Abstract
Over 4 days, more than 500 scientists involved in HIV persistence research shared their new unpublished data and designed future perspectives towards ART-free HIV remission. This 8th International Workshop on HIV Persistence followed the format of past conferences but further focused on encouraging participation of young investigators, especially through submission of oral and poster presentations. The topic of the workshop was HIV persistence. Consequently, issues of HIV reservoirs and HIV cure were also addressed. In this article, we report the discussions as closely as possible; however, all the workshop abstracts can be found online at www.viruseradication.com.
Keywords: HIV persistence, HIV reservoirs, HIV cure, HIV functional cure, HIV eradication
Opening keynote lecture
The opening keynote lecture about ‘Obstacles and Opportunities of Sustained ART-Free HIV Remission’ was given by Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases (NIAID). His talk outlined the differences between a ‘classic’ cure (complete eradication of the replication-competent viral reservoir) and sustained virological remission (control of HIV rebound in the absence of antiretroviral therapy [ART]) [1]. He discussed how potential pathways to a classic cure (latency-reversing agents [LRAs], modified antibodies, engineered T cells, stem cell transplantation, and gene editing) have yet to demonstrate robust reservoir eradication that would be as safe and scalable to a broad population as once-daily ART. In contrast, Dr Fauci discussed recent results that suggest that a sustained virological remission might be more readily achievable. He presented data on early ART-treated individuals that demonstrate some degree of control of viraemia in up to 40% of individuals 4 months after treatment cessation [2]. Importantly, the rate of viral rebound did not correlate with the size of the reservoir. He also discussed recent encouraging results in which passive transfer of either HIV-specific [3–5] or anti-α4β7 [6] antibodies have been successful in inducing immunological control of viral rebound for prolonged periods following ART cessation.
In a second opening talk, Ronald Desrosiers provided an update on his so-called ‘Miami monkey’, in which he achieved sustained virological remission by delivering two broadly neutralising monoclonal antibodies (bNAbs) using an adeno-associated virus (AAV) vector [7,8]. Despite the inability to recover virus for 20 months, Dr Desrosiers reported that his team were finally able to recover and characterise virus in three successful attempts, demonstrating that this was indeed a remission and not a complete eradication of the viral reservoir.
Satellite symposium: NIH Martin Delaney Collaboratories
A satellite symposium was convened by the National Institute of Allergy and Infectious Diseases (NIAID) to provide updates on research highlights from each of the six Martin Delaney Collaboratories (MDC) for HIV cure research. This is the second iteration of the MDC programme, under which the number of groups funded was expanded from the original three to the current six groups beginning in July 2016. The programme is co-funded by NIAID, the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). The programme was designed to facilitate partnerships between academia, industry, government, and community to move HIV cure research forward more rapidly than could be accomplished by individual groups working alone.
David Margolis presented highlights for the CARE Collaboratory based at the University of North Carolina, Chapel Hill. CARE is collaborating with partners at GSK to develop mimetics of the 2nd Mitochondrial Activator of Caspase (SMAC) as LRAs for HIV. SMAC mimetics activate a non-canonical NFkB pathway with fewer effects on host genes as compared to PKC agonists [9,10]. CARE is also collaborating with MacroGenics to develop engineered antibody-based agents termed DARTs [11] that would recognise HIV peptides in the context of HLA-E on the surface of reservoir cells after latency reversal. HLA-E is an attractive target because it is less polymorphic across populations. On the basic research front, CARE is beginning to explore the use of single-cell transcriptomics to more precisely characterise host gene expression in the context of latent HIV. These studies may provide clues for how to better target latently infected cells. Finally, CARE continues to refine its humanised mouse [12] and non-human primate (NHP) models for the assessment of efficacy of combinations of LRAs and reservoir targeting agents in vivo.
Dan Barouch provided an update on the overall research strategy of the I4C Collaboratory based at the Beth Israel Deaconess Medical Center (BIDMC). I4C is engaging in ongoing collaborations with Janssen and Gilead to determine the effects of therapeutic vaccination [13], or passive bNAb administration [14], combined with latency reversal on viral control and reservoir size after ART interruption. I4C has an ongoing controlled study of the bNAb PGT121 and a TLR7 agonist alone or in combination in non-human primates (NHP) (n=11 per group) to determine the effect of these agents on viral rebound and subsequent control of viraemia following ART interruption. I4C is also currently planning two clinical trials. One will be a controlled test of a conserved gag-pol peptide-stimulated dendritic cell (DC) vaccine in 28 acutely infected participants (Fiebig stage I–III) from the RV254 clade AE cohort in Thailand. An analytical treatment interruption (ATI) will determine the effect of the vaccine on viral rebound and control. This trial is expected to begin in late 2018. The other trial will test passive administration of a combination of two bNAbs (PGT121 + PGDM1400) in 30 participants from the clade C FRESH cohort of early-treated individuals (Fiebig stage I–III) in South Africa in a placebo controlled, randomised study. An ATI will determine the effect of the interventions on time to viral rebound and control of viraemia. The study is expected to begin in early 2019.
Steven Deeks gave an overview of highlights from the DARE Collaboratory based at the University of California, San Francisco. DARE investigators demonstrated that very early treatment of NHP or human participants [15] (days 1–6 post-infection) can dramatically affect the size of the HIV DNA reservoir and result in an extended period of viral control (200–600 days) before rebound, which presumably occurs due to eventual stochastic reactivation of a latently-infected cell. DARE also demonstrated that a CMV-based SIV vaccine that leads to sustained control of viraemia when given prior to infection fails to control viraemia when given after infection has been established [16,17]. DARE hypothesises that combining the CMV/SIV vaccine with another immune modulator will be necessary for efficacy in the SIV-infected NHP model. DARE is exploring the effects of immune checkpoint inhibitors (anti-PD-1 and anti-CTLA-4) alone or in combination on viral control in NHP. On the basic research front, DARE is exploring the role of HIV-specific CD8 T cells in persistence and whether disruption of the B cell follicle will enable CD8-mediated clearance or control of the reservoir [18].
Douglas Nixon and Brad Jones presented highlights from the BELIEVE Collaboratory, based at the George Washington University. BELIEVE is collaborating with partners Altor and Torque Therapeutics to engineer cytotoxic T cells to deliver IL-15 superagonist to HIV-infected cells in nanocapsules termed ‘backpacks’ [19]. BELIEVE is also developing a Hu-HIV-LAT mouse model in which reservoir cells are transplanted into mice from participants on ART [20]. This model can be used in preclinical studies to evaluate the efficacy of reservoir activation and elimination strategies. BELIEVE is pursuing two strategies to increase CD8 T cell concentration in B cell follicles: (1) transient treatment with IL-15 superagonist; and (2) engineering chimeric antigen receptor (CAR) T cells to express CXCR5. BELIEVE will initiate a clinical trial of their HST-NEETs strategy in early 2018 in which T cells are isolated from participants, stimulated ex vivo with peptides representing a mosaic of conserved HIV-specific T cell epitopes, and then re-infused into the participant.
Keith Jerome provided an update from the defeatHIV Collaboratory based at the Fred Hutchinson Cancer Research Center (FHCRC). defeatHIV is pursuing three approaches towards control or elimination of reservoirs [21]. In the first approach, defeatHIV successfully engrafted anti-SHIV CAR T cells in NHP, but found relatively low persistence of dual allele CCR5 knockout cells. A subsequent study will utilise a cell-surface C46 HIV fusion inhibitor peptide to protect CAR T cells from SHIV infection instead of CCR5 knockout to see if this improves expansion and persistence of engineered cells. In the second approach, defeatHIV is optimising an eCD4-Ig chimeric antibody-based therapeutic to maximise antibody-dependent cellular cytotoxicty (ADCC) [22] and avoid anti-drug antibody. The resulting construct will be delivered to NHP in an AAV vector either alone or in combination with a TLR7 agonist from Gilead. In the third approach, defeatHIV is testing a conserved HIV DNA prime-boost therapeutic vaccine alone or in combination with engineered CCR5 knockout T cells in SHIV infected NHP (n=8 per arm). Future studies will combine the most promising approaches to test for synergistic effects.
Luis Montaner presented highlights from the BEAT-HIV Collaboratory, based at the Wistar Institute. On the basic research front, BEAT-HIV investigators launched a multi-pronged effort that ruled out CD32 as a biomarker for latently infected cells. The data were presented in multiple presentations during the main workshop. On the clinical front, BEAT-HIV is planning two trials to begin in the first half of 2018. The first trial will determine the immunological and virological effects of adding IFNα2b treatment to a two-bNAb regimen (3BNC117 + 10-1074) following ART cessation. The study will include an ATI to determine the rate of viral rebound and control. The second trial will determine the effect of HIV-resistant, CD4-based CAR T cell infusion [23] on viral rebound/control after ART cessation. The study will utilise two approaches to render the CAR T cells resistant to HIV infection: (1) zinc finger nuclease (ZFN)-mediated knockout of CCR5 [24]; and (2) expression of an engineered CXCR4-linked to a C34 HIV fusion inhibitor peptide [25].
Session 1 and Session 2: Basic science of HIV latency I and II
The Conference on HIV Persistence During Therapy serves as an international forum to showcase the latest advances in research pursuing a cure for HIV infection and, in particular, the reservoirs that support viral persistence. Two of the eight sessions focused on the research aimed at understanding the nature of the viral reservoirs that maintain HIV in the face of therapy as well as strategies being developed for the elimination of those reservoirs. Robert Siliciano presented an overview on the current understanding of viral latency [26]. Three processes have been proposed to maintain HIV persistence in the face of effective ART; latency, homeostatic proliferation and cryptic viral replication. Of these, viral latency has received the most attention and is considered the single largest obstacle in finding the cure for HIV infection. In latent HIV infection, the virus establishes a transcriptionally silent infection in quiescent, memory CD4 T cells and, as such, is hidden from immune surveillance. HIV persistence in the latent reservoir is principally a consequence of the intrinsic stability of the memory CD4 T cell, which may have the capacity to support life-long persistence of the virus. This reservoir may further be maintained through the process of homeostatic proliferation where latent proviruses may be duplicated in daughter cells formed at mitosis. The vast majority of proviruses are defective in that they contain inactivating mutations (owing to, for example, mutations in essential regulatory or enzymatic processes of the virus that eliminate the function of that element) or deletions in the viral genome. As a consequence, homeostatic proliferation would be expected to lead to the duplication of inactive proviruses. It is also likely that homeostatic proliferation would result in selection against intact proviruses. If the process of mitosis led to reactivation of the provirus, renewed viral gene expression could trigger viral cytopathic effects and/or clearance of the infected cell through cell mediated immunity. The extent by which homeostatic proliferation promotes expansion of intact proviruses is not yet well understood.
There have been significant technical challenges to developing assays that reliably measure the size of the latent virus reservoir and, as a consequence, monitoring the efficacy of approaches aimed at reducing the latent reservoir has been challenging. Because of the large population of defective proviruses, PCR-based assays that enumerate the number of proviruses greatly overestimate the size of the latent reservoir. Some researchers have focused on the use of viral outgrowth assays that directly quantitate inducible HIV. However, these assays are labour intensive, require large numbers of CD4 T cells, and lack precision. Dr Siliciano [26] described the use of a digital droplet PCR assay that can be used to quantify intact and defective proviruses. Such approaches may prove valuable for the understanding of the nature of the latent reservoir, but also provide accurate assessment of the efficacy of reservoir depletion approaches.
Some proviral insertion sites can result in insertional activation, whereby a cellular gene is placed under control of the transcriptional apparatus of the provirus. If that cellular gene were involved in cell cycle or growth, this could lead to a proliferative advantage to the host cell and clonal expansion of the provirus. This has been demonstrated to be the case in infected individuals where clonally expanded proviruses representing a significant percentage of the total proviral population can be observed [27]. Expanded proviruses also appear capable of producing replication-competent virus [28]. This theme was further expanded by Varabyou et al. [29]. The authors developed an HIV SortSeq approach to isolate cells from infected individuals harbouring inducible HIV genomes and then used RNAseq to identify RNA transcripts. Some transcripts were found to be chimeric-harbouring HIV and host sequences that resulted from HIV integration into cancer genes. This illustrates the process of insertional activation that has been long recognised for cancer-related retroviruses including murine leukaemia virus. Although HIV integration into cancer genes could confer a proliferative advantage to the cell, there has been no clear demonstration of malignancies caused by insertional activation of an HIV provirus into a cancer gene.
Strategies for the elimination of the latent reservoir have been based primarily on latency reversal, with the premise that reversing latency and renewing viral gene expression would render the cell vulnerable to elimination – either through viral cytopathicity or through cell-mediated immune clearance. This approach has been referred to as ‘kick and kill’ and numerous agents have been described that have the capacity to reverse HIV latency in vitro. The challenge has been to identify agents that can induce HIV gene expression leading to antigen production while at the same time avoiding overt activation of the infected cell. However, the majority of latency-reversing agents identified to date have neither potent kick nor potent kill activity. As a result, these agents have yielded poor results when used in infected individuals in order to reduce the size of the viral reservoirs. Gramatica et al. [30] described results with two new latency-reversing agents. The authors previously demonstrated that activators of the AKT/mTOR pathway reverse HIV latency. They focused on two small molecules, SB-216763 and Tideglusib, that by inhibiting glycogen synthase kinase-3 can stimulate the AKT/mTOR pathway. These agents were assessed in a tissue-based model of viral latency and on CD4 T cells from individuals with HIV. Both of them were found to activate latent HIV to levels exceeding that achieved by CD3/CD28 costimulation. Importantly, latency reversal was not accompanied by T cell activation. These agents offer promise for approaches reducing viral reservoir size through latency reversal.
The true nature of HIV latency, as it exists in CD4 T cells in vivo, remains ill-defined. Latency is generally believed to reflect a provirus in a transcriptionally silent, yet inducible state. However, several lines of evidence suggest that the reservoirs that persist in the face of effective ART may not be as quiescent as originally believed. For example, there is a direct correlation between the levels of cell-associated viral RNA and time to viral rebound following ATI. It is unclear whether the transcriptionally active cells that persist in ART-suppressed individuals are latently infected cells. Browne and colleagues conducted single cell analysis of latency models and identified a subset of latently infected cells that can transcribe viral RNA in the absence of stimulation [31]. Interestingly, approximatively 500 RNA copies/cell were needed before antigen could be detected. The results suggest that latency may be a dynamic state represented by cells at various levels of basal transcription. However, a threshold of transcription needs to be crossed before antigen is expressed. Although not surprising, the study indicates that some integration sites would allow leaky latency. Furthermore, the presence of viral transcripts in latently infected cells could activate innate sensors that facilitate detection of these cells.
The identification of markers that distinguish latently infected cells from uninfected cells could potentially represent an important advance in the HIV cure field. Such markers could allow specific targeting of vectors to the latent reservoir or selective killing of reservoir cells by CAR T cells or cytolytic antibodies. Early studies proposed HIV latency to be a transcriptionally silent state and as such, the presence of a provirus was the only distinguishing feature between a latently infected cell and an uninfected cell. As alluded to above, it appears that latency may not be a rigid state and that transcription could occur to varying degrees within the reservoir. As such, one could speculate that there may be other markers, such as nucleic acid sensors, that could be used to identify latently infected cells. In the past year, CD32a has been identified as a marker that is selectively expressed on quiescent cells harbouring replication-competent proviruses [32]. The identification of such a marker was important in that it could allow selective targeting and elimination of the latent reservoir. However, studies presented at the conference [33–36] argued that CD32a does not mark latently infected cells. Osuna et al. [33], on behalf of the BELIEVE Collaboratory, found no difference in total and replication-competent proviral DNA levels in cell populations that included or excluded the CD32a+ cell population. Bertagnolli et al. [34] demonstrated that the majority of latently infected cells did not express CD32 while Spivak et al. [35] demonstrated that CD32 expression on CD4 T cells was associated with the activation marker HLA-DR and did not identify cells harbouring higher levels of replication-competent virus. Abdel-Mohsen et al. [36] presented evidence that CD32a is preferentially expressed on activated CD4 T cells that contain transcriptionally active provirus. Therefore, the search continues for factors expressed selectively on latently-infected, quiescent CD4 T cells.
While most of the studies regarding the reservoirs that maintain HIV persistence has focused on CD4 T cells, non-CD4 T cell reservoirs have received little attention. Clements et al. [37] presented evidence that brain macrophages harbour latent SIV in ART-suppressed macaques. ART-suppressed animals did not have detectable levels of viral RNA in the brain, although viral DNA was evident in all suppressed animals. The authors demonstrated the presence of latent virus in macrophages using a macrophage viral outgrowth assay. This study provides intriguing evidence that brain macrophages harbour latent virus in macaques. Evidence that infected macrophages maintain viral persistence in ART-suppressed individuals remains elusive.
Session 3: In vitro and animal model studies of HIV persistence
The session began with an overview from Guido Silvestri, (Emory University and Yerkes National Primate Research Center, Atlanta) regarding the testing cure approaches in non-human primates at Emory [38]. The first step forward in recent years has been the definition of potent cART employed in human participants that can also be durably used in rhesus macaques to suppress SIV viraemia to undetectable levels (<20–40 copies of SIV RNA/mL plasma). Numerous investigators (Paiardini, Chahroudi, Lifson, Del Prete, Savarino, Luciw, North, Clements, Barouch, Okoye, Picker, Mason and Whitney were cited) have described this. Research foci have recently moved from studies that ask why SIV does not cause disease in African monkeys, to questions on the causes of residual disease despite ART, if viral reservoirs can be cleared, or if full immune recovery can be achieved.
Silvestri pointed to recent tissue-based studies that suggested that CTLA-4+/PD-1- CD4+ T cells with features of Tregs are critical for viral persistence in lymphoid tissues and should be targeted as part of HIV cure strategies [39], as well as studies using CD8 depletion in SIV-infected macaques on ART [40], suggesting that CD8 cells play an ongoing role in viral control even during suppressive ART. Finally, recent studies using IL-21 and interferon alpha have modulated viral rebound and improved immune control in infected animals after ART interruption [41]. In summary, Silvestri advocated combinatorial immune interventions (blockade of immune checkpoint receptors, therapeutic vaccines, latency-reversing agents, inflammatory pathway blockade, modulation of T cell differentiation and targeted cellular depletion strategies) with the goal of induced ART-free remission or even viral clearance in the SIV model.
Won-Bin Young (Temple University, Philadelphia) proposed that ‘seeing is believing,’ in a presentation entitled ‘Visualization and quantification of HIV dissemination and reservoirs using in vivo imaging’ [42]. Using a replication-competent HIV-BaL strain engineered to carry a firefly luciferase or improved deep sea shrimp luciferase reporter gene, the group was able to show that the labelled virus grew with comparable kinetics to the unmodified virus. When inoculated in the humanised bone marrow-liver-thymus (BLT) mouse model, the dissemination of infected cells expressing the imaging reporter could follow viral dissemination using bioluminescence imaging (BLI). After 2 weeks of antiretroviral therapy, a complete suppression of HIV-1 infection was observed, and 25 days after ART withdrawal the onset sites of viraemia rebound were visualised. This offered the experimental potential of monitoring viral rebound in animal model systems in real time, without any invasive procedures. For some imaging modalities, a minimum of 10,000 infected cells were needed to allow detection.
Chris Peterson (Fred Hutchinson Cancer Research Center and University of Washington, Seattle) presented two studies seeking to create engineered cells that were HIV-resistant and used the NHP model to study the potential of marrow grafts to exert an anti-viral-reservoir effect [43,44]. They reported improved safety with conditioning regimens ‘making room’ for transplanted cells; however, the negative effects of these broadly cytotoxic conditioning regimens, such as myeloablative irradiation, outweigh their impact on reservoir size, reinforcing the need for genetic protection strategies and safer conditioning [43]. Transplantation with CCR5-edited stem cells leads to a successful engraftment of about 3–5% of CCR5-modified cells for up to a year post-transplant in NHPs. However, the conditioning did not affect SHIV (hybrid SIV with human envelope gene) reservoir size, and CCR5-modified cells transplanted under ART achieved only a modest decrease of persisting SHIV DNA and RNA in tissues. A future goal is to edit CCR5, to stitch in a gene encoding a CAR in order to target other SHIV-infected cells, and to express a T20-like HIV entry inhibitor to further protect cells.
In the second study, Peterson reported that following transplant, despite nearly 100% donor chimerism, there was not a sufficiently potent graft-versus-SHIV-reservoir effect to significantly deplete persistent infection in ART-treated animals [44]. Animals were infected with SHIV-1157ipd3N4 for up to 6 months, suppressed by cART for at least 6 months, then received haplo-identical and/or MHC-matched stem cells following myeloablative total body irradiation with concurrent ART. Analysis of whole blood and gated CD4 T cells showed acquisition of 100% donor chimerism in peripheral blood at day 29 post-transplant, but viral reservoirs persisted in multiple tissues, including lymphoid and hematopoietic organs, gut and CNS.
Adam Ward (George Washington University, Washington DC) presented preliminary data on a revised effort to study HIV infection in an immunodeficient mouse model [45]. Ward criticised the use of fully humanised models such as the BLT, claiming that fatal graft-versus-host disease (GvHD) limits the utility of existing models for cure studies by precluding the generation of fully quiescent reservoirs through long-term antiretroviral (ARV) suppression. In this study, immunodeficient NSG mice were injected with 10–50 million total CD4 cells or memory CD4 T cells from ARV-treated participants with HIV (pre-established HIV reservoir xenografts). In the model, termed CD4-ARV-Treated (CATmouse), one might expect that 5–50 infected cells were transferred, allowing virus to grow in however many of the 10–50 million CD4 cells that survived transfer. When total CD4 cells were transferred, Ward reported robust engraftment of up to 40% human CD4 cells in total circulating PBMC 14 days after transfer. In mice given ART (PMPA, FTC, DTG) for up to 25 days after transfer of total CD4 cells, viraemia was seen after ART interruption in 16 of 17 mice. However, the experiments could not be carried on for more than 7 weeks due to GvHD in this NSG-total CD4 T cell model.
In contrast, the majority of animals that had been injected with selected memory CD4 T cells remained free of signs of GvHD (measured by weight loss) up to 30 weeks post-engraftment. In five animals, circulating CD4 cells were detected at a frequency of a peak of 1000 cells/μL blood (when on ART) and 0 cells (when viraemic off ART). In this cohort, it appeared that all five animals were viraemic prior to ART at 10 weeks. However, spontaneous viraemia was less frequent in a second cohort of mice not on ART (2 out of 24 at 6–8 weeks). When the PKC agonist bryostatin was injected twice to 19 of these 24 mice, transient viraemia was seen in five out of 19 mice, with viraemia at more than one timepoint in at least two mice. In another part of this cohort (n=34) that was under ART during 10 weeks after cell transplant, viraemia was seen in seven of 19 after bryostatin at week 13 and 20, although one appeared viraemic prior to bryostatin. While the BLT has thus provided useful insights in persistent HIV infection, Ward suggested that the engraftment of NSG mice with memory CD4 T cells from ARV-treated participants might serve as a model for testing in vivo latency reversal.
Afam Okoye (Oregon Health and Science University) discussed the effect of alemtuzumab-induced CD4 T cell depletion in the SIV model [46]. Alemtuzumab (ATM) is a lymphocyte-depleting, humanised anti-CD52 monoclonal antibody used for the treatment of multiple malignancies and licensed for relapsing-remitting multiple sclerosis. Okoye tested whether ATM can deplete long-lived latently infected CD4+ memory T cells in SIV-infected rhesus macaques on ART. Nine animals were intravenously infected with the highly pathogenic SIVmac239 strain, and treated 12 days after the infection with tenofovir/emtricitabine/dolutegravir. Once suppression (<15 RNA copies/mL) was achieved, six of nine animals received ATM at a dose of 5 mg/kg on days 0, 7, 14 and 29. Lymph node and GALT tissues were collected at 10 and 20 weeks. ATM induced rapid depletion of circulating T cells, NK cells, B cells and monocytes, although depletion was more significant in CD4+ memory and naïve T cells in the blood (>95%) than in the tissue samples (50%). Subsequent recovery from ATM-induced massive memory T cell proliferation peaked around 3–4 weeks post-ATM. However, full recovery was not seen at 7 months (naïve, central memory, and effector memory cells representing, respectively, 51%, 60% and 100% of cells at baseline), and while plasma SIV RNA on ART was detectable, it remained below 15 copies/mL. With this profound cell depletion at 10 weeks post-ATM, total SIV DNA in peripheral blood mononuclear cells (PBMC) decreased from a mean of 2.4 to 1.4 log10 copies per million blood cells (P=0.03), but very strikingly DNA in peripheral LN and GALT was unchanged. Peripheral lymphodepletion seemed much more profound than in tissue in this study, and did not affect viral rebound post-ATI, which is not surprising given the detection of persistent low-level viraemia and tissue SIV DNA.
The final presentation was given by Denise Hsu of the US Military HIV Research Program, and the Armed Forces Research Institute of Medical Sciences, Bangkok [47]. She studied sites of viral recrudescence after ART interruption in Indian-origin rhesus macaques. Animals were infected by intrarectal inoculation with the SHIV strain 1157ipd3N4. This was a CCR5-tropic, mucosally transmissible virus, encoding an HIV subtype C env derived from a Zambian infant. Macaques were then treated using daily tenofovir/emtricitabine/dolutegravir from weeks 2 to 18. Peripheral blood, cerebral spinal fluid (CSF) and tissue samples (colon and inguinal lymph node) were collected pre-infection, at week 2 (peak viremia), week 18 (on ART), and when animals were euthanased at week 34–36. This study was followed until 12 weeks after the detection of rebound viraemia, as median time to rebound was 3 weeks (range 17–28 days).
Median SHIV-RNA at peak was highest in colon, 24,633 (470–115,356) copies/mg, followed by lymph node, 2732 (119–17,205) copies/mg, and CSF 2050 (53–4720) copies/mL. SHIV-RNA was undetectable in all lymph node and colon samples on ART. However, after rebound, SHIV-RNA was detected in three out of five animals (15–50,813 copies/mg) in lymph node, in one out of five animals (11 copies/mg) in colon and none out of five animals in CSF. While on ART, viral particles were seen in lymph nodes via RNAscope. After rebound, infected cells were detected in the colon in one out of five and in lymph node in three out of five animals. Overall, the timing of rebound was remarkably reproducible in this cohort, and closely recapitulated most acutely HIV-infected individuals. Despite higher viral burden in colon at peak viraemia, viral rebound post ART occurred preferentially in lymph nodes as measured by both PCR and RNAscope. Surprisingly, at this point (12 weeks) SHIV RNA is undetectable in the CSF, and biomarkers of immune activation are not grossly elevated in the CSF. Despite higher viral burden in colon at peak viraemia, viral rebound after ART cessation occurred preferentially in lymph nodes on RT-qPCR and RNAscope ISH. It will be important to determine if the first site of rebound is typically the lymph node in durably treated, chronically infected humans.
Session 4: Virology of HIV persistence
An overview on the current ‘Barriers to HIV eradication’ opened the Virology Session of the meeting and was delivered by Daria Hazuda (Merck Research Labs, USA) [48]. She articulated her presentation on the dynamic interaction between the pool of cells latently infected with HIV (i.e. carrying replication-competent proviruses) and other forms of viral persistence (that she referred to as ‘active HIV reservoir’ to indicate ongoing virus and/or viral antigen expression) as revealed by the evidence of viral RNA and/or protein expression in rectal tissue biopsies of cART-suppressed individuals as well as by the observation that HIV expression following cART suspension occurs at multiple anatomical sites [49]. Hazuda underscored that among other factors contributing to antiviral drug distribution in tissue, such as metabolism, inflammation and fibrosis, the local microbiota has emerged as a relevant variable [50,51]. After advocating for the need for more sensitive and specific assays to size the viral reservoir, she described the features of MK-8591 (EFdA), a potent NNRTI broadly active against HIV-1, HIV-2 and MDR strains, which is also of potential interest for HIV persistence studies in that it is characterised by a very slow metabolism leading to long-lasting intracellular concentrations as already validated by different in vivo studies conducted both in animal models and in human volunteers.
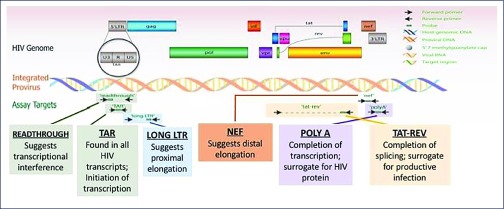
On the issue of comparing different anatomical sites, Sushama Telwatte (San Francisco Veterans Affairs Health Care System and University of California, USA) investigated the state of proviral transcription (see Figure 1) in both freshly isolated, FACS-sorted CD4 T cells from dissociated rectal biopsies and blood versus flash frozen rectal biopsies and PMBC obtained from 16 individuals having suppressed viraemia by cART [52]. She concluded that there are differences in the mechanisms governing HIV transcription and latency in the two anatomical sites, but that there were also lower levels of HIV transcription ongoing in the gut-associated cells compared to PBMC of cART-treated individuals. Thus, ‘Infected gut cells that are activated, but do not produce virus, could serve as a model for therapies designed to prevent reactivation from latency’, concluded Telwatte.
Figure 1.
Use of ‘transcription profiling’ to investigate the mechanisms that regulate HIV transcription in vivo (after Yukl et al., Sci Transl Med 2017, in press)
The issue of proviral latency versus ongoing HIV-1 replication was also addressed by Michael J Bale (HIV Dynamics and Replication Program, NCI-Frederick, USA), who studied four individuals under cART for ≥4 years [53]. Both variations in proviral genetics and integration sites were compared in paired samples of lymph nodes (LN) and peripheral blood. Bale observed no evidence of compartmentalisation of proviral DNA between blood and LN obtained from different anatomical sites that also harboured identical expanded CD4 T cell clones, leading to the conclusion that ‘the HIV reservoir is likely maintained by the proliferation of cells infected prior to cART and not by ongoing cycles of virus replication’. In this regard, Rémi Fromentin (CHUM and University of Montreal, Canada) described the ‘in vivo massive expansion of a T cell clone carrying a defective HIV genome’ leading to a 60-fold increase in the number of cells carrying integrated proviral DNA versus a median value established from cells of 17 infected individuals receiving cART [54]. Fromentin reported that around 97% of the infected cells in this peculiar individual showed an ‘effector memory (EM)’ phenotype; indeed, 50% of the patient's EM cells harboured proviral DNA in the same integration site in chromosome 14 (gene RPS6KA5), with evidence of large deletions. Fromentin concluded that ‘massive clonal expansion of defective genomes can occur in vivo, leading to a >30,000-fold difference between PCR and QVOA measurements.’
The potential consequences of proviral integration were also discussed by Daniela Cesana (San Raffaele Telethon Institute for Gene Therapy, Italy) who specifically underlined the relevance of ‘HIV-1 mediated insertional activation of STAT5B and BACH2’ in the potential ‘formation of a viral reservoir in T regulatory (Treg) cells’ [55]. After the initial report by Ikeda et al. [56], two seminal papers published in Science in 2014 [57, 58] definitively proved that insertion of the HIV-1 provirus in specific sites, including MKL2 and BACH2, may result in the expansion of CD4 T cell clones, therefore contributing to the perpetuation and potential expansion of the proviral reservoir. Furthermore, certain CD4 T cell clones can persist in the host for 10 years or more. In the context of a study that she carried out by characterising around 200 integration sites in 54 participants, she reported that an additional site of preferential insertion is represented by STAT5B. Of interest, both BACH2 and STAT5B gene products play a relevant role in the differentiation of the regulatory (i.e. immunosuppressive) subset of CD4 T cells. In addition, Cesana described the presence of chimeric mRNAs generated by the splicing of HIV sequences and BACH2 or STAT5B RNAs that she demonstrated to skew the in vitro differentiation of CD4 T cells towards the Treg phenotype, as recently published [59].
Shifting gear from CD4 T cells to tissue-associated macrophages (Mø), Morgane Bomsel (Institut Cochin, France) described the existence of a potential viral reservoir in the penile tissue of 20 cART-treated infected individuals undergoing surgery for gender re-assortment [60]. According to Bomsel (and others) Mø qualify for contributing to the viral reservoir as long-lived cells capable of self-renewal, resistance to viral cytopathicity, and capacity of accumulating nascent virions in subcellular ‘virus-containing compartments’ (VCC) [61]. Bomsel described that CD68+ urethral Mø, and not CD4 T cells, carried integrated HIV DNA and expressed viral RNA and p24 Gag protein in VCC, as also confirmed by ultrastructural studies and additional imaging approaches. Replication-competent virus could be reactivated by stimulation of tissue-derived cell suspensions by bacterial endotoxin (LPS), but not by phytohaemagglutinin, a mitogen more effective on T lymphocytes than on Mø. Conversely, Mø depletion leads to a greatly diminished capacity of the cell suspension to release virus upon LPS stimulation. The cells that were more frequently infected in the penile tissue were phenotypically defined by Bomsel as ‘transitional M1/M2’, expressing the interleukin-4 receptor as most distinctive marker. Thus, urethral Mø from infected individuals should be taken into account in ‘shock and kill’ or ‘block and lock’ strategies, as discussed at the meeting.
Session 5: Immunology of HIV persistence
Elizabeth Connick [62] opened the session with an overview on the role of B cell follicles in HIV replication and persistence [62]. While it has been established since the 1990s that most HIV replication occurs in secondary lymphoid tissues [63], novel insights in the biology of the cells found in these tissues, and particularly of T follicular helper (Tfh) cells, bring new perspectives in this area. Tfh cells, which are primarily found in germinal centres of the B cell follicles, are highly permissive to HIV infection [64]. However, novel data from Dr Connick's laboratory indicate that the phenotype of these cells is altered during productive infection. Using microscopy, she found that cytotoxic T lymphocytes (CTL) failed to accumulate in follicles of untreated individuals with HIV. This was also observed in a NHP model in which productively infected cells (SIV RNA+) were more frequently detected in the follicle than in the extra follicular region, and these frequencies of infected cells were inversely related to the frequencies of CTL. Indeed, only a few SIV-specific CTL exhibited a follicular homing phenotype (CXCR5+CCR7-). The findings were supported by measurements performed in lymph nodes and spleens from individuals living with HIV on ART: B-cell follicles were identified as preferential reservoirs in virally suppressed individuals. In addition, although the number of HIV virions decreased with ART, persistent viral particles were detected at the surface of follicular dendritic cells in these participants. Dr Connick concluded her presentation by highlighting the essential role of B cell follicles in HIV persistence through at least three mechanisms: persistence of virions on FDC, heightened permissivity of Tfh cells and diminished antiviral immunity.
Morgane Bomsel [65] presented an elegant and innovative study in which she identified a potential role for platelets in HIV persistence during ART. Platelets are anucleated and short-lived and cannot serve as a reservoir for HIV directly. Nonetheless, platelets can serve as carriers to transmit infection towards tissue macrophages. Dr Bomsel presented several pieces of evidence supporting this model: HIV RNA could be detected in platelets purified from a subset of individuals living with HIV. These platelets contained infectious viral particles that could be internalised by macrophages. Interestingly, individuals with HIV who had detectable HIV in their platelets displayed lower CD4 T cell counts and the presence of HIV in platelets was strongly associated with a non-immune responder phenotype. Overall, individuals with evidence for sheltering of HIV in platelets had a >20-fold higher likelihood of failing to restore their CD4 compartment during ART when compared to individuals without detectable HIV in platelets.
Fatima Laher [66] presented results from a study suggesting a role for the relatively new subset of follicular regulatory T cells (Tfr) [67] in the pathogenesis of HIV infection, and potentially in HIV persistence. Using blood and lymph node samples from the FRESH acute infection cohort (South Africa), she observed that Tfr cells were present at relatively low frequencies in germinal centres when compared to other regions of the lymph node. The absence of Tfr in this critical site may contribute to the excessive expansion of Tfh cells in germinal centres during acute infection. Indeed, early ART initiation was associated with an increase in the frequencies of Tfr cells that migrate into the germinal centres as measured by flow cytometry and immunofluorescence staining.
Latently infected cells are not a uniform entity: each individual cell has diverse characteristics. To characterise the heterogeneity of the reservoir, Edward Browne [68] applied single cell assay to in vitro cellular models of HIV latency. The concurrent measures of vRNA and viral proteins revealed heterogeneity in the levels of expression of these markers between different cells. To determine whether the heterogeneous levels of vRNA expression were related to underlying heterogeneity of the host cell itself, he used single-cell RNAseq. Overall, there were minor differences between infected and uninfected cells; however, a few genes were differentially expressed. Strikingly, within the infected cell population, the level of vRNA expression was highly correlated to the transcriptome of the infected cells. Viral RNA expression was clearly concentrated in a subset of cells, and other clusters were enriched for cells with more transcriptionally silent viruses. This suggested that HIV silencing and expression were most probably connected to the underlying cellular gene expression. There was a gradient in HIV silencing across subsets of CD4 T cells: naïve and central memory cells showed lower levels of HIV expression, suggesting that HIV is preferentially silenced in these cells. Finally, higher viral gene expression was associated with higher expression of activation and effector markers.
John Huang [69] presented the results of a study aimed at characterising the effect of the bcl-2 inhibitor ABT-199 on the latent HIV reservoir. Using a killing assay called ‘HIV eradication assay’ (HIVE), he previously observed that a wide range of latency-reversing agents (LRAs) can induce CD8-mediated killing of cells harbouring HIV DNA. However, the frequency of cells harbouring replication-competent HIV was minimally affected in these experiments. Results from a series of elegant experiments demonstrated that the inability to eliminate the infectious reservoir was not due to immune escape, CD8 dysfunction or lack of latency reversal. In a recent study, Cummins et al. [70] demonstrated that the maintenance of the HIV reservoir can be antagonised by selective inhibition of the anti-apoptotic factor bcl-2. Huang used the bcl-2 inhibitor ABT-199 in the HIVE assay, which resulted in a more pronounced decrease in the frequency of cells harbouring replication-competent HIV. He concluded that the bcl-2 inhibitor ABT-199 sensitises the latent reservoir to CTL-mediated elimination.
Michael R Betts [71] gave the third presentation of this session on lymph nodes and their potential role in HIV pathogenesis and persistence. Although it is clear that CD8 T cells control viral replication in lymph nodes of SIV-infected NHP elite controllers [72], the potential role of CTL in the control observed in human elite controllers remains unknown. Strikingly, cytolytic CD8+ T cells were nearly absent from lymph nodes obtained from elite controllers. Perforin and granzyme B were rarely detected in either total or CD8+ T cells isolated from these tissues. In contrast, high frequencies of cytolytic CD8+ T cells were detected in chronic progressors. Using an in vitro killing assay, it was demonstrated that CD8+ T cells from lymph nodes generally have minimal killing ability, despite high expression of specific cytokines and low expression of inhibitory receptors. Altogether, these results suggested that elite control is likely mediated by non-cytolytic CD8+ T cells in human lymphoid tissues.
Yanqin Ren [73] closed the session by presenting an extensive characterisation of the breadth and potency of broadly neutralising antibodies (bNAbs) against HIV reactivated from the latent reservoirs of virally suppressed individuals. Using viruses recovered from the latent reservoir from eight individuals on ART, she found that the V3-glycan and the V1V2-specific bNAb exhibited potent but relatively narrow neutralisation and binding activities. In contrast, the CD4 binding site (CD4bs)-specific antibodies exhibited broader but less potent activity. Importantly, combination of two antibodies was able to provide up to 83% coverage of neutralisation and up to 100% binding coverage of reservoir viruses.
Session 6: Human studies
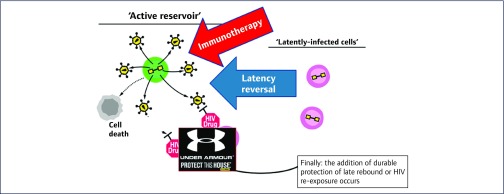
David M Margolis (University of North Carolina HIV Cure Center, UNC at Chapel Hill, Chapel Hill, USA) opened Session 6 on Human studies describing Clinical Studies at the UNC HIV Cure Center [74]. Even if cell-associated HIV-RNA [75] or p24 are increased following clinical administration of some HDACis and HIV-1 specific T cell responses are stable and broadly detectable in durably suppressed adults [76], immune response has to be harnessed and new technologies are needed to assist in the clearance of persistently infected cells (Figure 2). Furthermore, the ability of vorinostat and an associated immunotherapy to reduce the frequency of latently infected resting CD4 T cells has yet to be proven.
Figure 2.
Steps to eliminate HIV infection (after DM Margolis)
Maria Salgado (AIDS Research Institute, IrsiCaixa, Badalona, Spain) presented data regarding the longitudinal serostatus of HIV-positive participants after allogeneic stem cell transplantation (allo-SCT) on behalf of the IciStem Consortium [77]. Allo-SCT in subjects living with HIV is known to reduce HIV latent reservoirs in blood and tissues [78], but HIV-seroreversion dynamics has not yet been established. Longitudinal plasma samples from 13 HIV-positive allo-transplanted participants under cART were analysed using the standard and low-sensitive versions of the VITROS Enzyme anti-HIV-1 Immunoassay (Ortho-Clinical Diagnostics, Rochester, NY, USA), as well as the Lag Avidity Assay (Sedia Biosciences, Portland, OR, USA). Evolution of the HIV-specific antibodies in plasma of these participants showed that even if bulk antibody levels persist after allo-SCT in most individuals, detuned antibody levels and antibody maturity are reduced. They also observed that p24 and/or p31 disappeared in nine out of 13 participants, and seroreversion was evidenced in two patients a few years after allo-SCT, independently of CCR5 mutation.
Una O’Doherty (Department of Pathology and Cellular Therapeutics, University of Pennsylvania, Philadelphia, USA) investigated the dynamics and character of HIV proviruses from two HIV-infected participants for up to 9 years after ART initiation by means of sequencing, within the BELIEVE Martin Delaney Collaboratory [79]. Notably, intact proviruses appear to decay faster than defective ones, even if occasional episodes of reservoir expansion may affect overall decay. In patient 1, the fraction of intact proviruses decreased over time on ART (about 85% of the intact reservoir was cleared over 8 years). The decay appeared exponential with a half-life of 2.32 years, exponential decay rate -0.23/year, slope that was less than 0, P<0.05. Patient 2 displayed a similar pattern of viral decay at the first three timepoints (half-life 2.46 years), but a remarkable increase in intact proviruses was observed at the last collection. These data suggest that sequencing proviruses longitudinally provides an interesting alternative method to measure reservoir decline, because HIV DNA is apparently more stable than the true reservoir measured by sequencing.
Katharine J Bar (University of Pennsylvania, Philadelphia, USA) studied the effect of analytical treatment interruptions (ATI) on the size and diversity of the latent reservoir [80]. A5340 was a clinical trial investigating the effect of VRC01 during ATI, allowing evaluation of the impact of transient viraemia on the reservoir, as well as assessment of relatedness of viruses sampled pre-ATI and at rebound. Nine participants infused with VRC01 underwent ATI and were monitored for viral rebound. All of them experienced a viral rebound presenting a modest delay compared with historical controls. Participants’ median ART duration prior to ATI was 4.8 years. Total DNA, cell-associated RNA and infectious units per million cells by QVOA were not statistically different pre- and post-ATI (P>0.3, Wilcoxon signed rank test), with median log10 change of 0.3 copies, 0.08 copies, and -0.05 infectious units per million cells, respectively. Phylogenetic analyses of QVOA supernatant viruses and plasma viruses showed no evidence for enrichment of rebound viruses post-trial, and pre-trial QVOA sequences failed to predict the identity of rebound virus.
Thomas A Rasmussen (Peter Doherty Institute for Infection and Immunity, University of Melbourne and Royal Melbourne Hospital, Melbourne, Australia) displayed results of a randomised, placebo-controlled trial of dolutegravir intensification during 56 days, in terms of residual viral replication (RVR) while on suppressive ART [81]. Forty participants with HIV were enrolled and were comparable between the dolutegravir and the placebo groups. There was no significant difference in the primary endpoint, namely the 2-LTR circles in peripheral blood CD4 T cells, as assessed by repeated-measures ANOVA at day 7 or any other time point. They found no consistent difference in the levels of total and integrated HIV DNA, cell-associated or plasma RNA, T cell activation or inflammation markers (sCD14, d-dimer, IL-6, hsCRP).
Julio C Lorenzi (Rockefeller University, New York, USA) presented results of a Phase 2 trial evaluating the impact of 3BNC117 infusions in addition to ART on the latent reservoir and viral rebound after analytical treatment interruption (ATI) [82]. 3BNC117 is a broadly neutralising antibody administrated in 15 long-term stable ART-treated participants who were not selected by 3BNC117 sensitivity. These participants received four intravenous infusions on day 0, week 12, week 24 and week 27, while ATI was initiated after the third infusion. No significant changes were observed in the size of the latent reservoir after two doses of 3BNC117 over 6 months in participants on suppressive ART. Reservoir landscape, as observed by clonal size fluctuation, changed significantly over a 6-month period in four out of nine patients, but there was no correlation between the changes in clone frequency and sensitivity to 3BNC117. 3BNC117 treatment significantly delayed viral rebound in participants sensitive to this antibody compared with historical controls, and promoted viral selection in eight out of 10 patients. Isolated latent viruses were highly similar but generally not identical to rebound viruses.
Deborah McMahon (University of Pittsburgh, Pittsburgh, USA) presented the A5315 Phase I/II study of romidepsin (RMD) use in order to assess safety, tolerability and activation of HIV-1 expression [83]. Forty-three participants (36 RMD; seven placebo) enrolled in the study. No significant dose-dependent changes in any virological measurement (SCA, CA-DNA, CA-RNA) or in histone-3 acetylation were observed, but researchers saw changes in immune activation in the RMD 5 mg/m2 cohort compared to placebo and a satisfactory safety and tolerance profile overall.
Sessions 7 and 8: New therapeutic approaches I and II
As previously proposed by community representatives and ethicists, the metaphor ‘shock and kill strategy’ should be replaced with a non-military metaphor [84] such as ‘wake me up, before I go’ as proposed by Chomont [85]. Community representatives, as well as Sharon Lewin from the University of Melbourne, agree that the term ‘participants’ should now be replaced with ‘subjects’ or ‘individuals participating in such clinical trials’. In order to increase exchange between scientists and community each oral presentation was summarised by a community slide that wrapped up key study findings and future directions.
Ole Søgaard (Aarhus University, Denmark) opened the sessions by highlighting the initial hopes and limitations associated with the ‘shock and kill strategies’ [86].
Based on disappointing results from clinical trials, it is now suggested to include combined administration of an LRA to reactivate the latent reservoirs as well as an intervention to enhance cytotoxic functions for reservoir clearance. Both arms of this strategy seem to be inefficient due to: (1) low level of induced antigen expression; (2) low cytotoxic T cell function; (3) late administration of LRAs, during exhausted HIV-specific CD8 T cell response. Very early ART initiation combined with LRA may overcome such limitation. New directions include combining LRAs with vaccines and immune activators like TLR agonists.
Javier Martinez-Picado's group reviewed strategies for an HIV cure including cytokine and gene therapy. Interestingly, his Barcelona team identified a new subgroup of chronically treated subjects that presented with extremely low HIV-proviral load, called ‘low viral reservoir-treated’ (LoViRet) participants [87]. They are characterised by a low viral load before ART and enhanced latency decay while on ART. A report from the International Consortium on allogeneic STEM cell transplant for leukemic/lymphoma HIV-Infected participants (ICISTEM) showed that viral reservoir was undetectable in five out of six patients. The sole participant with detectable reservoir received a cord blood transplant and did not achieve a full donor chimera by 1 year post transplant. Such results showed the contribution of full chimerism for obtaining an undetectable reservoir following all stem cell transplants.
Overcoming CD8 T cell dysfunction by using modified recombinant IL-15
A significant hurdle to clearing HIV reservoirs is the dysfunction and exclusion of CD8 T cells from B cell follicles. Heterodimeric, superagonist IL-15 activates and expands cytotoxic T cells and NK cells. This suggests that IL-15, used in cancer clinical trials, could play a role in the HIV eradication strategy [88].
Pavlakis et al. from National Cancer institute at Frederick [89] and Webb et al. [90] from Oregon Health and Science University presented on intravenous IL-15 administrated in SIV-infected macaques. Massive proliferation of NK and CD8 T cells and reactivation of quiescent SIV were observed. Interestingly, activated cytotoxic cells and SIV-specific CD8 T cells entered B cell follicles harbouring latently infected CD4 TFH. The ability of IL-15 to grant CD8 T cell access to lymph node sanctuary sites may represent a new therapeutic avenue.
Anti PD-1 pathway in patients with HIV and cancer: rejuvenating immune fight
Anti PD-1 and anti PD-L1 have revolutionised the treatment of certain cancers by reverting T cell exhaustion and serving as a strategy for purging HIV reservoirs. Since autoimmune toxicity of anti-PD-1 antibodies remains a significant issue, Uldrick et al. assessed the safety of anti PD-1 pembrolizumab in HIV-infected patients with advanced cancers [91]. For these 17 ART-treated participants, acceptable safety profiles were observed without evidence of increased viral load. Influence of pembrolizumab on HIV reservoir size has not yet been established at the time of the presentation. Encouragingly, a case reported by Guihot et al. showed a significant decrease in the size of HIV reservoir [92]. As in cancer, predictors of anti-PD-1 therapy response, including gut microbial composition, must be identified to select best study candidates [93]. Furthermore, as McGary et al. have shown, major checkpoint CTLA-4+ in PD-1 negative CD4 T cells also contributes to viral persistence. Combination of immune checkpoint inhibitors will be needed, while avoiding severe autoimmune toxicity [94].
Immune boosters
Macedo et al. (George Washington University, Washington DC) presented on the effects of synthetic dual TLR-2 and TLR-7 agonists on latent HIV [95]. This team demonstrated the ability of TLR-2 agonists (directly) and TLR-7 agonists (via cytokines produced by plasmacytoid dendritic cells) to reactivate the latent HIV in resting CD4 T cells. They have now generated several dual agonists composed by TLR-2 and seven ligands to trigger viral reactivation. Using modified J-Lat clones that express TLR-2 on the cell surface and the primary cell model of HIV latency and PBMCs from virologically suppressed participants, three out of four dual ligands retain their ability to reactivate latent HIV. Furthermore, these agonists induced production of cytokines and activated both NK and T cells, promoting an antiviral immunity. Study findings motivate further exploration for preclinical studies in animals.
New antiviral strategies: anti Rev and anti Tat
Anti Rev
Steens et al. (AbivaxINC, France) along with investigators from Barcelona reported on ABX464, a first-in-class orally available antiviral small molecule that blocks HIV replication by preventing Rev-mediated export of unspliced HIV-1 transcripts to the cytoplasm [96].
ART-treated participants, in addition to their boosted darunavir-based regimen, received either ABX464 (50 or 150 mg p.o. daily) or placebo. Treatment was tolerated and a 150 mg dose of ABX464 for 28 days was associated with a decrease in HIV-1 DNA in CD4 T cells as compared to baseline. These data support the continued development of ABX464 for cure eradication strategies.
Anti Tat
Kessing CF et al. (Scripps Research Institute, Jupiter, Florida) presented on suppression of HIV reservoir with a Tat inhibitor [97]. HIV-1 Tat activates viral transcription and limited Tat-transactivation correlates with latency establishment. Investigators proposed to use a Tat inhibitor, didehydro-Cortistatin A (dCA) to reduce HIV reservoir size. This ‘block-and-lock’ hypothesis was investigated in CD4 T cells from virologically suppressed, ART-treated participants and in the bone marrow-liver-thymus mouse model of HIV latency and persistence. Encouragingly, adding dCA to ART-suppressed BLT mice systemically reduced viral mRNA in tissues and significantly delayed and reduced viral rebound levels upon treatment interruption. Potential clinical benefits for dCA may include rapid control of viral load and further reduction of size of HIV reservoir. The continued pre-clinical development of an anti Tat is warranted for cure eradication strategies.
Conclusion
The wealth of new information provided and exchanged during this 2017 workshop clearly defined new strategies to further control viral replication, to reverse latency, and boost immune response in order to build a scalable approach to open the next frontier of long-term ART discontinuation for a functional cure.
References
- 1. Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 2015; 16: 584– 589. [DOI] [PubMed] [Google Scholar]
- 2. Sneller MC, Justement JS, Gittens KR et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9: pii: eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheid JF, Horwitz JA, Bar-On Y et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 2016; 535: 556– 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar KJ, Sneller MC, Harrison LJ et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 2016; 375: 2037– 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishimura Y, Gautam R, Chun TW et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017; 543: 559– 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byrareddy SN, Arthos J, Cicala C et al. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science 2016; 354: 197– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez-Navio JM, Fuchs SP, Pedreño-López S et al. Host anti-antibody responses following adeno-associated virus-mediated delivery of antibodies against HIV and SIV in rhesus monkeys. Mol Ther 2016; 24: 76– 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs SP, Desrosiers RC. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol Ther Methods Clin Dev 2016; 3: 16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampey GC, Browne EP, Irlbeck DM et al. SMAC mimetics reverse latency by selective activation of the non-canonical NF-KB pathway. J Virus Erad 2017; 3( Suppl 5): abstract PP 7.7 [Google Scholar]
- 10. Dunham RM, Sampey GC, Irlbeck DM et al. SMAC mimetics are potent latency reversal agents with single agent and combination activity ex vivo. J Virus Erad 2017; 3( Suppl 5): abstract PP 7.9 [Google Scholar]
- 11. Sung JA, Pickeral J, Liu L et al. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest 2015; 125: 4077– 4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessing CF, Nixon CC, Li C et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a ‘Block-and-Lock’ Strategy for HIV-1 Treatment. Cell Rep 2017; 21: 600– 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borducchi EN, Cabral C, Stephenson KE et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016; 540: 284– 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barouch DH, Whitney JB, Moldt B et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013; 503: 224– 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henrich TJ, Hatano H, Bacon O et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med 2017; 14: e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen SG, Ford JC, Lewis MS et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473: 523– 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen SG, Piatak M Jr, Ventura AB et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502: 100– 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukazawa Y, Lum R, Okoye AA et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21: 132– 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones RB, Mueller S, Kumari S et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials 2017; 117: 44– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flerin N, Korom M, Lynch R et al. Evaluation of the in vivo capacity of broadly neutralizing anti-HIV antibodies to eliminate latently infected cells from HIV-infected individuals using a novel humanized mouse model. J Virus Erad 2017; 3( Suppl 5): abstract PP 3.1 [Google Scholar]
- 21. Spragg C, De Silva Feelixge H, Jerome KR. Cell and gene therapy strategies to eradicate HIV reservoirs. Curr Opin HIV AIDS 2016; 11: 442– 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis-Gardner ME, Gardner MR, Alfant B, Farzan M. eCD4-Ig promotes ADCC activity of sera from HIV-1-infected patients. PLoS Pathog 2017; 13: e1006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leibman RS, Richardson MW, Ellebrecht CT et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog 2017; 13: e1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tebas P, Stein D, Tang WW et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 2014; 370: 901– 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leslie GJ, Wang J, Richardson MW et al. Potent and broad inhibition of HIV-1 by a peptide from the gp41 heptad repeat-2 domain conjugated to the CXCR4 amino terminus. PLoS Pathog 2016; 12: e1005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siliciano R. Understanding persistence of the latent reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 1.0. [Google Scholar]
- 27. Maldarelli F, Wu X, Su L et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345: 179– 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bui JK, Sobolewski MD, Keele BF et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog 2017; 13: e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varabyou A, Talbot C Jr., Zhang H et al. HIV-1 proviruses which are integrated into cancer-related genes are inducible. J Virus Erad 2017; 3( Suppl 5): abstract OP 1.1. [Google Scholar]
- 30. Gramatica A, Greene W, Schwarzer R et al. Identification of a promising new class of latency reversing agents. J Virus Erad 2017; 3( Suppl 5): abstract OP 1.3. [Google Scholar]
- 31. Browne EP, Bradley T, Ferrari G et al. High-throughput single-cell transcriptome analysis of immune cells from HIV-1 infected individuals before and after therapy. J Virus Erad 2017; 3( Suppl 5): abstract OP 1.4. [Google Scholar]
- 32. Descours B, Petitjean G, López-Zaragoza JL et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 2017 Mar 23; 543: 564– 567. [DOI] [PubMed] [Google Scholar]
- 33. Osuna CE, Apps R, Lim SY et al. CD32 does not mark the HIV-1/SIV latent reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 1.5. [Google Scholar]
- 34. Bertagnolli LN, White JA, Beg SA et al. Majority of the latent reservoir resides in CD32a negative CD4+ T cells. J Virus Erad 2017; 3( Suppl 5): abstract OP 2.2. [Google Scholar]
- 35. Spivak AM, Nell RA, Coletti ML et al. CD4+ T cells expressing CD32 from HIV-1+ patients are not enriched for proviral DNA. J Virus Erad 2017; 3( Suppl 5): abstract OP 2.4. [Google Scholar]
- 36. Abdel-Mohsen M, Tomescu C, Vadrevu S et al. CD32+ CD4+ T cells are HIV transcriptionally active rather than a resting reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 2.6. [Google Scholar]
- 37. Clements J, Abreu C, Mac Gabhann F et al. Brain macrophages in SIV-infected ART-suppressed macaques represent a functional latent reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 2.3. [Google Scholar]
- 38. Silvestri G. Testing cure approaches in NHPs: the Emory experience. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.0. [Google Scholar]
- 39. McGary CS, Deleage C, Harper J et al. CTLA-4+PD-1–Memory CD4+ T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 2017; 47: 776– 788. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cartwright EK, Spicer L, Smith SA et al. CD8(+) Lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity 2016; 45: 656– 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Micci L, Ryan ES, Fromentin R et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest 2015; 125: 4497– 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young WB, Qu X, Wu G. Visualization and quantification of HIV dissemination and reservoirs using in vivo imaging. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.1. [Google Scholar]
- 43. Peterson CW, Zhen A, Deleage C et al. Enhancing infection-resistant cells for HIV Cure in the non-human primate model. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.2. [Google Scholar]
- 44. Peterson CW, Colonna L, Schell JB et al. Modeling the graft-versus-viral-reservoir effect in a non-human primate model of HIV persistence. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.3. [Google Scholar]
- 45. Ward A, Charleus E, Karandish S et al. Patient-derived HIV reservoirs can be stably engrafted into NSG mice and reactivated by latency-reversing agents in vivo. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.4. [Google Scholar]
- 46. Okay AA, Lewin SR, Xu CH et al. SIV persists in lymphoid tissues despite alemtuzumab-induced CD4+ T cell depletion. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.5. [Google Scholar]
- 47. Hsu DC, Silsorn D, Inthawong D et al. Differential viral rebound between lymph node and colon after treatment interruption in SHIV-infected rhesus macaques. J Virus Erad 2017; 3( Suppl 5): abstract OP 3.6. [Google Scholar]
- 48. Hazuda D. Barriers to HIV eradication. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.0. [Google Scholar]
- 49. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS 2016; 11: 417– 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson CG, Gay CL, Kashuba ADM. HIV persistence in gut-associated lymphoid tissues: pharmacological challenges and opportunities. AIDS Res Hum Retroviruses 2017; 33: 513– 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klatt NR, Cheu R, Birse K et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356: 938– 945. [DOI] [PubMed] [Google Scholar]
- 52. Telwatte S, Lee S, Somsouk M et al. Gut and blood differ in mechanisms governing HIV Transcription/Latency. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.6. [Google Scholar]
- 53. McManus WR, Bale MJ, Spindler J et al. No evidence for ongoing HIV replication in lymph nodes during suppressive ART. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.3. [Google Scholar]
- 54. Fromentin R, Massanella M, Vandergeeten C et al. In vivo massive expansion of a T-cell clone carrying a defective hiv genome: implication for the measurement of the HIV reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.5. [Google Scholar]
- 55. Cesana D, Santoni de Sio F, Rudilosso L et al. HIV-1 Mediated insertional activation of STAT5B and BACH2 promotes the formation of a viral reservoir in T regulatory cells. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ikeda T, Shibata J, Yoshimura K et al. Recurrent HIV-1 integration at the BACH2 locus in resting CD4+ T cell populations during effective highly active antiretroviral therapy. J Infect Dis 2007; 195: 716– 725. [DOI] [PubMed] [Google Scholar]
- 57. Maldarelli F, Wu X, Su L et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345: 179– 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner TA, McLaughlin S, Garg K et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345: 570– 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cesana D, Santoni de Sio FR, Rudilosso L et al. HIV-1-mediated insertional activation of STAT5B and BACH2 trigger viral reservoir in T regulatory cells. Nat Commun 2017; 8: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bomsel M, Ganor Y, Sennepin A et al. Tissue macrophages are a major viral reservoir in male urethra of HIV-1-infected individuals under suppressive antiretroviral therapy. J Virus Erad 2017; 3( Suppl 5): abstract OP 4.4. [Google Scholar]
- 61. Graziano F, Vicenzi E, Poli G. Immuno-pharmacological targeting of virus-containing compartments in HIV-1-infected macrophages. Trends Microbiol 2016; 24: 558– 567. [DOI] [PubMed] [Google Scholar]
- 62. Connick E. The role of B cell follicles in HIV replication and Persistence. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.0. [Google Scholar]
- 63. Pantaleo G, Graziosi C, Butini L et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A 1991; 88: 9838– 9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perreau M, Savoye AL, De Crignis E et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 2013; 210: 143– 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bomsel M, Real F, Capron C et al. Platelets from HIV-infected cART-treated patients carry infectious viruses and predict poor immunological recovery. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.1. [Google Scholar]
- 66. Laher F, Ndhlovu ZM, Baiyegunhi O et al. Follicular regulatory T cell dynamics in peripheral blood and lymphoid tissue during very early treatment initiation in HIV-1 clade C infection. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.2. [Google Scholar]
- 67. Chung Y, Tanaka S, Chu F et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011; 17: 983– 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Browne EP, Bradley T, Ferrari G, Haynes BF and Margolis DM. Single cell analysis of HIV latency reveals diverse proviral and host cell behavior. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.3. [Google Scholar]
- 69. Huang SH, Ren Y, Macedo Y et al. BCL-2 Inhibitor sensitizes the latent HIV reservoir to elimination by CTLs. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.4. [Google Scholar]
- 70. Cummins NW, Sainski-Nguyen AM, Natesampillai S et al. Maintenance of the HIV reservoir Is antagonized by selective BCL2 inhibition. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Betts M, Nguyen S, Deleage C et al. Defining the nature of protective CD8+ T-cell response in lymph nodes of HIV elite controllers. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.5. [Google Scholar]
- 72. Fukazawa Y, Lum R, Okoye AA et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21: 132– 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ren Y, Korom M, Truong M et al. Susceptibility to neutralization by bnAbs correlates with infected cell binding for a panel of clade B HIV reactivated from latent reservoirs. J Virus Erad 2017; 3( Suppl 5): abstract OP 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Margolis D. Current efforts in latency reversal and clearance. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.0. [Google Scholar]
- 75. Archin NM, Kirchherr JL, Sung JAM et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 2017; 127: 3126– 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu G, Swanson M, Talla A et al. HDAC inhibition induces HIV-1 protein and enables immune-based clearance following latency reversal. JCI Insight 2017; 2: e92901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Salgado M, González V, Rivaya B et al. HIV-seroreversion dynamics after allogeneic stem cell transplantation. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.1. [Google Scholar]
- 78. Bosman K, Nijhuis M, Bruns A et al. Persistence of HIV DNA in tissues early after transplantation with CCR5Δ32 stem cells. Conference on Retroviruses and Opportunistic Infections. February 2017. Seattle, Washington. Abstract 320.
- 79. VanBelzen DJ, Weissman S, Hwang WT et al. Sequencing HIV proviruses over time provides new insights into reservoir decay. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.2. [Google Scholar]
- 80. Bar KJ, Salantes B, Zheng Y et al. Brief ATI does not alter the size or composition of the latent HIV-1 reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.3. [Google Scholar]
- 81. Rasmussen TA, McMahon J, Chang J et al. No residual virus replication in a randomised trial of dolutegravir intensification. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.4. [Google Scholar]
- 82. Lorenzi J, Cohen Y, Burke L et al. A phase 2 trial to evaluate the effects of 3BNC117 in addition to antiretroviral therapy on the latent reservoir and viral rebound. J Virus Erad 2017; 3( Suppl 5): abstract OP 6.5. [Google Scholar]
- 83. McMahon D, Zheng L, Cyktor J et al. Single romidepsin infusions do not increase HIV expression in persons on ART (A5315). J Virus Erad 2017; 3( Suppl 5): abstract OP 6.6. [Google Scholar]
- 84. Nie JB, Gilbertson A, Roubaix M et al. Healing Without Waging War: Beyond Military Metaphors in Medicine and HIV Cure Research. Am J Bioeth 2016; 16: 3– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chomont N, Okay AA, Favre D et al. Wake me up before you go: a strategy to reduce the latent HIV reservoir. AIDS 2018; 32: 293– 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Søgaard OS. Early lessons from shock and kill trials. J Virus Erad 2017; 3( Suppl 5): abstract OP 7.0. [Google Scholar]
- 87. Galvez Celada C, Dalmau J, Garcia F et al. Chronically treated HIV+ subjects can naturally harbor extremely low viral reservoir. J Virus Erad 2017; 3( Suppl 5): abstract OP 8.6. [Google Scholar]
- 88. Miller JS, Morishima C, McNeel DG et al. A first-in-human phase 1 study of subcutaneous outpatient recombinant human IL-15 (rhIL-15) in adults with advanced solid tumors. Clin Cancer Res 2017 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pavlakis G, Watson DC, Moysi E et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes in lymph nodes and reduces SHIV RNA. J Virus Erad 2017; 3( Suppl 5): abstract OP 7.4. [Google Scholar]
- 90. Webb G, Li S, Mwakalundwa G et al. The human IL-15 superagonist complex ALT-803 drives SIV-specific CD8+ T cells into B cell follicles. J Virus Erad 2017; 3( Suppl 5): abstract OP 7.5. [Google Scholar]
- 91. Uldrich TS, ‘Mac’ Cheever MA, Gonçalves PH et al. Interim safety analysis of Cancer Immunotherapy Trials Network–12 (CITN-12): A phase 1 study of pembrolizumab in patients with hiv and cancer. J Virus Erad 2017; 3( Suppl 5): abstract OP 8.3. [Google Scholar]
- 92. Guihot A, Marcelin AG, Massiani MA et al. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol 2017 Dec 1. [DOI] [PubMed] [Google Scholar]
- 93. Routy B, Le Chatelier E, Derosa L et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91– 97. [DOI] [PubMed] [Google Scholar]
- 94. McGary CS, Deleage C, Harper J et al. CTLA-4+PD-1–memory CD4+ T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity; 47: 776– 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Macedo AB, Novis CL, Spivak AM et al. Direct and indirect effects of synthetic dual TLR-2 and TLR-7 agonists (Dual TLR-2/7) on latent HIV. J Virus Erad 2017; 3( Suppl 5): abstract OP 8.4. [Google Scholar]
- 96. Steens JM, Cranston R, Martinez-Picado J et al. Oral ABX464 reduces the HIV DNA reservoir in CD4+ peripheral blood T cells. J Virus Erad 2017; 3( Suppl 5): abstract OP 8.2. [Google Scholar]
- 97. Kessing CF, Nixon CC, Li C et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a ‘block-and-lock’ strategy for HIV-1 cure. J Virus Erad 2017; 3( Suppl 5): abstract OP 7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]