Abstract
The intramolecular hydroamination of a guanidine on an eneyne unit affords a guanidine-substituted diene capable of reacting with dienophiles. These substrates undergo [4+2]-cycloaddition reactions to generate a series of complex cyclic- and spirocyclic-guanidines. Select substrates can further undergo a ring opening-elimination cascade that ultimately reveals a vinyl-2-aminoimidazole. As such this cascade reaction may find application in the synthesis of oroidin-type natural products and their analogues
Keywords: Cycloaddition, Heterocycle, Alkaloids, 2-aminoimidazoles, Hydroamination
Graphical abstract

1. Introduction
Marine sponges have been a prolific source of pyrrole-2-aminoimidazole alkaloids (PAIs).1,2 Members of this family have been prized for their biological activities, especially as anti-tumors agents, anti-microbial or anti-biofilm agents.3 Besides their potential utility as therapeutics leads, the community has been fascinated with the genesis of the more structurally complex members of this family from the simple building blocks e.g. oroidin and hymenedin (Figure 1). Initial hypotheses suggested that these dimeric products were forged by electrocyclic reactions ([2+2] or [4+2] cycloadditions) of the vinyl-2-aminoimidazole fragment or from stepwise addition/isomerization sequences of the rapidly interconverting iminium ion tautomers of the 2-aminoimidazole core.4
Figure 1.
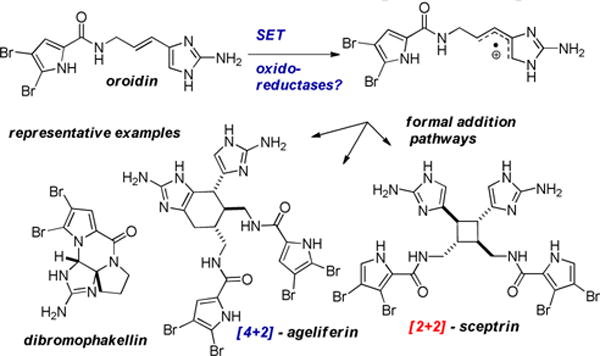
Biogenesis of complex pyrrole-2-aminoimidazole alkaloids form simple precursors.
However, Molinksi and Romo have recently shown that these cyclized and/or dimerized homologues arise from an enzymatic process. More specifically through the generation of a radical cation, via SET to an oxido-reductase.
We have previously demonstrated that propargylguanidines can be regiodivergently cyclized to the 5-exo product with Ag(I) or to the 6-endo product with Rh(II) catalysis (Scheme 1A).5 We became interested in extending the utility of this propargylguanidine hydroamination sequence to rapidly construct complex polycyclic guanidine scaffolds via the intermediacy of a guanidine-substituted diene, akin to the oroidin biosynthetic manifold. We envisioned that extension of the alkyne to an enyne would generate substrates at the same oxidation state as the vinyl-2-aminoimidazoles, albeit transposed (Scheme 1B). While the reactivity of many heteroatom substituted dienes have been studied, this represents an unknown class of guanidine-substituted dienes.
Scheme 1.
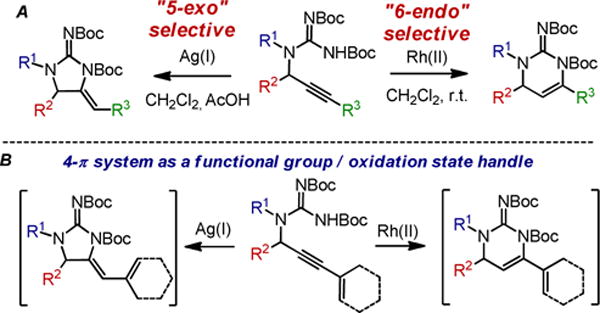
Applications of ene-ynes in the the guanidine hydroamination sequence.
Herein we explore the reactivity of these dienes to generate polycyclic guanidinium ion containing compounds and compounds that are structurally aligned with the PAIs. These complex structures arise from simple linear precursors via a cascade hydroamination-[4+2]-cycloaddition sequence.
2. Results and discussion
2.1 6-endo-selective hydroamination/[4+2] cycloaddition
We began by examining the hydraomination-[4+2]-cycloaddition cascade reaction with guanidine 1a (Scheme 2). Exposure of 1a to Rh(II) cleanly gave the 6-endo product 2a in 70% yield. This guanidine-substituted diene cleanly reacted with 4-phenyl-1,2,4-triazole-3,5-dione at room temperature to give 3a in 94% yield. Exploratory reactions showed that the diene 2a does not spontaneously react with electron poor dieneophiles (e.g. acrylates, quinones or fumarates) suggesting that the diacylated guanidine renders the diene relatively electron poor. The hydroamination-cycloaddition cascade can be carried out in a one-pot sequence to afford 3a in 74% isolated yield. Deprotection of the guanidine with trifluoroacetic acid followed by salt exchange gave 4a as a crystalline hydrochloride, the structure of which was confirmed by X-ray crystallography.
Scheme 2.
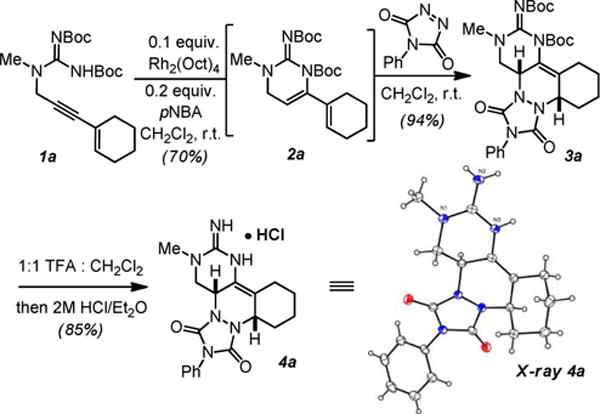
6-endo-selective hydroamination / [4+2]-cycloaddition sequence to access polyclicguanidines.
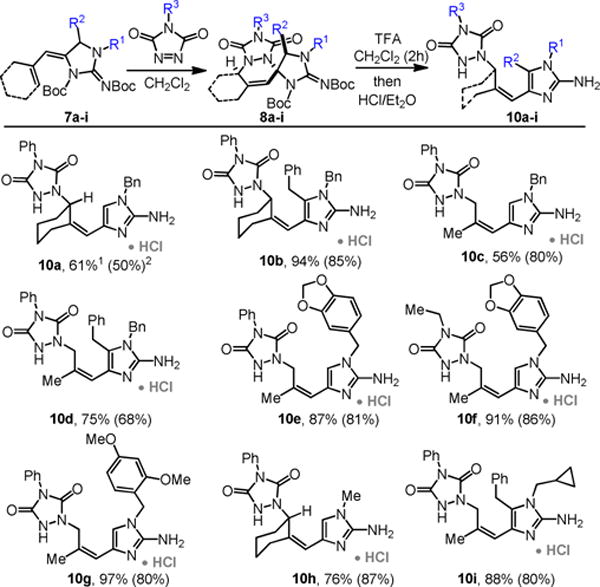
This reaction development permitted the synthesis of a focused library of these polycyclic guanidines (Scheme 3). Substitutions on the guanidine nitrogen (N1) are universally tolerated. As demonstrated, both cyclic and acyclic ene-ynes participate to give tetra- or tri-cyclic guanidines respecttively (e.g. 4b-4g). Other triazolinediones can be employed (4g). Substituents on the dihydroaminopyrimidine effectively control the approach of the dienophile to give the adducts 4h–i as single diastereomers.
Scheme 3.
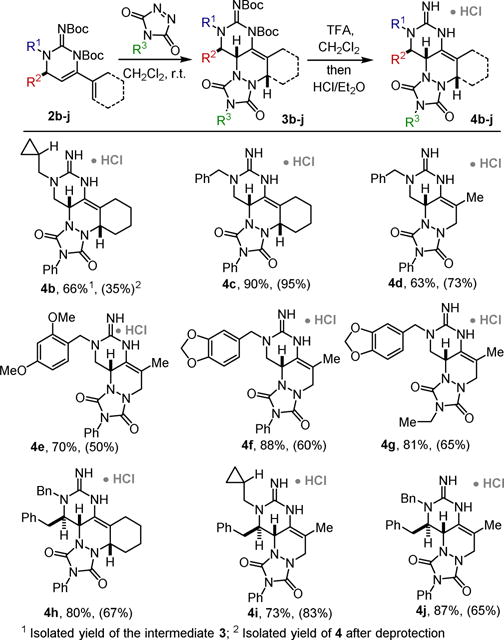
Scope of the 6-endo-selective hydroamination / [4+2]-cycloaddition sequence.
2.2 5-exo-selective hydroamination/[4+2] cycloaddition
We then investigated the [4+2]-cycloaddition reaction on the 5-exo-dig derived dienes (Scheme 4). Interestingly hydroamination of 1c under Ag(I) conditions gave 7a in poor yield as the minor regiosiomer All other substrates in this series cyclize with >10:1 selectivity favoring the expected 5-exo-dig product. Reaction of 7a with 4-phenyl-1,2,4-triazole-3,5-dione proceeded cleanly to give the protected spirocyclic guanidine 8a. Deprotection of 8a with TFA for 30 min followed by salt exchange gave 9a in good yield, the structure of which was ultimately confirmed by X-ray crystallography. On working with this compound we ultimately observed that it decomposed when stored in solution, to give a compound that appeared to be an isomer of 9a as suggested by an identical mass (ultimately proven to be compound 10a in Scheme 6).
Scheme 4.
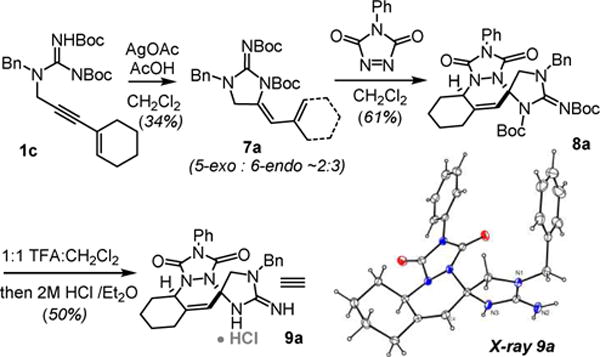
5-exo-selective hydroamination / [4+2]-cycloaddition sequence.
Scheme 6.
Scope of the [4+2]-Cycloaddition-fragmentation sequence to access vinyl-2-aminoimidazoles.
Insight into this decomposition was gained when studying the same reaction on substrate 1h (Scheme 5). Cyclization of 1h in the presence of Ag(I) gave 7b in good yield as a single regioisomer. Substitution at the alpha-position of the propargylguanidine favors the kinetically preferred 5-exo-dig product. Cycloaddition of the resultant diene gives 8b as a single diastereomer. Deprotection of 8b with TFA does not lead to the deprotected spirocycle but instead the allylic aminal fragments to reveal the vinyl-2-aminoimidazole 10b.
Scheme 5.
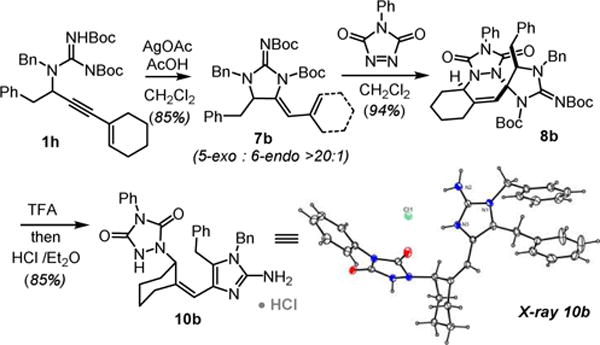
5-exo-selective hydroamination / [4+2]-cycloaddition-fragmentation sequence.
The structure of 10b was confirmed by X-ray crystallography and the spectral data were consistent with those of the isomer obtained from the decomposition of 9a. Presumably the more substituted 2-aminoimidazole ring in 8b fragments faster via stabilization of the intermediate N-acyliminium ion.
It was subsequently found that deprotection of the cycloadducts with 1:1 TFA:CH2Cl2 for >2 hours universally provides the ring-opened vinyl-2-aminoimidazoles 10a–i in good yield (Scheme 6).6
Having noted that the di-Boc-guanidine substituted dienes react with very reactive dienophiles, we also examined their participation in the acylnitroso Diels-Alder reaction (Scheme 7). Using Read de Alaniz’s conditions, we were able to generate the acylnitroso intermediate in-situ by oxidation of the N-hydroxycarbamate 11.7 This smoothly underwent cycloaddition with the dienes 2a,f,g to give the adducts 12a–c in moderate to good yield. These cycloadditions afford a single regio-isomer, confirmed in 12a by HMBC correlation of the C12 methine proton to the carbonyl of the benzylcarbamate. This is consistent with the more electron rich end of the diene reacting with the nitrogen of the acylnitroso system as previously demonstrated.7b These intermediates can be deprotected without reduction of the internal alkene to give 13a–c.
Scheme 7.
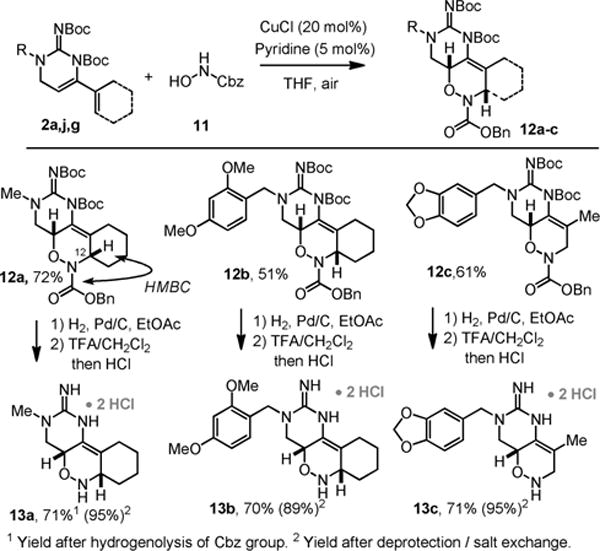
[4+2]-Cycloaddition sequence with an acylnitroso-dienophile.
We also examined a hydroamination-Michael addition-[4+2]-cycloaddition sequence (Scheme 8). Reaction of the guanidine 14 with Ag(I) delivers the bicyclic guanidine substituted diene 15 in good yield. Reaction with 4-phenyl-1,2,4-triazole-3,5-dione gave the spirocycle 16. This example further serves to illustrate that guanidine substituted dienes that are only mono-acylated are competent dienes as well.
Scheme 8.
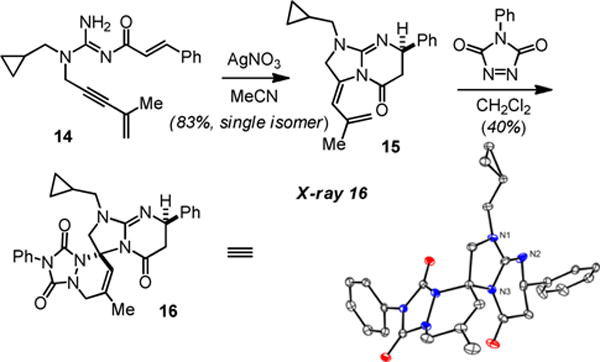
Hydroamination-Michael addition-[4+2]-cycloaddition sequence.
3. Conclusion
In conclusion, we have demonstrated that ene-yne containing di-Boc-guanidines generally undergo selective Rh(II)-mediated 6-endo-dig or Ag(I)-mediated 5-exo-dig hydroamination. The hydroamination products behave as electron poor dienes but can participate in [4+2]-cycloadditions with activated dienophiles. Cycloaddition of the 5-exo-dig products with a triazolinediones generates a spirocyclic allylic aminal which is prone to elimination to reveal a vinyl-2-aminoimidazole. As such, this sequence may find use in the preparation of PAI natural products or analogues. Given the successful reaction of monoacylsubstituted dienes, we are currently examining the ability of these more electron-rich systems to engage a wider variety of dieneophiles to access other complex and interesting structures.
4. Experimental section
4.1. General Experimental Considerations
Unless otherwise noted all starting materials were either known compounds or were obtained from commercial sources and used without purification. All reactions requiring anhydrous conditions were performed under a positive pressure of nitrogen using flame-dried glassware. Rhodium (II) octanoate, Silver nitrate, and Silver acetate were purchased from Sigma-Aldrich. Dichloromethane (CH2Cl2), acetonitrile (CH3CN), toluene (C6H5CH3), tetrahydrofuran (THF) and diethyl ether (Et2O) were degassed with argon and passed through a solvent purification system (J.C. Meyer of Glass Contour) containing either alumina or molecular sieves. Flash chromatography was performed by CombiFlash Rf (TELEDYNE ISCO) or on Merk silica gel Kieselgel 60 (230–400 mesh) from EM science with the indicated solvent.
1H NMR spectra were recorded on Varian Unity-300, Inova-400, or VXR-500 MHz spectrometers as indicated. The chemical shifts (δ) of proton resonances are reported relative to CDCl3, DMSO-d5, or CD3OD using the following format: chemical shift [multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, app = apparent), coupling constant(s) (J in Hz), integral].1,2 13C NMR spectra were recorded at 75, 100, or 125 MHz. The chemical shifts of carbon resonances are reported relative to the deuterated solvent peak.
Infrared spectra were recorded on a Nicolet 380-FT IR spectrometer fitted with a SmartOrbit sample system. All absorptions are reported in cm-1 relative to polystyrene (1601 cm-1).
Mass spectra were obtained at the University of Utah CIF on a Micromass Quattro II (ESI/APCI) for LRMS or an LCT XE premier (ESI/APCI-TOF) for HRMS.
4.2 Synthetic Procedures and Characterization Data
4.2.1.General Procedure 1 for the guanylation of propargyl amines S-2a–i to form 1a–i
To a stirring solution of S-methyl-N,N′-diBocpseudothiourea (1.1 equiv), HgO (1.1 equiv), and triethylamine (3.0 equiv) in CH2Cl2 was added the appropriate propargyl amine (1.0 equiv). The reaction was stirred at room temperature until complete as judged by TLC. The reaction mixture was filtered through a short plug of Celite, concentrated, and purified by column chromatography to yield the propargylguanidine.
4.2.1.1. 1-(3-(cyclohex-1-en-1-yl)prop-2-yn-1-yl)-1-methyl-di-Boc-guanidine (1a)
The general procedure 1 was used to yield the propargylguanidine as a pale yellow solid (0.549 g, 71% yield). Rf = 0.45 (20% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 10.07 (s. 1H), 6.09 (s, 1H), 4.39 (s, 2H), 3.09 (s, 3H), 2.09 (m, 4H), 1.61 (m, 2H), 1.57 (m, 2H), 1.49 (s, 18H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 163.3, 153.6, 150.2, 137.4, 120.5, 87.5, 82.7, 79.3, 77.6, 36.4, 32.2, 28.9, 28.4, 28.3, 26.5, 22.5, 21.8 ppm; LRMS (ESI) Calculated for C21H34N3O4 m/z (M+H): 392.3, Obsd 392.4.
4.2.1.2. 1-(3-(cyclohex-1-en-1-yl)prop-2-yn-1-yl)-1-(cyclopropylmethyl)-di-Boc-guanidine (1b)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 67% yield (0.573 g). Rf = 0.68 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 9.82 (s, 1H), 6.06 (s, 1H), 4.51 (s, 2H), 3.46 (s, 2H), 2.00 (m, 4H), 1.61 (m, 2H), 1.57 (m, 2H), 1.49 (s, 18H), 1.07 (m, 1H), 0.56 (m, 2H), 0.31 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.3, 154.6, 150.5, 135.1, 120.1, 86.8, 81.7, 80.6, 79.2, 52.1, 38.2, 29.0, 28.1, 28.0, 25.5, 22.1, 21.4, 8.9, 3.7 ppm; LRMS (ESI) Calculated for C24H38N3O4 m/z (M+H): 432.3, Obsd. 432.4.
4.2.1.3. 1-benzyl-1-(3-(cyclohex-1-en-1-yl)prop-2-yn-1-yl)-di-Boc-guanidine (1c)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 71% yield (0.657 g). Rf = 0.55 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 9.91 (s, 1H), 7.33-7.26 (m, 5H), 6.07 (s, 1H), 4.83 (s, 2H), 4.21 (s, 2H), 2.08 (m, 4H), 1.62 (m, 2H), 1.57 (m, 2H), 1.50 (s, 18H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 155.4, 153.8, 150.7, 136.1, 135.6, 128.7, 128.5, 127.8, 120.3, 87.5, 82.3, 80.3, 79.8, 51.4, 38.7, 29.2, 28.3, 25.7, 22.4, 21.6 ppm; LRMS (ESI) Calculated for C27H38N3O4 m/z (M+H): 468.3, Obsd. 468.4.
4.2.1.4. 1-(3-(cyclohex-1-en-1-yl)prop-2-yn-1-yl)-1-(2,4-dimethoxybenzyl)-di-Boc-guanidine (1d)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 83% yield (0.703 g). Rf = 0.71 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 9.97 (s, 1H), 7.33-7.27 (m, 5H), 5.27 (s, 1H), 5.22 (s, 1H), 4.83 (s, 2H), 4.24 (s, 2H), 1.86 (s, 3H), 1.51 (s, 9H), 1.49 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.4, 155.3, 150.6, 135.8, 128.6, 128.2, 127.7, 126.2, 122.4, 86.6, 82.4, 82.1, 79.6, 51.3, 38.4, 28.4, 28.3, 23.3 ppm; LRMS (ESI) Calculated for C24H34N3O4 m/z (M+H): 428.3, Obsd. 428.3.
4.2.1.5. 1-(3-(cyclohex-1-en-1-yl)prop-2-yn-1-yl)-1-(2,4-dimethoxybenzyl)-di-Boc-guanidine (1e)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 68% yield (0.700 g). Rf = 0.56 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 9.44 (s, 1H), 7.22 (m, 1H), 6.46 (m, 2H), 6.07 (s, 1H), 4.59 (br, 2H), 4.25 (s, 2H), 3.83 (s, 3H), 3.78 (s, 3H), 2.08 (m, 4H), 1.62 (m, 2H), 1.57 (m, 2H), 1.51 (s, 9H), 1.50 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.3, 160.8, 158.1, 152.5, 150.2, 134.6, 131.4, 120.0, 115.4, 103.9, 98.2, 86.3, 81.1, 80.6, 78.4, 77.5, 59.8, 55.0, 54.9, 46.2, 28.8, 27.9, 27.8, 25.2, 21.9, 21.2 ppm; LRMS (ESI) Calculated for C29H42N3O6 m/z (M+H): 528.3, Obsd. 528.3.
4.2.1.6. 1-(benzo[d][1,3]dioxol-5-ylmethyl)-1-(4-methylpent-4-en-2-yn-1-yl)-di-Boc-guanidine (1f)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 81% yield (0.756 g). Rf = 0.48 (20% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 10.0 (s, 1H), 6.83-6.74 (m, 3H), 5.93 (s, 2H), 5.28 (s, 1H), 5.23 (s, 1H), 4.72 (s, 2H), 4.23 (s, 2H), 1.87 (s, 9H), 1.50 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.3, 155.1, 150.5, 147.9, 147.2, 129.3, 126.1, 122.3, 121.8, 108.1, 101.0, 86.5, 82.2, 82.0, 79.5, 51.0, 38.1, 28.1, 28.0, 23.2 ppm; LRMS (ESI) Calculated for C25H34N3O6 m/z (M+H): 472.2, Obsd. 472.4.
4.2.1.7. 1-(2,4-dimethoxybenzyl)-1-(4-methylpent-4-en-2-yn-1-yl)-di-Boc-guanidine (1g)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 50% yield (0.483 g). Rf = 0.63 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 9.42 (s, 1H), 7.20 (m, 1H), 6.45 (m, 2H), 5.27 (s, 1H), 5.21 (s, 1H), 4.59 (s, 2H), 4.26 (s, 2H), 3.84 (s, 3H), 3.79 (s, 3H), 1.87 (s, 3H), 1.51 (s, 18H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.4, 160.9, 158.2, 150.4, 131.5, 126.2, 121.8, 115.4, 104.1, 98.4, 85.8, 82.7, 81.3, 78.7, 55.1, 46.5, 36.4, 28.0, 27.9, 23.2 ppm; LRMS (ESI) Calculated for C26H38N3O6 m/z (M+H): 488.3, Obsd. 488.5.
4.1.2.8. 1-(cyclopropylmethyl)-1-(5-methyl-1-phenylhex-5-en-3-yn-2-yl)-di-Boc-guanidine (1h)
Prepared according to the general procedure 1, to give the propargylguanidine as white solid in 22% yield (0.210 g). Rf = 0.50 (20% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 8.97 (s, 1H), 7.35-7.21 (m, 5H), 5.20 (s, 1H), 5.18 (s, 1H), 3.52 (m, 1H), 3.34 (dd, J = 14.7, 6.9 Hz, 1H), 3.27 (dd, J = 12.7, 4.9 Hz, 1H), 2.98 (dd, J = 12.7, 9.8 Hz, 1H), 1.80 (s, 3H), 1.51 (s, 9H), 1.49 (s, 9H), 1.24 (m, 1H), 0.59 (m, 2H), 0.35 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.9, 153.2, 150.8, 137.3, 129.9, 128.3, 126.9, 126.2, 122.1, 88.0, 85.7, 81.9, 79.3, 53.5, 51.5, 41.4, 28.3, 28.2, 23.3, 10.8, 5.7, 4.3 ppm; LRMS (ESI) Calculated for C28H40N3O4 m/z (M+H): 482.3, Obsd. 482.5.
4.1.2.9. 1-benzyl-1-(4-(cyclohex-1-en-1-yl)-1-phenylbut-3-yn-2-yl)-di-Boc-guanidine (1i)
Prepared according to the general procedure 1, to give the propargylguanidine as pale yellow solid in 78% yield (0.861 g). Rf = 0.51 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 8.85 (s, 1H), 7.36-7.18 (m, 10H), 5.94 (s, 1H), 4.97 (br, 1H), 4.62 (d, J = 16.7 Hz, 1H), 3.15 (dd, J = 12.7, 4.4 Hz, 1H), 2.76 (dd, J = 12.2, 9.8 Hz, 1H), 2.02 (m, 2H), 1.95 (m, 2H), 1.53 (m, 4H), 1.48 (s, 9H), 1.47 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.5, 154.3, 150.3, 138.4, 137.2, 128.6, 127.3, 127.1, 126.5, 126.3, 125.8, 122.3, 88.7, 84.8, 82.6, 78.9, 49.8, 46.3, 28.5, 28.3, 27.4, 24.7, 22.8, 21.7 ppm; LRMS (ESI) Calculated for C34H44N3O4 m/z (M+H): 558.3, Obsd. 558.5.
4.2.2.General Procedure 2 for the 6-endo cyclization of the Propargyl Guanidines 1a–g
To a stirring solution of the appropriate propargylguanidine (1.0 mmol) in CH2Cl2 (0.07 M) was added rhodium(II) octanoate dimer (10 mol%) and 4-nitrobenzoic acid (0.2 equiv). The reaction was stirred at room temperature until the reaction was complete by TLC. After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to yield 6-endo-dig cyclized guanidine.
4.2.2.1. tert-butyl (E)-2-((tert-butoxycarbonyl)imino)-6-(cyclohex-1-en-1-yl)-3-methyl-3,4-dihydropyrimidine-1(2H)-carboxylate (2a)
The general procedure 2 was used to yield 6-endo-dig cyclized guanidine as a white solid (6-endo-dig:5-exo-dig = >20:1), (0.274 g, total 70% yield). Rf = 0. 28 (60% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.35 (s. 1H), 5.61 (t, J = 5.2 Hz, 1H), 3.70 (d, J = 4.3 Hz, 2H), 3.05 (s, 3H), 2.16 (m, 4H), 1.69 (m, 2H), 1.60 (m, 2H), 1.54 (s, 9H), 1.41 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.4, 150.5, 149.6, 143.2, 132.4, 126.5, 110.0, 82.3, 78.7, 47.3, 37.7, 28.3, 28.1, 25.7, 25.6, 22.7, 22.3 ppm; LRMS (ESI) Calculated for C21H34N3O4 m/z (M+H): 392.3, Obsd. 392.3.
4.2.2.2. tert-butyl (E)-2-((tert-butoxycarbonyl)imino)-6-(cyclohex-1-en-1-yl)-3-(cyclopropylmethyl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2b)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 31% yield (6-endo-dig:5-exo-dig = >20:1), (0.134 g). Rf = 0.74 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.37 (s, 1H), 5.64 (t, J = 5.1 Hz, 1H), 3.78 (d, J = 4.9 Hz, 1H), 3.33 (d, J = 6.8 Hz, 1H), 2.17 (m, 4H), 1.70 (m, 2H), 1.60 (m, 2H), 1.54 (s, 9H), 1.41 (s, 9H), 1.06 (m, 1H), 0.50 (m, 2H), 0.20 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.5, 150.4, 149.5, 143.2, 132.2, 126.3, 110.3, 82.0, 78.4, 53.4, 44.4, 28.2, 27.9, 25.6, 25.5, 22.6, 22.1, 9.0, 3.4 ppm; LRMS (ESI) Calculated for C24H38N3O4 m/z (M+H): 432.3, Obsd. 432.4.
4.2.2.3. tert-butyl (E)-3-benzyl-2-((tert-butoxycarbonyl)imino)-6-(cyclohex-1-en-1-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2c)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 76% yield (6-endo-dig: 5-exo-dig = >20:1), (0.327 g). Rf = 0.25 (20% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.31-7.21 (m, 5H), 6.41 (s, 1H), 5.51 (t, J = 5.1 Hz, 1H), 4.70 (s, 2H), 3.55 (d, J = 4.1 Hz, 2H), 2.19 (m, 2H), 2.15 (m, 2H), 1.69 (m, 2H), 1.61 (m, 2H), 1.55 (s, 9H), 1.43 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.5, 151.0, 149.6, 143.3, 136.2, 132.2, 128.6, 127.9, 127.5, 126.5, 110.5, 82.3, 78.7, 52.1, 43.6, 28.2, 28.0, 25.6, 25.5, 22.6, 22.1 ppm; LRMS (ESI) Calculated for C27H38N3O4 m/z (M+H): 468.3, Obsd. 468.4.
4.2.2.4. tert-butyl (E)-3-benzyl-2-((tert-butoxycarbonyl)imino)-6-(prop-1-en-2-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2d)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 79% yield (6-endo-dig: 5-exo-dig = >20:1), (0.338 g). Rf = 0.57 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.30-7.21 (m, 5H), 5.72 (s, 1H), 5.65 (t, J = 4.3 Hz, 1H), 5.07 (s, 1H), 4.71 (s, 2H), 3.58 (d, J = 3.4 Hz, 2H), 1.93 (s, 3H), 1.55 (s, 9H), 1.43 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.4, 150.9, 149.4, 142.9, 138.3, 136.1, 128.6, 127.8, 127.5, 115.1, 112.9, 82.9, 78.7, 52.1, 43.6, 28.2, 27.8, 20.0 ppm; LRMS (ESI) Calculated for C24H34N3O4 m/z (M+H): 428.3, Obsd. 428.3.
4.2.2.5. tert-butyl (E)-2-((tert-butoxycarbonyl)imino)-3-(2,4-dimethoxybenzyl)-6-(prop-1-en-2-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2e)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a pale yellow solid in 70% yield (6-endo-dig: 5-exo-dig = >20:1), (0.341 g). Rf = 0.41 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.13 (m, 1H), 6.41 (m, 2H), 5.71 (s, 1H), 5.71 (s, 1H), 5.62 (t, J = 5.5 Hz, 1H), 5.05 (s, 1H), 4.64 (s, 2H), 3.77 (s, 3H), 3.75 (s, 3H), 3.60 (d, J = 5.0 Hz, 2H), 1.91 (s, 3H), 1.54 (s, 9H), 1.43 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.5, 160.4, 158.6, 150.9, 149.5, 142.9, 138.4, 130.6, 116.6, 114.9, 113.3, 104.3, 98.3, 82.6, 78.4, 55.3, 55.2, 46.7, 43.4, 28.3, 27.9, 20.0 ppm; LRMS (ESI) Calculated for C26H38N3O6 m/z (M+H): 488.3, Obsd. 488.3.
4.2.2.6. tert-butyl (E)-3-(benzo[d][1,3]dioxol-5-ylmethyl)-2-((tert-butoxycarbonyl)imino)-6-(prop-1-en-2-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2f)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a pale yellow solid in 76% yield (6-endo-dig: 5-exo-dig = 10:1), (0.358 g). Rf = 0.50 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.74-6.70 (m, 3H), 5.92 (s, 2H), 5.71 (s, 1H), 5.67 (t, J = 5.0 Hz, 1H), 5.06 (s, 1H), 4.60 (s, 2H), 3.58 (d, J = 3.9 Hz, 2H), 1.93 (s, 3H), 1.55 (s, 9H), 1.43 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.3, 150.8, 149.3, 147.9, 147.1, 142.8, 138.3, 129.8, 121.4, 115.0, 112.9, 108.4, 108.2, 101.0, 82.8, 78.6, 51.8, 28.2, 27.8, 19.9 ppm; LRMS (ESI) Calculated for C25H34N3O6 m/z (M+H): 472.2, Obsd. 472.3.
4.2.2.7. tert-butyl (E)-3,4-dibenzyl-2-((tert-butoxycarbonyl)imino)-6-(cyclohex-1-en-1-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2g)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 96% yield (6-endo-dig: 5-exo-dig = >20:1), (0.535 g). Rf = 0.56 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.31-7.07 (m, 10H), 6.44 (s, 1H), 5.32 (d, J = 6.9 Hz, 1H), 5.15 (d, J = 15.2 Hz, 1H), 4.12 (d, J = 15.2 Hz, 1H), 3.68 (m, 1H), 2.67 (m, 2H), 2.20 (m, 3H), 2.01 (m, 1H), 1.69 (m, 2H), 1.61 (m, 2H), 1.58 (s, 9H), 1.50 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.6, 150.8, 149.9, 142.1, 137.2, 136.3, 132.4, 129.4, 128.7, 128.5, 128.3, 127.7, 126.8, 126.7, 113.7, 82.3, 78.8, 56.3, 51.3, 39.5, 28.3, 28.1, 25.6, 25.5, 22.6, 22.2 ppm; LRMS (ESI) Calculated for C34H44N3O4 m/z (M+H): 558.3, Obsd 558.2.
4.2.2.8. tert-butyl (E)-4-benzyl-2-((tert-butoxycarbonyl)imino)-3-(cyclopropylmethyl)-6-(prop-1-en-2-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2h)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 54% yield (6-endo-dig:5-exo-dig = 3:2), (0.260 g). Rf = 0.26 (20% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.32-7.15 (m, 5H), 5.70 (s, 1H), 5.58 (d, J = 6.9 Hz, 1H), 5.07 (t, J = 1.5 Hz, 1H), 4.01 (m, 1H), 3.47 (dd, J = 14.3, 7.1 Hz, 1H), 3.19 (dd, J = 14.3, 6.5 Hz, 1H), 3.00 (dd, J = 12.6, 5.4 Hz, 1H), 2.71 (dd, J = 12.6, 10.5 Hz, 1H), 1.89 (s, 3H), 1.56 (s, 9H), 1.49 (s, 9H), 1.05 (m, 1H), 0.55 (m, 2H), 0.23 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.8, 150.5, 149.9, 141.8, 138.7, 137.4, 129.5, 128.7, 127.0, 116.8, 115.3, 82.9, 78.9, 57.5, 53.5, 40.1, 28.4, 28.2, 20.1, 9.6, 4.2, 3.8 ppm; LRMS (ESI) Calculated for C28H40N3O4 m/z (M+H): 482.3, Obsd. 482.4.
4.2.2.9. tert-butyl (E)-3,4-dibenzyl-2-((tert-butoxycarbonyl)imino)-6-(prop-1-en-2-yl)-3,4-dihydropyrimidine-1(2H)-carboxylate (2i)
Prepared according to the general procedure 2, to give 6-endo-dig cyclized guanidine as a white solid in 70% yield (6-endo-dig:5-exo-dig = >20:1), (0.362 g). Rf = 0.60 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.33-7.05 (m, 10H), 5.77 (s, 1H), 5.50 (d, J = 7.0 Hz, 1H), 5.18 (d, J = 15.1 Hz, 1H), 5.12 (s, 1H), 4.09 (d, J = 15.1 Hz, 1H), 3.74 (m, 1H), 2.71 (m, 2H), 1.91 (s, 3H), 1.60 (s, 9H), 1.53 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.6, 150.9, 149.8, 141.9, 138.6, 137.1, 136.3, 129.4, 128.6, 128.3, 127.8, 127.0, 116.7, 115.3, 83.0, 79.0, 56.4, 51.8, 39.5, 28.3, 28.1, 20.0 ppm; LRMS (ESI) Calculated for C31H40N3O4 m/z (M+H): 518.3, Obsd. 518.2.
4.2.3. General procedure 3 for the cycloaddition of the guanidine dienes 2a-i
To a stirring solution of the desired 6-endo-dig cyclic guanidine (diene, 1 equiv) in CH2Cl2 (0.07 M) was added the appropriate triazoledione (dienophile, 1.2 equiv). The reaction was stirred at room temperature until judged complete by TLC. After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to yield polycyclic guanidine.
4.2.3.1. tert-butyl (4aR,9aS,E)-2-((tert-butoxycarbonyl)imino)-3-methyl-6,8-dioxo-7-phenyl-3,4,4a,7,8,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-1(2H)-carboxylate (3a)
Prepared using the general procedure 3 to yield the polycyclic guanidine as a pale yellow solid, (0.160 g, 94% yield). Rf = 0.46 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.51-7.36 (m. 5H), 4.57 (m, 1H), 4.44 (m, 1H), 3.99 (dd, J =14.0, 3.0 Hz, 1H), 3.84 (dd, J =14.0, 7.5 Hz, 1H), 3.39 (m, 1H), 3.02 (s, 3H), 2.49 (m, 1H), 1.92 (m, 2H), 1.78 (m, 1H), 1.57 (m, 2H), 1.53 (s, 9H), 1.45 (s, 9H), 1.38 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.3, 154.5, 151.1, 149.5, 149.0, 134.3, 130.7, 129.1, 128.2, 125.3, 118.7, 82.9, 78.9, 55.2, 54.8, 50.1, 37.3, 30.8, 28.5, 28.2, 28.0, 26.6, 24.1 ppm; LRMS (ESI) Calculated for C29H39N6O6 m/z (M+H): 567.3, Obsd. 567.4.
4.2.3.2. tert-butyl (4aR,9aS,E)-2-((tert-butoxycarbonyl)imino)-3-(cyclopropylmethyl)-6,8-dioxo-7-phenyl-3,4,4a,7,8,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-1(2H)-carboxylate (3b)
Prepared according to the general procedure 3, to give the polycyclic guanidine as white solid in 35% yield (0.064 g). Rf = 0.45 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.49-7.34 (m, 5H), 4.60 (m, 1H), 4.43 (m, 1H), 4.35 (dd, J = 14.3, 1.5 Hz, 1H), 3.77 (dd, J = 14.3, 7.2 Hz, 1H), 3.46 (m, 1H), 3.42 (dd, J = 14.3, 7.8 Hz, 1H), 3.19 (dd, J = 14.3, 6.0 Hz, 1H), 2.45 (m, 1H), 1.89 (m, 2H), 1.72 (m, 1H), 1.54 (m, 1H), 1.49 (s, 9H), 1.45 (m, 1H), 1.42 (s, 9H), 1.33 (m, 1H), 1.05 (m, 1H), 0.52 (m, 1H), 0.45 (m, 1H), 0.21 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.6, 155.1, 151.6, 149.7, 149.3, 134.7, 130.9, 129.2, 128.4, 125.4, 119.4, 82.9, 79.0, 57.4, 54.8, 52.8, 47.1, 31.1, 28.6, 28.3, 28.1,27.0, 24.3, 9.2, 3.9, 3.2 ppm; LRMS (ESI) Calculated for C32H43N6O6 m/z (M+H): 607.3, Obsd. 607.3.
4.2.3.3 tert-butyl (4aR,9aS,E)-3-benzyl-2-((tert-butoxycarbonyl)imino)-6,8-dioxo-7-phenyl-3,4,4a,7,8,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-1(2H)-carboxylate (3c)
Prepared according to the general procedure 3, to give the polycyclic guanidine as white solid in 95% yield (0.183 g). Rf = 0.43 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.45-7.23 (m, 10H), 5.30 (d, J = 15.2 Hz, 1H), 4.55 (m, 1H), 4.40 (m, 1H), 4.06 (dd, J = 14.3, 1.6 Hz, 1H), 4.01 (d, J = 15.2 Hz, 1H), 3.75 (dd, J = 14.3, 7.3 Hz, 1H), 3.53 (m, 1H), 2.41 (m, 1H), 1.97 (m, 1H), 1.90 (m, 1H), 1.77 (m, 1H), 1.58 (m, 1H), 1.54 (s, 9H), 1.47 (s, 9H), 1.37 (m, 1H), 1.29 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.4, 154.3, 151.7, 149.6, 149.2, 136.5, 135.0, 130.8, 129.1, 128.5, 128.2, 128.1, 127.7, 125.2, 119.2, 83.0, 79.1, 56.4, 54.7, 51.6, 47.0, 30.9, 28.5, 28.3, 28.1, 27.0, 24.3 ppm; LRMS (ESI) Calculated for C35H43N6O6 m/z (M+H): 643.3, Obsd. 643.3.
4.2.3.4 tert-butyl (R,E)-2-benzyl-3-((tert-butoxycarbonyl)imino)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3d)
Prepared according to general procedure 3, to the give polycyclic guanidine as white solid in 73% yield (0.132 g). Rf = 0.39 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.48-7.25 (m, 10H), 4.86 (d, J = 15.2 Hz, 1H), 4.60 (m, 1H), 4.46 (d, J = 15.2 Hz, 1H), 4.27 (d, J = 16.3 Hz, 1H), 3.99 (d, J = 16.3 Hz, 1H), 3.74 (m, 2H), 2.10 (s, 3H), 1.55 (s, 9H), 1.49 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.0, 153.3, 151.3, 149.2, 136.1, 130.8, 129.4, 128.9, 128.6, 128.5, 127.9, 127.5, 125.5, 122.3, 114.6, 83.6, 79.5, 54.6, 52.4, 47.3, 46.1, 28.4, 28.3, 15.7 ppm; LRMS (ESI) Calculated for C32H39N6O6 m/z (M+H): 603.3, Obsd. 603.4.
4.2.3.5. tert-butyl (R,E)-3-((tert-butoxycarbonyl)imino)-2-(2,4-dimethoxybenzyl)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3e)
Prepared according to the general procedure 3, to give the polycyclic guanidine as pale yellow solid in 50% yield (0.099 g). Rf = 0.47 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.45-7.33 (m, 5H), 7.15 (m, 1H), 6.38 (m, 2H), 4.94 (d, J = 14.7 Hz, 1H), 4.55 (m, 1H), 4.22 (d, J = 16.5 Hz, 1H), 4.20 (d, J = 14.7 Hz, 1H), 3.72 (s, 3H), 3.71 (s, 3H), 3.69 (m, 1H), 2.05 (s, 3H), 1.53 (s, 9H), 1.46 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.7, 159.8, 158.9, 153.0, 151.1, 151.0, 149.1, 131.2, 130.8, 129.2, 128.4, 127.0, 125.3, 122.2, 116.4, 104.2, 98.3, 83.0, 78.9, 55.3, 55.2, 54.9, 46.6, 46.5, 45.9, 28.3, 28.1, 15.5 ppm; LRMS (ESI) Calculated for C34H43N6O8 m/z (M+H): 663.3, Obsd. 663.4.
4.2.3.6. tert-butyl (R,E)-2-(benzo[d][1,3]dioxol-5-ylmethyl)-3-((tert-butoxycarbonyl)imino)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3f)
Prepared according to the general procedure 3, to give the polycyclic guanidine as pale yellow solid in 60% yield (0.116 g). Rf = 0.35 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.48-7.36 (m, 5H), 6.76 (s, 1H), 6.72 (m, 2H), 5.90 (m, 2H), 4.76 (d, J = 15.1 Hz, 1H), 4.60 (m, 1H), 4.60 (m, 1H), 4.34 (d, J = 15.1 Hz, 1H), 4.28 (d, J = 16.1 Hz, 1H), 4.02 (d, J = 16.1 Hz, 1H), 3.77 (dd, J = 13.7, 4.0 Hz, 1H), 3.69 (dd, J = 13.7, 7.4 Hz, 1H), 2.09 (s, 3H), 1.55 (s, 9H), 1.48 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.9, 153.3, 151.3, 151.2, 149.2, 148.1, 147.4, 130.8, 129.8, 129.4, 128.6, 127.5, 125.4, 122.2, 122.1, 108.9, 108.4, 101.2, 83.5, 79.4, 54.6, 52.2, 46.9, 46.1, 28.4, 28.2, 15.7 ppm; LRMS (ESI) Calculated for C33H39N6O8 m/z (M+H): 647.3, Obsd. 647.3.
4.2.3.7. tert-butyl (R,E)-2-(benzo[d][1,3]dioxol-5-ylmethyl)-3-((tert-butoxycarbonyl)imino)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3g)
Prepared according to the general procedure 3, to give the polycyclic guanidine as pale yellow solid in 61% yield (0.110 g). Rf = 0.29 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.74-6.71 (m, 3H), 5.92 (s, 2H), 4.74 (d, J = 15.1 Hz, 1H), 4.48 (m, 1H), 4.34 (d, J = 15.1 Hz, 1H), 4.18 (dd, J = 16.1, 2.0 Hz, 1H), 3.92 (dd, J = 16.1, 2.0 Hz, 1H), 3.68 (qd, J = 7.3, 5.4 Hz, 2H), 2.05 (d, J = 1.5 Hz, 3H), 1.54 (s, 9H), 1.47 (s, 9H), 1.23 (t, J = 7.3 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.8, 154.5, 152.3, 151.2, 149.2, 148.0, 147.3, 129.7, 127.5, 122.3, 122.0, 108.8, 108.3, 101.1, 83.3, 79.3, 54.4, 52.1, 46.9, 45.8, 34.5, 28.3, 28.2, 15.6, 13.4 ppm; LRMS (ESI) Calculated for C29H39N6O8 m/z (M+H): 599.3, Obsd. 599.4.
4.2.3.8. tert-butyl (4aR,9aS,E)-3,4-dibenzyl-2-((tert-butoxycarbonyl)imino)-6,8-dioxo-7-phenyl-3,4,4a,7,8,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-1(2H)-carboxylate (3h)
Prepared according to the general procedure 3, to give the polycyclic guanidine as white solid in 67% yield (0.147 g). Rf = 0.67 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.47-7.19 (m, 13H), 7.03 (m, 2H), 5.12 (d, J = 15.0 Hz, 1H), 4.66 (dd, J = 11.0, 2.3 Hz, 1H), 4.38 (s, 1H), 4.36 (m, 1H), 3.61 (m, 1H), 3.27 (dd, J = 13.2, 2.8 Hz, 1H), 2.90 (dd, J = 12.3, 12.3 Hz, 1H), 2.84 (d, J = 15.0 Hz, 1H), 2.33 (m, 1H), 1.89 (m, 1H), 1.83 (m, 1H), 1.73 (m, 1H), 1.56 (s, 9H), 1.51 (s, 9H), 1.49 (m, 1H), 1.27 (m, 1H), 1.16 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.3, 154.7, 151.9, 149.6, 149.3, 137.1, 136.7, 134.8, 130.7, 129.7, 129.1, 128.9, 128.5, 128.2, 128.1, 127.5, 127.1, 125.3, 119.5, 82.9, 79.0, 63.0, 59.3, 54.4, 52.1, 43.3, 30.6, 28.3, 28.2, 28.1, 26.8, 24.2 ppm; LRMS (ESI) Calculated for C42H49N6O6 m/z (M+H): 733.4, Obsd. 733.4.
4.2.3.9. tert-butyl (11aR,E)-1-benzyl-3-((tert-butoxycarbonyl)imino)-2-(cyclopropylmethyl)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3i)
Prepared according to general procedure 3, to give the polycyclic guanidine as white solid in 83% yield (0.164 g). Rf = 0.10 (20% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.56-7.24 (m, 10H), 5.06 (dd, J = 10.4, 4.9 Hz, 1H), 4.55 (s, 1H), 4.40 (d, J = 16.6 Hz, 1H), 3.98 (d, J = 16.6 Hz, 1H), 3.68 (dd, J = 14.2, 6.3 Hz, 1H), 3.20 (dd, J = 13.2, 4.9 Hz, 1H), 2.99 (dd, J = 13.2, 10.3 Hz, 1H), 2.13 (s, 3H), 2.09 (dd, J = 14.2, 7.3 Hz, 1H), 1.60 (s, 9H), 1.56 (s, 9H), 0.78 (m, 1H), 0.50 (m, 1H), 0.36 (m, 1H), 0.05 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.1, 154.7, 151.9, 151.0, 149.6, 136.9, 130.8, 130.4, 129.5, 129.0, 128.7, 127.2, 125.7, 123.0, 83.2, 79.5, 63.1, 58.3, 53.2, 46.0, 43.7, 28.4, 28.3, 15.3, 9.5, 5.0, 2.1 ppm; LRMS (ESI) Calculated for C36H45N6O6 m/z (M+H): 657.3, Obsd. 657.3.
4.2.3.10. tert-butyl (11aR,E)-1,2-dibenzyl-3-((tert-butoxycarbonyl)imino)-5-methyl-8,10-dioxo-9-phenyl-2,3,6,9,10,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-4(1H)-carboxylate (3j)
Prepared according to general procedure 3, to give the polycyclic guanidine as white solid in 65% yield (0.135 g). Rf = 0.53 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.48-7.20 (m, 13H), 6.89 (m, 2H), 5.06 (d, J = 14.7 Hz, 1H), 4.42 (s, 1H), 4.41 (dd, J = 10.7, 3.9 Hz, 1H), 4.27 (d, J = 16.6 Hz, 1H), 3.83 (d, J = 16.6 Hz, 1H), 3.23 (dd, J = 13.7, 3.9 Hz, 1H), 3.00 (d, J = 14.7 Hz, 1H), 2.89 (dd, J = 13.7, 10.7 Hz, 1H), 2.13 (s, 3H), 1.58 (s, 9H), 1.52 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.8, 153.4, 151.9, 151.0, 149.6, 137.0, 136.4, 130.7, 129.9, 129.4, 129.0, 128.6, 128.5, 128.4, 127.8, 127.5, 127.3, 125.5, 123.0, 83.3, 79.6, 62.3, 58.6, 52.6, 45.9, 43.2, 28.4, 28.3, 15.3 ppm; LRMS (ESI) Calculated for C39H45N6O6 m/z (M+H): 693.3, Obsd. 693.4.
4.2.4 General procedure 4 for the deprotection and salt exchange of guanidines 3a–j
A 50 mL round-bottom flask was charged with the appropriate polycyclic guanidine (0.2 mmol). The CH2Cl2/TFA mixture (1:1, 4.0 mL) was added. The reaction mixture was stirred at room temperature for 2 hrs. The crude mixture was taken up in MeOH and 2 M HCl in ethyl ether was added. The mixture was again concentrated and the resulting solid triturated with ethyl ether. The solid was then collected to give the title compound.
4.2.4.1. (4aR,9aS)-2-imino-3-methyl-7-phenyl-1,2,3,4,4a,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-6,8(7H)-dione (4a)
Prepared according to the general procedure 4 to afford the HCl salt as a white powder (69 mg, 85%). 1H NMR (500 MHz, DMSO-d6) δ 10.84 (s, 1H), 8.20 (s, 2H), 7.50-7.42 (m. 5H), 4.67 (s, 1H), 4.39 (d, J = 8.8 Hz, 1H), 4.29 (d, J = 7.3 Hz, 1H), 3.63 (t, J = 8.6 Hz, 1H), 3.07 (s, 4H), 2.17 (m, 1H), 1.81 (m, 1H), 1.78 (m, 2H), 1.54 (m, 1H), 1.34 (m, 1H), 1.20 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 153.9, 151.5, 149.1, 130.8, 128.8, 128.1, 126.0, 117.6, 114.2, 53.2, 49.1, 49.0, 37.3, 30.2, 26.8, 26.3, 23.5 ppm; HRMS (ESI) Calculated for C19H23N6O2 m/z (M+H): 367.1882, Obsd. 367.1890.
4.2.4.2. (4aR,9aS)-3-(cyclopropylmethyl)-2-imino-7-phenyl-1,2,3,4,4a,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-6,8(7H)-dione (4b)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 66% yield (59 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.41 (s, 1H), 7.98 (s, 2H), 7.53-7.41 (m, 5H), 4.64 (m, 1H), 4.51 (dd, J = 12.4, 5.7 Hz, 1H), 4.42 (dd, J = 8.4, 2.0 Hz, 1H), 3.63 (t, J = 12.4 Hz, 1H), 3.39 (m, 2H), 2.94 (m, 1H), 2.17 (m, 1H), 1.91 (m, 1H), 1.80 (m, 2H), 1.55 (m, 1H), 1.37 (m, 1H), 1.24 (m, 1H), 1.04 (m, 1H), 0.54 (m, 1H), 0.31 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 156.1, 152.6, 151.3, 132.3, 130.2, 129.7, 127.5, 119.4, 117.0, 55.8, 55.5, 51.6, 49.7, 31.9, 28.3, 28.1, 25.3, 9.8, 4.2, 4.1 ppm; HRMS (ESI) Calculated for C22H28N6O2 m/z (M+H): 408.2274, Obsd. 408.2285.
4.2.4.3. (4aR,9aS)-3-benzyl-2-imino-7-phenyl-1,2,3,4,4a,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-6,8(7H)-dione (4c)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 90% yield (79 mg). 1H NMR (500 MHz, DMSO-d6) δ 11.00 (s, 1H), 8.53 (s, 2H), 7.49-7.34 (m, 10H), 4.82 (d, J = 16.2 Hz, 1H), 4.66 (d, J = 16.2 Hz, 1H), 4.63 (s, 1H), 4.40 (d, J = 10.4 Hz, 1H), 4.30 (dd, J = 12.2, 5.2 Hz, 1H), 3.56 (t, J = 11.9 Hz, 1H), 3.12 (m, 1H), 2.15 (m, 1H), 1.89 (m, 1H), 1.78 (m, 2H), 1.54 (m, 1H), 1.34 (m, 1H), 1.24 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 154.0, 151.5, 149.2, 134.5, 130.9, 128.9, 128.8, 128.2, 128.0, 127.4, 126.1, 117.7, 114.7, 53.3, 52.0, 49.2, 46.8, 30.2, 26.9, 26.4, 23.6 ppm; HRMS (ESI) Calculated for C25H27N6O2 m/z (M+H): 443.2195, Obsd. 443.2205.
4.2.4.4. (R)-2-benzyl-3-imino-5-methyl-9-phenyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4d)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 63% yield (55 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.83 (s, 1H), 8.38 (s, 2H), 7.49-7.32 (m, 10H), 4.70 (d, J = 15.9 Hz, 1H), 4.63 (m, 1H), 4.54 (d, J = 15.9 Hz, 1H), 4.22 (m, 2H), 3.94 (d, J = 15.3 Hz, 1H), 3.44 (t, J = 11.5 Hz, 1H), 1.88 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 152.9, 151.2, 150.6, 134.2, 130.7, 128.6, 127.9, 127.8, 127.1, 125.8, 119.9, 106.9, 51.8, 48.6, 46.4, 44.8, 13.8 ppm; HRMS (ESI) Calculated for C22H24N6O2 m/z (M+H): 404.1961, Obsd. 404.1971.
4.2.4.5. (R)-2-(2,4-dimethoxybenzyl)-3-imino-5-methyl-9-phenyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4e)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 70% yield (70 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.87 (s, 1H), 8.34 (s, 2H), 7.48-7.42 (m, 5H), 7.24 (m, 1H), 6.60 (s, 1H), 6.51 (m, 1H), 4.60 (d, J = 15.2 Hz, 1H), 4.54 (m, 1H), 4.47 (d, J = 15.2 Hz, 1H), 4.32 (d, J = 7.8 Hz, 1H), 4.20 (d, J = 14.7 Hz, 1H), 3.92 (d, J = 14.7 Hz, 1H), 3.79 (s, 3H), 3.74 (s, 3H), 3.36 (m, 1H), 1.88 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 160.7, 159.8, 158.9, 153.0, 151.1, 151.0, 149.1, 131.2, 130.8, 129.2, 128.4, 127.0, 125.3, 122.2, 116.4, 104.2, 98.3, 83.0, 78.9, 55.3, 55.2, 54.9, 46.6, 46.5, 45.9, 28.3, 28.1, 15.5 ppm; HRMS (ESI) Calculated for C24H27N6O4 m/z (M+H): 463.2094, Obsd. 463.2099.
4.2.4.6. (R)-2-(benzo[d][1,3]dioxol-5-ylmethyl)-3-imino-5-methyl-9-phenyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4f)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 88% yield (85 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.93 (s, 1H), 8.51 (s, 2H), 7.48-7.40 (m, 5H), 6.94-6.82 (m, 3H), 6.01 (s, 1H), 6.72 (m, 2H), 5.90 (m, 2H), 4.76 (d, J = 15.1 Hz, 1H), 4.60 (m, 1H), 4.60 (m, 1H), 4.34 (d, J = 15.1 Hz, 1H), 4.28 (d, J = 16.1 Hz, 1H), 4.02 (d, J = 16.1 Hz, 1H), 3.77 (dd, J = 13.7, 4.0 Hz, 1H), 3.69 (dd, J = 13.7, 7.4 Hz, 1H), 2.09 (s, 3H), 1.55 (s, 9H), 1.48 (s, 9H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 153.2, 151.1, 150.9, 147.7, 147.1, 131.0, 128.9, 128.2, 128.0, 126.1, 121.3, 120.1, 108.5, 108.1, 107.0, 101.2, 51.7, 48.8, 46.2, 45.0, 13.9 ppm; HRMS (ESI) Calculated for C23H23N6O4 m/z (M+H): 447.1781, Obsd. 447.1782.
4.2.4.7. (R)-2-(benzo[d][1,3]dioxol-5-ylmethyl)-9-ethyl-3-imino-5-methyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4g)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 81% yield (70 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 8.46 (s, 2H), 6.93-6.82 (m, 3H), 6.02 (s, 2H), 4.68 (d, J = 16.1 Hz, 1H), 4.55 (d, J = 16.1 Hz, 1H), 4.51 (m, 1H), 4.20 (dd, J = 11.7, 4.9 Hz, 1H), 4.12 (d, J = 15.7 Hz, 1H), 3.81 (d, J = 15.7 Hz, 1H), 3.39 (m, 3H), 1.85 (s, 3H), 1.10 (t, J = 7.1 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 154.3, 151.9, 151.2, 147.7, 147.1, 128.0, 121.3, 120.3, 108.4, 108.1, 107.1, 101.2, 51.7, 48.6, 46.3, 44.8, 33.7, 13.9, 12.9 ppm; HRMS (ESI) Calculated for C19H23N6O4 m/z (M+H): 399.1781, Obsd. 399.1783.
4.2.4.8. (4aR,9aS)-3,4-dibenzyl-2-imino-7-phenyl-1,2,3,4,4a,9a,10,11,12,13-decahydro-6H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]cinnoline-6,8(7H)-dione (4h)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 80% yield (91 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.20 (s, 1H), 8.54 (s, 2H), 7.51-7.14 (m, 15H), 5.00 (d, J = 16.6 Hz, 1H), 4.97 (s, 1H), 4.48 (s, 2H), 4.09 (d, J = 16.6 Hz, 1H), 3.44 (d, J = 12.7 Hz, 1H), 3.23 (d, J = 12.7 Hz, 1H), 2.94 (m, 1H), 2.27 (m, 1H), 1.83 (m, 2H), 1.78 (m, 1H), 1.55 (m, 1H), 1.32 (m, 1H), 1.02 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 165.7, 154.5, 149.7, 135.8, 135.4, 131.0, 130.3, 128.8, 128.6, 128.5, 128.2, 127.9, 127.1, 126.6, 126.2, 123.8, 117.1, 60.0, 59.5, 53.3, 50.7, 39.0, 31.0, 27.2, 26.7, 23.4 ppm; HRMS (ESI) Calculated for C32H33N6O2 m/z (M+H): 533.2665, Obsd. 533.2671.
4.2.4.9. (11aR)-1-benzyl-2-(cyclopropylmethyl)-3-imino-5-methyl-9-phenyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4i)
Prepared according to the general procedure 4, to give the polycyclic guanidine as white solid in 72% yield (71 mg). 1H NMR (500 MHz, CD3OD) δ 7.57-7.29 (m, 10H), 4.97 (s, 2H), 4.30 (d, J = 15.9 Hz, 1H), 4.06 (d, J = 15.9 Hz, 1H), 3.55 (dd, J = 14.6, 6.2 Hz, 1H), 3.39 (d, J = 14.6, 3.8 Hz, 1H), 3.32 (d, J = 14.6, 3.8 Hz, 1H), 2.87 (dd, J = 14.6, 7.5 Hz, 1H), 1.82 (s, 3H), 1.03 (m, 1H), 0.64 (m, 1H), 0.56 (m, 1H), 0.31 (m, 1H), 0.21 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CD3OD) δ 157.8, 155.9, 153.6, 137.1, 132.7, 131.6, 130.3, 130.1, 129.8, 128.7, 127.5, 121.2, 118.9, 61.2, 60.0, 54.2, 47.0, 40.2, 14.4, 10.1, 5.2, 3.3 ppm; HRMS (ESI) Calculated for C26H30N6O2 m/z (M+H): 458.2430, Obsd. 458.2436.
4.2.4.10. (11aR)-1,2-dibenzyl-3-imino-5-methyl-9-phenyl-1,2,3,4,6,11a-hexahydro-8H-pyrimido[5,4-c][1,2,4]triazolo[1,2-a]pyridazine-8,10(9H)-dione (4j)
Prepared according to general procedure 4, to give the polycyclic guanidine as white solid in 87% yield (92 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.20 (s, 1H), 8.39 (s, 2H), 7.53-7.13 (m, 15H), 5.03 (d, J = 16.5 Hz, 1H), 4.91 (s, 1H), 4.44 (q, J = 4.3 Hz, 1H), 4.37 (d, J = 16.5 Hz, 1H), 4.23 (d, J = 15.3 Hz, 1H), 4.04 (d, J = 15.3 Hz, 1H), 3.44 (dd, J = 14.3, 4.0 Hz, 1H), 3.28 (dd, J = 14.3, 4.6 Hz, 1H), 1.79 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 155.7, 153.1, 151.9, 135.3, 135.2, 131.1, 130.5, 128.9, 128.5, 128.2, 127.9, 127.2, 126.8, 126.3, 119.2, 116.1, 59.1, 57.0, 50.1, 45.8, 37.4, 14.3 ppm; HRMS (ESI) Calculated for C29H29N6O2 m/z (M+H): 493.2352, Obsd. 493.2355.
4.2.5 General Procedure 5 for the cyclization of propargylguanidines using silver (I)
To a stirring solution of the preferred propargylguanidine (1.0 mmol) in CH2Cl2 (0.07 M) was added silver acetate (10 mol%) and acetic acid (3.0 equiv). After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to give 5-exo-dig cyclized guanidine.
4.2.5.1. tert-butyl (2E,5Z)-3-benzyl-2-((tert-butoxycarbonyl)imino)-5-(cyclohex-1-en-1-ylmethylene)imidazolidine-1-carboxylate (7a)
The general procedure 5 was used to give 5-exo-dig cyclized guanidine as a white solid in 85% yield (5-exo-dig:6-endo-dig = 2:3), (0.398 g). Rf = 0.50 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.34-7.26 (m, 5H), 5.75 (s, 1H), 5.49 (s, 1H), 4.58 (s, 2H), 3.70 (s, 2H), 2.22 (m, 2H), 2.13 (m, 2H), 1.63 (m, 4H), 1.54 (s, 9H), 1.47 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.2, 151.9, 149.3, 135.6, 134.1, 129.3, 128.8, 128.4, 127.9, 126.0, 120.3, 83.5, 79.2, 49.7, 48.4, 28.3, 28.1, 27.0, 25.8, 22.7, 22.2 ppm; LRMS (ESI) Calculated for C27H38N3O4 m/z (M+H): 468.3, Obsd. 468.3.
4.2.5.2. tert-butyl (2E,5Z)-3,4-dibenzyl-2-((tert-butoxycarbonyl)imino)-5-(cyclohex-1-en-1-ylmethylene)imidazolidine-1-carboxylate (7b)
Prepared according to the general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 85% yield (5-exo-dig:6-endo-dig = >20:1), (0.474 g). Rf = 0.50 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.35-7.01 (m, 10H), 5.52 (s, 1H), 5.07 (d, J = 15.1 Hz, 1H), 4.72 (s, 1H), 4.13 (d, J = 15.1 Hz, 1H), 3.63 (dd, J = 9.4, 4.2 Hz, 1H), 2.86 (dd, J = 13.0, 9.4 Hz, 1H), 2.41 (dd, J = 13.0, 9.4 Hz, 1H), 2.20 (m, 1H), 2.07 (m, 3H), 1.72 (m, 2H), 1.64 (m, 1H), 1.55 (s, 9H), 1.52 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.6, 152.1, 149.8, 136.2, 136.1, 134.1, 130.0, 129.7, 129.0, 128.5, 128.4, 128.1, 127.1, 122.3, 83.4, 79.4, 61.7, 46.9, 38.8, 28.4, 28.3, 26.9, 25.9, 22.9, 22.4 ppm; LRMS (ESI) Calculated for C34H44N3O4 m/z (M+H): 558.3, Obsd. 558.3.
4.2.5.3. tert-butyl (2E,5Z)-3-benzyl-2-((tert-butoxycarbonyl)imino)-5-(2-methylallylidene)imidazolidine-1-carboxylate (7c)
Prepared according to the general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 54% yield (5-exo-dig:6-endo-dig = 10:1), (0.231 g). Rf = 0.49 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.35-7.27 (m, 5H), 5.60 (s, 1H), 5.00 (s, 2H), 4.60 (s, 2H), 3.72 (d, J = 1.0 Hz, 2H), 1.94 (s, 3H), 1.54 (s, 9H), 1.46 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.5, 152.1, 149.2, 140.3, 135.5, 128.9, 128.6, 128.5, 128.0, 119.1, 117.5, 84.2, 79.4, 49.6, 48.5, 28.4, 28.1, 21.2 ppm; LRMS (ESI) Calculated for C24H34N3O4 m/z (M+H): 428.3, Obsd. 428.3.
4.2.5.4. tert-butyl (2E,5Z)-3,4-dibenzyl-2-((tert-butoxycarbonyl)imino)-5-(2-methylallylidene)imidazolidine-1-carboxylate (7d)
Prepared according to the general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 90% yield (5-exo-dig:6-endo-dig = >20:1), (0.466 g). Rf = 0.47 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.36-7.00 (m, 10H), 5.07 (d, J = 15.1 Hz, 1H), 4.92 (s, 1H), 4.83 (s, 1H), 4.78 (s, 1H), 4.16 (d, J = 15.1 Hz, 1H), 3.66 (dd, J = 9.7, 4.6 Hz, 1H), 2.86 (dd, J = 13.1, 4.6 Hz, 1H), 2.46 (dd, J = 13.1, 9.7 Hz, 1H), 1.56 (s, 3H), 1.56 (s, 9H), 1.51 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.7, 152.0, 149.4, 140.0, 135.9, 135.8, 130.6, 129.9, 129.0, 128.5, 128.4, 128.1, 127.1, 83.8, 79.4, 61.5, 46.9, 38.8, 28.3, 28.2, 20.9 ppm; LRMS (ESI) Calculated for C31H40N3O4 m/z (M+H): 518.3, Obsd. 518.4.
4.2.5.5. tert-butyl (2E,5Z)-3-(benzo[d][1,3]dioxol-5-ylmethyl)-2-((tert-butoxycarbonyl)imino)-5-(2-methylallylidene)imidazolidine-1-carboxylate (7e)
Prepared according to general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 80% yield (5-exo-dig:6-endo-dig = 10:1), (0.377 g). Rf = 0.45 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.79-6.74 (m, 3H), 5.94 (s, 2H), 5.61 (s, 1H), 5.01 (s, 2H), 4.49 (s, 2H), 3.74 (s, 2H), 1.94 (s, 3H), 1.54 (s, 9H), 1.46 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.3, 151.8, 149.0, 148.0, 147.4, 140.0, 129.0, 128.3, 121.9, 118.9, 117.3, 108.9, 108.3, 101.1, 84.0, 79.2, 49.3, 48.1, 28.2, 28.0, 21.0 ppm; LRMS (ESI) Calculated for C25H34N3O6 m/z (M+H): 472.2, Obsd. 472.3.
4.2.5.6. tert-butyl (2E,5Z)-2-((tert-butoxycarbonyl)imino)-3-(2,4-dimethoxybenzyl)-5-(2-methylallylidene)imidazolidine-1-carboxylate (7f)
Prepared according to general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 60% yield (5-exo-dig:6-endo-dig = 9:1), (0.377 g). Rf = 0.39 (40% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.20 (m, 1H), 6.44 (m, 2H), 5.59 (s, 1H), 4.99 (s, 1H), 4.98 (s, 1H), 4.54 (s, 2H), 3.79 (s, 3H), 3.78 (s, 3H), 3.75 (s, 2H), 1.93 (s, 3H), 1.54 (s, 9H), 1.46 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.7, 159.2, 158.6, 151.7, 148.9, 140.0, 131.4, 128.8, 118.3, 116.8, 115.6, 104.3, 98.2, 83.5, 78.7, 55.1, 49.4, 42.4, 28.1, 28.0, 20.8 ppm; LRMS (ESI) Calculated for C26H38N3O6 m/z (M+H): 488.3, Obsd. 488.4.
4.2.5.7. tert-butyl (2E,5Z)-2-((tert-butoxycarbonyl)imino)-5-(cyclohex-1-en-1-ylmethylene)-3-methylimidazolidine-1-carboxylate (7g)
Prepared according to the general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid, (5-exo-dig:6-endo-dig = 1:1), (0.211 g, total 54% yield). Rf = 0.21 (60% ethyl acetate/N-hexanes). 1H NMR (500 MHz, CDCl3) δ 5.76 (s. 1H), 5.58 (s, 1H), 3.90 (s, 2H), 2.97 (s, 3H), 2.20 (m, 2H), 2.14 (m, 2H), 1.63 (m, 4H), 1.51 (s, 9H), 1.46 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.9, 151.8, 149.2, 134.0, 129.1, 125.9, 119.9, 83.3, 78.9, 52.5,31.6, 28.1, 27.9, 26.8, 25.7, 22.6, 22.1 ppm; LRMS (ESI) Calculated for C21H34N3O4 m/z (M+H): 392.3, Obsd. 392.4.
4.2.5.8. tert-butyl (2E,5Z)-4-benzyl-2-((tert-butoxycarbonyl)imino)-3-(cyclopropylmethyl)-5-(2-methylallylidene)imidazolidine-1-carboxylate (7h)
Prepared according to the general procedure 5, to give the 5-exo-dig cyclized guanidine as a white solid in 79% yield (5-exo-dig:6-endo-dig = >20:1), (0.381 g). Rf = 0.16 (20% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.29 (m, 3H), 7.15 (m, 2H), 4.92 (s, 1H), 4.87 (s, 1H), 4.80 (s, 1H), 4.06 (dd, J = 9.8, 3.8 Hz, 1H), 3.70 (dd, J = 14.6, 6.6 Hz, 1H), 3.11 (dd, J = 13.0, 3.8 Hz, 1H), 3.00 (dd, J = 14.6, 7.5 Hz, 1H), 2.48 (dd, J = 13.0, 9.9 Hz, 1H), 1.88 (s, 3H), 1.54 (s, 9H), 1.51 (s, 9H), 1.03 (m, 1H), 0.64 (m, 1H), 0.56 (m, 1H), 0.30 (m, 1H), 0.26 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.7, 151.6, 149.4, 140.0, 135.9, 130.7, 129.2, 128.4, 127.1, 121.0, 117.4, 83.6, 79.1. 62.1, 47.7, 38.8, 28.2, 28.1, 20.8, 9.4, 4.8, 3.3 ppm; LRMS (ESI) Calculated for C28H40N3O4 m/z (M+H): 482.3, Obsd. 482.3.
4.2.6 General procedure 6 to form the cycloaddition of guanidine dienes 7a–h
To a stirring solution of the desired 5-exo-dig cyclic guanidine (diene, 1 equiv) in CH2Cl2 (0.07 M) was added the appropriate triazoledione (dienophile, 1.2 equiv). The reaction was stirred at room temperature until judged complete by TLC. After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to yield polycyclic guanidine.
4.2.6.1. tert-butyl (4R,10a′R,E)-1-benzyl-2-((tert-butoxycarbonyl)imino)-1′,3′-dioxo-2′-phenyl-2′,3′,8′,9′,10′,10a′-hexahydro-1′H,7′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]cinnoline]-3-carboxylate (8a)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as white solid in 61% yield (0.118 g). Rf = 0.32 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.61-7.27 (m, 10H), 5.35 (s, 1H), 5.22 (d, J = 14.9 Hz, 1H), 4.40 (dd, J = 11.4, 3.5 Hz, 1H), 4.20 (d, J = 14.9 Hz, 1H), 3.88 (d, J = 11.2 Hz, 1H), 3.50 (d, J = 11.2 Hz, 1H), 2.46 (m, 1H), 2.40 (m, 1H), 2.14 (m, 1H), 1.96 (m, 1H), 1.90 (m, 1H), 1.60 (m, 1H), 1.49 (s, 9H), 1.42 (s, 9H), 1.37 (m, 1H), 1.30 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.1, 153.2, 151.6, 149.6, 148.7, 142.5, 135.6, 131.1, 129.2, 128.8, 128.7, 128.4, 127.8, 126.2, 115.8, 83.4, 78.5, 73.8, 56.8, 54.4, 49.9, 34.2, 31.1, 28.5, 28.2, 24.3 ppm; LRMS (ESI) Calculated for C35H43N6O6 m/z (M+H): 643.3, Obsd. 643.3.
4.2.6.2. tert-butyl (4R,5R,10a′R,E)-1,5-dibenzyl-2-((tert-butoxycarbonyl)imino)-1′,3′-dioxo-2′-phenyl-2′,3′,8′,9′,10′,10a′-hexahydro-1′H,7′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]cinnoline]-3-carboxylate (8b)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as white solid in 94% yield (0.207 g). Rf = 0.54 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.59-7.35 (m, 5H), 7.23-7.14 (m, 8H), 6.95 (m, 2H), 5.49 (s, 1H), 4.95 (dd, J = 9.2, 5.2 Hz, 1H), 4.76 (d, J = 15.6 Hz, 1H), 4.68 (d, J = 15.6 Hz, 1H), 4.22 (dd, J = 11.6, 3.6 Hz, 1H), 2.96 (dd, J = 15.6, 5.2 Hz, 1H), 2.75 (dd, J = 15.6, 9.2 Hz, 1H), 2.30 (m, 1H), 2.20 (m, 1H), 2.00 (m, 1H), 1.69 (m, 1H), 1.61 (m, 1H), 1.49 (s, 9H), 1.40(s, 9H), 1.28 (m, 1H), 0.79 (m, 1H), 0.68 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.0, 152.9, 151.0, 149.8, 148.2, 141.4, 136.2, 135.8, 131.0, 129.1, 128.7, 128.4, 128.3, 128.2, 127.4, 126.8, 126.0, 112.2, 83.6, 78.5, 77.3, 63.7, 54.1, 48.8, 35.7, 34.2, 30.5, 28.5, 28.1, 26.8, 23.9 ppm; LRMS (ESI) Calculated for C42H49N6O6 m/z (M+H): 733.4, Obsd. 733.4.
4.2.6.3. tert-butyl (R,E)-1-benzyl-2-((tert-butoxycarbonyl)imino)-7′-methyl-1′,3′-dioxo-2′-phenyl-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8c)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as white solid in 56% yield (0.101 g). Rf = 0.16 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.58-7.26 (m, 10H), 5.45 (s, 1H), 5.14 (d, J = 14.9 Hz, 1H), 4.24 (d, J = 16.8 Hz, 1H), 4.22 (d, J = 14.9 Hz, 1H), 3.99 (d, J = 16.8 Hz, 1H), 3.80 (d, J = 11.3 Hz, 1H), 3.47 (d, J = 11.3 Hz, 1H), 1.90 (s, 3H), 1.49 (s, 9H), 1.42 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.0, 152.7, 151.1, 150.0, 149.4, 135.4, 133.5, 130.9, 129.2, 128.7, 128.6, 128.4, 127.8, 126.0, 120.6, 83.3, 78.5, 73.7, 56.0, 49.6, 45.0, 28.4, 28.1, 19.9 ppm; LRMS (ESI) Calculated for C32H39N6O6 m/z (M+H): 603.3, Obsd. 603.4.
4.2.6.4. tert-butyl (4R,5R,E)-1,5-dibenzyl-2-((tert-butoxycarbonyl)imino)-7′-methyl-1′,3′-dioxo-2′-phenyl-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8d)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as pale yellow solid in 75% yield (0.156 g). Rf = 0.23 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.48-7.12 (m, 13H), 5.61 (s, 1H), 5.04 (d, J = 15.6 Hz, 1H), 4.88 (dd, J = 11.2, 4.9 Hz, 1H), 4.49 (d, J = 15.6 Hz, 1H), 3.88 (d, J = 16.6 Hz, 1H), 3.14 (dd, J = 14.7, 4.4 Hz, 1H), 2.80 (d, J = 16.6 Hz, 1H), 2.54 (dd, J = 14.7, 11.2 Hz, 1H), 1.77 (s, 3H), 1.50 (s, 9H), 1.36 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.0, 151.9, 149.7, 149.5, 149.1, 135.4, 135.3, 134.1, 130.8, 129.2, 128.8, 128.7, 128.4, 128.3, 128.2, 127.7, 126.8, 125.6, 117.3, 83.6, 78.9, 77.2, 62.0, 47.5, 43.9, 34.7, 28.5, 28.0, 20.1 ppm; LRMS (ESI) Calculated for C39H45N6O6 m/z (M+H): 693.3, Obsd. 693.4.
4.2.6.5. tert-butyl (R,E)-1-(benzo[d][1,3]dioxol-5-ylmethyl)-2-((tert-butoxycarbonyl)imino)-7′-methyl-1′,3′-dioxo-2′-phenyl-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8e)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as pale yellow solid in 87% yield (0.169 g). Rf = 0.17 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.58-7.34 (m, 5H), 6.83 (s, 1H), 6.74 (m, 2H), 5.92 (s, 2H), 5.46 (s, 1H), 5.01 (d, J = 14.6 Hz, 1H), 4.24 (d, J = 17.1 Hz, 1H), 4.13 (d, J = 14.6 Hz, 1H), 3.90 (d, J = 17.1 Hz, 1H), 3.78 (d, J = 11.4 Hz, 1H), 3.48 (d, J = 11.4 Hz, 1H), 1.90 (s, 3H), 1.49 (s, 9H), 1.42 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.9, 152.5, 151.0, 150.0, 149.3, 148.0, 147.3, 133.6, 130.9, 129.1, 129.0, 128.4, 125.9, 122.0, 120.5, 109.0, 108.2, 101.1, 83.2, 78.5, 73.6, 55.9, 49.3, 45.0, 28.4, 28.0, 20.0 ppm; LRMS (ESI) Calculated for C33H39N6O8 m/z (M+H): 647.3, Obsd. 647.4.
4.2.6.6. tert-butyl (R,E)-1-(benzo[d][1,3]dioxol-5-ylmethyl)-2-((tert-butoxycarbonyl)imino)-2′-ethyl-7′-methyl-1′,3′-dioxo-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8f)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as pale yellow solid in 91% yield (0.163 g). Rf = 0.17 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 6.84 (s, 1H), 6.76 (m, 2H), 5.94 (s, 2H), 5.41 (s, H), 4.98 (d, J = 14.7 Hz, 1H), 4.17 (d, J = 14.7 Hz, 1H), 4.14 (d, J = 17.1 Hz, 1H), 3.81 (d, J = 17.1 Hz, 1H), 3.72 (d, J = 11.2 Hz, 1H), 3.63 (qd, J = 7.3, 2.1 Hz, 2H), 3.42 (d, J = 11.2 Hz, 1H), 1.88 (s, 3H), 1.48 (s, 9H), 1.39 (s, 9H), 1.28 (t, J = 7.3 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.0, 152.2, 152.0, 151.4, 149.5, 148.1, 147.3, 133.4, 129.2, 122.1, 120.9, 109.1, 108.3, 101.2, 83.1, 78.6, 73.4, 55.8, 49.3, 45.0, 34.5, 28.5, 28.1, 20.0, 13.5 ppm; LRMS (ESI) Calculated for C29H39N6O8 m/z (M+H): 599.3, Obsd. 599.3.
4.2.6.7. tert-butyl (R,E)-2-((tert-butoxycarbonyl)imino)-1-(2,4-dimethoxybenzyl)-7′-methyl-1′,3′-dioxo-2′-phenyl-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8g)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as pale yellow solid in 97% yield (0.193 g). Rf = 0.13 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.57-7.25 (m, 6H), 6.41 (m, 2H), 5.44 (s, 1H), 4.85 (d, J = 14.5 Hz, 1H), 4.39 (d, J = 14.5 Hz, 1H), 4.21 (d, J = 16.9 Hz, 1H), 3.89 (d, J = 16.9 Hz, 1H), 3.87 (d, J = 11.3 Hz, 1H), 3.75 (s, 6H), 3.47 (d, J = 11.3 Hz, 1H), 1.88 (s, 3H), 1.48 (s, 9H), 1.40 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.6, 158.9, 158.7, 152.3, 150.7, 149.9, 149.4, 132.9, 131.5, 131.0, 129.0, 128.2, 125.8, 120.7, 115.6, 104.3, 98.2, 82.9, 78.1, 73.6, 55.7, 55.3, 55.2, 44.8, 43.2, 28.4, 27.9, 19.8 ppm; LRMS (ESI) Calculated for C34H43N6O8 m/z (M+H): 663.3, Obsd. 663.4.
4.2.6.8. tert-butyl (4R,10a′R,E)-2-((tert-butoxycarbonyl)imino)-1-methyl-1′,3′-dioxo-2′-phenyl-2′,3′,8′,9′,10′,10a′-hexahydro-1′H,7′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]cinnoline]-3-carboxylate (8h)
Prepared according to the general procedure 6, to give the spirocyclic guanidine as pale yellow solid in 76% yield (0.127 g). Rf = 0.13 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.59-7.33 (m, 5H), 5.43 (s, 1H), 4.42 (dd, J = 11.3, 3.4 Hz, 1H), 4.00 (d, J = 11.0 Hz, 1H), 3.82 (d, J = 11.0 Hz, 1H), 3.00 (s, 3H), 2.53 (m, 1H), 2.42 (m, 1H), 2.16 (m, 1H), 2.00 (m, 1H), 1.94 (m, 1H), 1.61 (m, 1H), 1.49 (m, 1H), 1.46 (s, 9H), 1.41 (s, 9H), 1.36 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.8, 153.1, 151.5, 149.3, 148.5, 142.6, 130.9, 129.0, 128.2, 126.0, 115.5, 83.0, 78.0, 73.6, 58.7, 54.2, 34.2, 32.2, 30.9, 28.3, 28.1, 28.0, 24.2 ppm; LRMS (ESI) Calculated for C29H39N6O6 m/z (M+H): 567.3, Obsd. 567.4.
4.2.6.9. tert-butyl (4R,5R,E)-5-benzyl-2-((tert-butoxycarbonyl)imino)-1-(cyclopropylmethyl)-7′-methyl-1′,3′-dioxo-2′-phenyl-2′,3′-dihydro-1′H,8′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]pyridazine]-3-carboxylate (8i)
Prepared according to general procedure 6, to give the spirocyclic guanidine as white solid in 88% yield (0.173 g). Rf = 0.30 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.51-7.13 (m, 10H), 5.57 (s, 1H), 5.24 (dd, J = 11.3, 4.3 Hz, 1H), 4.04 (dd, J = 14.6, 5.4 Hz, 1H), 3.92 (d, J = 16.6 Hz, 1H), 3.37 (dd, J = 14.6, 4.2 Hz, 1H), 2.88 (d, J = 16.6 Hz, 1H), 2.79 (dd, J = 14.6, 8.2 Hz, 1H), 2.67 (dd, J = 14.6, 11.3 Hz, 1H), 1.77 (s, 3H), 1.48 (s, 9H), 1.35 (s, 9H), 1.13 (m, 1H), 0.63 (m, 1H), 0.51 (m, 1H), 0.22 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.0, 151.8, 150.0, 149.8, 149.3, 135.9, 134.2, 131.0, 129.3, 129.0, 128.6, 128.4, 127.0, 125.8, 117.7, 83.4, 78.6, 77.2, 61.8, 48.0, 44.0, 34.8, 28.5, 28.1, 20.2, 8.5, 5.2, 2.5 ppm; LRMS (ESI) Calculated for C36H45N6O6 m/z (M+H): 657.3, Obsd. 657.4.
4.2.7. (4R,10a′R)-1-benzyl-2-imino-2′-phenyl-8′,9′,10′,10a′-tetrahydro-1′H,7′H-spiro[imidazolidine-4,5′-[1,2,4]triazolo[1,2-a]cinnoline]-1′,3′ (2′H)-dione (9a)
A 50 mL round-bottom flask was charged with the appropriate polycyclic guanidine (0.2 mmol). The CH2Cl2/TFA mixture (1:1, 4.0 mL) was added. The reaction mixture was stirred at room temperature for 2 hrs. The crude mixture was taken up in MeOH and 2 M HCl in ethyl ether was added. The mixture was again concentrated and the resulting solid triturated with ethyl ether. The solid was then collected to give the title compound as white solid in 50% yield (48 mg). 1H NMR (500 MHz, DMSO-d6) δ 9.81 (s, 1H), 8.56 (s, 2H), 7.52-7.32 (m, 10H), 5.56 (s, 1H), 4.79 (d, J = 12.4 Hz, 1H), 4.56 (d, J = 12.4 Hz, 1H), 4.42 (m, 1H), 4.15 (s, 1H), 3.79 (s, 1H), 2.37 (m, 1H), 2.18 (m, 2H), 1.83 (m, 1H), 1.75 (m, 1H), 1.55 (m, 1H), 1.37 (m, 1H), 1.21 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 156.7, 152.0, 149.1, 140.8, 134.1, 130.4, 128.9, 128.4, 128.3, 127.8, 127.6, 126.0, 116.5, 72.6, 57.0, 53.7, 47.8, 32.7, 29.8, 27.3, 23.2 ppm; HRMS (ESI) Calculated for C25H27N6O2 m/z (M+H): 443.2195, Obsd. 443.2197.
4.2.8 General procedure 7 for the deprotection and fragmentation of 8a–i
A 50 mL round-bottom flask was charged with the appropriate polycyclic guanidine (0.2 mmol). The CH2Cl2/TFA mixture (1:1, 4.0 mL) was added. The reaction mixture was stirred at room temperature for 2 hrs. The crude mixture was taken up in MeOH and 2 M HCl in ethyl ether was added. The mixture was again concentrated and the resulting solid triturated with ethyl ether. The solid was then collected to give the title compound.
4.2.8.1. (R,Z)-1-(2-((2-amino-1-benzyl-1H-imidazol-4-yl)methylene)cyclohexyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10a)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 50% yield (48 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.61 (s, 1H), 10.51 (s, 1H), 7.96 (s, 2H), 7.51-7.30 (m, 10H), 7.08 (s, 1H), 6.09 (s, 1H), 5.15 (d, J = 14.6 Hz, 1H), 5.10 (d, J = 14.6 Hz, 1H), 4.80 (s, 1H), 2.61 (m, 1H), 2.30 (m, 1H), 2.23 (m, 1H), 1.79 (m, 2H), 1.54 (m, 2H), 1.36 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 153.1, 152.8, 145.6, 140.1, 134.8, 131.3, 128.5, 127.8, 127.6, 127.3, 125.9, 121.7, 113.9, 113.1, 55.0, 47.4, 32.7, 29.2, 26.3, 20.2 ppm; HRMS (ESI) Calculated for C25H28N6O2 m/z (M+H): 443.2195, Obsd. 443.2197.
4.2.8.2. (R,Z)-1-(2-((2-amino-1,5-dibenzyl-1H-imidazol-4-yl)methylene)cyclohexyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10b)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 85% yield (97 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.59 (s, 1H), 10.53 (s, 1H), 7.99 (s, 2H), 7.46-6.99 (m, 15H), 6.17 (s, 1H), 5.06 (d, J = 17.5 Hz, 1H), 4.99 (d, J = 17.5 Hz, 1H), 4.89 (s, 1H), 3.82 (d, J = 17.5 Hz, 1H), 3.78 (d, J = 17.5 Hz, 1H), 2.54 (m, 1H), 2.42 (m, 1H), 2.28 (m, 1H), 1.82 (m, 2H), 1.64 (m, 1H), 1.54 (s, 9H), 1.36 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 153.5, 153.2, 147.2, 142.5, 136.7, 134.7, 131.8, 128.7, 128.6, 128.5, 127.9, 127.8, 127.6, 126.6, 126.4, 126.1, 123.4, 119.3, 118.9, 55.4, 45.3, 33.0, 30.1, 27.8, 27.3, 20.5 ppm; HRMS (ESI) Calculated for C32H34N6O2 m/z (M+H): 533.2665, Obsd. 533.2669.
4.2.8.3. (Z)-1-(3-(2-amino-1-benzyl-1H-imidazol-4-yl)-2-methylallyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10c)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 80% yield (70 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.63 (s, 1H), 11.02 (s, 1H), 7.99 (s, 2H), 7.50-7.31 (m, 10H), 7.25 (s, 1H), 6.16, (s, 1H), 5.17 (s, 2H), 4.35 (s, 2H), 1.84 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 152.6, 152.1, 145.8, 136.0, 135.2, 131.6, 128.8, 128.7, 128.0, 127.8, 127.5, 126.0, 122.0, 114.6, 114.5, 47.6, 47.2, 21.1 ppm; HRMS (ESI) Calculated for C22H23N6O2 m/z (M+H): 403.1882, Obsd. 403.1888.
4.2.8.4. (Z)-1-(3-(2-amino-1,5-dibenzyl-1H-imidazol-4-yl)-2-methylallyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10d)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 68% yield (72 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.73 (s, 1H), 11.05 (s, 1H), 7.96 (s, 2H), 7.52-7.02 (m, 15H), 6.30 (s, 1H), 5.05 (s, 2H), 4.12 (s, 2H), 3.82 (s, 2H), 1.83 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 152.8, 152.3, 147.2, 136.7, 136.6, 134.8, 131.8, 128.9, 128.7, 128.6, 128.0, 127.9, 127.7, 126.7, 126.2, 126.1, 123.6, 119.4, 114.9, 47.2, 45.3, 28.0, 21.3 ppm; HRMS (ESI) Calculated for C29H29N6O2 m/z (M+H): 493.2352, Obsd. 493.2363.
4.2.8.5. (Z)-1-(3-(2-amino-1-(benzo[d][1,3]dioxol-5-ylmethyl)-1H-imidazol-4-yl)-2-methylallyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10e)
Prepared according to the general procedure 7, to give the polycyclic guanidine as pale yellow solid in 81% yield (78 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.40 (s, 1H), 10.91 (s, 1H), 7.85 (s, 2H), 7.30-7.18 (m, 5H), 7.09 (s, 1H), 6.87 (s, 1H), 6.76-6.69 (m, 2H), 5.95 (s, 1H), 5.80 (s, 2H), 4.87 (s, 2H), 4.16 (s, 2H), 1.63 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 152.7, 152.3, 147.2, 145.7, 136.0, 131.7, 128.9, 128.8, 127.9, 126.1, 122.0, 121.8, 114.8, 114.4, 108.6, 108.4, 101.2, 47.5, 47.3, 21.2 ppm; HRMS (ESI) Calculated for C23H23N6O4 m/z (M+H): 447.1781, Obsd. 447.1785.
4.2.8.6. (Z)-1-(3-(2-amino-1-(benzo[d][1,3]dioxol-5-ylmethyl)-1H-imidazol-4-yl)-2-methylallyl)-4-ethyl-1,2,4-triazolidine-3,5-dione (10f)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 86% yield (75 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.58 (s, 1H), 10.63 (s, 1H), 8.01 (s, 2H), 7.24 (s, 1H), 7.05-6.88 (m, 3H), 6.09 (s, 1H), 6.00 (s, 2H), 5.05 (s, 2H), 4.24 (s, 2H), 3.42 (q, J = 7.3 Hz, 2H), 1.72 (s, 3H), 1.10 (t, J = 6.8 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 154.0, 153.6, 147.5, 147.2, 145.7, 136.2, 128.8, 122.0, 121.8, 114.5, 114.3, 108.6, 108.4, 101.2, 47.5, 47.1, 33.5, 21.1, 13.1 ppm; HRMS (ESI) Calculated for C19H23N6O4 m/z (M+H): 399.1781, Obsd. 399.1786.
4.2.8.7. (Z)-1-(3-(2-amino-1-(2,4-dimethoxybenzyl)-1H-imidazol-4-yl)-2-methylallyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10g)
Prepared according to the general procedure 7, to give the polycyclic guanidine as pale yellow solid in 80% yield (80 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.63 (s, 1H), 11.07 (s, 1H), 7.88 (s, 2H), 7.48-7.23 (m, 6H), 6.96 (s, 1H), 6.56-6.50 (m, 2H), 6.14 (s, 1H), 4.97 (s, 2H), 4.33 (s, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 1.82 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 160.9, 158.0, 152.7, 152.2, 145.8, 135.7, 131.6, 130.2, 128.8, 127.8, 126.1, 126.0, 121.5, 114.9, 114.8, 114.5, 104.6, 98.5, 55.5, 55.3, 47.2, 43.5, 21.2 ppm; HRMS (ESI) Calculated for C24H27N6O4 m/z (M+H): 463.2094, Obsd. 463.2094.
4.2.8.8. (R,Z)-1-(2-((2-amino-1-methyl-1H-imidazol-4-yl)methylene)cyclohexyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10h)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 83% yield (67 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.57 (s, 1H), 10.59 (s, 1H), 7.77 (s, 2H), 7.46-7.37 (m, 5H), 7.02 (s, 1H), 6.09 (s, 1H), 4.81 (s, 1H), 3.43 (s, 3H), 2.63 (m, 1H), 2.37 (m, 1H), 2.23 (m, 1H), 1.82 (m, 2H), 1.53 (m, 2H), 1.37 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 153.1, 152.8, 145.8, 139.9, 131.4, 128.5, 127.6, 126.0, 121.1, 115.0, 113.0, 55.2, 32.8, 32.1, 29.4, 26.6, 20.3 ppm; HRMS (ESI) Calculated for C19H23N6O2 m/z (M+H): 367.1882, Obsd. 367.1880.
4.2.8.9. (Z)-1-(3-(2-amino-5-benzyl-1-(cyclopropylmethyl)-1H-imidazol-4-yl)-2-methylallyl)-4-phenyl-1,2,4-triazolidine-3,5-dione (10i)
Prepared according to the general procedure 7, to give the polycyclic guanidine as white solid in 80% yield (79 mg). 1H NMR (500 MHz, DMSO-d6) δ 12.53 (s, 1H), 10.96 (s, 1H), 7.76 (s, 2H), 7.50-7.19 (m, 10H), 6.28 (s, 1H), 4.49 (s, 2H), 4.00 (s, 2H), 3.66 (d, J = 4.2 Hz, 2H), 1.82 (s, 3H), 0.89 (m, 1H), 0.35 (m, 2H), 0.27 (m, 2H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 152.8, 152.2, 146.7, 137.0, 136.1, 131.7, 128.8, 128.7, 128.0, 127.8, 126.7, 126.1, 123.4, 119.0, 114.8, 47.3, 46.0, 27.8, 21.2, 10.0, 3.3 ppm; HRMS (ESI) Calculated for C26H30N6O2 m/z (M+H): 458.2430, Obsd. 458.2430.
4.2.9. General procedure 8 for the hetero cycloaddition of cyclic guanidines
To a stirring solution of the preferred cyclic guanidine (diene,1 equiv) and benzyl hydroxycarbamate 11 (dienophile, 1.2 equiv) in THF (0.07 M) was added CuCl (20 mol%) and pyridine (5 mol%). The reaction was stirred at room temperature open to the air until completed by TLC. Upon completion, the reaction was quenched with EDTA (0.5 M, pH 7.0), diluted with ethyl acetate and stirred for 30 min. The reaction was extracted with ethyl acetate three times and the combined organic layers were then dried over Na2SO4, filtered, and condensed by rotary evaporation. Purification was accomplished by flash chromatography to give the polycyclic guanidine.
4.2.9.1. 6-benzyl 1-(tert-butyl) (4aR,6aS,E)-2-((tert-butoxycarbonyl)imino)-3-methyl-3,4,4a,6a,7,8,9,10-octahydro-1H-benzo[c]pyrimido[4,5-e][1,2]oxazine-1,6(2H)-dicarboxylate (12a)
The general procedure 8 was used to give the polycyclic guanidine as pale yellow solid. (0.160 g, 72% yield). Rf = 0.52 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.37-7.31 (m. 5H), 5.25 (d, J = 12.7 Hz, 1H), 5.20 (d, J = 12.7 Hz, 1H), 4.80 (br, 1H), 3.59 (dd, J = 14.07, 7.3 Hz, 1H), 3.25 (d, J = 13.7 Hz, 1H), 3.16 (d, J = 14.2 Hz, 1H), 2.98 (s, 3H), 2.10 (m, 1H), 1.85 (m, 2H), 1.68 (m, 2H), 1.51 (s, 9H), 1.45 (m, 1H), 1.41 (s, 9H), 1.32 (m, 1H), 1.27 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.4, 154.2, 151.7, 149.2, 136.0, 134.0, 128.7, 128.6, 128.4, 128.2, 82.6, 78.6, 76.6, 67.7, 56.4, 49.9, 37.5, 32.2, 28.3, 28.1, 28.0, 26.3, 24.8 ppm; LRMS (ESI) Calculated for C29H41N4O7 m/z (M+H): 567.3, Obsd. 567.4.
4.2.9.2. 6-benzyl 1-(tert-butyl) (4aR,6aS,E)-2-((tert-butoxycarbonyl)imino)-3-(2,4-dimethoxybenzyl)-3,4,4a,6a,7,8,9,10-octahydro-1H-benzo[c]pyrimido[4,5-e][1,2]oxazine-1,6(2H)-dicarboxylate (12b)
Prepared according to the general procedure 8, to give the polycyclic guanidine as pale yellow solid in 51% yield (0.141 g). Rf = 0.33 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.31 (m, 5H), 7.13 (m, 1H), 6.39 (m, 2H), 5.17 (d, J = 14.2 Hz, 1H), 5.14 (d, J = 14.2 Hz, 1H), 5.05 (d, J = 14.7 Hz, 1H), 4.76 (br, 1H), 4.07 (d, J = 14.7 Hz, 1H), 3.76 (s, 3H), 3.73 (s, 3H), 3.47 (dd, J = 14.7, 7.3 Hz, 1H), 3.32 (m, 1H), 3.22 (d, J = 14.7 Hz, 1H), 2.03 (m, 1H), 1.83 (m, 2H), 1.68 (m, 1H), 1.49 (s, 9H), 1.46 (m, 1H), 1.39 (s, 9H), 1.37 (m, 1H), 1.25 (m, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 160.6, 159.6, 159.0, 158.7, 154.4, 152.3, 149.5, 135.8, 130.5, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 117.0, 104.4, 98.5, 82.8, 79.0, 77.2, 67.6, 55.5, 55.4, 47.2, 46.2, 32.4, 28.5, 28.4, 28.3, 28.1, 26.6, 24.9 ppm; LRMS (ESI) Calculated for C37H49N4O9 m/z (M+H): 693.3, Obsd. 693.5.
4.2.9.3. 2-benzyl 5-(tert-butyl) (R,E)-7-(benzo[d][1,3]dioxol-5-ylmethyl)-6-((tert-butoxycarbonyl)imino)-4-methyl-6,7,8,8a-tetrahydro-2H-pyrimido[4,5-e][1,2]oxazine-2,5(3H)-dicarboxylate (12c)
Prepared according to the general procedure 8, to give the polycyclic guanidine as white solid in 61% yield (0.155 g). Rf = 0.38 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.33 (m, 5H), 6.74-6.67 (m, 3H), 5.90 (s, 2H), 5.22 (d, J = 12.2 Hz, 1H), 5.15 (d, J = 12.2 Hz, 1H), 4.81 (br, 1H), 4.68 (d, J = 15.2 Hz, 1H), 4.36 (d, J = 15.7 Hz, 1H), 4.33 (d, J = 15.2 Hz, 1H), 3.92 (d, J = 15.7 Hz, 1H), 3.38 (dd, J = 14.7, 7.3 Hz, 1H), 3.10 (d, J = 14.7 Hz, 1H), 1.95 (s, 3H), 1.52 (s, 9H), 1.43 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.9, 155.0, 151.8, 149.3, 148.0, 147.3, 135.8, 129.7, 128.7, 128.6, 128.3, 127.6, 123.9, 121.9, 108.9, 108.3, 101.1, 82.9, 79.3, 75.8, 68.1, 52.0, 48.3, 46.5, 28.3, 28.1, 14.8 ppm; LRMS (ESI) Calculated for C33H41N4O9 m/z (M+H): 637.3, Obsd. 637.4.
4.2.10 General procedure 9 for the deprotection and salt exchange of 12a–c
A 50 mL round-bottom flask was charged with the appropriate polycyclic guanidine (0.2 mmol). The CH2Cl2/TFA mixture (1:1, 4.0 mL) was added. The reaction mixture was stirred at room temperature for 2 hrs, then the solvent removed under reduced pressure. The crude mixture was taken up in MeOH and 2 M HCl in ethyl ether was added. The mixture was again concentrated and the resulting solid triturated with ethyl ether. The solid was then collected to give the title compound.
4.2.10.1. (4aR,6aS)-3-methyl-4,4a,6,6a,7,8,9,10-octahydro-1H-benzo[c]pyrimido[4,5-e][1,2]oxazin-2(3H)-imine (13a)
Prepared according to general procedure 9 (0.3 mmol scale), to give polycyclic guanidine as yellow solid in 95% yield (74 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 8.08 (s, 2H), 5.07 (s, 1H), 3.78 (s, 1H), 3.69 (s, 1H), 3.30 (d, J = 12.2 Hz, 1H), 3.01 (s, 3H), 2.92 (d, J = 12.2 Hz, 1H), 2.10 (m, 1H), 1.78 (m, 3H), 1.71 (m, 1H), 1.51 (m, 2H), 1.15 (m, 1H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 151.7, 119.5, 111.1, 66.3, 54.7, 47.4, 37.2, 30.2, 26.1, 25.9, 23.7 ppm; HRMS (ESI) Calculated for C11H19N4O m/z (M+H): 223.1559, Obsd. 223.1561.
4.2.10.2. (4aR,6aS)-3-(2,4-dimethoxybenzyl)-4,4a,6,6a,7,8,9,10-octahydro-1H-benzo[c]pyrimido[4,5-e][1,2]oxazin-2(3H)-imine (13b)
Prepared according to the general procedure 9 (0.3 mmol scale), to give polycyclic guanidine as pale yellow solid in 89% yield (105 mg). 1H NMR (500 MHz, DMSO-d6) δ 11.02 (s, 1H), 8.28 (s, 2H), 7.21 (m, 1H), 6.61-6.52 (m, 2H), 4.98 (m, 1H), 4.55 (d, J = 15.7 Hz, 1H), 4.47 (d, J = 15.7 Hz, 1H), 3.80 (s, 3H), 3.76 (s, 3H), 3.55 (m, 1H), 3.15 (t, J = 10.7 Hz, 1H), 2.95 (m, 1H), 2.10 (m, 1H), 1.77 (m, 3H), 1.50 (m, 2H), 1.17 (m, 1H); 13C{1H} NMR (100 MHz, DMSO-d6) δ 160.8, 158.3, 151.5, 130.4, 119.7, 113.7, 104.7, 98.6, 66.4, 55.6, 55.3, 54.7, 48.0, 45.0, 30.2, 26.2, 25.9, 23.8 ppm; HRMS (ESI) Calculated for C19H27N4O3 m/z (M+H): 359.2083, Obsd. 359.2092.
4.2.10.3. (R)-7-(benzo[d][1,3]dioxol-5-ylmethyl)-4-methyl-3,7,8,8a-tetrahydro-2H-pyrimido[4,5-e][1,2]oxazin-6(5H)-imine (13c)
Prepared according to general procedure 9 (0.3 mmol scale), to give polycyclic guanidine as yellow solid in 95% yield (96 mg). 1H NMR (500 MHz, DMSO-d6) δ 11.02 (s, 1H), 8.40 (s, 2H), 6.94-6.82 (m, 3H), 6.01 (s, 2H), 4.97 (m, 1H), 4.59 (d, J = 16.1 Hz, 1H), 4.53 (d, J = 16.1 Hz, 1H), 3.77 (d, J = 15.7 Hz, 1H), 3.62 (m, 1H), 3.59 (d, J = 15.7 Hz, 1H), 3.13 (t, J = 10.3 Hz, 1H), 1.77 (s, 3H) ppm; 13C{1H} NMR (100 MHz, DMSO-d6) δ 151.3, 147.6, 147.1, 127.9, 121.9, 121.5, 108.4, 108.3, 104.4, 101.2, 66.6, 51.9, 46.4, 45.4, 13.4 ppm; HRMS (ESI) Calculated for C15H19N4O3 m/z (M+H): 303.11457, Obsd. 303.1458.
4.2.11. N-((E)-amino((cyclopropylmethyl)(4-methylpent-4-en-2-yn-1-yl)amino)methylene)cinnamamide (14)
N-cyanocinnamamide (0.172 g, 1.0 mmol) was stirred in CH2Cl2 (10 mL). Chlorotrimethylsilane (0.152 mL, 1.2 mmol) and NEt3 (0.209 mL, 1.5 mmol) were then added and the mixture stirred for 15 min. Secondary amine S-2k (0.149 g, 1.0 mmol) was then added. Upon completion (as judged by TLC on a basified reaction aliquot), the reaction mixture was washed 3 times with NaHCO3 and then washed with a sat. brine solution. The combined organic layers were dried over Na2SO4, filtered and concentrated by rotary evaporation. Purification was accomplished by flash chromatography to yield the propagylguaniline as a pale yellow oil (0.209 g, 65% yield). Rf = 0.44 (60% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 16.2 Hz, 1H), 7.55 (m, 2H), 7.36-7.29 (m. 3H), 6.67 (d, J = 16.2 Hz, 1H), 5.29 (s, 1H), 5.24 (s, 1H), 4.50 (s, 2H), 3.46 (s, 2H), 1.86 (s, 3H), 1.08 (m, 1H), 0.59 (m, 2H), 0.34 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.8, 160.6, 139.9, 136.0, 129.2, 129.0, 128.7, 127.9, 126.1, 122.7, 86.1, 82.7, 51.4, 37.2, 23.4, 9.6, 4.0 ppm; LRMS (ESI) Calculated for C20H24N3O m/z (M+H): 322.2, Obsd. 322.2.
4.2.12 (R,Z)-1-(cyclopropylmethyl)-3-(2-methylallylidene)-7-phenyl-2,3,6,7-tetrahydroimidazo[1,2-a]pyrimidin-5(1H)-one (15)
To a stirring solution of propargylguanidine 14 (0.5 mmol) in acetonitrile (5.0 mL) was added silver nitrate (0.05 mmol, 10 mol%). After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to yield the bicyclic guanidine as pale yellow solid in 83% yield (0.133 g). Rf = 0.43 (60% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.39-7.22 (m, 5H), 5.67 (s, 1H), 4.99 (s, 1H), 4.75 (s, 1H), 4.74 (dd, J = 10.8, 4.4 Hz, 1H), 4.13 (d, J = 11.9 Hz, 1H), 4.05 (d, J = 11.9 Hz, 1H), 3.33 (dd, J = 14.0, 6.5 Hz, 1H), 3.22 (dd, J = 14.0, 6.5 Hz, 1H), 2.84 (dd, J = 17.7, 4.4 Hz, 1H), 2.47 (dd, J = 17.7, 10.8 Hz, 1H), 1.83 (s, 3H), 1.01 (m, 1H), 0.59 (m, 2H), 0.26 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 166.3, 151.6, 143.5, 141.1, 128.6, 127.2, 126.5, 126.4, 116.1, 114.9, 56.4, 50.9, 49.1, 40.6, 22.0, 8.8, 3.7, 3.5 ppm; LRMS (ESI) Calculated for C20H24N3O m/z (M+H): 322.2, Obsd. 322.2.
4.2.13. (3R,7R)-1-(cyclopropylmethyl)-7′-methyl-2′,7-diphenyl-1,2,2′,6,7-pentahydro-1′H,5H,8′H-spiro[imidazo[1,2-a]pyrimidine-3,5′-[1,2,4]triazolo[1,2-a]pyridazine]-1′,3′,5-trione (16)
To a stirring solution of the desired 5-exo-dig cyclic guanidine (diene, 1 equiv) in CH2Cl2 (0.07 M) was added the appropriate triazoledione (dienophile, 1.2 equiv). After completion, the reaction mixture was concentrated by rotary evaporation, and purified by column chromatography to give the polycyclic guanidine as white solid in 40% yield (0.060 g). Rf = 0.33 (40% ethyl acetate/n-hexanes). 1H NMR (500 MHz, CDCl3) δ 7.51-7.22 (m, 10H), 5.54 (d, J = 1.5 Hz, 1H), 4.78 (dd, J = 11.7, 4.9 Hz, 1H), 4.37 (d, J = 16.6 Hz, 1H), 4.17 (d, J = 9.8 Hz, 1H), 4.07 (d, J = 16.6 Hz, 1H), 3.73 (d, J = 9.8 Hz, 1H), 3.43 (dd, J = 14.9, 6.9 Hz, 1H), 3.16 (dd, J = 14.9, 6.9 Hz, 1H), 2.73 (dd, J = 16.6, 4.9 Hz, 1H), 2.40 (dd, J = 16.6, 11.7 Hz, 1H), 1.94 (s, 3H), 1.01 (m, 1H), 0.55 (m, 2H), 0.23 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 167.9, 151.1, 150.2, 149.4, 144.2, 132.5, 131.1, 129.4, 128.7, 128.5, 127.2, 126.5, 125.7, 119.7, 72.0, 57.0, 56.5, 49.8, 45.4, 40.2, 20.1, 8.8, 3.7, 3.6 ppm; HRMS (ESI) Calculated for C28H29N6O3 m/z (M+H): 497.2301, Obsd. 497.2309.
Supplementary Material
Acknowledgments
We are very grateful to Prof. J. Rainier and M. Sigman for helpful discussions. REL thanks the NIH, General Medical Sciences (2R01 GM090082-A1, P41 GM08915) Amgen and Eli Lilly for financial support. We thank Dr. Atta Aarif (U. of U. Chemistry) for help with X-ray crystallography analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Experimental procedures and spectroscopic data for all new compounds. X-ray crystallography data for compounds 4a, 9a, 10b and 16.
References
- 1.Forte B, Malgesini B, Piutti C, Quartieri F, Scolaro A, Papeo G. A Submarine Journey: The Pyrrole-Imidazole Alkaloids. Marine Drugs. 2009;7(4):705–753. doi: 10.3390/md7040705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Ma Z, Wang X, De S, Ma Y, Chen C. Dimeric pyrrole-imidazole alkaloids: Synthetic approaches and biosynthetic hypotheses. Chemical communications (Cambridge, England) 2014;50(63):8628–8639. doi: 10.1039/c4cc02290d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Harris TL, Worthington RJ, Melander C. Potent Small-Molecule Suppression of Oxacillin Resistance in Methicillin-Resistant Staphylococcus aureus. Angew Chem Int Ed. 2012;51(45):11254–11257. doi: 10.1002/anie.201206911. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yeagley AA, Su Z, McCullough KD, Worthington RJ, Melander C. N-Substituted 2-aminoimidazole inhibitors of MRSA biofilm formation accessed through direct 1,3-bis(tert-butoxycarbonyl)guanidine cyclization. Org Biomol Chem. 2013;11(1):130–137. doi: 10.1039/c2ob26469b. [DOI] [PubMed] [Google Scholar]; (c) Worthington RJ, Richards JJ, Melander C. Non-Microbicidal Control of Bacterial Biofilms with Small Molecules. Anti-Infect Agents. 2014;12(1):120–138. [Google Scholar]
- 4.Al Mourabit A, Potier P. Sponge’s Molecular Diversity Through the Ambivalent Reactivity of 2-Aminoimidazole: A Universal Chemical Pathway to the Oroidin-Based Pyrrole-Imidazole Alkaloids and Their Palau’amine Congeners. European Journal of Organic Chemistry. 2001;2001(2):237–243. [Google Scholar]
- 5.Gainer MJ, Bennett NR, Takahashi Y, Looper RE. Regioselective Rhodium (II)‐Catalyzed Hydroaminations of Propargylguanidines. Angew Chem. 2011;123(3):710–713. doi: 10.1002/anie.201006087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroif-Gregoire C, Travert N, Zaparucha A, Al-Mourabit A. Direct Access to Marine Pyrrole-2-aminoimidazoles, Oroidin, and Derivatives, via New Acyl-1,2-dihydropyridin Intermediates. Org Lett. 2006;8(14):2961–2964. doi: 10.1021/ol0608451. [DOI] [PubMed] [Google Scholar]
- 7.(a) Frazier CP, Engelking JR, Read de Alaniz J. Copper-Catalyzed Aerobic Oxidation of Hydroxamic Acids Leads to a Mild and Versatile Acylnitroso Ene Reaction. J Am Chem Soc. 2011;133(27):10430–10433. doi: 10.1021/ja204603u. [DOI] [PubMed] [Google Scholar]; (b) Frazier CP, Bugarin A, Engelking JR, Read de Alaniz J. Copper-Catalyzed Aerobic Oxidation of N-Substituted Hydroxylamines: Efficient and Practical Access to Nitroso Compounds. Org Lett. 2012;14(14):3620–3623. doi: 10.1021/ol301414k. [DOI] [PubMed] [Google Scholar]; (c) Chaiyaveij D, Cleary L, Batsanov AS, Marder TB, Shea KJ, Whiting A. Copper(II)-Catalyzed Room Temperature Aerobic Oxidation of Hydroxamic Acids and Hydrazides to Acyl-Nitroso and Azo Intermediates, and Their Diels–Alder Trapping. Org Lett. 2011;13(13):3442–3443. doi: 10.1021/ol201188d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


