Abstract
Purpose
Neoadjuvant chemotherapy followed by radical cystectomy (RC) is a standard of care for the management of muscle-invasive bladder cancer (MIBC). Dose-dense cisplatin-based regimens have yielded favorable outcomes compared with standard-dose chemotherapy, yet the optimal neoadjuvant regimen remains undefined. We assessed the efficacy and tolerability of six cycles of neoadjuvant dose-dense gemcitabine and cisplatin (ddGC) in patients with MIBC.
Patients and Methods
In this prospective, multicenter phase II study, patients received ddGC (gemcitabine 2,500 mg/m2 on day 1 and cisplatin 35 mg/m2 on days 1 and 2) every 2 weeks for 6 cycles followed by RC. The primary end point was pathologic downstaging to non–muscle-invasive disease (< pT2N0). Patients who did not undergo RC were deemed nonresponders. Pretreatment tumors underwent next-generation sequencing to identify predictors of chemosensitivity.
Results
Forty-nine patients were enrolled from three institutions. The primary end point was met, with 57% of 46 evaluable patients downstaged to < pT2N0. Pathologic response correlated with improved recurrence-free survival and overall survival. Nineteen patients (39%) required toxicity-related dose modifications. Sixty-seven percent of patients completed all six planned cycles. No patient failed to undergo RC as a result of chemotherapy-associated toxicities. The most frequent treatment-related toxicity was anemia (12%; grade 3). The presence of a presumed deleterious DNA damage response (DDR) gene alteration was associated with chemosensitivity (positive predictive value for < pT2N0 [89%]). No patient with a deleterious DDR gene alteration has experienced recurrence at a median follow-up of 2 years.
Conclusion
Six cycles of ddGC is an active, well-tolerated neoadjuvant regimen for the treatment of patients with MIBC. The presence of a putative deleterious DDR gene alteration in pretreatment tumor tissue strongly predicted for chemosensitivity, durable response, and superior long-term survival.
INTRODUCTION
Randomized trials in patients with muscle-invasive bladder cancer (MIBC) have established that neoadjuvant cisplatin-based chemotherapy before radical cystectomy–pelvic lymph node dissection (RC-PLND) improves long-term survival compared with surgery alone, providing level 1 evidence that supports multimodality management as a standard of care.1 A pivotal trial that used neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) demonstrated that three cycles of chemotherapy over 12 weeks reduced the positive surgical margin rate, did not compromise surgical management, and could achieve pathologic downstaging at RC-PLND, which was associated with long-term survival.1 Subsequent trials modified the chemotherapy component via dose intensification,2 increased dose density,2-5 or by substituting newer cisplatin-based regimens, such as gemcitabine and cisplatin (GC), using standard or increased dose density and schedules.6,7 However, consensus is lacking with regard to the optimal cisplatin regimen, drug dose, number of cycles, and preoperative chemotherapy duration.
Neoadjuvant chemotherapy has been underused predominantly because of concerns that clinical benefit is restricted to a subset of patients while all patients are exposed to chemotherapy toxicity.8 These concerns have prompted studies to identify the patients who are most likely to benefit from chemotherapy by evaluating potential biomarkers that are predictive of chemosensitivity.9-12 Next-generation sequencing (NGS), which facilitates the genomic profiling of transurethral resection (TUR) specimens and correlation with pathologic response and long-term survival, has accelerated these efforts. In one study, mutations within the nucleotide excision repair (NER) DNA helicase, ERCC2, were enriched within patients who achieved a pathologic complete response (pT0) or were downstaged to noninvasive disease after neoadjuvant chemotherapy.10 Another study identified alterations within the DNA damage response (DDR) genes, ATM, FANCC, and RB1, as associated with heightened neoadjuvant chemosensitivity.11
We assessed the efficacy of neoadjuvant dose-dense (dd) GC in a multicenter phase II trial. This regimen was chosen on the basis of a randomized trial that compared ddGC with ddM-VAC—both administered for six cycles over 12 weeks—for the treatment of patients with metastatic disease that showed similar survival but less toxicity with ddGC. Moreover, the response rates and median survival that were observed with both dose-dense regimens were superior to results reported with standard-dose GC.13,14 The gemcitabine dose (2,500 mg/m2) has been demonstrated to be well tolerated in the metastatic setting in combination chemotherapy regimens.15 The primary end point of the trial was pathologic response rate—downstaging to non–muscle-invasive disease (< pT2) —with secondary end points of safety and recurrence-free survival (RFS). Patients’ bladders were serially imaged to identify the time to maximal chemotherapy response. Pretreatment TUR specimens were sequenced to validate previously identified, putative predictive biomarkers of chemosensitivity.
PATIENTS AND METHODS
Eligibility
Patients with clinical stage T2 to T4aN0M0 disease who were candidates for RC-PLND were enrolled. Cystoscopy with TUR was performed within 60 days of enrollment for stage confirmation. Whereas repeat TUR at study sites was not required, pathologic confirmation of MIBC was mandated. Exam under anesthesia was encouraged to inform clinical staging. Chest X-ray or chest computed tomography (CT) scan and a CT scan or magnetic resonance imaging of the abdomen and pelvis—diffusion-weighted magnetic resonance imaging preferred—were required within 30 days of enrollment. Cisplatin candidacy was defined as an estimated glomerular filtration rate ≥ 60 mL/min per 1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration formula); Karnofsky performance status (KPS) of ≥ 70%; and no pre-existing grade 2 peripheral neuropathy or hearing impairment, New York Heart Association class III or IV heart failure, or recent cardiovascular event.
This study was approved by the institutional review boards of participating sites and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before study entry.
Procedures
This was a nonrandomized, multi-institutional, open-label phase II study. Patients received six 14-day cycles of ddGC: gemcitabine 2,500 mg/m2 on day 1, cisplatin 35 mg/m2 on days 1 and 2 (achieving a planned dose intensity of 1.875 times and 1.5 times standard gemcitabine and cisplatin, respectively), and pegfilgrastim on day 3. Intravenous mannitol was administered precisplatin. Intravenous hydration and antiemetics were administered according to institutional guidelines.
Clinical Assessment
Patients underwent a history, physical examination, and toxicity assessment using National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) on day 1 of each cycle. Imaging was performed after four and six chemotherapy cycles. RC-PLND was recommended within 4 to 8 weeks after completing chemotherapy.
Correlative Assessments
Radiographic features of primary bladder tumors were compared between baseline and postcycles 4 and 6 scans (Data Supplement).
NGS using the Integrated Mutation Profiling of Actionable Cancer Targets platform was performed as previously described using DNA from pretreatment TUR and matched normal specimens (Data Supplement).16 Mutation status of 29 DDR genes—selected post hoc—was correlated with response (Data Supplement). Genes were chosen on the basis of their presence within canonical DDR pathways, a reported association with chemosensitivity in urothelial carcinoma (UC), or those genes altered significantly within The Cancer Genome Atlas (TCGA) for urothelial bladder carcinoma.
Statistical Analysis
The primary end point was pathologic response to neoadjuvant ddGC, which was defined as the absence of muscle-invasive disease (< pT2) and lymph node metastases (N0) within the RC-PLND specimen as assessed by institutional pathologists using the American Joint Committee on Cancer 7th edition criteria.17 Null hypothesis pathologic response rate was 35% on the basis of a retrospective study of standard-dose neoadjuvant GC and two prospective randomized trials.1,6,18 A response rate improvement from 35% to 55% was considered promising. Patients who developed progressive metastatic disease on chemotherapy or who were unable to or refused to undergo RC-PLND were considered nonresponders for the primary end point analysis. Patients who received fewer than three cycles of chemotherapy were inevaluable for the primary end point and replaced. If 22 or more of 46 evaluable patients were < pT2N0, then the treatment regimen would be considered worthy of additional study. The study design was based on an exact binomial one-sided test with a type I error of 5% and power of 87%.
The secondary end point of RFS was assessed using the Kaplan-Meier method and measured from treatment initiation until disease recurrence, which was defined as clinically or radiographically documented progression before RC-PLND, or local or metastatic recurrence after RC-PLND. Patients without documented recurrence were censored at last follow-up. Pathologic response and DDR mutation status were correlated with survival using a log-rank test. In these analyses, RFS was measured from RC-PLND to the date of disease recurrence. Association between response and DDR mutation status was analyzed using a one-sided Fisher exact test as the direction of the potential effect was determined on the basis of previous findings. Mutation burden between responders and nonresponders was tested using the Mann-Whitney test.
RESULTS
Forty-nine patients—40 male and nine female—were enrolled between June 2012 and July 2015 (Table 1). Median Karnofsky performance status was 90% and median age 64 years. Thirty-four percent of patients had cT3 to 4 disease at enrollment. Three patients who received two or fewer cycles of chemotherapy—one experienced a vascular access complication, and two patients had elevated creatinine levels that precluded safe cisplatin administration—were inevaluable for the primary end point and were replaced.
Table 1.
Patient Demographics and Clinical Characteristics (N = 49)

Of 49 patients, 19 (39%) required toxicity-related dose modifications or early treatment discontinuation. Dose delays or reductions were predominantly a result of thrombocytopenia (18%) and creatinine elevation (6%). Treatment-related grade 3 and 4 toxicity occurred in 37% of patients (Table 2). The most common toxicity was anemia (12%; grade 3). The most common nonhematologic toxicity was hyperglycemia (39% [grades 1 and 2] and 12% [grades 3 and 4]). The thromboembolic event rate—arterial or venous—was 6%, which occurred during chemotherapy. The median number of days from the completion of ddGC to RC-PLND was 45 (range, 30 to 78 days). No patient failed to undergo RC-PLND as a result of treatment toxicity. Eight patients underwent RC-PLND > 8 weeks after ddGC completion secondary to scheduling availability.
Table 2.
Treatment-Related Toxicities Occurring During Chemotherapy and Within 30 Days of Radical Cystectomy

Forty-six of 49 patients were evaluable for the primary end point. The study met the primary end point with a pathologic response rate of 57% (95% CI, 42% to 70%) with 26 responders. In an exploratory, intent-to-treat analysis of all patients, the response rate was 53% (26 of 49 patients; 95% CI, 0.39 to 0.66). Five patients who completed three or more cycles did not undergo RC and were designated nonresponders—one refused surgery, one developed metastatic progression after four cycles, one withdrew consent, one was lost to follow-up, and one patient was incidentally diagnosed with moyamoya and discontinued the study because of the risk for vascular thrombotic events. Among the 41 patients who underwent RC and the patient who experienced progression with metastatic disease during therapy, pathologic response rate was 62% (95% CI, 48% to 76%), including 21 patients who were downstaged to < pT1 (Table 3). pT0 rate was 15% (95% CI, 0.08 to 0.28). The median number of nodes retrieved at RC was 24 (range, four to 56 nodes), and two patients had N1 disease. Median RFS and overall survival were not reached at a median follow-up for living patients of 25.6 months (Figs 1A and 1B). Among 46 eligible patients, nine have died (seven from disease), and eight have experienced disease progression. Of 41 patients who underwent RC, chemotherapy responders (< pT2N0) had significantly better RFS compared with nonresponders (≥ pT2N0 disease; hazard ratio, 0.088; 95% CI, 0.01 to 0.73; log rank P = .004; Fig 2A). The 2-year RFS rate responders and nonresponders was 96% and 52%, respectively, and the 2-year overall survival rate for responders versus nonresponders was 96% and 84%, respectively (hazard ratio, 0.15; 95% CI, 0.02 to 1.26; log-rank P = .043; Fig 2B).
Table 3.
Pathologic Response at Radical Cystectomy

Fig 1.
(A) Recurrence-free survival (RFS) and (B) overall survival (OS) in 46 patients who were treated with dose-dense gemcitabine and cisplatin. The dashed lines refer to 95% confidence intervals.
Fig 2.
(A) Recurrence-free survival (RFS) and (B) overall survival (OS) stratified by pathologic response (< pT2 v ≥ pT2) for 41 patients who underwent radical cystectomy (RC).
Twenty-seven patients (59%) were evaluable for comparison of imaging of the primary bladder tumor pretreatment, postcycle 4, and postcycle 6 (Data Supplement). Twenty-four patients demonstrated an evaluable primary tumor mass and/or bladder wall thickening that was consistent with residual disease after four cycles. Ten patients had an additional reduction of the radiographic lesion on the scan obtained after cycle 6 (42%). Nine of 10 patients underwent RC-PLND, and seven of nine patients exhibited downstaging of disease postchemotherapy. Of the remaining 14 patients, nine had a reduction in lesions after four cycles, but not after six cycles, and seven of nine patients had corresponding pathologic downstaging.
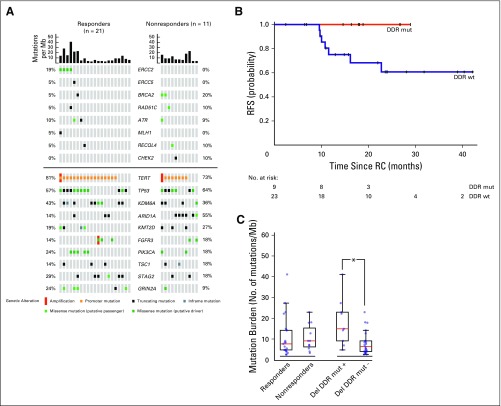
Adequate pretreatment tumor and germline DNA was available for NGS analyses in 32 patients, including those who received three or more cycles of ddGC followed by RC-PLND and one patient who experienced progression systemically during chemotherapy. For one patient, matched germline DNA was unavailable and a pooled normal control was used. Missense variants reported in dbSNP or the 1000 Genomes project were excluded for this sample. Median tumor and germline coverage were 691X and 467X, respectively. Somatic alterations identified per tumor sample are listed in the Data Supplement. Frequency and type of somatic alterations resembled the urothelial TCGA (Data Supplement), except for the TERT promoter region (not sequenced in TCGA). Alterations within a panel of 29 DDR genes were correlated with chemotherapy response. Deleterious DDR gene alterations, which were defined as nonsense, frameshift, or splice site alterations, or ERCC2 missense mutations, were identified in nine patients, with ERCC2 mutations being most common (n = 4). Eight of these nine patients were chemotherapy responders (Fig 3A). The positive predictive value of a somatic deleterious DDR gene alteration for response was 89%, and 2-year RFS was higher in patients whose tumors had a deleterious DDR gene alteration compared with those without an alteration (100% v 61%; log-rank P = .07; Fig 3B).
Fig 3.
(A) Onco-print showing the alterations identified in 32 pretreatment transurethral resection specimens from patients who underwent radical cystectomy (RC) –pelvic lymph node dissection after treatment with dose-dense gemcitabine and cisplatin. Samples are organized into responders or nonresponders with mutation burden displayed for each sample. (Top) Alterations within DNA damage response (DDR) genes. (Bottom) Alterations within the top 10 most frequently altered genes within this tumor cohort. (B) Two-year recurrence-free survival (RFS) in patients with or without DDR mutant tumors (DDR mut [mutant] v DDR wt [wild type]). (C) Mutation burden in responders versus nonresponders and in any patient with deleterious DDR gene alterations (Del DDR mut+) compared with those without (Del DDR mut-). Mb, megabase. (*)P < .05.
Deleterious DDR gene–altered tumors have been associated with a higher mutation burden.19,20 In this study, patients with a deleterious DDR gene alteration had a higher mutation burden than did patients without an alteration, regardless of response status (median 15.2 mutations v 5.8 mutations/megabase; P < .01); however, mutation burden between responders and nonresponders was not statistically significant (median 7.9 mutations v 9.4 mutations/megabase; P = .6; Fig 3C).
DISCUSSION
The current study sought to maximize GC dose density and to explore six cycles of neoadjuvant chemotherapy using a dose and schedule that demonstrated encouraging clinical outcomes for patients with metastatic disease. With a pathologic response rate of 57%, the study met its primary end point. The regimen delivered twice the total cisplatin dose density that was intended in SWOG 8710, which used standard-dose M-VAC. This improvement in drug delivery is relevant as UC trials have consistently shown that dose-dense chemotherapy is associated with higher response rates in neoadjuvant and metastatic settings.3-5,13 Whereas grades 3 and 4 toxicity were observed in 37% of patients, no patient experienced toxicity-related delays to RC-PLND.
The 57% pathologic response rate is comparable to other dose-dense neoadjuvant regimens (49% to 53% < pT2 rate).4,5 Whereas the pT0 rate was lower (15%) than that reported in other neoadjuvant studies, we have shown that patients with pT0 and those with < pT2 responses have a similar 5-year survival of > 90%.21 Moreover, < pT2 is the primary efficacy end point in an ongoing intergroup neoadjuvant trial (SWOG S1314). Finally, although the pathologic response rate in patients who underwent RC-PLND was not a prespecified end point, the 62% rate of downstaging to < pT2 and the improved survival rate within this patient population underscores the efficacy of the ddGC regimen.
A controversial aspect of this trial was the use of six cycles of neoadjuvant chemotherapy; the optimal number of cycles has never been formally studied. Limiting treatment to three to four cycles originated empirically from concerns that potentially ineffective chemotherapy might delay curative surgery. Of 49 patients, 33 (67%) completed all six cycles, which allowed for the assessment of the relationship between cycle number and efficacy. Ten of 24 patients exhibited continued reduction of a radiographic abnormality between four and six cycles, and seven patients achieved downstaging from pretreatment clinical stage. In addition, only 4% of patients (two of 41) who underwent RC-PLND had metastatic node involvement—a median of 24 nodes retrieved—compared with a node-positive rate between 21% and 26% in retrospective analyses of standard neoadjuvant GC.21 These findings challenge the paradigm of three to four cycles as the standard for neoadjuvant treatment.
The higher than normal gemcitabine dose (2,500 mg/m2) selected for this study, although it was based on a previous randomized trial, is controversial. From a pharmacokinetics perspective, the plasma area under the concentration-time curve of gemcitabine increases linearly from 40 mg/m2 to 3,650 mg/m2, and its predominant active and inactive metabolites—2′, 2′-difluoro-2′-deoxycytidine triphosphate (dFdCTP) and 2′, 2′-difluorodeoxyuridine (dFdU), respectively—also display linear area under the concentration-time curve relationships with higher doses.22,23 Both dFdCTP and dFdU are also cytotoxic. Conversely, human studies that validate higher intratumoral concentrations of gemcitabine and its metabolites with higher administered doses are currently lacking. Multiple groups have reported venous thromboembolism rates of 13% to 18% in patients who receive cisplatin-based chemotherapy.24 In another trial of neoadjuvant ddGC, two of the first 13 enrolled patients experienced a myocardial infarction and two had venous thromboembolic events, with an overall 9% incidence of grades 3 and 4 vascular events.7 In that study, cisplatin 70 mg/m2 was administered on day 1. We did not observe a higher than anticipated rate of thromboembolic events (6%) in this study, which may be related to splitting cisplatin over days 1 and 2 and the upfront exclusion of patients who had experienced cardiovascular events or who had a history of angina or stroke within 6 months of chemotherapy administration. The possibility for significant toxicities that preclude potentially curative RC-PLND underscores the need to identify predictive biomarkers of neoadjuvant chemosensitivity.
Published retrospective studies suggest that deleterious alterations in DDR genes might represent predictive biomarkers of chemosensitivity. In this study, eight of nine patients whose tumors harbored a putative, loss-of-function DDR gene alteration exhibited either a pathologic complete response or downstaging to < pT1 at RC-PLND. To date, no patient with a deleterious DDR gene alteration has experienced a metastatic recurrence, including the CHEK2 mutant nonresponder. Whereas the NER pathway was most frequently altered, several other DDR pathway genes had potentially deleterious alterations, including BRCA2 and RAD51C (homologous recombination), ATR and CHEK2 (DNA damage sensing), and RECQL4 (double-strand break repair), and correlated with chemosensitivity. The association between high mutation burden and deleterious DDR gene alterations is in agreement with other studies that correlated the disruption of key DDR pathways with increased genomic instability. It highlights the need for a stringent definition for loss-of-function DDR gene alterations to discriminate known or likely deleterious mutations from passenger events and the need for functional validation of such alterations.25 Finally, most responders (62%) did not harbor deleterious DDR gene alterations, which suggest that additional factors affect chemosensitivity. SWOG S1314 is examining the use of gene expression profiling to predict for neoadjuvant chemotherapy response. These data may provide validation for our findings and identify novel biomarkers of chemosensitivity in DDR pathway wild-type responders.
Limitations of our study include the lack of a comparator arm and the relatively small sample size. Moreover, potentially deleterious DDR gene alterations were analyzed together. Larger studies are needed to determine whether chemosensitivity varies on the basis of specific mutant alleles within individual genes or which DDR gene is mutated. Unbiased genomic profiling—not feasible because of limited tumor tissue—may have identified additional putative biomarkers of chemosensitivity, particularly in DDR gene wild-type patients. Such profiling could also detect and correlate genome-wide mutation signatures with response, including the APOBEC signature, homologous recombination deficiency signatures that are associated with BRCA1/2 functional impairment, or the signature 5*, which is associated with ERCC2 alterations and NER deficiency.26-28 Moreover, whereas germline DNA was used to delineate somatic alterations, this study did not include consent to review this data for potential heritable alterations that could confer cisplatin chemosensitivity as observed in other tumor types.29-31
The concept of organ sparing has been adopted for the management of breast, rectal, and laryngeal cancers,32,33 but not MIBC, despite the fact that RC-PLND significantly affects body image and quality of life.34 Trimodality bladder sparing, which consists of chemoradiation after a maximal TUR, results in favorable outcomes for highly selected patients but is associated with local toxicities, and up to 29% of patients may ultimately require RC-PLND.35 Retrospective data from three institutions indicate that patients with MIBC who achieve a clinical complete response to neoadjuvant chemotherapy can be managed with surveillance without RC-PLND.36-38 The 10-year survival for patients from two of these institutions was 68% to 75%, with a bladder-intact survival rate of 47% to 61%. A refinement of this approach may be possible: Prospective detection of deleterious DDR gene alterations may allow for the selection of patients who are most likely to experience durable responses to chemotherapy through eradication of bladder-confined and micrometastatic disease. TUR plus definitive chemotherapy may suffice for patients with deleterious DDR gene alterations who display exquisite chemosensitivity. Bladder preservation is reasonable in this context as six cycles of ddGC can cure patients with metastatic disease, with a median survival that is more favorable than reported with standard-dose therapy. These results challenge the orthodoxy that bladder extirpation is required for all patients with MIBC. Consequently, the Alliance trial A031701 will test this concept prospectively using neoadjuvant ddGC in MIBC, performing real-time genomic profiling, and offering bladder preservation in responding patients with deleterious DDR gene alterations.
Footnotes
Funded by the Zena and Michael A. Wiener Research and Therapeutic Program in Bladder Cancer, Marie-Josée and Henry R. Kravis Center for Molecular Oncology, Cycle for Survival, National Cancer Institute Cancer Center Core Grant No. P30-CA008748.
Clinical trial information: NCT01589094.
AUTHOR CONTRIBUTIONS
Conception and design: Gopa Iyer, Arjun V. Balar, Bernard H. Bochner, Guido Dalbagni, Harry W. Herr, Irina Ostrovnaya, Jamie C. Riches, Jonathan E. Rosenberg, Dean F. Bajorin
Financial support: David B. Solit
Administrative support: David B. Solit
Provision of study materials or patients: Gopa Iyer, Arjun V. Balar, Matthew I. Milowsky, Bernard H. Bochner, Guido Dalbagni, S. Machele Donat, Harry W. Herr, William C. Huang, Samir Taneja, Michael Woods, Tracy L. Rose, William Y. Kim, David B. Solit, Jonathan E. Rosenberg, Dean F. Bajorin
Collection and assembly of data: Gopa Iyer, Caitlin Bourque, Maha Shady, Helen Won, William Y. Kim, Brooke E. Kania, Mariel E. Boyd, Catharine K. Cipolla, Ashley M. Regazzi, Daniela Delbeau, Asia S. McCoy, Hebert Alberto Vargas, David B. Solit
Data analysis and interpretation: Gopa Iyer, Arjun V. Balar, Matthew I. Milowsky, Bernard H. Bochner, Guido Dalbagni, S. Machele Donat, Harry W. Herr, William C. Huang, Samir S. Taneja, Michael Woods, Irina Ostrovnaya, Hikmat Al-Ahmadie, Maria E. Arcila, Andreas Meier, Maha Shady, Helen Won, Tracy L. Rose, William Y. Kim, Ashley M. Regazzi, Hebert Alberto Vargas, Michael F. Berger, David B. Solit, Jonathan E. Rosenberg, Dean F. Bajorin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Gopa Iyer
No relationship to disclose
Arjun V. Balar
Honoraria: Merck
Consulting or Advisory Role: Genentech, Merck, Cerulean Pharma, AstraZeneca, MedImmune
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), MedImmune (Inst)
Matthew I. Milowsky
Research Funding: Mirati Therapeutics (Inst), Pfizer (Inst), Cerulean Pharma (Inst), Merck (Inst), Seattle Genetics (Inst), Acerta Pharma (Inst), BioClin Therapeutics (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), X4 Pharma (Inst), MedImmune (Inst), Incyte (Inst), Innocrin Pharma (Inst), Inovio Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Genentech
Bernard H. Bochner
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Guido Dalbagni
No relationship to disclose
S. Machele Donat
No relationship to disclose
Harry W. Herr
No relationship to disclose
William C. Huang
No relationship to disclose
Samir S. Taneja
Consulting or Advisory Role: InSightec
Research Funding: MDxHealth (Inst), Trod Medical (Inst), Sophiris Bio (Inst)
Travel, Accommodations, Expenses: Biobot Surgical
Michael Woods
No relationship to disclose
Irina Ostrovnaya
No relationship to disclose
Hikmat Al-Ahmadie
Consulting or Advisory Role: EMD Serono, Bristol-Myers Squibb
Maria E. Arcila
No relationship to disclose
Jamie C. Riches
No relationship to disclose
Andreas Meier
No relationship to disclose
Caitlin Bourque
No relationship to disclose
Maha Shady
No relationship to disclose
Helen Won
No relationship to disclose
Tracy L. Rose
Research Funding: X4 Pharmaceuticals (Inst), Genentech (Inst)
William Y. Kim
No relationship to disclose
Brooke E. Kania
No relationship to disclose
Mariel E. Boyd
No relationship to disclose
Catharine K. Cipolla
No relationship to disclose
Ashley M. Regazzi
No relationship to disclose
Daniela Delbeau
No relationship to disclose
Asia S. McCoy
No relationship to disclose
Hebert Alberto Vargas
No relationship to disclose
Michael F. Berger
No relationship to disclose
David B. Solit
Honoraria: Loxo, Pfizer
Consulting or Advisory Role: Pfizer, Loxo
Jonathan E. Rosenberg
Stock or Other Ownership: Merck, Illumina
Honoraria: UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview
Consulting or Advisory Role: Eli Lilly, Merck, Agensys, Genentech, AstraZeneca, MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetics, Bayer, Inovio Pharmaceuticals
Research Funding: Genentech (Inst), Oncogenex (Inst), Agensys (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Viralytics (Inst), Genentech (Inst), Incyte
Patents, Royalties, Other Intellectual Property: Gene mutation predicting cisplatin sensitivity
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb
Dean F. Bajorin
Honoraria: Merck Sharp & Dohme, Genentech
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Genentech, Merck, Roche, Eli Lilly, Fidia Farmaceutici SpA, Eisai, Urogen Pharma
Research Funding: Dendreon (Inst), Novartis (Inst), Amgen (Inst), Genentech (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Genentech, Merck, Bristol-Myers Squibb, Eli Lilly
REFERENCES
- 1.Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859-866, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Seidman AD, Scher HI, Gabrilove JL, et al. : Dose-intensification of MVAC with recombinant granulocyte colony-stimulating factor as initial therapy in advanced urothelial cancer. J Clin Oncol 11:408-414, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, de Mulder PH, Schornagel JH, et al. : Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19:2638-2646, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Jacobus S, Bellmunt J, et al. : Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: Pathologic, radiologic, and biomarker correlates. J Clin Oncol 32:1889-1894, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32:1895-1901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dash A, Pettus JA, IV, Herr HW, et al. : A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer 113:2471-2477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plimack ER, Hoffman-Censits JH, Kutikov A, et al. : Neoadjuvant dose-dense gemcitabine and cisplatin (DDGC) in patients (pts) with muscle-invasive bladder cancer (MIBC): Final results of a multicenter phase II study. J Clin Oncol 32:5s, 2014. (suppl; abstr 4513) [Google Scholar]
- 8.David KA, Milowsky MI, Ritchey J, et al. : Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: A report from the National Cancer Data Base. J Urol 178:451-454, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Sun JM, Sung JY, Park SH, et al. : ERCC1 as a biomarker for bladder cancer patients likely to benefit from adjuvant chemotherapy. BMC Cancer 12:187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Allen EM, Mouw KW, Kim P, et al. : Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 4:1140-1153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plimack ER, Dunbrack RL, Brennan TA, et al. : Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 68:959-967, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadler WM, Lerner SP, Groshen S, et al. : Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol 29:3443-3449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamias A, Dafni U, Karadimou A, et al. : Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 24:1011-1017, 2013 [DOI] [PubMed] [Google Scholar]
- 14.von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068-3077, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Sternberg CN, Calabrò F, Pizzocaro G, et al. : Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 92:2993-2998, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC: The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471-1474, 2010 [DOI] [PubMed] [Google Scholar]
- 18.International Collaboration of Trialists. Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group) European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group. et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 29:2171-2177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YK, Anker JF, Carneiro BA, et al. : Genomic landscape of DNA repair genes in cancer. Oncotarget 7:23312-23321, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeb LA: A mutator phenotype in cancer. Cancer Res 61:3230-3239, 2001 [PubMed] [Google Scholar]
- 21.Tully CM BB, Dalbagni G, Zabor EC, et al. : Gemcitabine-cisplatin (GC) plus radical cystectomy-pelvic lymph node dissection (RC-PLND) for patients (pts) with muscle-invasive bladder cancer (MIBC): Assessing impacts of neoadjuvant chemotherapy (NAC) and the PLND. J Clin Oncol 32, 2014. (suppl; abstr 355) [Google Scholar]
- 22.Ciccolini J, Serdjebi C, Peters GJ, et al. : Pharmacokinetics and pharmacogenetics of gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother Pharmacol 78:1-12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters GJ, Clavel M, Noordhuis P, et al. : Clinical phase I and pharmacology study of gemcitabine (2′, 2′-difluorodeoxycytidine) administered in a two-weekly schedule. J Chemother 19:212-221, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moore RA, Adel N, Riedel E, et al. : High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol 29:3466-3473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helleday T, Eshtad S, Nik-Zainal S: Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 15:585-598, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Mouw KW, Polak P, et al. : Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet 48:600-606, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507:315-322, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng G, Chun-Jen Lin C, Mo W, et al. : Genome-wide transcriptome profiling of homologous recombination DNA repair. Nat Commun 5:3361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyd J, Sonoda Y, Federici MG, et al. : Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA 283:2260-2265, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Byrski T, Gronwald J, Huzarski T, et al. : Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375-379, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Golan T, Kanji ZS, Epelbaum R, et al. : Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111:1132-1138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Aguilar J, Renfro LA, Chow OS, et al. : Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): Results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16:1537-1546, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefebvre JL, Pointreau Y, Rolland F, et al. : Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. J Clin Oncol 31:853-859, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Henningsohn L, Steven K, Kallestrup EB, et al. : Distressful symptoms and well-being after radical cystectomy and orthotopic bladder substitution compared with a matched control population. J Urol 168:168-174, discussion 174-175, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Efstathiou JA, Spiegel DY, Shipley WU, et al. : Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol 61:705-711, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Herr HW, Bajorin DF, Scher HI: Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: Ten-year outcome. J Clin Oncol 16:1298-1301, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Sternberg CN, Pansadoro V, Calabrò F, et al. : Can patient selection for bladder preservation be based on response to chemotherapy? Cancer 97:1644-1652, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Meyer A, Ghandour R, Bergman A, et al. : The natural history of clinically complete responders to neoadjuvant chemotherapy for urothelial carcinoma of the bladder. J Urol 192:696-701, 2014 [DOI] [PubMed] [Google Scholar]