Abstract
Cholinesterase inhibitors (ChEIs) are the mainstays of symptomatic treatment of Alzheimer’s disease (AD); however, their efficacy is limited, and their use was associated with deaths in some groups of patients. The aim of the current study was to assess the impact of the long-term use of ChEIs on mortality in patients with AD. This observational, longitudinal study included 1171 adult patients with a diagnosis of AD treated with donepezil or rivastigmine. Each patient was observed for 24 months or until death. The cognitive and functional assessments, the use of ChEIs, memantine, antipsychotics, antidepressants, and anxiolytics were recorded. The total number of deaths at the end of the observational period was 99 (8.45%). The patients who had received rivastigmine treatment were at an increased risk of death in the follow-up period. The higher risk of death in the rivastigmine group remained significant in multivariate Cox regression models.
Keywords: Alzheimer’s disease, dementia, donepezil, mortality, rivastigmine, risk factors
Background
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases and accounts for more than 70% of dementia cases in elderly people worldwide. It leads to the progressive mental, behavioral, and functional decline. 1
An estimated 33 million people worldwide and 5.4 million Americans of all ages have AD. Today, one new case of AD develops every 67 seconds, and the number of patients is set to triple by 2050. 2 Cholinesterase inhibitors (ChEIs) are the mainstays of symptomatic treatment for patients with AD. The use of these drugs is associated with statistically significant improvements in cognition and daily functioning of patients; however, these effects are temporary and do not stop the global clinical deterioration. 3 There are also several experimental studies which have investigated the efficacy and safety of ChEIs in the treatment of vascular dementia, dementia in Huntington disease, Parkinson disease dementia (PDD), multiple sclerosis, and postoperative delirium. 4 According to these studies, there is insufficient evidence to recommend for or against the use of ChEIs for the treatment of above-mentioned disorders, although some advantages of the use of ChEIs in the treatment of PDD have been described. 5 However, one of the randomized, controlled trials assessing the use of rivastigmine in patients with delirium was stopped due to increased mortality in the active treatment group. 6 Taking into account limited efficacy of ChEIs in the treatment of AD and other types of dementia, the safety and the impact of ChEIs on cardiovascular system, the risk of death, long-term prognosis, and benefit to harm ratio are crucial issues for drug prescription. Kröger et al conducted the analysis of adverse drug reactions (ADRs) reported with ChEIs. 7 The analysis included 18 955 reports (43 753 ADRs) from 58 countries submitted to VigiBase between 1998 and 2013. Use of rivastigmine and donepezil was involved in the majority of reports (41.4% each). The most frequently reported ADRs were neuropsychiatric (31.4%), gastrointestinal (15.9%), and cardiovascular (11.7%) disorders. Cholinesterase inhibitor therapy was associated with a small but significant increase in the risk of bradycardia, hypotension, cardiac arrhythmia, and syncope when compared to nontreated patients. In addition, serious ADRs were more frequent than nonserious ones. Death occurred in 2.3% of the reports. Conversely, there is a growing body of evidence indicating favorable cardiovascular effects of ChEIs in patients with dementia. 8 -10 Nordström et al assessed the cohort of 7073 patients from the Swedish Dementia Registry with the diagnosis of AD. 10 Of these patients, 5159 patients received ChEIs at least once. After adjustment for confounders, patients who used ChEIs had a 34% lower risk for composite outcome of myocardial infarction (MI) or death than those who did not. In other study, Meguro et al revealed the lifetime expectancies among patients with AD after disease onset to be higher in patients treated with donepezil as compared to nondonepezil group (7.9 vs 5.3 years, respectively). 11 The major limitation of the above studies is their retrospective design. It is also possible that patients qualified to ChEIs therapy were generally healthier at baseline, had no history of cardiovascular disease (CVD), had better and more advanced medical care, and received cardioprotective drugs more frequently. Wattmo et al included into analysis 1021 patients from a 3-year, prospective multicenter study (The Swedish Alzheimer Treatment Study [SATS]). 12 In the multivariate models designed in this study, longer survival was associated with higher ChEI dose and longer duration of treatment. The patients receiving 3 types of ChEIs (donepezil, galantamine, and rivastigmine) were prospectively observed during a 3-year period; however, the death ratio was estimated after 16 years of follow-up on the basis of Swedish population register. No differences regarding the influence of ChEIs type on mortality were observed. The aim of the current study was to compare the impact of the 2 most frequently prescribed ChEIs—rivastigmine and donepezil on all-cause mortality in patients with AD after a 2-year treatment period.
Methods
This is a prospective, longitudinal study conducted between January 2000 and May 2014 at the Memory Clinic, 3rd Department of Neurology, Thessaloniki, Greece. The study included consecutive adult patients with a diagnosis of dementia due to AD followed at the Memory Clinic of Thessaloniki and day centers of Alzheimer Hellas during the study period. The study was approved by the Ethics Committee Alzheimer Hellas and performed in accordance with the ethical standards of the Declaration of Helsinki. The patients together with their guardians provided informed written consents for the participation in the study. The study procedures were performed by the team of qualified and experienced neuropsychologists, neurologists, and psychiatrists. Each patient was examined at a baseline visit and in the 6-month intervals until the completion of a 24-month observation period or death. During the initial recruitment period, the diagnosis of dementia due to AD was verified (for patients who had already attended the clinic) or established (for newly admitted patients) based upon the criteria of the National Institute on Aging and Alzheimer’s Association. 13 If any uncertainty was found, the final decision was made at a consensus meeting. The first and following clinical assessments included Mini-Mental State Examination (MMSE; total score 30; cutoff score for dementia screening ≤23) test, 14 Functional Rating Scale for Symptoms of Dementia (FRSSD), 15 and Geriatric Depression Scale (GDS; total score 15; cutoff ≥5) 16 neurologic and psychiatric examination. Functional Rating Scale for Symptoms of Dementia is a brief diagnostic scale which examines patients’ ability to carry out instrumental routine tasks such as eating, dressing, and toileting (total score 42). Functional Rating Scale for Symptoms of Dementia total score over 7 reveals functional difficulties in everyday living, while total score 5 to 7 is regarded as borderline. At baseline, patients who had received the ChEIs therapy for at least 1 preceding month in the following doses: either 5 mg or more of oral donepezil or 6 mg or more of oral rivastigmine or 4.6 mg transdermal rivastigmine were included in the study. Before inclusion, magnetic resonance imaging in each patient was performed. At each assessment, the data on the use of ChEIs, memantine, antipsychotics, antidepressants, and anxiolytics were recorded. Patients who discontinued ChEIs therapy for 30 days or more or changed the type of the ChEI during the observational period were excluded from the study. The physicians changed the type of ChEI in exceptional cases only (usually very rapid progress of the disease despite appropriate treatment). Memantine, antipsychotics, antidepressants, and anxiolytics were recorded if the patients had received these medications for at least 7 random or consecutive days in the month preceding the assessment. Moreover, patients were classified into 4 categories with regard to their physical condition. Category 1 included patients with no concomitant diseases, category 2 included patients with 1 or 2 concomitant diseases, category 3 included patients with 3 or 4 concomitant diseases, and category 4 included patients with 5 or more concomitant diseases. Since category 1 included 1 patient only, for statistical analysis, these categories were grouped into 3: category 1 (maximum 2 comorbid diseases), category 2 (3 or 4 comorbid diseases), and category 3 (5 or more comorbid diseases). As the patients had regular routine cognitive program and visits to the clinic (from 1 to 5 visits per week) or were regularly visited in their houses, a nurse or medical secretary contacted the patient and the patient’s family in case of any longer absence to the clinic, when longer intervals between appointments occurred, or when the patient omitted the scheduled visit. The patients’ death and the cause of death were reported by the internists who took medical care of patients in the Memory Clinic, or general practitioners who took medical care of patients in their society. These data and patients’ status were recorded both in the patients’ medical history and in the electronic database.
Statistical Analysis
The SPSS package (version 24) and STATISTICA PL (version 12.5) were used to conduct all the statistical analysis. Categorical variables are expressed as the number of observations (n) and fraction (%). Normality of quantitative variables was tested using the Shapiro-Wilk test for normality. Since the distributions were different from normal, medians and interquartile ranges (IQRs) were presented for quantitative variables. Differences between 2 independent samples for categorical variables were analyzed with the chi-square test, or chi-square test with Yates’ adjustment. Bonferroni correction was used for multiple comparisons. For continuous data, statistical analysis was based on Mann-Whitney U test. Univariate Cox proportional hazard models were used to estimate separately the effects of different risk factors on survival time. Backward stepwise elimination Cox regression models were used to estimate simultaneously the association of all potential predictors with patients’ mortality. Variables with P >.05 were removed from the stepwise models. There was no violation of the assumption of proportional hazards.
Results
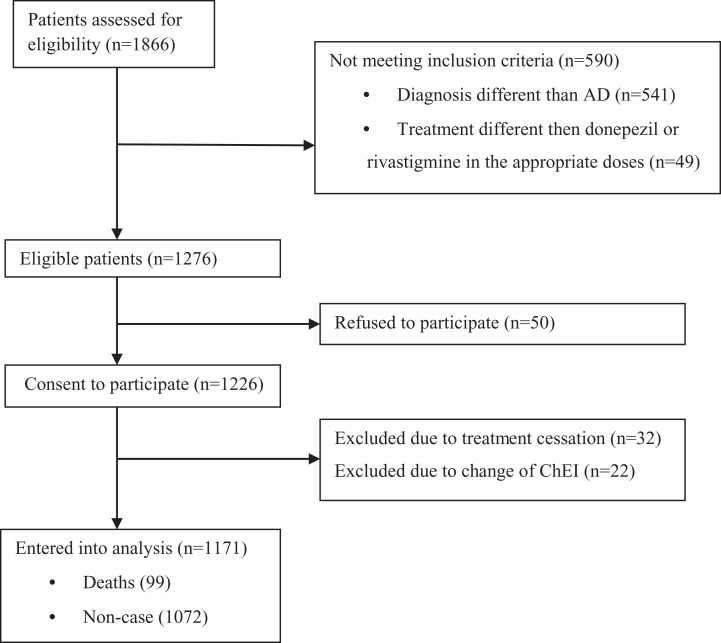
During the observational period, 1276 patients with the median age of 76 years (IQR 71-80) at baseline were eligible for the study. Finally, 1171 patients were included into analysis. The process of the patients’ recruitment and completion of the study is displayed in Figure 1. The rivastigmine group consisted of 737 (63%) patients, whereas the donepezil group included 434 (37%) individuals. There were no differences between these groups with regard to age, gender, and the cause of death (Table 1 and Figure 2). In univariate comparisons, the patients treated with donepezil had better performance in MMSE, revealed more depressive symptoms in GDS, and had better level of functioning measured with FRSSD (Table 1). Furthermore, significant differences regarding comorbidity between patients treated with donepezil and rivastigmine were observed (Table 1). The total number of deaths at the end of the observational period was 99 (8.45%). Demographic and clinical characteristics of patients who died and were still alive after the observational period are shown in Table 2. Application of univariate Cox proportional hazards model revealed that the risk of death was associated with male gender, younger age, and higher number of concomitant diseases at baseline (Table 3). Moreover, the patients who received rivastigmine treatment were at increased risk of death in the follow-up period (Table 3). The use of rivastigmine remained significant in multivariate backward stepwise elimination Cox regression model (Table 3). In addition, significant predictors of shorter survival were male gender, lower educational status, and higher comorbidity (Table 3).
Figure 1.
Number of patients excluded and included into data analysis.
Table 1.
Characteristics of Donepezil and Rivastigmine Groups With Regard to Demographic and Clinical Variables.a
| Variable | Donepezil | Rivastigmine | P Value |
|---|---|---|---|
| Gender male | 168 (38.71%) | 278 (37.72%) | .74 |
| Age (years) | 76 (71-82) | 76 (71-81) | .36 |
| Years of education | 6 (5-9) | 6 (4-8) | .26 |
| MMSE | 20 (16-24) | 17 (12-21) | <.001 |
| FRSSD | 9 (6-13) | 11 (7-16) | <.001 |
| GDS | 3 (1-6) | 3 (1-5) | .021 |
| Death rate | 6 (1.38%) | 93 (12.62%) | <.001 |
| ≤2 comorbid diseases | 427 (98.39%) | 655 (94.38%) | .003b |
| 3-4 comorbid diseases | 6 (1.38%) | 25 (3.6%) | .080b |
| ≥5 comorbid diseases | 1 (0.23%) | 14 (2.02) | .067b |
Abbreviations: FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; IQR, interquartile range; MMSE, Mini-Mental State Examination.
a Values are presented as n (%) or medians (IQR).
b With Bonferroni correction for multiple comparisons.
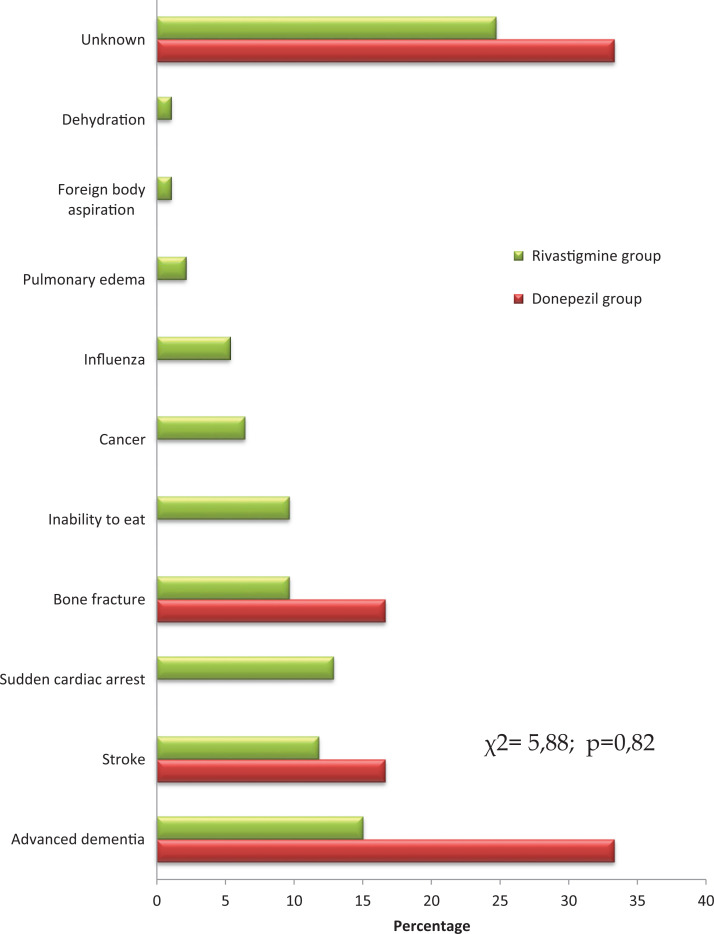
Figure 2.
Causes of death in patients treated with donepezil and rivastigmine.
Table 2.
Demographic and Clinical Variables Analyzed in Univariate Analysis.a
| Variable | Noncase | Deaths | P Value | |
|---|---|---|---|---|
| Gender male | 398 (37.1%) | 48 (48.5%) | .026 | |
| Age | 76 (71-81) | 73 (70-79) | .006 | |
| Years of education | 6 (5-8) | 6 (3-8) | .121 | |
| Type of drugs: | Rivastigmine | 644 (60.07%) | 93 (93.9%) | <.001 |
| Donepezil | 428 (39.93%) | 6 (6.1%) | ||
| ≤2 comorbid diseases | 1047 (97.76%) | 35 (61.4%) | <.001b | |
| 3-4 comorbid diseases | 14 (1.31%) | 17 (29.82%) | <.001b | |
| ≥5 comorbid diseases | 10 (0.93%) | 5 (8.77%) | .001b | |
| FRSSD | 10 (7-15) | 12 (8-12) | .018 | |
| GDS | 3 (1-6) | 3 (0-5) | .029 | |
| MMSE | 18 (13-22) | 16 (13-21) | .099 | |
| Moderate to severe dementiac | 758 (70.7%) | 77 (77.8%) | .137 | |
| The use of antidepressants | 335 (31.3%) | 27 (27.3%) | .409 | |
| The use of anxiolytics | 148 (13.8%) | 8 (8.1%) | .088 | |
| The use of antipsychotics | 155 (14.5%) | 17 (17.2%) | .468 | |
| Memantine use | 200 (18.7%) | 14 (14.1%) | .266 | |
Abbreviations: FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; IQR, interquartile range; MMSE, Mini-Mental State Examination.
a Values are presented as n (%) or medians (IQR).
b With Bonferroni correction for multiple comparisons.
c Moderate to severe dementia: Mini-Mental Examination score ≤ 19.
Table 3.
Cox Proportional Hazards Modeling of Time to Death.
| Variable | Univariate | Multivariate (Significant Predictors) | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Gender male (0-no, 1-yes) | 1.513 | 1.017-2.249 | .041 | 2.130 | 1.227-3.697 | .007 |
| Age (in years) | 0.972 | 0.948-0.998 | .032 | |||
| Years of education | 0.960 | 0.912-1.010 | .114 | 0.898 | 0.835-0.966 | .004 |
| The use of rivastigminea (0-no, 1-yes) | 9.047 | 3.957-20.686 | .000 | 4.475 | 1.897-10.557 | .001 |
| 3-4 comorbid diseasesb (0-no, 1-yes) | 18.300 | 10.346-32.367 | .000 | 26.778 | 13.994-51.238 | .000 |
| ≥5 comorbid diseasesb (0-no, 1-yes) | 8.714 | 3.479-21.831 | .000 | 11.820 | 4.436-31.493 | .000 |
| MMSE (at baseline) | 0.986 | 0.958-1.014 | .314 | |||
| GDS (at baseline) | 0.952 | 0.887-1.021 | .167 | |||
| FRSSD (at baseline) | 1.018 | 0.994-1.042 | .138 | |||
| Moderate to severe dementiac (0-no, 1-yes) | 1.335 | 0.828-2.152 | .235 | |||
| The use of antidepressants (0-no, 1-yes) | 0.904 | 0.578-1.413 | .657 | |||
| The use of anxiolytics (0-no, 1-yes) | 0.594 | 0.288-1.226 | .159 | |||
| The use of antipsychotics (0-no, 1-yes) | 1.119 | 0.655-1.914 | .680 | |||
| Memantine use (0-no, 1-yes) | 0.775 | 0.440-1.368 | .380 | |||
Abbreviations: CI, confidence interval; FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; HR, hazard ratio; MMSE, Mini-Mental State Examination.
a Reference category = donepezil.
b Reference category ≤2 comorbid diseases.
c Moderate to severe dementia: Mini-Mental Examination score ≤ 19.
Discussion
The present study has shown the association between the use of rivastigmine and increased risk of death comparing to donepezil among the population of patients with AD. This higher risk was significant after controlling for other potential predictors in backward stepwise elimination Cox regression models. In previous studies, the effect of rivastigmine on duration of delirium and mortality in critically ill patients was also investigated. In the study conducted by van Eijk et al (2010), active treatment did not decrease duration of delirium; however, a greater risk of death was found with rivastigmine treatment comparing to placebo. 17 Although a sample size of 440 patients had been planned, the trial was halted after inclusion of 104 eligible patients due to increased mortality in the rivastigmine group.
Zaslavsky et al designed a clinical trial to examine whether preoperative administration of transdermal rivastigmine reduced the incidence of delirium in older patients undergoing major surgery. 18 This study was terminated prematurely because of a warning letter issued by the rivastigmine manufacturer indicating the possibility of increased mortality associated with the oral administration of the drug in critically ill patients. However, the analysis did not demonstrate a significant difference between rivastigmine and placebo groups in the occurrence of delirium.
In another study, the hypothesis that prophylactic short-term administration of oral rivastigmine reduces the incidence of delirium in elderly patients during the first 6 days after elective cardiac surgery was tested. 19 No treatment effect on the incidence of delirium among patients after surgical intervention was observed. The first of the above studies conducted in frail and critically ill population showed increased mortality associated with rivastigmine use. Two other studies in the same field failed to reveal a positive effect of rivastigmine in novel clinical indication.
Nordström et al investigated the association between ChEI use (rivastigmine, donepezil, and galantamine) and the risk of MI and death in patients diagnosed with AD. 10 In this retrospective cohort study, data were obtained from the Swedish Dementia Registry. The analysis revealed that patients who had used ChEIs at least once had a 34% lower risk for the composite outcome of MI or death during the follow-up than those who had not (hazard ratio 0.66). Nevertheless, the impact of specific ChEIs use on the risk of death was not compared. The authors speculate that anti-inflammatory properties of ChEIs can underlay cardiovascular effects associated with their use. Other possible mechanisms include vagal nerve stimulation after MI resulting in an improved cardiac function and survival. On the other hand, the association revealed in the above study can be related to the fact that ChEIs treatment is not favorably considered in patients with CVD in clinical practice. Therefore, it is possible that patients with AD who received ChEIs were physically healthier and less likely to develop MI in later years.
Wattmo et al conducted a study which included a considerable number of patients treated due to AD (n = 1021). The analysis was derived from a 3-year, prospective multicenter study (SATS); however, the death ratio was estimated after 16 years since the study onset. 12 The authors included into analysis a number of potential predictors, finally longer survival of individuals was associated with higher ChEI dose and longer duration of treatment. There were no differences regarding the influence of ChEIs type (rivastigmine, donepezil, and galantamine) on mortality. Of note, the results of the current study are different, which can be due to the different study design. Namely, in the present study, the mortality was assessed during a 2-year observational period and regular visits to the clinic, therefore, the continuity of treatment was closely monitored from baseline to the end of the study or death.
It should be also noticed that long-term observational AD studies often report conflicting results regarding the use of ChEIs and longer survival. 20,21 In the Lopez et al study, the effects of ChEIs use were evaluated in 135 patients with AD and compared to 135 patients who were never exposed to ChEIs. 20 Cognitive and functional status of patients treated with ChEIs were better when compared to those who never used medications. There was no association, however, between the use of ChEIs and time to death. On the other hand, in the Zhu et al study, ChEIs use was associated with delayed time in reaching functional end point and death. 21 In this study, majority of patients reported treatment with donepezil (61.7%), 13.6% with galantamine, and only 4.8% with rivastigmine. Finally, Ali et al conducted analysis of 2 national pharmacovigilance databases (Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Database ) in order to determine the major adverse reactions observed with the use of ChEIs in dementia. A disproportionately higher frequency of reports of death as an adverse event for rivastigmine, compared to the other ChEIs, was observed in both data sets. 22 Other studies in the field included short observational periods, or did not compare mortality between specific ChEI groups or were cross-sectional database analyses. 3,7,16 One of the international pharmacovigilance study analyzed ADRs induced by ChEIs (donepezil, rivastigmine, and galantamine) based on individual case safety reports. 7 During a 16-year period of reporting, neuropsychiatric events were the most frequent (31.4%) ADRs. Serious cardiovascular events were also common (11.7%), which suggests that their significance had been probably previously underestimated. Death occurred in 2.3% of the reports. Unfortunately, the comparisons between different ChEI groups were not conducted, thus, the impact of specific agents on the patients’ mortality could not be determined. Hansen et al conducted meta-analysis among patients with AD treated with donepezil, galantamine, and rivastigmine. 3 The analysis included 33 articles based on 26 trials. On average, 76% of participants randomized to active treatment arm reported at least one adverse event. The most frequently reported adverse events were nausea, vomiting, diarrhea, dizziness, and weight loss. The mean frequency of these events was the lowest for donepezil and the highest for the rivastigmine group. Overall, 13% of patients withdrew from the trials due to adverse events. The frequency of withdrawals was the lowest for donepezil trials and the highest for rivastigmine ones. The data concerning patients’ mortality was not reported. Chinese researchers conducted meta-analysis of 10 trials (5 donepezil, 2 rivastigmine, and 3 memantine trials) including patients with PDD and Lewy body dementia. 23 The sample size was different across the included trials, from 14 to 550 patients, and eligible studies varied on the treatment duration, ranging from 10 to 24 weeks. The number of dropouts due to any reason was significantly higher in rivastigmine 12 mg-treated groups than the one in placebo-treated groups. Commonly reported adverse events were anorexia, nausea, vomiting, diarrhea, aggravation of Parkinson and psychiatric symptoms, dizziness, urinary tract infection, and respiratory tract infection. No data on mortality rate was presented. Summing up, to our knowledge, the present prospective observational study is the first one of this design to compare the impact of long-term rivastigmine and donepezil treatment on all-cause mortality in patients with AD.
Biological basis of increased risk of death in patients treated with rivastigmine can be only speculated. It is important to note that rivastigmine, contrary to donepezil, has dual mechanism of action as it inhibits both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Moreover, previous studies showed that rivastigmine use was associated with a significantly higher rate of adverse events when compared to other ChEIs. 3 In one study, the role of BuChE activity as a predictive biomarker in acute ChEI poisoning in patients admitted to Toxicology Clinic was investigated. 24 The reason for exposures was intentional in 70% of cases and accidental in 30%. Previous psychiatric disorders were reported in 4.3% of the cases while 5.8% patients had a history of suicide attempt. The analysis revealed that complications occurred in patients with BuChE-activity levels below 1.4 mL NaOH N/100. A positive correlation between mortality rate (3.8% of patients) and the lowest BuChE-activity level on admission (0.89 mL NaOH N/100) was found. The authors conclude that BuChE activity on admission and its level during hospital stay is an important predictive factor for acute ChEIs poisoning. 24 It is possible that rivastigmine, which represents the ChEI with dual mechanism of action, is more likely to contribute to chronic ChEI poisoning. This condition results from the accumulation of excessive levels of acetylcholine in the synapses, glands, smooth, and skeletal muscles where cholinergic muscarinic and nicotinic receptors are found. 25 Excessive stimulation of these receptors in patients treated with rivastigmine, a dual BuChE and AChE inhibitor, comparing to patients treated with donepezil, a selective AChE inhibitor, can result in impaired cardiac and smooth muscles activity (manifested as bronchospasm, nauseas, or diarrhea), as well as fasciculations, myotonic jerks, weakness, and paresis. 25 Unfortunately, the latter symptoms can be misdiagnosed with neurological symptoms of advanced AD. To conclude, the presence of cholinergic poisoning symptoms can result in life-threatening events and worsen the course of comorbid diseases and general prognosis.
In the present analysis, the difference in causes of death between donepezil and rivastigmine groups did not reach the level of significance; however, such events as sudden cardiac deaths and inability to eat cases were present only in the rivastigmine group. Since rivastigmine is characterized with higher potency to produce extrapyramidal symptoms, 26 this feature could contribute to more severe swallowing problems. Furthermore, such adverse symptoms as bradycardia and heart conduction disorders could increase the mortality rate in rivastigmine-treated individuals.
According to current analysis, a higher number of concomitant diseases was significantly associated with the risk of death in the population studied. This finding is in line with the results of other studies in the field. 27,28 Villarejo et al evaluated mortality rate in a nationwide sample of persons with dementia. Factors associated with higher mortality in Cox proportional hazard models included comorbidity, male gender, and older age. 27 In other study, concurrent physical illness at time of diagnosis was revealed to influence survival in patients with early-onset AD. 28 In the present study, the severity of patients’ comorbidities was not included into analysis, which may constitute the study limitation. Previous studies conducted in both individuals with and without dementia pointed that the severity of illness is related to mortality. 29,30 However, there are also studies which did not confirm this observation in multivariate models. 31
Another finding of the current analysis was the association between the male gender and the risk of death in the follow-up period. The survival advantage of women with AD comparing to men may occur to be a result of fewer comorbid conditions. Studies show that at any level of cognitive impairment, the prevalence of such diseases as arrhythmia, chronic obstructive pulmonary disease, and cancer is higher among men with AD than in women with this diagnosis. 32 Women are also less likely to be hospitalized and receive fewer medications. 32
According to current analysis, lower educational status was also a predictor of shorter survival. However, available studies investigating the correlation between education and mortality show inconsistent results. 33 -36 Some of the studies report shorter lifespan for patients with AD who are better educated, 33,34 whereas other revealed similar mortality rates among highly and less educated individuals. 35,36 Lower death rates associated with lower educational levels may be explained with a reserve hypothesis, which suggests that clinical symptoms of AD begin earlier in individuals with less education. As a result, patients with lower educational level might survive longer after diagnosis than those who are better educated.
In previous studies, the baseline functional status as well as the worsening of functional ability were associated with shorter survival. 12,31 However, the current analysis did not reveal the baseline instrumental functioning measured with FRSSD to be related to mortality. This difference may be the result of different diagnostic tools used to evaluate the level of patients’ independence. In previous mortality studies, functional status was usually assessed with the use of Activity of Daily Living or Blessed Dementia Rating Scale, 12,21 while in the current report the FRSSD was used.
It should be noticed that mortality rate revealed in the current study was low when compared to studies evaluating patients with dementia. In Gambassi et al study, a 2-year mortality rate of patients with AD living in nursing homes was 50%. 37 In other 1 year follow-up study, patients taking ChEIs had mortality rate of 13%, while patients who had never taken ChEIs was 38.5%. 20 The lower mortality revealed in the current study may be associated with homogenous sample of participants (only patients with AD) since short survival is overrepresented in non-AD dementias like vascular dementia, frontotemporal dementia, and AD dementia with concomitant cerebrovascular disease. 11,38,39 Moreover, in available reports, survival was evaluated both in treated and untreated, hospitalized, as well as institutionalized patients. 40,41 Since the treatment with ChEIs (treated as a group) prolongs the lifespan, individuals without treatment may have shorter survival. Furthermore, hospitalized or institutionalized patients have poorer prognosis compared to community-dwelling individuals, thus, death rates estimated in the former groups may be increased. In addition, participants of the current study were routinely engaged in intensive and regular nonpharmacological interventions (holistic cognitive rehabilitation program conducted in the Memory Clinic of Thessaloniki), which was proven to enhance cognitive and functional performance. 42 Finally, since different ChEIs may have different impact on patients’ survival, the overrepresentation of specific ChEI type use could influence mortality rate revealed in previous studies. On the other hand, the randomized controlled trials reporting low death rates among patients with dementia exist. In the study by Román et al, during a 6-month follow-up period, 1.7% of deaths occurred in the donepezil group, whereas no deaths in the placebo group were observed. 43 Unfortunately, we cannot exclude that the present study was burdened with potential selection bias. It is possible that patients who refused to participate were in poorer general condition and were more likely to have fatal prognosis. Of note, current analysis showed that patients from the donepezil group had lower comorbidity, better functional status, and higher GDS score at baseline. Hypothetically, clinicians preferred to use donepezil in individuals who complained of apathy and depression, and rivastigmine in patients who were agitated and had psychotic symptoms.
In order to avoid potential selection bias, all the participants available in the study site who fulfilled the inclusion criteria were included. As a result of this methodology, the rivastigmine group was larger than the sample of patients with donepezil. It is possible that transdermal form of rivastigmine constituted the advantage when the decision on the ChEIs type was made. Nevertheless, after controlling for confounders, the association between rivastigmine and risk of death remained significant in multivariate model.
In the current observational study, the impact of specific treatment on prognosis could be revealed due to a long period of observation, prospective design, and a considerable number of participants entered into analysis. The impact of rivastigmine use on the risk of death was controlled for comorbidity and other potential cofounders; however, the potential influence of physical condition on the association between rivastigmine use and mortality cannot be excluded. Similarly, higher level of single disease severity could contribute to the elevated risk of death in the rivastigmine group and interfered with the final study results. It is worthwhile to notice, that on the baseline, the patients were not drug-naive and previous time of treatment could also influence the final study results. Therefore, it would be of value to confirm the present findings in long-term randomized controlled trials. Moreover, the comparison of cholinesterase plasma activity among individuals treated with different ChEIs and its association with the number of deaths in each group would constitute a novel input to the field in future studies.
Conclusions
Present findings suggest that rivastigmine use is associated with higher mortality ratio among patients with AD when compared to treatment with donepezil. Therefore, rivastigmine should be used with special caution among patients burdened with comorbid diseases, cardiac problems, extrapyramidal symptoms, and having more advanced dementia stages.
Footnotes
Authors’ Note: The study was approved by the Ethics Committee of Alzheimer Hellas and was performed in accordance with the ethical standards of the Declaration of Helsinki. The participants provided informed consent for the participation in the study. The manuscript is the result of international cooperation within the Healthy Aging Research Centre (HARC) Project.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jakub Kazmierski  http://orcid.org/0000-0001-6002-7123
http://orcid.org/0000-0001-6002-7123
References
- 1. Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology. 2014;76 (pt A):27–50. [DOI] [PubMed] [Google Scholar]
- 2. Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. [DOI] [PubMed] [Google Scholar]
- 3. Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Hai S, Zhou Y, Dong BR. Cholinesterase inhibitors for rarer dementias associated with neurological conditions. Cochrane Database Syst Rev. 2015;(3):CD009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herrmann N, Lanctôt KL, Hogan DB. Pharmacological recommendations for the symptomatic treatment of dementia: the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Alzheimers Res Ther. 2013;5(suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheldon T. Study of rivastigmine for delirium in intensive care is stopped after deaths. BMJ. 2010;340:c2895. [DOI] [PubMed] [Google Scholar]
- 7. Kröger E, Mouls M, Wilchesky M, et al. Adverse drug reactions reported with cholinesterase inhibitors: an analysis of 16 years of individual case safety reports from Vigibase. Ann Pharmacother. 2015;49(11):1197–1206. [DOI] [PubMed] [Google Scholar]
- 8. Ballard C, Lane R, Barone P, Ferrara R, Tekin S. Cardiac safety of rivastigmine in Lewy body and Parkinson’s disease dementias. Int J Clin Pract. 2006;60(6):639–645. [DOI] [PubMed] [Google Scholar]
- 9. Sato K, Urbano R, Yu C, et al. The effect of donepezil treatment on cardiovascular mortality. Clin Pharmacol Ther. 2010;88(3):335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordström P, Religa D, Wimo A, Winblad B, Eriksdotter M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer’s disease. Eur Heart J. 2013;34(33):2585–2591. [DOI] [PubMed] [Google Scholar]
- 11. Meguro K, Kasai M, Akanuma K, Meguro M, Ishii H, Yamaguchi S. Donepezil and life expectancy in Alzheimer’s disease: a retrospective analysis in the Tajiri Project. BMC Neurol. 2014:14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wattmo C, Londos E, Minthon L. Longitudinal associations between survival in Alzheimer’s disease and cholinesterase inhibitor use, progression, and community-based services. Dement Geriatr Cogn Disord. 2015;40(5-6):297–310. doi:10.1159/000437050. [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 15. Hutton TJ: Alzheimer’s disease. In: Rakel RE, ed. Conn’s Current Therapy. Philadelphia: WB Saunders; 1990;780. [Google Scholar]
- 16. Fountoulakis KN, Tsolaki M, Iacovides A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano). 1999;11(6):367–372. [DOI] [PubMed] [Google Scholar]
- 17. van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet 2010;376(9755):1829–1837. [DOI] [PubMed] [Google Scholar]
- 18. Zaslavsky A, Haile M, Kline R, Iospa A, Frempong-Boadu A, Bekker A. Rivastigmine in the treatment of postoperative delirium: a pilot clinical trial. Int J Geriatr Psychiatry. 2012;27(9):986–988. [DOI] [PubMed] [Google Scholar]
- 19. Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—a randomized controlled trial. Crit Care Med. 2009;37(5):1762–1768. [DOI] [PubMed] [Google Scholar]
- 20. Lopez OL, Becker JT, Wisniewski S, Saxton J, Kaufer DI, DeKosky ST. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72(3):310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu CW, Livote EE, Scarmeas N, et al. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement. 2013;9(6):733–740. doi:10.1016/j.jalz.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ali TB, Schleret TR, Reilly BM, Chen WY, Abagyan R. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS One. 2015;10(12):e0144337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan CC, Yu JT, Wang HF, Jiang T, Tan CC, Meng XF. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–631. [DOI] [PubMed] [Google Scholar]
- 24. Gazzi EN, Sorodoc V, Petris O, et al. Butyrylcholinesterase activity-biomarker for predicting the outcome in acute cholinesterase inhibitor poisoning—a 30-year retrospective analysis. Rev Med Chir Soc Med Nat Iasi. 2014;118(4):971–978. [PubMed] [Google Scholar]
- 25. Li P, Yin YL, Zhu ML, et al. Chronic administration of isocarbophos induces vascular cognitive impairment in rats. J Cell Mol Med. 2016;20(4):731–739. doi:10.1111/jcmm.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;(127):45–63. [PubMed] [Google Scholar]
- 27. Villarejo A, Benito-León J, Trincado R, et al. Dementia-associated mortality at thirteen years in the NEDICES Cohort Study. J Alzheimers Dis. 2011;26(3):543–551. [DOI] [PubMed] [Google Scholar]
- 28. Ueki A, Shinjo H, Shimode H, Nakajima T, Morita Y. Factors associated with mortality in patients with early-onset Alzheimer’s disease: a five-year longitudinal study. Int J Geriatr Psychiatry. 2001;16(8):810–815. [DOI] [PubMed] [Google Scholar]
- 29. Zekry D, Herrmann FR, Graf CE, et al. High levels of comorbidity and disability cancel out the dementia effect in predictions of long-term mortality after discharge in the very old. Dement Geriatr Cogn Disord. 2011;32(2):103–110. doi:10.1159/000326950. [DOI] [PubMed] [Google Scholar]
- 30. Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004. 6;140(7):501. [DOI] [PubMed] [Google Scholar]
- 31. Rountree SD, Chan W, Pavlik VN, Darby EJ, Doody RS. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimers Res Ther. 2012;4(3):16. doi:10.1186/alzrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gambassi G, Lapane KL, Landi F, Sgadari A, Mor V, Bernabie R. Gender differences in the relation between comorbidity and mortality of patients with Alzheimer’s disease. Systematic Assessment of Geriatric drug use via Epidemiology (SAGE) Study Group. Neurology. 1999;53(3):508–516. [DOI] [PubMed] [Google Scholar]
- 33. Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer’s disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37(5):590–595. [DOI] [PubMed] [Google Scholar]
- 34. Wattmo C, Londos E, Minthon L. Response to cholinesterase inhibitors affects lifespan in Alzheimer’s disease. BMC Neurol. 2014;14:173. doi:10.1186/s12883-014-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruandet A, Richard F, Bombois S, et al. Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord. 2008;25(1):74–80. [DOI] [PubMed] [Google Scholar]
- 36. Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22(4):367–377. [DOI] [PubMed] [Google Scholar]
- 37. Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer’s disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. 2012;24(7):1034–1045. [DOI] [PubMed] [Google Scholar]
- 39. Kim JH, Go SM, Seo SW, et al. Survival in subcortical vascular dementia: predictors and comparison to probable Alzheimer’s disease in a tertiary memory clinic population. Dement Geriatr Cogn Disord. 2015;40(3-4):210–221. [DOI] [PubMed] [Google Scholar]
- 40. Navarro-Gil P, González-Vélez AE, Ayala A, Martín-García S, Martínez-Martín P, Forjaz MJ; Spanish Research Group on Quality of Life and Ageing. Which factors are associated with mortality in institutionalized older adults with dementia? Arch Gerontol Geriatr. 2014;59(3):522–527. [DOI] [PubMed] [Google Scholar]
- 41. Tsai MC, Weng HH, Chou SY, Tsai CS, Hung TH, Su JA. One-year mortality of elderly inpatients with delirium, dementia, or depression seen by a consultation-liaison service. Psychosomatics. 2012;53(5):433–438. [DOI] [PubMed] [Google Scholar]
- 42. Tsolaki M, Kounti F, Agogiatou C, et al. Effectiveness of nonpharmacological approaches in patients with mild cognitive impairment. Neurodegener Dis. 2011;8(3):138–145. [DOI] [PubMed] [Google Scholar]
- 43. Román GC, Salloway S, Black SE, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41(6):1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]