Abstract
Aerobic life brings with it a need to respond to external redox stress in ways that preserve key processes. Suppressor of copper sensitivity (Scs) proteins contribute to this response in some bacteria, but have poorly defined molecular functions. Furlong et al. now demonstrate that two Scs proteins from Proteus mirabilis provide a redox relay functionally equivalent to, but structurally distinct from, the Dsb proteins that orchestrate disulfide bonding in Escherichia coli, emphasizing the wide prevalence of this mechanism in bacteria.
Introduction
In the 1950s, experiments by Christian Anfinsen and colleagues on the relationship between protein folding and the correct formation of disulfide bonds were crucial in developing the fundamental concept that the amino acid sequence of a protein determines its native structure. Since then, we have learned that, in vivo, sophisticated control systems exist to orchestrate correct disulfide bond formation. Because the cytoplasm of cells maintains a reducing environment, cysteine residues remain reduced, and disulfide bonds are not formed. Instead, disulfide bonds are formed in specialized compartments: the endoplasmic reticulum in eukaryotes and the periplasm in Gram-negative bacteria. The bacterial system that orchestrates this process has been best characterized for Escherichia coli, in which the Dsb family of proteins (1) operates to oxidize disulfide bonds within newly formed proteins (the DsbA/DsbB system) or to reduce and reform disulfide bonds in proteins that have failed to fold correctly (the DsbD/DsbC/DsbG system).
Homologs of Dsb proteins can be found encoded in the genomes of many bacteria, but wide sequence variations often make their biological roles unclear. A classic example is found in a group of proteins referred to as Suppressor of copper sensitivity (Scs) proteins. First identified in Salmonella typhimurium, the Scs proteins confer resistance to copper stress and are co-located as a four-gene cassette (ScsABCD) (2). Intriguingly, these proteins possess many characteristic features of the Dsb proteins, including paired-Cys CXXC motifs and widespread use of thioredoxin domains. Confounding these similarities, however, individual Scs proteins vary widely between organisms. For example, ScsC from Caulobacter crescentus is a dimeric isomerase like DsbC (3) and can partner with ScsB. ScsC from S. typhimurium is monomeric, however, with no isomerase activity (4), and ScsC from Proteus mirabilis is a trimeric isomerase (5). Do these systems operate in ways similar to the Dsb system of E. coli? Do they have similar partner proteins? Or do they have different substrates and roles unrelated to disulfide bond formation? A new article by Furlong et al. (6) presents a beautiful analysis that answers some of these questions, providing strong evidence that the Scs proteins are functional replacements of the Dsb system and presenting structural insights that demonstrate how ScsC diversity across species allows the fundamental mechanisms to be maintained.
In their study, Furlong et al. (6) focus on the P. mirabilis proteins ScsB and ScsC. ScsB, like E. coli DsbD, has a central transmembrane domain (β) with N-terminal (α) and C-terminal (γ) periplasmic domain extensions. In E. coli, electrons pass from the cytoplasmic thioredoxin/thioredoxin reductase system via DsbDβ to DsbDγ and then to DsbDα, which ultimately reduces and activates the isomerase DsbC. To test the functional relationship between ScsBα and ScsC, the authors purified the ScsB α-domain and used the refolding of disulfide-scrambled RNase A to assay the isomerase activity of ScsC. Only reduced (but not oxidized) ScsC could shuffle the RNase A disulfides. When reduced ScsBα was added to oxidized ScsC, however, ScsC was reduced, and its refolding activity was restored. These experiments showed that the electron transfer is unidirectional, thus preventing backflow of electrons into the cytoplasm and establishing that these proteins do indeed form a functional redox relay.
Furlong et al. (6) then determined the crystal structure of reduced ScsBα (Fig. 1A). This brought a major surprise, in that ScsBα turns out to be a duplicated version of DsbDα despite almost no sequence identity. Whereas DsbDα comprises a single immunoglobulin (Ig)-fold domain with a CXXC active site motif (7), ScsBα has two tandem Ig-fold domains, one of which (subdomain A) has a paired-Cys CXXXC motif. ScsBα subdomain A and DsbDα have very similar folds and similarly positioned catalytic cysteines. Attention then turned to the question of how ScsBα interacts with its redox partner ScsC. Using inactive variants of ScsC and ScsBα, a stable 3:1 complex (ScsC trimer + ScsBα monomer) could be prepared and analyzed by solution scattering. Here, innovative use was made of small-angle neutron scattering (SANS) with contrast matching (8), a technique more commonly applied to protein–nucleic acid complexes. Using deuterated ScsBα complexed with nondeuterated ScsC, the deuterium level of the solvent can be manipulated to “match out” one or other component of the complex and so obtain a useful, albeit low-resolution, model of the assembly.
Figure 1.
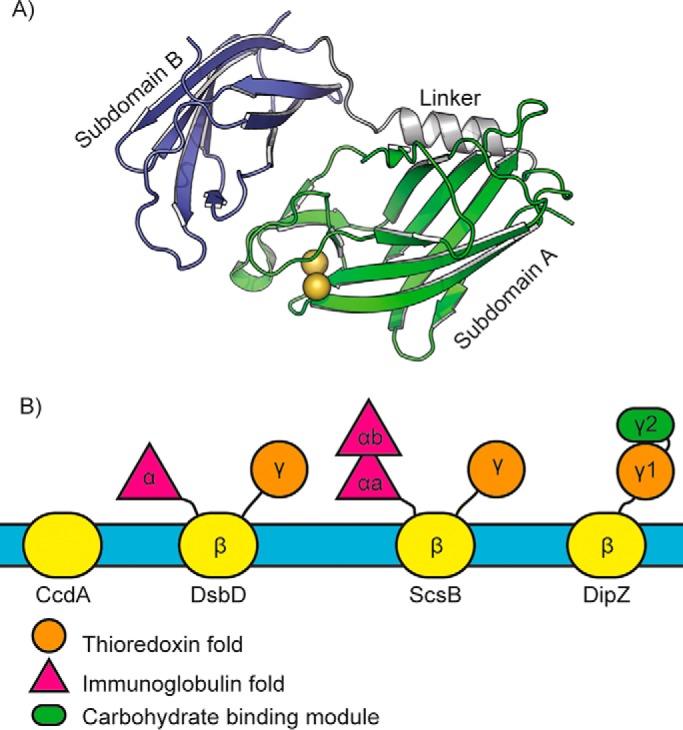
Structural variability in redox relay proteins. A, the ScsBα domain, shown here, comprises two Ig-fold subdomains, in contrast to DsbDα, which has only one. Subdomain A contains the two redox-active cysteine residues (gold spheres). B, schematic depiction of the variation in the periplasmic domains attached to the electron transporters Rhodobacter capsulatus CcdA, E. coli DsbD, P. mirabilis ScsB, and M. tuberculosis DipZ.
With an ScsB–ScsC redox relay now established for both P. mirabilis and C. crescentus, it is likely that it is present in all organisms with the four-gene scs cassette and functions as the Dsb system in E. coli does to orchestrate disulfide bond formation. How does this relate to the original Scs annotation? Due to the strong oxidizing nature of copper, sensitivity to growth in the presence of copper has often been used to identify proteins involved in the maintenance of redox pathways in the periplasm. In a sense, then, the Scs annotation for these proteins could be seen as a surrogate for the wider role of redox control.
What this research highlights is that functional mechanisms can be maintained despite apparently major structural differences. Sequence analysis indicates that P. mirabilis, C. crescentus, and S. typhimurium have similar ScsB α-domains, yet their SbsC partners are trimeric, dimeric, and monomeric, respectively. The modeled ScsBα–ScsC interaction here suggests that the second Ig-subdomain of ScsBα may provide additional interactions that enable productive complex formation with ScsCs irrespective of oligomeric state, although this has yet to shown by further structural studies. Looking more widely at the transmembrane electron transporters and their homologs, it is intriguing that so much variation exists (Fig. 1B). Some, such as CcdA, have no appended domains (9), whereas others have N- or C-terminal ectodomains. In Mycobacterium tuberculosis, DipZ (a DsbD homolog) has a C-terminal domain comprising a thioredoxin-like subdomain combined with a carbohydrate-binding module and may act in cell wall modulation (10). S. typhimurium ScsB, with its tandem-Ig α-domain and thioredoxin-like γ-domain, reduces ScsC, but also a cell-envelope peroxiredoxin and possibly other substrates (3). This diversity of form and function is likely evident in many bacteria, with much still to be discovered.
Footnotes
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1. Heras B., Shouldice S. R., Totsika M., Scanlon M. J., Schembri M. A., and Martin J. L. (2009) DSB proteins and bacterial pathogenicity. Nat. Rev. Microbiol. 7, 215–225 10.1038/nrmicro2087 [DOI] [PubMed] [Google Scholar]
- 2. Gupta S. D., Wu H. C., and Rick P. D. (1997) A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J. Bacteriol. 179, 4977–4984 10.1128/jb.179.16.4977-4984.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho S.-H., Parsonage D., Thurston C., Dutton R. J., Poole L. B., Collet J.-F., and Beckwith J. (2012) A new family of membrane electron transporters and its substrates, including a new cell envelope peroxiredoxin, reveal a broadened reductive capacity of the oxidative bacterial cell envelope. mBio 3, e00291–11 10.3391/mbi.2012.3.1.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepherd M., Heras B., Achard M. E., King G. J., Argente M. P., Kurth F., Taylor S. L., Howard M. J., King N. P., Schembri M. A., and McEwan A. G. (2013) Structural and functional characterization of ScsC, a periplasmic thioredoxin-like protein from Salmonella enterica serovar Typhimurium. Antioxid. Redox Signal. 19, 1494–1506 10.1089/ars.2012.4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furlong E. J., Lo A. W., Kurth F., Premkumar L., Totsika M., Achard M. E. S., Halili M. A., Heras B., Whitten A. E., Choudhury H. G., Schembri M. A., and Martin J. L. (2017) A shape-shifting redox foldase contributes to Proteus mirabilis copper resistance. Nat. Commun. 8, 16065 10.1038/ncomms16065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furlong E. J., Choudhury H. G., Kurth F., Duff A. P., Whitten A. E., and Martin J. L. (2018) Disulfide isomerase activity of the dynamic, trimeric Proteus mirabilis ScsC protein is primed by the tandem immunoglobulin-fold domain of ScsB. J. Biol. Chem. 293, 5793–5805 10.1074/jbc.RA118.001860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haebel P. W., Goldstone D., Katzen F., Beckwith J., and Metcalf P. (2002) The disulphide bond isomerase DsbC is activated by an immunoglobulin-fold thiol oxidoreductase: Crystal structure of the DsbC-DsbDα complex. EMBO J. 21, 4774–4784 10.1093/emboj/cdf489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heller W. T. (2010) Small angle neutron scattering and contrast variation: A powerful combination for studying biological structures. Acta Crystallogr. D Biol. Crystallogr. 66, 1213–1217 10.1107/S0907444910017658 [DOI] [PubMed] [Google Scholar]
- 9. Williamson J. A., Cho S.-H., Ye J., Collet J.-F., Beckwith J. R., and Chou J. J. (2015) Structure and multistate function of the transmembrane electron transporter CcdA. Nat. Struct. Mol. Biol. 22, 809–814 10.1038/nsmb.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstone D. C., Metcalf P., and Baker E. N. (2016) Structure of the ectodomain of the electron transporter Rv2874 from Mycobacterium tuberculosis reveals a thioredoxin-like domain combined with a carbohydrate binding module. Acta Crystallogr. D Struct. Biol. 72, 40–48 10.1107/S2059798315021488 [DOI] [PubMed] [Google Scholar]


