single subject traces of the average H-reflex amplitude during 10% contraction, SR nerve conditioning, and MED nerve conditioning. Solid traces indicate control averages, whereas dotted traces indicate inhibited compression averages. B: group average of M-wave amplitude across conditions indicating same input provided. C: group average of H-reflex amplitude with and without compression. *Significant reduction in H-reflex amplitude while wearing compression sleeve across all three conditions. Values are means ± SE (P < 0.05).
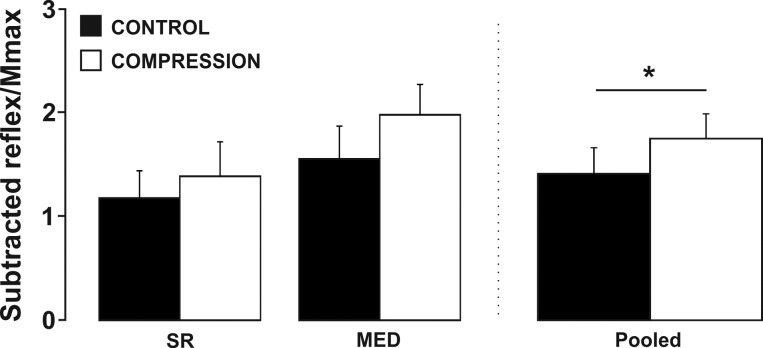
Effect of the compression sleeve on long-latency cutaneous reflex amplitudes.
Wearing the compression sleeve resulted in a general increase in long-latency cutaneous reflex amplitude, regardless of the nerve stimulated (Fig. 6). A 2 (condition) × 2 (stimulation site) factorial ANOVA revealed that there was a significant main effect for condition (F1,12 = 5.390, P = 0.039). The peak long-latency response occurred at an average latency of 135 ms after stimulation. An alteration in long-latency excitability indicates that the compression sleeve altered sensory transmission within multiple pathways including supraspinal excitability.
Fig. 6.
Effects of compression on long-latency cutaneous reflex amplitude. *Significant increase in long-latency cutaneous reflex amplitude while wearing the compression sleeve. Values are means ± SE (P < 0.05). MED, distal median; SR, superficial radial.
Experiment 2: Effects of Compression on Afferent Transmission and Performance During Movement
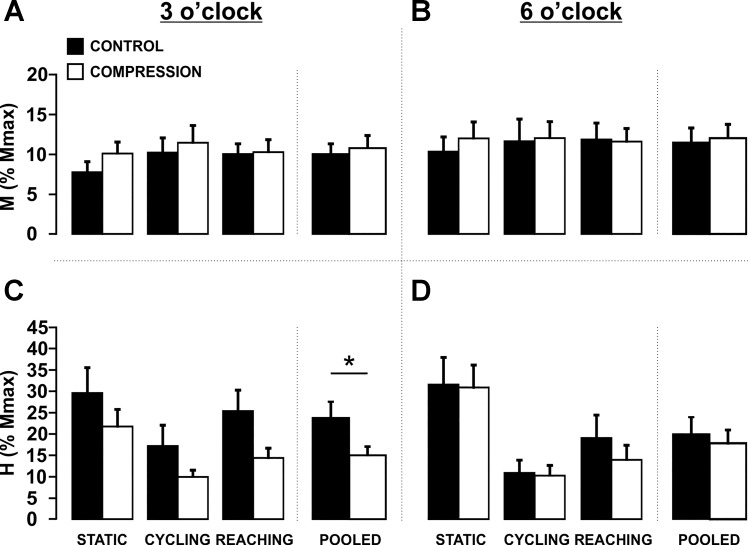
Effects of compression sleeve on maximally evoked M-wave and H-reflexes during movement.
Similar to experiment 1, wearing the compression sleeve did not alter Mmax or Hmax values during static, cycling, or reaching task recruitment curves (P > 0.05). This is consistent with findings above that the ability to maximally recruit motor and primarily Ia afferent axons was not affected by a compression garment.
Effects of the compression sleeve on H-reflex amplitudes during movement.
Wearing the compression sleeve caused a general suppression of M-wave matched H-reflexes evoked at the 3 o’clock, but not the 6 o’clock, position across all tasks (Fig. 7, C and D). Evoked M-wave amplitudes ranged from 7.3 to 11.4% of Mmax and were not statistically different between conditions or tasks (P > 0.05) (Fig. 7, A and B). At the 6 o’clock position, H-reflex amplitudes were not significantly different between condition (P > 0.05) (Fig. 7D). However, a 2 (condition) × 3 (task) factorial ANOVA revealed a significant main effect for condition (F1,11 = 7.523, P = 0.019), indicating that there was a similar reduction in H-reflex amplitude across each of the tasks at the 3 o’clock position (Fig. 7C).
Fig. 7.
Effects of compression sleeve across movement tasks and positions in experiment 2. A: maintenance of M-wave across condition and movement tasks at the 3 o’clock elbow extension position. B: maintenance of M-wave across condition and movement tasks at the 6 o’clock elbow flexion position. C: effect of compression on H-reflex amplitude across movement tasks at the 3 o’clock elbow extension position. *Significant reduction in H-reflex amplitude pooled across movement task. D: effect of compression on H-reflex amplitude across movement tasks at the 6 o’clock elbow flexion position. No differences were present at this position. Values are means ± SE (P < 0.05).
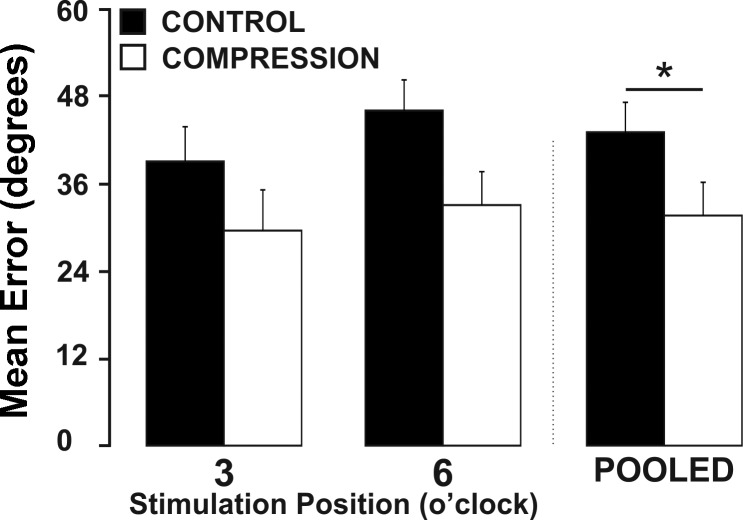
Effects of the compression sleeve on discrete reaching accuracy.
Wearing the compression sleeve reduced the average magnitude of errors during the discrete reaching task (Fig. 8). Regardless of the position at which the participants received the stimulation during the reaching task, a similar improvement in reaching accuracy was seen while wearing compression. A 2 (condition) × 2 (stimulation position) factorial ANOVA revealed that there was a significant main effect for condition (F1,12 = 6.713, P = 0.024). The reduced magnitude of error was similar across two distinct positions of stimulation indicating the compression sleeve may impact performance on a discrete reaching task.
Fig. 8.
Reaching accuracy across stimulation positions. *Significant reduction in the deviation from the intended target while wearing the compression apparel. Values are means ± SE (P < 0.05).
DISCUSSION
The major finding is that a customized compression sleeve worn around the elbow joint alters reflex excitability and improves reaching accuracy. This alteration in excitability occurs in cutaneous and muscle afferent sensory pathways and across multiple movement tasks.
Experiment 1: Effects of Compression on Afferent Transmission During a Static Task
Altered sensory transmission during a static task.
The main result from experiment 1 is expressed in Fig. 5. To ensure stability of the afferent test volley between conditions, M-wave amplitudes elicited in the FCR were maintained while the corresponding H-reflex amplitudes were observed (Fig. 5B). There were no significant differences in M-wave between CONTROL and COMPRESSION or across condition, suggesting the same relative input was provided to sensory and motor axons (Zehr 2002). Interestingly, with the same relative input, a consistent decrease in H-reflex amplitude was identified while the compression sleeve was worn regardless of paradigm. This indicates wearing a compression sleeve alters spinal cord excitability by inhibiting Ia afferent transmission presynaptically or motoneuron excitability postsynaptically (Knikou 2008; Misiaszek 2003; Zehr 2002). The effects of compression on H-reflex amplitude are clearly expressed in the individual subject traces shown in Fig. 5A.
A reduction in H-reflex excitability while wearing the compression sleeve may seem counterintuitive but may not be all together that surprising. An interesting proxy for compression apparel is the use of tape applied under tension across joints or muscles. Tape applied in the direction of the muscle fibers has been suggested to alter activity in the underlying muscle (Morrissey 2000). Potential contributions to this effect included cutaneous afferent input altering motoneuron excitability (McNair and Heine 1999; Simoneau et al. 1997). This question was explored in a series of two experiments in which the H-reflex was employed to assess changes in excitability with different taping techniques in the trapezius muscle. The first study (Alexander et al. 2003) used an average of 50 reflex sweeps at a consistent level of stimulation and matched M-wave amplitude to compare four conditions: control, under tape (no tension), rigid tape (Endura sports tape), and after removal (no tape). The results showed a 22% reduction in H-reflex amplitude only during the rigid sports tape condition. When the under tape was applied or the tape was removed, the H-reflex amplitude remained unchanged from the control condition. Our results using a compression sleeve worn across the elbow joint are similar in principle to these results at the shoulder.
A follow-up study was then completed in the gastrocnemius muscle via stimulation of the tibial nerve under four conditions: 1) before taping, 2) under tape aligned across or along the direction of the medial gastrocnemius fibers, 3) rigid tape application, and 4) both tapes removed (Alexander et al. 2008). Once again, there was a 19% reduction in H-reflex amplitude when the rigid sports tape was applied in parallel to the fibers of the medial gastrocnemius. Interestingly, when the rigid tape was applied across the muscle fibers there was no change in H-reflex amplitude. While a compression sleeve cannot be said to be applied in parallel or across the muscle fibers, the similarity of results may provide an indication that activating specific cutaneous mechanoreceptors with either tape or compression apparel can directly alter sensory feedback transmission and thus motor output. This may be related to sensory information only being altered at a phase of movement when significant stretch would have occurred and been functionally relevant to proprioception.
Effectiveness of conditioning paradigm.
A conditioning paradigm was employed in an attempt to uncover whether differential effects occurred when testing multiple afferent pathways during CONTROL and COMPRESSION. Figure 4 illustrates the conditioning paradigm was effective at altering excitability of the Ia reflex pathway as assessed by the H-reflex. At rest, stimulation of the median nerve activates both motor and sensory nerve fibers which produces the M-wave and H-reflex, respectively. Figure 4B indicates that the maximally evoked motor response (Mmax) did not differ across conditioning paradigm or between CONTROL and COMPRESSION. This indicates that the relative number of motor units recruited across conditions was similar. Furthermore, Fig. 4, A and C clearly shows that, with the same relative input to the spinal cord, more motor units were depolarized during a contraction and during SR conditioning, demonstrated by a significant increase in Hmax amplitude. The 10% FCR contraction increased the excitability of the motoneuron pool above REST as motor units are brought closer to their subliminal fringe. SR conditioning is known to reduce presynaptic inhibition on the Ia afferent pathway within the forearm (Nakajima et al. 2013). This was confirmed in the current experiment as the same relative input significantly increased Hmax amplitude (Fig. 4C). Single subject data provided in Fig. 4A shows a clear waveform example of the effects that the conditioning paradigm had on averaged H-reflex sweeps.
The conditioning paradigm was employed to potentially identify whether specific presynaptic contributions to the altered sensory transmission could be identified (Nakajima et al. 2013). However, there were no differential results between unconditioned and conditioned H-reflexes. While this result does not provide specific information on presynaptic contributions to the inhibition, the similarity in amplitude of effect across paradigms provides important information on the robust nature of the inhibition. A schematic representation of possible integration sites for the compression sleeve is provided in Fig. 2, which has been modified from Nakajima et al. (2013). Overall, wearing a compression sleeve at the elbow during a static task corresponds with a robust but discrete reduction in excitability across conditioning paradigm.
Role of cutaneous mechanoreceptors in altered sensory transmission.
Wearing a compression sleeve altered excitability of long-latency cutaneous reflexes as shown in Fig. 6. Cutaneous reflexes provide information on the relative contribution of sensory information from the skin being incorporated into ongoing motor output (Zehr and Stein 1999). The convergence of excitatory and inhibitory effects on FCR motoneurons depends on the nerve being stimulated and the latency at which the response is measured. Similar to previous studies, long-latency responses to SR nerve stimulation in the FCR produce a large facilitation (Zehr et al. 2001). Interestingly, when the compression sleeve was applied, there was a significant facilitation of the long-latency response. Contributions to the facilitation of ongoing muscle activity at this latency can occur at multiple levels of the nervous system including segmental reflex, brain stem reflex, or cortical descending inputs, which ultimately converge on the motoneuron of the FCR (Aimonetti et al. 1999; Birmingham et al. 1998; Iles 1996; Zehr and Stein 1999).
Previous work has highlighted the important role of skin stretch to proprioception in the upper limb (Edin and Abbs 1991). Induced skin strain near an anesthetized joint produces perceived joint movement. However, when the skin strain is eliminated, movement in the anesthetized joint is not perceived indicating its possible importance to joint position (Edin and Johansson 1995). Importantly, ensemble activity in human cutaneous sensory afferents evoked by the stretching of the skin over and around a joint contributes to the conscious perception of movement. In a previous series of experiments, cutaneous afferents were activated by mechanical stretching of the skin over and around multiple joints. Perceived movements were then mimicked by voluntary movements of the contralateral limb. The authors found a mismatch between actual joint position and perceived joint position, which indicated that input from the skin stretched during movement contributed to the conscious perception of movement for the joint under the stimulated skin (Collins and Prochazka 1996; Collins et al. 2000, 2005). Together, these studies illustrate that cutaneous feedback provides accurate perceptual information about joint position and movement and is integrated with feedback from muscle spindles to provide judgments of position and movement for joints throughout the body.
Muscle contractions cause skin on one side of the joint to be stretched while skin on the other side becomes slackened or possibly folded. It is possible that wearing a compression sleeve around a joint tightens the skin at each end of the garment. Therefore, during a given muscle contraction, relatively more skin stretch occurs when wearing the compression sleeve. This increase in skin stretch could be identified in the nervous system as a greater discharge rate of the appropriate mechanoreceptors. A greater discharge rate of cutaneous mechanoreceptors while wearing a compression sleeve could alter excitability at multiple levels of the nervous system (Gandevia et al. 2002). Inputs are combined, not just of individual afferent responses from one muscle but of pooled responses from combinations of muscles acting at a joint (Proske and Gandevia 2012). The current investigation indicates there is an excitatory input acting on the forearm flexor motoneuron pool, which is seen as an increase in long-latency reflex amplitude (Nielsen et al. 1997). The enhanced skin stretch from the compression garment may be unconsciously higher due to a greater discharge rates of cutaneous receptors. A schematic representation of possible integration sites for the compression sleeve is provided in Fig. 2.
Experiment 2: Effects of Compression on Sensory Transmission and Performance with Movement
Wearing a compression sleeve produced a similar effect as previously shown in experiment 1 across multiple types of movement tasks (Fig. 7B). When the stimulation was provided at the 3 o’clock elbow extension position, there was a significant reduction in H-reflex amplitude across all movement conditions (Fig. 7C). Figure 7, A and B provide evidence of a consistent level of input being provided to the motor and sensory axons. Similar to experiment 1 there was a robust reduction in excitability regardless of the type of task that was being completed at the 3 o’clock extension position. The combined results of the experiment provide an indication that the robust reduction in H-reflex amplitude across conditioning and movement paradigms is likely due to an increase in presynaptic inhibition of the Ia afferent (Rudomin 2009). If a postsynaptic input was responsible for the robust inhibitory effect of compression apparel, it would likely inhibit the long-latency cutaneous reflex along with Ia afferent transmission (Zehr and Stein 1999; Zehr 2002, 2006). Since the long-latency cutaneous reflex was facilitated while wearing compression apparel it is likely that compression apparel is having differential effects at multiple levels of the nervous system. Further investigations will be needed to confirm the sites of adaptation and mechanism responsible for the effect.
Phase specific alterations in sensory transmission are similar across movement tasks.
An interesting result in experiment 2 is the presence of phase-specific difference between the 3 and 6 o’clock positions for the effect of a compression garment. Phase dependency is a hallmark of the human nervous system (Zehr and Kido 2001; Zehr et al. 2003). There were no differences in reflex amplitude between CONTROL and COMPRESSION conditions across any of the movement tasks when the stimulation was provided at the 6 o’clock elbow flexion position. The reduction in H-reflex amplitude specific to the 3 o’clock position is the first evidence of a position specific effect of compression apparel on sensory feedback transmission in the upper limb.
Phase specific differences may be accounted for due to the greater amount of stretch being applied to the skin on the medial aspects of the elbow and forearm by the compression garment while at full extension (3 o’clock). Cutaneous sensory information at the elbow has previously been shown to provide accurate perceptual information about joint position and movement and this is integrated with feedback from muscle spindles to provide judgments of position and movement around the joint (Collins et al. 2005). Phase-specific differences in the relative contribution of cutaneous mechanoreceptors could differentially alter the contribution of compression apparel across movement phase. Greater skin stretch due to the compression sleeve at an extended position would increase the discharge rate of cutaneous receptors and may provide a larger contribution to an ongoing motor task. This is compared with the 6 o’clock position near maximal flexion when less skin stretch would be provided to the medial aspects of the elbow and forearm as the compression sleeve would be stretched over the olecranon. It is important to note that the current investigation did not specifically assess ischemic or thermal effects that could potentially influence H-reflex amplitude. However, such influences would not explain phase-specific effects as demonstrated across multiple movement tasks in the current investigation. Overall, this result highlights the importance of functionally specific sensory information that is constantly being incorporated into ongoing movement.
Are alterations in sensory transmission with compression related to functional improvements in performance?
The most functionally relevant finding from the current investigation is that reaching accuracy was improved when participants wore the compression sleeve compared with the control condition (Fig. 8). Participants had their deviation from the intended target measured during a discrete reaching task with stimulation provided at multiple positions. Across stimulation position there was a significant reduction in the magnitude of deviation from the intended target, which is preliminary evidence of improved task performance. While this is the first study to highlight possible adaptations in sensorimotor control while wearing compression apparel, previous investigations have noted performance benefits during tasks or conditions that include a large proprioceptive component including improved power output, joint position sense (Birmingham et al. 1998; Kraemer et al. 1998), and one-leg balance (Michael et al. 2014).
Summary
In summary, wearing a compression sleeve alters the excitability of multiple pathways within the central nervous system regardless of conditioning input or movement task. The overall effect appears to be a reduced gain of the sensory feedback transmission at functionally relevant phases of movement. The compression sleeve appears to increase precision and sensitivity at the joint where the sleeve is applied. Therefore, compression apparel may function as a filter of irrelevant mechanoreceptor information allowing the nervous system to obtain “enhanced” sensory information related to proprioception. The most functionally relevant finding from the current investigation is that reaching accuracy was improved in the COMPRESSION condition compared with CONTROL. An important distinction is that the reduced magnitude of errors was similar regardless of the position at which the stimulation was delivered. Thus providing compression around a joint may alter the availability of sensory information relevant to proprioception but further exploration is needed to refine our understanding. This is one of the first studies to show that compression apparel can alter fundamental properties of sensorimotor control within the nervous system.
GRANTS
Support for this research was provided by a doctoral fellowship from the Heart and Stroke Foundation of Canada (British Columbia and Yukon) and the Canadian Stroke Network (to T. S. Barss). Support was also provided by doctoral fellowships from the Natural Sciences and Engineering Research Council of Canada (to T. S. Barss and G. Pearcey). This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Nike Sport Research Laboratory (to E. P. Zehr).
DISCLOSURES
A portion of the funding for this project was obtained from a research contract-for-hire from NIKE Inc. One author (E. P. Zehr) has worked in the capacity as a consultant for NIKE Inc. and two authors (B. Munro and J. L. Bishop) are Senior Research Scientists at the NIKE Sport Research Laboratory. We further certify and declare that none of these competing interests had any impact on the analysis, interpretation of results, or conclusions derived within the manuscript.
AUTHOR CONTRIBUTIONS
T.S.B., G.E.P., B.M., J.L.B., and E.P.Z. conceived and designed research; T.S.B. and G.E.P. performed experiments; T.S.B. and G.E.P. analyzed data; T.S.B., G.E.P., and E.P.Z. interpreted results of experiments; T.S.B. and G.E.P. prepared figures; T.S.B. drafted manuscript; T.S.B., G.E.P., B.M., J.L.B., and E.P.Z. edited and revised manuscript; T.S.B., G.E.P., B.M., J.L.B., and E.P.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the participants for their contributions during data acquisition.
REFERENCES
- Aimonetti JM, Schmied A, Vedel JP, Pagni S. Ia presynaptic inhibition in human wrist extensor muscles: effects of motor task and cutaneous afferent activity. J Physiol Paris 93: 395–401, 1999. doi: 10.1016/S0928-4257(00)80067-4. [DOI] [PubMed] [Google Scholar]
- Alexander CM, McMullan M, Harrison PJ. What is the effect of taping along or across a muscle on motoneurone excitability? A study using triceps surae. Man Ther 13: 57–62, 2008. doi: 10.1016/j.math.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Stynes S, Thomas A, Lewis J, Harrison PJ. Does tape facilitate or inhibit the lower fibres of trapezius? Man Ther 8: 37–41, 2003. doi: 10.1054/math.2002.0485. [DOI] [PubMed] [Google Scholar]
- Beliard S, Chauveau M, Moscatiello T, Cros F, Ecarnot F, Becker F. Compression garments and exercise: no influence of pressure applied. J Sports Sci Med 14: 75–83, 2015. [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, McMurray RG. Effects of graduated compression stockings on blood lactate following an exhaustive bout of exercise. Am J Phys Med 66: 121–132, 1987. [PubMed] [Google Scholar]
- Birmingham T. Barss, Kramer JF, Inglis JT, Mooney CA, Murray LJ, Fowler PJ, Kirkley S. Effect of a neoprene sleeve on knee joint position sense during sitting open kinetic chain and supine closed kinetic chain tests. Am J Sports Med 26: 562–566, 1998. doi: 10.1177/03635465980260041601. [DOI] [PubMed] [Google Scholar]
- Born DP, Sperlich B, Holmberg HC. Bringing light into the dark: effects of compression clothing on performance and recovery. Int J Sports Physiol Perform 8: 4–18, 2013. doi: 10.1123/ijspp.8.1.4. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol 496: 857–871, 1996. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Sensory integration in the perception of movements at the human metacarpophalangeal joint. J Physiol 529: 505–515, 2000. doi: 10.1111/j.1469-7793.2000.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94: 1699–1706, 2005. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol 67: 1105–1113, 1992. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol 92: 3233–3243, 2004. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol 487: 243–251, 1995. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Refshauge KM, Collins DF.. Proprioception: peripheral inputs and perceptual interactions. In: Sensorimotor Control of Movement and Posture. Advances in Experimental Medicine and Biology, edited by Gandevia SC, Proske U, Stuart DG. Boston, MA: Springer, 2002, vol. 508, p. 61–68. [DOI] [PubMed] [Google Scholar]
- Gandhi DB, Palmar JR, Lewis B, Schraibman IG. Clinical comparison of elastic supports for venous diseases of the lower limb. Postgrad Med J 60: 349–352, 1984. doi: 10.1136/pgmj.60.703.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hill J, Howatson G, van Someren K, Leeder J, Pedlar C. Compression garments and recovery from exercise-induced muscle damage: a meta-analysis. Br J Sports Med 48: 1340–1346, 2014. doi: 10.1136/bjsports-2013-092456. [DOI] [PubMed] [Google Scholar]
- Hundza SR, Zehr EP. Muscle activation and cutaneous reflex modulation during rhythmic and discrete arm tasks in orthopaedic shoulder instability. Exp Brain Res 179: 339–351, 2007. doi: 10.1007/s00221-006-0793-z. [DOI] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491: 197–207, 1996. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171: 1–12, 2008. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Bush JA, Newton RU, Duncan ND, Volek JS, Denegar CR, Canavan P, Johnston J, Putukian M, Sebastianelli WJ. Influence of a compression garment on repetitive power output production before and after different types of muscle fatigue. Sports Med Train Rehabil 8: 163–184, 1998. doi: 10.1080/15438629809512525. [DOI] [Google Scholar]
- Kraemer WJ, Volek JS, Bush JA, Gotshalk LA, Wagner PR, Gómez AL, Zatsiorsky VM, Duarte M, Ratamess NA, Mazzetti SA, Selle BJ. Influence of compression hosiery on physiological responses to standing fatigue in women. Med Sci Sports Exerc 32: 1849–1858, 2000. doi: 10.1097/00005768-200011000-00006. [DOI] [PubMed] [Google Scholar]
- MacRae BA, Cotter JD, Laing RM. Compression garments and exercise: garment considerations, physiology and performance. Sports Med 41: 815–843, 2011. doi: 10.2165/11591420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- MacRae BA, Laing RM, Niven BE, Cotter JD. Pressure and coverage effects of sporting compression garments on cardiovascular function, thermoregulatory function, and exercise performance. Eur J Appl Physiol 112: 1783–1795, 2012. doi: 10.1007/s00421-011-2146-2. [DOI] [PubMed] [Google Scholar]
- McNair PJ, Heine PJ. Trunk proprioception: enhancement through lumbar bracing. Arch Phys Med Rehabil 80: 96–99, 1999. doi: 10.1016/S0003-9993(99)90314-3. [DOI] [PubMed] [Google Scholar]
- Michael JS, Dogramaci SN, Steel KA, Graham KS. What is the effect of compression garments on a balance task in female athletes? Gait Posture 39: 804–809, 2014. doi: 10.1016/j.gaitpost.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28: 144–160, 2003. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- Morrissey D. Proprioceptive shoulder taping. J Bodyw Mov Ther 4: 189–194, 2000. doi: 10.1054/jbmt.2000.0156. [DOI] [Google Scholar]
- Nakajima T, Mezzarane RA, Klarner T, Barss TS, Hundza SR, Komiyama T, Zehr EP. Neural mechanisms influencing interlimb coordination during locomotion in humans: presynaptic modulation of forearm H-reflexes during leg cycling. PLoS One 8: e76313, 2013. doi: 10.1371/journal.pone.0076313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. J Physiol 501: 473–484, 1997. doi: 10.1111/j.1469-7793.1997.473bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey S. Compression garments: evidence for their physiological effects. In: The Engineering of Sport, edited by Estivalet M, Brisson P. Paris: Springer, 2009, vol. 7, p. 319–328. doi: 10.1007/978-2-287-09413-2_40. [DOI] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol 587: 4139–4146, 2009. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Rudomin P. In search of lost presynaptic inhibition. Exp Brain Res 196: 139–151, 2009. doi: 10.1007/s00221-009-1758-9. [DOI] [PubMed] [Google Scholar]
- Simoneau GG, Degner RM, Kramper CA, Kittleson KH. Changes in ankle joint proprioception resulting from strips of athletic tape applied over the skin. J Athl Train 32: 141–147, 1997. [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EVL, Zehr EP. Multi-frequency arm cycling reveals bilateral locomotor coupling to increase movement symmetry. Exp Brain Res 211: 299–312, 2011. doi: 10.1007/s00221-011-2687-y. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86: 455–468, 2002. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol (1985) 101: 1783–1794, 2006. doi: 10.1152/japplphysiol.00540.2006. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140: 495–504, 2001. doi: 10.1007/s002210100857. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Frigon A, Hoogenboom N. Neural control of rhythmic human arm movement: phase dependence and task modulation of Hoffmann reflexes in forearm muscles. J Neurophysiol 89: 12–21, 2003. doi: 10.1152/jn.00416.2002. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hundza SR. Forward and backward arm cycling are regulated by equivalent neural mechanisms. J Neurophysiol 93: 633–640, 2005. doi: 10.1152/jn.00525.2004. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537: 1033–1045, 2001. doi: 10.1113/jphysiol.2001.012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Klimstra M, Johnson EA, Carroll TJ. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett 419: 10–14, 2007. doi: 10.1016/j.neulet.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. doi: 10.1016/S0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]