Abstract
Rationale:
Neonatal gastric perforation is a rare and life-threatening disorder in neonates and is associated with high morbidity and mortality. However, the exact mechanisms of neonatal gastric perforation remain unknown.
Patient concerns:
In this study, we reported 2 cases of neonatal gastric perforation and conducted a systematic review to analyze the prognostic factors for mortality.
Diagnoses:
Two neonates received a diagnosis of gastric perforation based on clinical presentation and imaging studies. The 2 patients underwent emergent surgery, which yielded favorable outcomes.
Interventions:
We reviewed 168 cases from the literature as well as our 2 cases to analyze whether mortality in neonatal gastric perforation is associated with sex, gestational age, operation type, perforation location, or timing of perforation.
Outcomes:
The results revealed that mortality was significantly higher in preterm neonates (n = 80, P < .01) and the mortality group had a lower birth weight (n = 73, P < .05). The timing of perforation in the preterm subgroup was significantly earlier than that in the full-term subgroup (n = 90, P < .05). The outcomes about mortality of gastric perforation were significantly associated with preterm neonates (adjusted odds ratio: 4.21, 95% confidence interval: 1.28–13.88, P < .05).
Lessons:
This study shows the prognostic factor of gastric perforation was significantly associated with preterm neonates. Furthermore, low-birth-weight full-term neonates had a relatively higher mortality rate than the normal-birth-weight full-term neonates. In addition, preterm neonates have an earlier timing of perforation.
Keywords: mortality, neonatal gastric perforation, preterm, timing of perforation
1. Introduction
Gastric perforation accounts for approximately 7% of all gastrointestinal perforations in neonates; it has poor outcomes and a high mortality rate.[1,2] The probable causes of gastric perforation include asphyxia, vigorous respiratory resuscitative measures, ventilator use, increased intragastric pressure, gastric acidity changes, and anatomical abnormalities of the stomach (eg, congenital agenesis of the gastric musculature) secondary to iatrogenic traumas (eg, endotracheal and feeding tube insertion) and steroid use (possibly in combination with cyclooxygenase inhibitors for ductal closure).[2–5] However, the exact mechanisms of neonatal gastric perforation remain unclear. In this report, we presented 2 cases of neonatal gastric perforation and conducted a systematic review on 168 cases to analyze the relationship of mortality with sex, gestational age, operation type, perforation location, and timing of perforation.
2. Case report no 1
A 4-day-old full-term male neonate was delivered through caesarean section, with a birth weight of 3320 g and Apgar scores of 6 and 8 at 1- and 5-minute assessments, respectively, and without birth insult. Prenatal examination was uneventful. At 4 days after birth, he was admitted to our pediatric emergency department due to severe abdominal distention, progressive shortness of breath accompanied with grunting, and yellowish skin discoloration for 1 day (Fig. 1). His feeding condition was satisfactory during the 4 days, and he had defecated one time after feeding. Upon admission, tachycardia (approximately 200 beats/minute) and hypotension (systolic/diastolic blood pressure: 71/59 mm Hg) were observed. Prominent abdominal distension was visible, and bowel sounds were hypoactive. Laboratory results revealed leukocytosis with left shifting (white blood count: 25.8 × 109/L, band: 2.5%, myelocytes: 3%, metamyelocytes: 3%, and neutrophils: 73%), hyperbilirubinemia (total bilirubin: 358 μmol/L), and serum sodium of 132 mmol/L (normal value: 135–148 mmol/L). Plain abdominal radiography showed football signs (Fig. 2), and the lateral decubitus abdominal radiograph revealed pneumoperitoneum. Because hollow organ perforation with sepsis was suspected, emergent surgery was arranged. Empiric antibiotics with ampicillin (YF Chemical Corp, Taiwan), cefotaxime (Zentiva Saglik Urenleri San, Turkey), and metronidazole (Taiwan Biotech, Taiwan) were administered. The surgical findings were as follows: perforation at the anterior wall of the stomach body, approximately 200 mL of milk-like material within the peritoneum, and malrotation with a small bowel volvulus of approximately 360°. Gastrorrhaphy and the Ladd procedure were performed. Meckel diverticulum was 30 cm above the ileocecal valve but remained unresected to reduce operation time. The neonate was started on oral milk feeding with an uneventful hospitalized course, and the 2nd operation for wedge resection of Meckel diverticulum was arranged subsequently.
Figure 1.
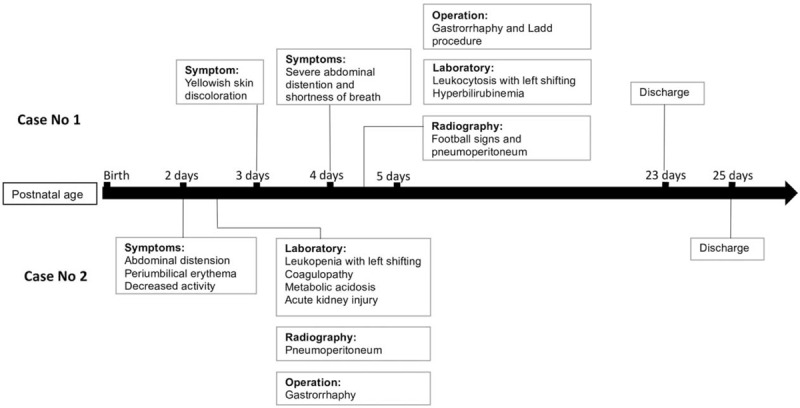
Clinical courses of gastric perforation in the 2 cases.
Figure 2.
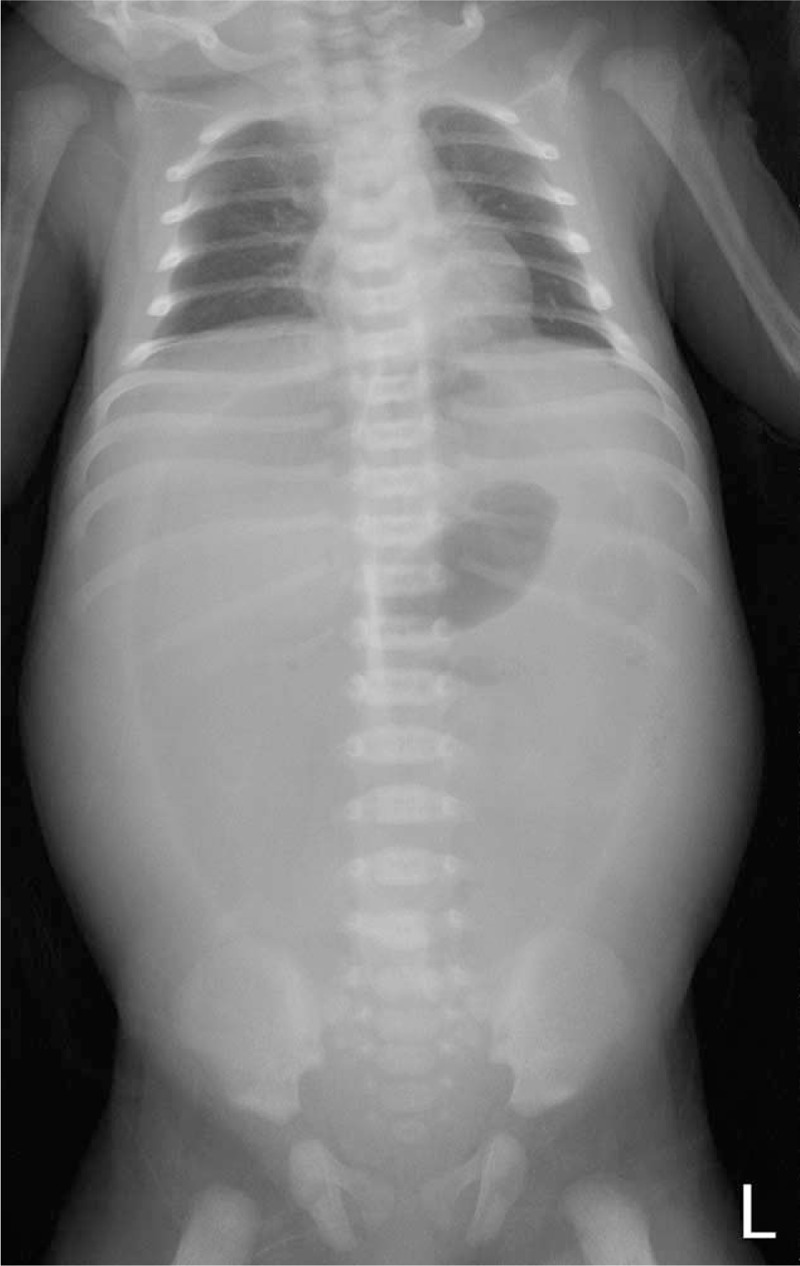
Plain abdominal radiography in case no 1 showed football signs.
3. Case report no 2
A 2-day-old preterm male neonate was delivered through caesarean section at a gestational age of 35 weeks and 3 days, with a birth weight of 2240 g and Apgar scores of 7 and 8 at 1- and 5-minute assessments, respectively. Progressive abdominal distension accompanied with periumbilical erythema, decreased activity, minimal amount meconium passage, and hypoactive bowel sounds were observed during the 2 days (Fig. 1). Therefore, he was transferred to our hospital. Blood examination showed leukopenia with left shifting (white blood count: 4.91 × 109/L, band: 1%, myelocytes: 3%, metamyelocytes: 7%, neutrophils: 10%), elevated C-reactive protein levels of 156.2 nmol/L (normal levels: 7.6–150.4 nmol/L), coagulopathy (prothrombin time: 22.1 seconds; activated partial thromboplastin time: 104.1 seconds, international normalized ratio: 2.1), metabolic acidosis (pH: 7.3, PO2: 66.0 mm Hg, PCO2: 26.1 mm Hg, HCO3−: 11.3 mmol/L), acute kidney injury (creatinine: 167.9 μmol/L; normal value: 2.65–44.2 μmol/L), blood urea nitrogen: 13.8 mmol/L (normal value: 1.1–9.0 mmol/L), and serum sodium: 127 mmol/L. Plain abdomen radiography revealed pneumoperitoneum (Fig. 3). Under unstable hemodynamic conditions, septic shock caused by hollow organ perforation was suspected initially. Fluid resuscitation was performed, and an inotropic agent with dopamine was administered. Empirical antibiotics were prescribed similar to those in case no 1. Emergent laparotomy showed gastric perforation, and gastrorrhaphy was performed. Upon surgery, a 3-cm perforation over the anterior wall of the stomach with necrosis changes of the surrounding tissue and fibrin coating and approximately 50 mL of turbid whitish fluid inside the peritoneum were observed. We maintained his nutritional status with parenteral nutrition, which was gradually tapered after satisfactory feeding. He was discharged with uneventful hospitalization course afterwards.
Figure 3.
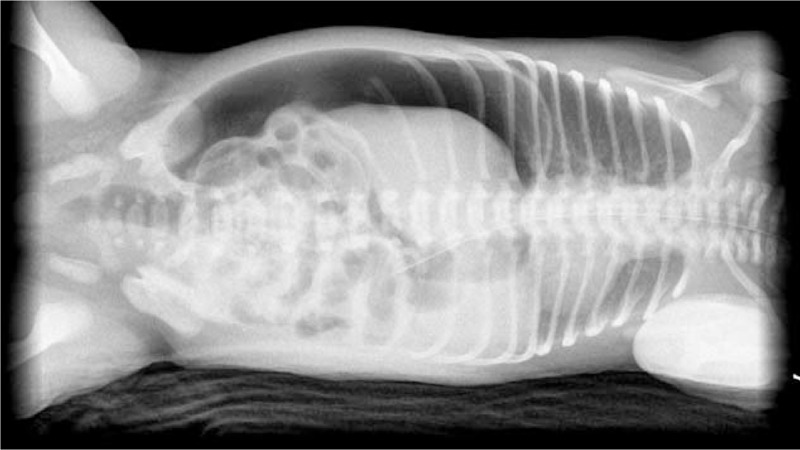
Plain abdominal plain radiography in case no 2 showed pneumoperitoneum.
4. Discussion
The clinical manifestation of neonatal gastric perforation includes abdominal distention, feeding intolerance, respiratory distress, poor activity, gastrointestinal bleeding, abdominal erythema, and hemodynamic changes as shock.
Some poor prognostic factors for survival, including male sex, hyponatremia (serum sodium < 130 mmol/L), and metabolic acidosis (pH < 7.3), have been reported.[1,2,6] However, reviews in recent 10 years have suggested that an early diagnosis of neonatal gastric perforation has favorable effects on outcomes because many aspects of critical care have enhanced our ability to rectify metabolic and electrolyte abnormalities before they become irreversible.[7] In this report, the 1st case showed acidemia on initial arterial gas analysis, whereas the 2nd case showed hyponatremia and acidemia. The 2 cases separately met 2 and 3 of the aforementioned poor prognostic factors for survival retrospectively but both patients survived due to early disease management.
Although several theories for causes of neonatal gastric perforation have been proposed, the exact mechanisms remain unknown.[2–5,8] To assess the prognostic factors for mortality, we searched PubMed for English language papers published during 1950 to 2017. We used the search terms “gastric perforation” and “neonate.” We then assessed the resulting 14 articles for relevance to our topic. The cases identified from the articles found during the initial search were reviewed for relevance. We obtained 168 cases from systematic review and enrolled 2 cases in our hospital for further analysis (Table 1).[1,6,9–20] The protocol was approved by the Institutional Review Board of E-Da Hospital (approval number: EMRP55106N). Statistical analysis of the results was performed by using Student t test, Pearson chi-squared test, Mann–Whitney U test, or logistic regression analysis. In all cases, differences were considered significant when P < .05. This review included 104 male (61.9%) and 64 female (38.1%); 72 preterm (57.1%) and 54 full-term (42.9%) neonates after 42 cases from the studies by Sato et al were excluded due to the unavailability of data on their gestational age.[19] The perforation locations included the greater curvature (n = 90; 73.8%), lesser curvature (n = 16; 13.1%), anterior wall (n = 11; 9.0%), and posterior wall (n = 5; 4.1%) after 3, 1, and 42 cases from the studies by Shashikumar et al, Kiesewetter et al, and Sato et al were excluded, respectively, due to the unavailability of data on the location of perforation.[11,14,19] Three cases in study of Rosser et al and 1 case in study of Amadeo et al, which the perforation was identified at fundus, were included into group of greater curvature for statistical analysis-based anatomical position.[15,18] Furthermore, 18 cases from the studies by Houck et al and Rosser et al were excluded from survival analysis due to the lack of survival records in this analysis.[13,15] The overall survival rate of these 150 cases of neonatal gastric perforation was 64% (death, n = 54; survival, n = 96). In addition, we used the Student t test to analyze the outcomes and birth weights after 95 cases from the studies by Houck et al, Kiesewetter et al, Rosser et al, Hwang et al, Holgersen et al, and Sato et al were excluded due to the lack of birth weights in this analysis.[13–17,19] The mean birth weights of the mortality and survival groups were 2328 ± 805 g (n = 37) and 2800 ± 740 g (n = 36), respectively. The results revealed that the birth weight of mortality group was significantly lower than survival group (P < .05).
Table 1.
Systematic review on neonatal gastric perforation.

Holgersen et al mentioned the gross and microscopic finding of perforation site and location of perforation, which was assumed those perforations were caused by mechanical disruptions.[17] However, the location of perforation with related mortality has never been discussed in previous study. We divided 49 cases into 4 groups based the location of perforation, including greater curvature, lesser curvature, anterior wall, and posterior wall. Of these 168 cases, 15 cases in Lin et al, 28 cases in Holgersen et al, and 12 cases in Chung et al were excluded due to lack of detail description upon location of perforation.[1,6,17] Also, 3, 1, and 42 cases from the studies by Shashikumar et al, Kiesewetter et al, and Sato et al were excluded, respectively, due to the unavailability of data on the location of perforation.[11,14,19] Also, 18 cases from the studies by Houck et al and Rosser et al were excluded from survival analysis due to the lack of survival records in this analysis.[13,15] The relationship between mortality and location of perforation was analyzed using the Pearson chi-squared test (Table 2). The results revealed that the mortality rate was not significant in different location of perforation. (Of the total 49 cases, anterior wall 4 cases, lesser curvature 13 cases, posterior wall 2 cases, greater curvature 30 cases, survival 24 cases, and death 25 cases, P > .05).
Table 2.
Outcomes of the 2 present cases and review cases.
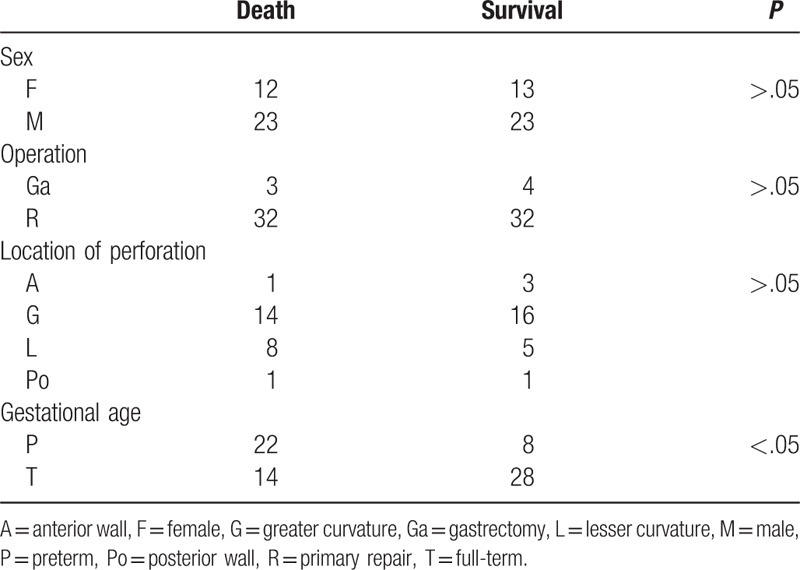
The relationship between mortality and various parameters (sex, operation type, or gestational age) was also analyzed using the Pearson chi-squared test (Table 2). The results revealed that the mortality rate was significantly higher in preterm neonates than in full-term neonates (of the total 80 cases, 22/31 preterm cases and 18/49 full-term cases died; P < .05) after 18 cases from the studies by Houck et al and Rosser et al were excluded from survival analysis due to the lack of survival records, and 28 and 42 cases from the studies by Holgersen et al and Sato et al, respectively, were excluded from subgroup analysis due to incomplete details of the original data.[13,15,17,19] The results were similar to those of a previous study.[1] However, the mortality rate was not significantly different between the male and female subgroups. Additionally, the mortality rate was not significantly different between different types of operations after 3 cases from the studies by Hwang et al due to the lack of surgical detail and 2, 1, and 2 cases from the studies by Wilson et al, Shashikumar et al, and Kiesewetter et al, respectively, were excluded because of death before surgery.[16,10,11,14] In this study, the higher mortality rate in preterm neonates and low birth weight neonates might be associated with the poor conditions of these subgroups that can easily develop sepsis and organ failure, eventually resulting in mortality. Furthermore, preterm and low birth weight neonates have a higher probability of ischemia episodes, such as hypotension and perinatal asphyxia, which may increase the risk of intestinal ischemia, thereby resulting in higher morbidity and mortality of gastric perforation.[4]
Of all 168 cases, 28 and 42 from the studies by Holgersen et al and Sato et al, respectively, were excluded from subgroup analysis due to the incomplete details of the original data.[17,19] Furthermore, 2 cases each from the studies by Wilson et al and Shashikumar et al and one case from the study by Kiesewetter et al were excluded because the diagnosis of gastric perforation was assigned upon autopsy.[10,11,14] In addition, 3 cases from the study by Hwang et al were excluded due to the unavailability of adequately detailed descriptions of the date of disease onset and body birth weights.[16] On the basis of 90 cases after excluding aforementioned incomplete data, the mean timing of perforation was 3.5 days. We used the Mann–Whitney U test to analyze the timing of perforation for the preterm and full-term subgroups (n = 37 and 53, separately). The 2 subgroups differed significantly (mean of rank: 38.22 vs 49.60, P < .05), indicating that gastric perforation occurred earlier in the preterm subgroup than in the full-term subgroup.
The outcomes were further analyzed using logistic regression analysis. To control the potential confounding effects, gestational age, birth weight, and timing of perforation were used as independent variables for adjustment. The outcomes were significantly associated with gestational age (preterm or full term) when adjusted for birth weight, timing of perforation (adjusted odds ratio: 4.21, 95% confidence interval: 1.28–13.88, P < .05). The mortality rates in the preterm and term subgroups were 72.4% and 33.3%, respectively. Therefore, preterm neonates with gastric perforations have 4.21 times higher risk of mortality than full-term neonates. Furthermore, because the birth weights of the subgroup of death in the full-term was significantly lower than the birth weights of those who survived (2645 ± 691 vs 3077 ± 525 g, n = 14 and 28, respectively, P < .05). Hence, the outcomes between birth weights of <2500 and ≥2500 g in the full-term only subgroup were further analyzed using the Pearson chi-square test. The mortality rate was significantly higher in the full-term neonates with birth weights of <2500 g (low birth weight) than in those with birth weights of ≥2500 g (P < .05).
According to previous studies, the peak incidence of gastric perforation is on the 2nd to 7th day of life, which is consistent with our analysis.[20–22] Miller et al found that gastric acidity in newborns is equal to that in adults, with a maximal acidity at 24 hours of age. The acidity decreased over the subsequent 9 days, approaching the normal level for a child.[3] Therefore, gastric acidity is exceptionally high during the first week of life, which is the peak incidence period of gastric perforation. In addition, poor nutritional support, gastric mucus secretion, or digestion ability, which are highly common in preterm neonates, may increase the risk of gastric perforation. However, further studies are required to elucidate the reasons for the earlier timing of perforation in preterm neonates.
5. Conclusions
Early diagnosis, hemodynamic monitoring, and appropriate management were achieved in our 2 cases, which may have contributed to the favorable outcomes. This systematic review showed that prognostic factor of gastric perforation was significantly associated with preterm neonates, who have 4.21 times higher risk of mortality than the full-term neonates. Furthermore, low-birth-weight full-term neonates had a relatively higher mortality rate than the normal-birth-weight full-term neonates. In addition, preterm neonates have an earlier timing of perforation.
Acknowledgements
The authors thank Tzu-Shan Chen for statistical assistance (Department of Medical Research, E-Da hospital). The authors also thank intramural funding provided by the E-Da hospital (EDAHP106014 and EDAHP106072).
Author contributions
Data collection: Tsung-Yen Chen, Hsien-Kuan Liu, Ming-Chun Yang, Yung-Ning Yang, Po-Jui Ko, Yu-Tsun Su.
Formal analysis: Tsung-Yen Chen, Hsien-Kuan Liu, Ching-Chung Tsai, Ru-Yi Huang.
Methodology: Ching-Chung Tsai.
Project administration: Ching-Chung Tsai.
Supervision: Ching-Chung Tsai.
Writing – original draft: Tsung-Yen Chen.
Writing – review & editing: Hsien-Kuan Liu, Ru-Yi Huang, Ching-Chung Tsai.
Footnotes
Ethics: Written informed consent was obtained from each patient guardian for publication of this case reports and any accompanying images.
Funding/support: The study was supported by intramural funding provided by the E-Da Hospital (EDAHP106014 and EDAHP106072).
The authors have no conflicts of interest to disclose.
References
- [1].Lin CM, Lee HC, Kao HA, et al. Neonatal gastric perforation: report of 15 cases and review of the literature. Pediatr Neonatol 2008;49:65–70. [DOI] [PubMed] [Google Scholar]
- [2].Byun J, Kim HY, Noh SY, et al. Neonatal gastric perforation: a single center experience. World J Gastrointest Surg 2014;6:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miller RA. Observation on the gastric acidity during the first month of life. Arch Dis Child 1941;16:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Touloukian RJ, Posch JN, Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate selective gut mucosal ischemia in asphyxiated piglets. J Pediatr Surg 1972;7:194. [DOI] [PubMed] [Google Scholar]
- [5].Shaker IJ, Schaffer JA, James AE, et al. Aerophagia, a mechanism for spontaneous rupture of the stomach in newborn. Am Surg 1973;39:619–23. [PubMed] [Google Scholar]
- [6].Chung MT, Kuo CY, Wang JW, et al. Gastric perforation in the neonate: clinical analysis of 12 cases. Acta Paediatr Sin 1994;35:360–5. [PubMed] [Google Scholar]
- [7].Yang CY, Lien R, Fu RH, et al. Prognostic factors and concomitant anomalies in neonatal gastric perforation. J Pediatr Surg 2015;50:1278–82. [DOI] [PubMed] [Google Scholar]
- [8].Shaw A, Blanc WA, Santulli TV, et al. Spontaneous rupture of the stomach in the newborn: a clinical and experimental study. Surgery 1965;58:561–71. [PubMed] [Google Scholar]
- [9].Leone RJ, Jr, Krasna IH. Spontaneous neonatal gastric perforation: is it really spontaneous? J Pediatr Surg 2000;35:1066–9. [DOI] [PubMed] [Google Scholar]
- [10].Wilson ES., Jr Neonatal gastric perforation. Am J Roentgenol Radium Ther Nucl Med 1968;103:307–9. [DOI] [PubMed] [Google Scholar]
- [11].Shashikumar VL, Bassuk A, Pilling GP, et al. Spontaneous gastric rupture in the newborn: a clinical review of nineteen cases. Ann Surg 1975;182:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jones TB, Kirchner SG, Lee FA, et al. Stomach rupture associated with esophageal atresia, tracheoesophageal fistula and ventilatory assistance. AJR Am J Roentgenol 1980;134:675–7. [DOI] [PubMed] [Google Scholar]
- [13].Houck WS, Jr, Griffin JA., 3rd Spontaneous linear tears of the stomach in the newborn infant. Ann Surg 1981;193:763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kiesewetter WB. Spontaneous rupture of the stomach in the newborn. AMA J Dis Child 1956;91:162–7. [DOI] [PubMed] [Google Scholar]
- [15].Rosser SB, Clark CH, Elechi EN. Spontaneous neonatal gastric perforation. J Pediatr Surg 1982;17:390–4. [DOI] [PubMed] [Google Scholar]
- [16].Hwang RC, Chen BS, Cho CC, et al. Rupture of the stomach in newborn infants: report of 3 cases. Acta Paediatr Sin 1982;23:178–84. [Google Scholar]
- [17].Holgersen LO. The etiology of spontaneous gastric perforation of the newborn: a reevaluation. J Pediatr Surg 1981;16:608–13. [DOI] [PubMed] [Google Scholar]
- [18].Amadeo JH, Ashmore HW, Aponte GE. Neonatal gastric perforation caused by congenital defects of the gastric musculature. Surgery 1960;47:1010–7. [PubMed] [Google Scholar]
- [19].Sato M, Hamada Y, Kohno M, et al. Neonatal gastrointestinal perforation in Japan: a nationwide survey. Pediatr Surg Int 2017;33:33–41. [DOI] [PubMed] [Google Scholar]
- [20].Gillesby WJ, Wheeler JR. Acute gastric dilatation. Am Surg 1956;22:1154–62. [PubMed] [Google Scholar]
- [21].Inouye WY, Evans G. Neonatal gastric perforation. Arch Surg 1964;88:471–85. [DOI] [PubMed] [Google Scholar]
- [22].Saracli T, Mann M, French DM, et al. Rupture of the stomach in the newborn infant: report of three cases and review of the world literature. Clin Pediatr 1967;6:583–8. [DOI] [PubMed] [Google Scholar]


