This article reviews the evolution of the global biosimilar landscape and provides examples of current discrepancies among biosimilar regulatory frameworks in different regions. In addition, efforts to address regulatory inconsistencies are reviewed, and the importance of healthcare professional and patient education in acceptance of biosimilars is highlighted.
Keywords: Antineoplastic agents, Biosimilar pharmaceuticals, Monoclonal antibodies, Neoplasms, Oncologists
Abstract
Globally, biosimilars are expected to have a key role in improving patient access to biological therapies and addressing concerns regarding the escalating cost of health care. Indeed, in Europe, increased use of biologics and reduced drug prices have been observed after the introduction of biosimilars. Recently, several monoclonal antibody biosimilars of anticancer therapies have been approved, and numerous others are in various stages of clinical development. Biosimilars are authorized via a regulatory pathway separate from that used for generic drugs; they are also regulated separately from novel biologics. Biosimilar approval pathways in many major regulatory regions worldwide are, to a broad degree, scientifically aligned. However, owing to regional differences in health care priorities, policies, and resources, some important regulatory inconsistencies are evident. Acceptance of biosimilars by health care systems, health care professionals, and patients will be a key factor in the uptake of these therapies, and such regulatory variations could contribute to confusion and diminished confidence regarding the quality, efficacy, and reliability of these agents. Furthermore, the need for manufacturers to account for regulatory inconsistencies introduces inefficiencies and delays into biosimilar development programs. These issues should be addressed if biosimilars are to attain their maximal global potential. This review summarizes the evolution of the global biosimilar landscape and provides examples of inconsistencies between regulatory requirements in different regions. In addition, we review ongoing efforts to improve regulatory alignment and highlight the importance of education as a crucial factor in generating trust in, and acceptance of, biosimilars on a worldwide scale.
Implications for Practice.
Biosimilars of monoclonal antibody anticancer therapies are beginning to emerge, and more are likely to become available for clinical use in the near future. The extent to which biosimilars can contribute to cancer care will depend on their level of acceptance by health care systems, health care professionals, and patients. A better understanding of the regulatory basis for the approval of biosimilars may enhance confidence and trust in these agents. In order to have informed discussions about treatment choices with their patients, oncologists should familiarize themselves with the biosimilar paradigm.
摘要
在全球范围内,生物仿制药有望在改善患者获得生物疗法以及解决医疗费用不断上升等问题方面发挥关键作用。事实上,在欧洲引入生物仿制药之后,生物制剂的使用就有所增加,药品价格也有所下降。近年来已批准了数种用于抗癌治疗的单克隆抗体生物仿制药,其他许多单克隆抗体也正处于不同的临床开发阶段。生物仿制药会采用一种有别于普通药物的监管途径获得授权;它们也与新型生物制剂分开监管。在世界各地的许多主要监管地区,生物仿制药的批准途径在很大程度上都具有科学上的一致性。然而,由于卫生保健优先级、政策和资源方面的地区差异,也显而易见地存在着重要的监管不一致现象。医疗保健系统、卫生保健专业人员和患者对生物仿制药的接受程度将是采用这些疗法的一个关键因素,而这种监管的不一致可能会让人们对这些制剂的质量、疗效和可靠性产生困扰或失去信心。此外,由于制造商需要对监管的不一致性予以解释,也导致生物仿制药开发计划出现低效和延迟的问题。如果生物仿制药想要在全球发挥最大的潜力,这些问题必须得到解决。本文概述了全球生物仿制药的演变,并提供了不同地区监管要求不一致的案例。此外,我们还回顾了目前为改善监管协调正在做出的努力,并强调了为在全球范围内达到信任和接受生物制药的目标,教育作为其中一个关键因素的重要性。
实践意义
单克隆抗体抗癌治疗的生物仿制药开始出现,在不久的将来可能会有更多的药物用于临床。生物仿制药对癌症治疗的贡献程度将取决于它们被医疗系统、卫生保健专业人员和患者的接受程度。更好地理解生物仿制药审批的监管基础,可以增强对这些药物的信心和信任。为了能与患者就治疗选择进行知情讨论,肿瘤科医生应熟悉生物仿制药的治疗模式。
Introduction
Biopharmaceuticals, or biologics, are medicinal products manufactured using living systems [1]. Biologics include complex macromolecular products with biotechnology‐derived proteins as active substances. Such therapies have a key role in the treatment of life‐threatening and chronic conditions, including cancer, diabetes, rheumatoid arthritis, and inflammatory bowel disease (IBD). In oncology, monoclonal antibody (mAb) therapies, such as bevacizumab, rituximab, and trastuzumab, have been shown to extend patient survival in the context of various solid or hematologic malignancies [2], [3], [4]. Despite their clinical benefits, however, access to biologics remains a significant problem for patients, even in developed countries [5], [6], [7], [8], [9]. Access barriers include issues related to insurance coverage, health care system reimbursement, formulary inclusion, drug availability, and out‐of‐pocket cost to the patient [6], [7], [8], [9].
Loss of exclusivity and patent expiries for commonly used biologics have, however, provided the opportunity to develop biosimilars. A biosimilar is a biotherapeutic product that is highly similar to an already licensed biologic (hereafter, the “reference” or “originator”) product, with no clinically meaningful differences in quality, efficacy, or safety [10], [11], [12]. Biosimilars provide additional treatment options, and evidence suggests that their introduction can provide savings for health care systems and expand access to biologics [13], [14], [15].
The inherent complexity of biologics and their manufacturing processes means it is highly unlikely to be possible to create truly identical copies [16]. As a result, the regulatory framework established for chemically derived, small‐molecule generic drugs is not appropriate for evaluating biosimilars [11], [12]. Instead, biosimilars are licensed via a separate process based on rigorous head‐to‐head comparisons with the corresponding originator product, including comparative clinical studies [12], [17]. Establishing appropriate standards for biosimilarity remains an important area for scientific, legislative, and regulatory debate [18]. Furthermore, there are regional variations in health care priorities and resources, intellectual property rights, and regulatory policies [19]. The net result is discrepancies and gaps in the regulatory framework for biosimilar approval at a global level [20].
Combined with a need for education about biosimilars [21], [22], such discrepancies may be confusing or worrying for health care professionals, patients, and other stakeholders, and could contribute to reduced confidence in the quality, efficacy, and reliability of these biological agents. Moreover, manufacturers must balance the need to account for regulatory variations against the costs of the studies required to seek biosimilar approvals in all geographic regions [19]. Hence, a lack of regulatory consistency may ultimately stifle the potential of biosimilars. It should be noted that global regulatory inconsistency is not unique to biosimilars. In recent years, for example, the International Generic Drug Regulators Programme was established to promote international convergence and collaboration in the assessment of generic drugs against a backdrop of increasing numbers of generic drug applications, globalization, and the increasing complexity of generic products [23].
This article reviews the evolution of the global biosimilar landscape and provides examples of current discrepancies among biosimilar regulatory frameworks in different regions. In addition, we review efforts to address regulatory inconsistencies and highlight the importance of health care professional and patient education in generating trust in, and acceptance of, biosimilars.
Evolution of the Global Biosimilar Landscape
The European Medicines Agency (EMA) was the first regulatory authority to establish a framework for biosimilar approval, issuing guidelines in 2005 [24]. Since that time, the agency has published additional overarching and product‐specific biosimilar guidelines and has approved >30 biosimilar products [25]. In the past decade, biosimilar guidelines were issued in other stringently regulated markets, such as Australia, Canada, Japan, the Republic of Korea, and the U.S. [10], [26], [27], [28], [29]. Furthermore, in 2009, the World Health Organization (WHO) published guidance aimed at providing “globally acceptable principles” for the evaluation of biosimilars [12]. Intended to assist national regulatory authorities in other regions in licensing proposed biosimilars, the WHO guidelines are regarded as a step toward global harmonization of biosimilar approval requirements [19], [30].
The biosimilar guidelines cited above are, broadly speaking, scientifically aligned and share many key features. In all cases, development of a biosimilar involves a stepwise, head‐to‐head comparison exercise that begins with comprehensive analytical assessments of structural and functional attributes of the potential biosimilar compared with the originator product, followed by nonclinical and clinical assessments [10], [11], [12], [26], [27], [28], [29]. Clinical assessments usually comprise a pharmacodynamics/pharmacokinetics comparison followed by at least one comparative safety and efficacy trial [10], [12], [27], [28], [29], [31]. The aim of the comparison exercise is to establish a high degree of similarity between the biosimilar and originator, rather than to demonstrate the safety and efficacy of the biosimilar per se [32]. Establishing biosimilarity allows the biosimilar manufacturer to rely on the extensive safety and efficacy profile of the originator, hence enabling licensing based on an abbreviated nonclinical and clinical data package [12], [27], [29]. A determination of biosimilarity is based on the totality of the evidence from all stages of the comparison exercise [10], [11], [12], [27], [28], [29].
The aim in the clinical phase of the biosimilarity exercise is to exclude any potential clinically significant differences between a biosimilar and the originator. It is preferred that safety and efficacy trials have an equivalence design, with upper and lower biosimilarity margins, to establish that the proposed biosimilar is neither inferior nor superior to the originator [10], [12], [27], [29], [31]. Biosimilarity trials should be designed so that parameters such as study population, endpoints, and time points are appropriately sensitive for the detection of product‐related differences between the potential biosimilar and originator [10], [11], [12], [27], [29]. In the case of biosimilar anticancer mAbs, for example, activity‐based endpoints such as overall response rate are likely to be more sensitive than survival‐based endpoints [33].
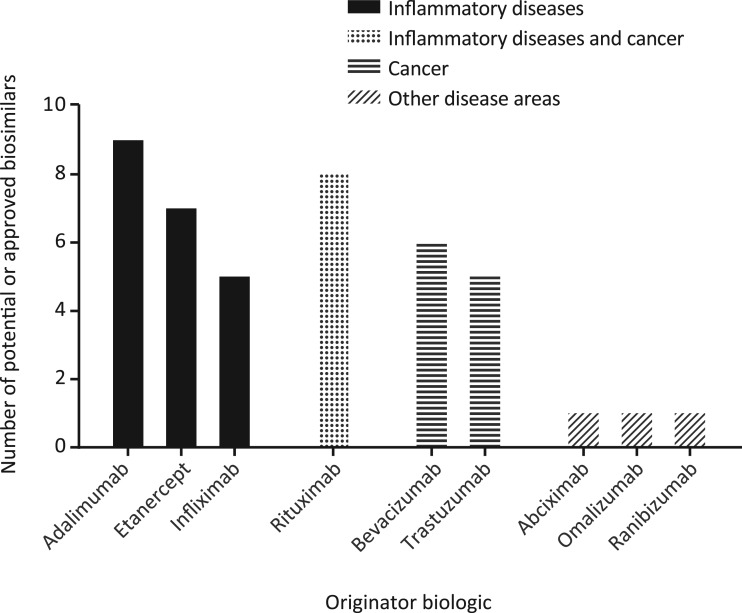
It has been estimated that >150 potential biosimilars are in clinical development [34]. Many of these reference “blockbuster” mAb products for the treatment of inflammatory diseases or cancer, such as adalimumab, bevacizumab, infliximab, rituximab, and trastuzumab (Fig. 1) [35], [36], [37]. Indeed, in 2017, the EMA authorized two biosimilar rituximab molecules (CT‐P10 and GP2013), which represented the first anticancer mAb biosimilars to be approved in Europe [38], [39]. Moreover, in September 2017, the U.S. Food and Drug Administration (FDA) licensed a biosimilar of bevacizumab (ABP 215), which was the first anticancer biosimilar to be approved in the U.S. [40]. ABP 215 has since been authorized in Europe [41]. Additionally, as of February 2018, two trastuzumab biosimilars (CT‐P6 and SB3) have been authorized in Europe [42], [43], with a third (MYL‐1401O) licensed in the U.S. [44]. Several of these anticancer biosimilars have also been approved in other regions, including Brazil (MYL‐1401O), India (MYL‐1401O), and the Republic of Korea (CT‐P6, CT‐P10, SB3), with regulatory reviews underway in countries including Australia, Canada, and Japan [45], [46], [47], [48].
Figure 1.
Monoclonal antibody and fusion protein biosimilar development pipeline. Based on named potential or approved biosimilars identified in the systematic literature review by Jacobs et al. [35], [36], [37]. Note that noncomparable biotherapeutic products (i.e., products intended to “copy” an already licensed product that are authorized without a complete exercise of head‐to‐head comparison with the originator) are not included, and the cutoff date for the literature review was September 2015.
Governments, health care systems, and other health care decision makers have a pressing need to manage increasing health care costs while maintaining quality and clinical effectiveness, and have thus shown significant interest in the potential of biosimilars [16], [49], [50]. In the U.S., for example, expenditure on mAb therapies in oncology reportedly reached $13.6 billion in 2015, accounting for 35% of the total spending on cancer medicines [51]. In one recent report, estimates for the potential savings that could result from the introduction of biosimilars for eight originator biologics across five European countries and the U.S. combined ranged from €49 billion to €98 billion (assuming reductions in treatment‐day prices of 20% to 40%, respectively) for the 5‐year period 2016–2020 [52]. Accordingly, health economic matters are particularly relevant to the global use and acceptance of biosimilars. Pricing and value must be considered alongside other potential benefits and challenges associated with biosimilar approvals.
Recognizing that biosimilars could help increase access to treatment and address escalating health care expenditure, the WHO recently announced a pilot project in which it will invite manufacturers to submit applications for the prequalification of biosimilars of rituximab and trastuzumab [53]. Prequalification is a quality assurance process wherein the WHO evaluates products for quality, efficacy, and safety and is relied upon by many low‐ and middle‐income countries to guide decisions on drug procurement [53].
The implementation of biosimilars is perhaps best illustrated in Europe, the region with the longest experience with biosimilars and that has a variety of health care systems and economies. A 2017 report examined the effect of biosimilar competition in several product classes, based on volume estimates and list price data across countries of the European Economic Area [13]. It was noted that biosimilar competition drives price reductions, although the magnitude of reductions varied significantly among countries. Interestingly, the correlation between biosimilar market share and price reductions was weak in many instances. Finally, increases in the consumption of biologics, used by the investigators as a surrogate measure of increased access to treatment, were observed after the introduction of biosimilars [13].
It was noted that biosimilar competition drives price reductions, although the magnitude of reductions varied significantly among countries. Interestingly, the correlation between biosimilar market share and price reductions was weak in many instances.
Overall, the uptake of biosimilars appears to be highly sensitive to country‐specific factors such as market characteristics, payer archetypes, incentive policies, and policies on substitution [54], [55]. In the U.S., for example, it has been proposed that a so‐called “rebate trap” (whereby rebates offered by the manufacturer of an originator could be withdrawn in response to biosimilar competition) may create the incentive for payers to favor originators over less‐costly biosimilars, and thus act as a barrier to uptake [56]. In contrast, significant penetration of biosimilars has been achieved in Norway via initiatives such as competitive drug tendering and commitment from the government to fund the NOR‐SWITCH study, which aimed to provide evidence about the effects of switching from originator infliximab to an infliximab biosimilar [57], [58], [59]. Such examples illustrate the importance of considering the role of health care systems, policymakers, and payers in the uptake of biosimilars. These issues will be better understood as the biosimilar landscape continues to evolve.
Regulatory Inconsistencies Between Regions
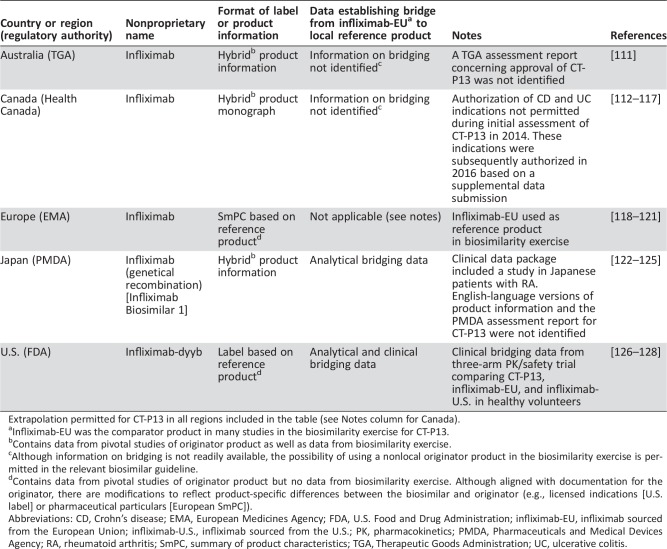
Although biosimilar approval requirements in stringently regulated regions of the world are scientifically aligned on many principles, some important regulatory inconsistencies are evident. Below, we provide examples of key regulatory variations or gaps; Table 1 illustrates these using the example of CT‐P13, a mAb biosimilar of infliximab that is approved for use in 80 countries [46]. It should be noted that there are additional areas in which further regulatory alignment may be necessary, such as in relation to requirements concerning animal studies [60], [61].
Table 1. Attributes of biosimilar infliximab CT‐P13 in different regulatory regions.
Extrapolation permitted for CT‐P13 in all regions included in the table (see Notes column for Canada).
Infliximab‐EU was the comparator product in many studies in the biosimilarity exercise for CT‐P13.
Contains data from pivotal studies of originator product as well as data from biosimilarity exercise.
Although information on bridging is not readily available, the possibility of using a nonlocal originator product in the biosimilarity exercise is permitted in the relevant biosimilar guideline.
Contains data from pivotal studies of originator product but no data from biosimilarity exercise. Although aligned with documentation for the originator, there are modifications to reflect product‐specific differences between the biosimilar and originator (e.g., licensed indications [U.S. label] or pharmaceutical particulars [European SmPC]).
Abbreviations: CD, Crohn's disease; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; infliximab‐EU, infliximab sourced from the European Union; infliximab‐U.S., infliximab sourced from the U.S.; PK, pharmacokinetics; PMDA, Pharmaceuticals and Medical Devices Agency; RA, rheumatoid arthritis; SmPC, summary of product characteristics; TGA, Therapeutic Goods Administration; UC, ulcerative colitis.
Choice of Reference Product
Several biosimilar regulatory guidelines specify that the reference product used during the comparison exercise should be licensed in that country or region [10], [11], [26], [28]. The first biosimilar guideline from the EMA, for example, stated “the chosen reference medicinal product, defined on the basis of its marketing authorisation in the [European] Community, should be used throughout the comparability program for quality, safety and efficacy studies” [24].
Such restrictions are potentially problematic for manufacturers, because developing a biosimilar that can be commercialized globally would require duplicative trial programs, vastly increasing the time and cost of development [19]. However, with the aim of facilitating the global development of biosimilars, regulators have indicated their support for the concept of “bridging” studies to demonstrate that originator products sourced in different areas are representative of each other. For example, although biosimilar guidance from the U.S. FDA states that “a sponsor must demonstrate that the proposed product is biosimilar to a single reference product that previously has been licensed by FDA,” it allows the sponsor to compare a proposed biosimilar with a non‐U.S.‐licensed originator in certain clinical and animal studies, provided that this can be scientifically justified and that an acceptable bridge to the U.S.‐licensed product can be established [10]. Furthermore, the EMA guideline was updated in 2014 to include similar provisions regarding the choice of reference product [11].
In practice, biosimilar development programs often incorporate three‐arm bridging studies during the analytical and clinical pharmacology phases of development to make pairwise comparisons between the proposed biosimilar, a Europe‐sourced originator, and a U.S.‐sourced originator (Table 1).
Extrapolation
Another feature common to regulatory frameworks is that approval of a biosimilar for indications held by the originator but not studied during the biosimilarity exercise (“extrapolation”) may be possible, provided that there is adequate scientific justification [10], [11], [12], [27], [28], [29]. Extrapolation is a core aspect of the abbreviated regulatory pathway because it avoids the need for a biosimilar manufacturer to conduct clinical trials in all indications for which licensure is sought. The specific wording of considerations relevant to extrapolation differs among the various regulatory guidelines, although there are clear similarities. In the FDA guideline, for example, the scientific rationale for extrapolation must address factors such as the mechanism of action and target receptors in each indication, product pharmacokinetics in different patient populations, and the immunogenicity and toxicity profiles of the product in different conditions of use [10]. Differences in such factors between indications do not necessarily preclude extrapolation but may necessitate additional supportive data.
Despite the similar regulatory basis for extrapolation, decisions are made on a case‐by‐case and agency‐by‐agency basis. Consequently, different agencies may make different decisions regarding extrapolation when reviewing the same biosimilar (Table 1) [62]. This likely reflects differences in how regulators interpret and weigh evidence regarding biosimilarity, and it may ultimately undermine the acceptance of biosimilars because the existence of biosimilars with different authorized indications to the originator, or to other biosimilars, creates uncertainty [63].
Naming and Product Labeling
Biosimilar nomenclature has important implications for both safety monitoring and patient access to treatment [64]. An inability to accurately distinguish between related biologics that share the same nonproprietary name could, for example, result in misattribution of adverse events or inadvertent substitution [64], [65]. Conversely, distinct nonproprietary names for biosimilars and originators may cause confusion and could hamper biosimilar uptake by making substitution of interchangeable biologics by pharmacists (where permitted) less likely [66], [67].
In Europe, biosimilars authorized by the EMA generally share the International Nonproprietary Name of the corresponding originator (Table 1), although, to support pharmacovigilance, the brand name and batch number should be included when reporting adverse events for any biologic [11]. In contrast, the FDA requires that all biological products bear a nonproprietary name consisting of a core name and a unique, agency‐designated, distinguishing suffix comprising four lower‐case letters [65]. Other naming conventions have been used elsewhere, for example in Japan and Australia [68], and some regulators have yet to decide their approach [69].
Regulatory authorities have also taken divergent positions on the format of product labels or summaries of product characteristics (SmPCs). In Europe, biosimilar SmPCs do not contain data from the biosimilarity exercise; instead, they include a statement that the product is a biosimilar but are otherwise aligned with the SmPC of the originator [70], [71]. Modifications may be necessary to reflect product‐specific differences; for example, the approved indications of the biosimilar and originator may differ [70]. The FDA has advocated a similar approach in its draft guidance on labeling for biosimilars [72]. This approach may help prevent confusion about the risk–benefit profile of the biosimilar and avoid the perception that biosimilars differ from the corresponding originator in any meaningful way [63], [72]. On the other hand, not including data from the biosimilarity exercise in the label may make it more difficult for health care professionals to communicate with patients about the evidence underpinning biosimilar authorization [66]. As a result, in some countries, such as Canada and Australia, a “hybrid” label is used for biosimilars, including information based on studies of the originator and data from the biosimilarity exercise (Table 1) [63].
Interchangeability
The meaning of “interchangeability” differs according to region [73]. In Europe, for example, interchangeability has been defined as the “medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patient on the initiative, or with the agreement of the prescriber” [74]. In its evaluation of biosimilars, the EMA does not provide recommendations on interchangeability with originators, and delegates this responsibility to individual member states [11]. Regulators in a number of European countries have endorsed interchanging under the supervision of the prescriber [73]. In a similar manner, authorization of a biosimilar by Health Canada is “not a declaration of equivalence,” and decisions on interchangeability are made by the province or territory [69].
In contrast, the FDA can designate a biosimilar as interchangeable with a reference product if certain additional standards are met. As well as demonstrating biosimilarity to a reference product, an interchangeable product “can be expected to produce the same clinical result as the reference product in any given patient,” and “for a biological product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch” [75]. At the time of writing, the agency had released only draft guidance on the requirements for demonstrating interchangeability [75], and the biosimilars licensed in the U.S. are, accordingly, not designated as interchangeable.
Design of Comparative Clinical Trials
At present, regulatory authorities provide broad guidance on comparative clinical trials, but generally they require applicants to scientifically justify the design specifics of their studies [10], [12], [27], [28], [31]. A recent systematic review of assessment reports for biosimilars approved in Europe found significant variability between clinical development strategies and that, even for biosimilars referencing the same originator, there was heterogeneity in the endpoints, statistical model, and sample sizes employed in clinical trials [76]. Furthermore, the FDA and EMA have differed in their interpretations of the appropriateness of the biosimilarity margins used in certain trials [77]. It has been suggested that regulatory authorities could agree upon and standardize various design features for clinical trials of all potential biosimilars referencing the same originator in a given disease [78], which might aid interpretation of the results of different trials.
Noncomparable Biotherapeutic Products
Outside of the regulatory regions discussed above, another key global inconsistency is highlighted by the fact that in some countries, biologics intended to “copy” an already licensed product have been authorized via less‐stringent approval pathways, without a complete exercise of head‐to‐head comparison with the originator [19], [79]. Although a full consideration of such “noncomparable biotherapeutic products” is outside the scope of this review, it should be noted that these agents should not be termed biosimilars, as they have not been developed and assessed in line with the principles of a strictly comparative development program, and their approval may ultimately carry safety and efficacy concerns, as the risk–benefit balance is often unknown [17], [68], [79]. As a result, health care professionals should not conflate noncomparable biotherapeutics with “true” biosimilars.
Noncomparable biotherapeutics are reportedly available in countries and regions such as China, India, and Latin America [68]. However, there have been calls for such products to be reassessed according to current regulations for biosimilars [80]. To this end, the WHO has issued recommendations for national regulatory authorities on the stepwise identification and reevaluation of biologics that no longer meet current regulatory expectations, including, for example, those that were authorized on the basis of limited data or via a generic pathway [81]. This may require manufacturers to generate and submit additional data to national regulatory authorities [81].
Ongoing Efforts to Promote Global Regulatory Alignment
Clearly, globally consistent approval processes and decision making would facilitate a less‐complex regulatory landscape for biosimilars and could affect issues such as the breadth of development programs (streamlining would be possible), clinical trial designs, extrapolation (maximizing appropriate extrapolation could help minimize the number of requisite comparative clinical studies), access to treatment for patients, and postmarketing surveillance (e.g., through consistent approaches to naming). Thus, there is the potential to save substantial time and resources, as well as to ensure that standards are sufficiently high in all areas of the world. As noted earlier, closer alignment would also help drive confidence in the biosimilar concept both within the health care community and among patients [63], [78].
Steps toward regulatory alignment have been taken in several areas in recent years, with regulators continuing to refine and update guidance documents as the biosimilar landscape evolves [82]. One example is the case of Health Canada, which updated its biosimilar guideline in 2016, based in part on international developments in biosimilar regulation [69]; several of the revisions brought the document into closer alignment with the regulatory approach followed in other regions. Among other updates, the revised document uses the term “biosimilar” rather than the previous phrase “subsequent entry biologic,” and wording was added to clarify that the basis for biosimilar authorization is a demonstration of similarity based on the entirety of the submission (i.e., a totality of the evidence approach) [69].
Moreover, there are ongoing collaborative efforts between regulatory authorities in different regions. For example, the International Pharmaceutical Regulators Forum has established a biosimilars working group to discuss and harmonize the issues and challenges in regulation of biosimilars among member countries [83]. The group's recent work includes the publication of a reflection paper that “compiles common features of various [national regulatory authority] biosimilar guidelines and highlights harmonized scientific considerations on extrapolation for biosimilar products” [84]. Furthermore, the working group has published a template to assist regulatory authorities in making available English‐language summaries of their assessments of biosimilar applications, given that assessment reports are often only available in the local language and hence not accessible to the wider global community [85]. In additional examples of collaboration, four agencies (EMA, FDA, Health Canada, and Japan's Pharmaceuticals and Medical Devices Agency) have formed a “biosimilars cluster” to foster global interactions on biosimilars, and the EMA and FDA have introduced a joint program to provide manufacturers with parallel scientific advice [77], [86]. The four‐agency cluster was established in 2011 and convenes three times per year with the objective of achieving alignment on scientific approaches to the evaluation of biosimilars, so that “data developed for one regulatory authority could be acceptable to another” [87].
More recently, the concept of a single “global reference” comparator for biosimilar development has been proposed. A 2017 publication by Webster and Woollett argued that bridging studies designed to demonstrate that local and foreign versions of an originator product are representative of one another (in order to justify use of the foreign version as a comparator during the biosimilarity exercise) are “usually redundant” [88]. This is because versions of the originator approved in different jurisdictions normally share common development data, and any subsequent manufacturing changes have been justified as a result of a rigorous comparability assessment. The authors stated, therefore, that bridging studies during biosimilar development add “substantial unnecessary development time and cost for biosimilar sponsors, particularly since the same data are required from each sponsor, and by multiple jurisdictions” [88]. Instead, a set of criteria were proposed under which a single reference version of the originator could be used for the global development of a biosimilar [88].
Although it seems unrealistic to expect that a central, global regulatory framework for the evaluation and approval of biosimilars will be established, it is hoped that initiatives and proposals such as those outlined above will foster closer regulatory alignment between regions, thus preventing major divergence and hence supporting the potential of these products to provide cost efficiencies and improved access to important medications.
Health Care Professional and Patient Perceptions: Trust and Education
Trust is a critical component in the real‐life implementation of medicines, whether in cancer or other diseases. Furthermore, trust on the part of the patient is not merely related to the fact that a drug is approved by regulatory bodies. Patients must trust that prescribed drugs are reliable and safe, and were produced according to high quality standards. Studies exploring patients’ attitudes toward biosimilars have identified issues such as a low awareness of these treatments, a desire to be involved in treatment decisions, and the belief that cost savings should not be prioritized above efficacy and safety [22], [89], [90]. Therefore, there exists an immediate need for effective patient education on biosimilars. Furthermore, it is key that health care professionals are able to communicate and reinforce the biosimilar concept endorsed by leading regulators, such as the EMA and FDA.
Trust and confidence in biosimilars are also crucial among health care professionals [52]. This may be particularly true in oncology, given that cancer is complex to treat and often catastrophic for patients [91]. Regrettably, as demonstrated in published surveys, some physicians are not well informed about biosimilars. A recent survey among U.S.‐based specialty physicians who already prescribe biologics revealed that, although the majority of respondents had heard of biosimilars, in‐depth knowledge of fundamental concepts such as the “totality of the evidence” was low [21]. Other physician surveys have revealed general positivity toward biosimilars but uncertainty regarding issues such as extrapolation [8], [92], [93]. Similar knowledge gaps are likely among pharmacists, nurses, and other health care practitioners [91], [94].
Health care professionals are accustomed to evaluating therapeutics on the basis of data from clinical trials in each approved indication, and it is understandable that they may have uncertainties related to the emphasis on analytical assessments in the biosimilar paradigm [16]. Regulatory authorities have recognized the importance of providing educational materials on biosimilars [95], [96], along with a need for patients and health care professionals to trust in the science that underpins biosimilar approval pathways [97]. Expert bodies such as the National Comprehensive Cancer Network have also identified the need to educate practitioners on the scientific principles concerning biologic manufacturing processes and pharmacovigilance [91].
Clinical experience with biosimilars will be another important element in their acceptance. In the past decade, no relevant differences between biosimilars and respective originators have been identified via the safety monitoring system in the European Union, and none of the approved biosimilars has been withdrawn as a result of safety or efficacy concerns [70], thus providing reassurance and validation concerning the biosimilar approval pathway in stringently regulated regions. Furthermore, the legitimacy of extrapolation has been supported by real‐world data collected in indications not studied during initial biosimilar development. There was initial debate within the gastroenterology community regarding authorization of CT‐P13 for the treatment of IBD without supporting clinical trial results, for example [98]. However, data collected in patients with Crohn's disease or ulcerative colitis have revealed no significant safety, efficacy, or immunogenicity concerns [99], [100], and, as experience has accrued, confidence with this biosimilar has increased among IBD specialists [101].
In the past decade, no relevant differences between biosimilars and respective originators have been identified via the safety monitoring system in the European Union, and none of the approved biosimilars has been withdrawn as a result of safety or efficacy concerns, thus providing reassurance and validation concerning the biosimilar approval pathway in stringently regulated regions.
Finally, although not a major focus of our review, it should be noted that payers and other decision makers also require education on biosimilars if the potential of these products is to be maximized. Although marked price reductions have been achieved through the introduction of biosimilars in certain notable cases (for example, in a 2015 Norwegian national hospital tender for biologics, CT‐P13 was offered at a price 69% lower than that offered for the originator [102]), several commentators have cautioned that focusing on short‐term cost savings only, without appropriately incentivizing health care professionals and manufacturers, may ultimately prove detrimental [52], [59]. The investment required for biosimilar development is significant compared with that needed for generic, chemically derived products. It has been estimated that biosimilar development may take 5–9 years and cost in excess of U.S. $100 million; in contrast, development of a generic version of a small‐molecule drug may cost in the order of U.S. $1–4 million [103], [104], [105]. Manufacturing costs for biologics are also significantly higher than those for chemically derived products [59]. These and other factors help explain why the price differential between biosimilars and originator products has tended to be smaller than the discounts observed for generic drugs [59]. In short, a “race to the bottom” in terms of pricing may discourage manufacturers from investing in biosimilars, hence hindering the longer‐term development of a sustainable, competitive biosimilars market [52], [59], [106]. As the biosimilars industry matures, how such issues develop will be of significant interest to all stakeholders. More generally, pricing and availability of medications, not least anticancer treatments, are global health care challenges, and innovative solutions must be encouraged [107], [108]. In certain markets, for example, one option may be public–private partnerships, including collaborations designed specifically to promote access to biosimilars [109], [110].
Conclusion
At a time of rapidly increasing health care expenditure, there is an urgent need to generate savings while maintaining quality and clinical effectiveness. Biosimilars are consistent with this concept and represent an important tool for making medicines more accessible [18], [49]. Anticancer mAb biosimilars have recently been authorized in Europe and the U.S., and numerous others are in development. For biosimilars to have maximal impact, it is essential that they are accepted by health care professionals and patients [18] and that health care systems are amenable to biosimilar uptake. Education on, and trust in, the regulatory pathway for these agents will play an important part. A greater degree of global alignment in regulatory requirements and decision making would likely increase confidence in the biosimilar concept and enable streamlined development programs. Ideally, a globally consistent approach to biosimilar approval would take into consideration the existing regional differences in regulations, as well as ensuring that the concept of “head‐to‐head” biosimilarity at the core of robust guidelines from the EMA, FDA, and WHO is retained. Ultimately, global adoption of guidelines modeled on existing templates that have proven successful could expedite approval processes and facilitate improvements in patient care. Should the establishment of a central, global framework for biosimilar evaluation and approval prove impractical, then continued efforts to promote regulatory alignment between regions should be supported.
Acknowledgments
Medical writing support was provided by Paul Shepherd of Engage Scientific Solutions and was funded by Pfizer. This support included assistance with drafting, copyediting, and other editorial and production tasks.
Footnotes
For Further Reading: Fadi Farhat, Alfredo Torres, Wungki Park et al. The Concept of Biosimilars: From Characterization to Evolution—A Narrative Review. The Oncologist 2018;23:346–352.
Implications for Practice: This article highlights the importance of biosimilars, as a cost‐cutting strategy, in the delivery of state‐of‐the‐art health care in developing countries, at a fraction of what a reference biological agent would cost.
Author Contributions
Conception/design: Eduardo Cazap, Ira Jacobs, Ali McBride, Robert Popovian, Karol Sikora
Manuscript writing: Eduardo Cazap, Ira Jacobs, Ali McBride, Robert Popovian, Karol Sikora
Final approval of manuscript: Eduardo Cazap, Ira Jacobs, Ali McBride, Robert Popovian, Karol Sikora
Disclosures
Eduardo Cazap: Bayer, Schering Pharma, Roche, Pfizer, Tuvuga Inc. (C/A), Bayer, Bristol‐Myers Squibb, Fresenius (H), Poniard Pharmaceuticals, Daiichi Sankyo Pharma, Breast Cancer Research Foundation (RF); Ira Jacobs: Pfizer (E, OI); Ali McBride: Pfizer (H); Sandoz (SAB); Robert Popovian: Pfizer (E, OI). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Rader RA. (Re)defining biopharmaceutical. Nat Biotechnol 2008;26:743–751. [DOI] [PubMed] [Google Scholar]
- 2. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 3. Hiddemann W, Kneba M, Dreyling M et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2005;106:3725–3732. [DOI] [PubMed] [Google Scholar]
- 4. Slamon DJ, Leyland‐Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 5. Socinski MA, Curigliano G, Jacobs I et al. Clinical considerations for the development of biosimilars in oncology. MAbs 2015;7:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lammers P, Criscitiello C, Curigliano G et al. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: A physician survey in the United States and emerging markets. Pharmaceuticals (Basel) 2014;7:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baer WH, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non‐Hodgkin's lymphoma and chronic lymphocytic leukemia: A physician survey. Pharmaceuticals (Basel) 2014;7:530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monk BJ, Lammers PE, Cartwright T et al. Barriers to the access of bevacizumab in patients with solid tumors and the potential impact of biosimilars: A physician survey. Pharmaceuticals (Basel) 2017;10:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherny N, Sullivan R, Torode J et al. ESMO European Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol 2016;27:1423–1443. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry Silver Spring, MD: U.S. Food and Drug Administration; April 2015. Available at http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed October 26, 2017.
- 11.Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on Similar Biological Medicinal Products London, U.K.: European Medicines Agency; October 23, 2014. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed June 20, 2017.
- 12.Expert Committee on Biological Standardization, World Health Organization. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs) Geneva, Switzerland: World Health Organization; October 23, 2009. Available at http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed June 20, 2017.
- 13.QuintilesIMS, European Commission. The impact of biosimilar competition on price, volume, and market share ‐ update 2017. European Commission Web site. Available at http://ec.europa.eu/growth/tools-databases/newsroom/cf/itemdetail.cfm?item_id=9146&lang=en. Published May 5, 2017. Accessed October 26, 2017.
- 14. Gascon P, Tesch H, Verpoort K et al. Clinical experience with Zarzio in Europe: What have we learned? Support Care Cancer 2013;21:2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aladul MI, Fitzpatrick RW, Chapman SR. Impact of infliximab and etanercept biosimilars on biological disease‐modifying antirheumatic drugs utilisation and NHS budget in the UK. BioDrugs 2017;31:533–544. [DOI] [PubMed] [Google Scholar]
- 16. Christl LA, Woodcock J, Kozlowski S. Biosimilars: The US regulatory framework. Annu Rev Med 2017;68:423–454. [DOI] [PubMed] [Google Scholar]
- 17. Weise M, Bielsky MC, De Smet K et al. Biosimilars ‐ why terminology matters. Nat Biotechnol 2011;29:690–693. [DOI] [PubMed] [Google Scholar]
- 18. Schellekens H, Smolen JS, Dicato M et al. Safety and efficacy of biosimilars in oncology. Lancet Oncol 2016;17:e502–e509. [DOI] [PubMed] [Google Scholar]
- 19. McCamish M, Woollett G. Worldwide experience with biosimilar development. MAbs 2011;3:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinemann L, Khatami H, McKinnon R et al. An overview of current regulatory requirements for approval of biosimilar insulins. Diabetes Technol Ther 2015;17:510–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen H, Beydoun D, Chien D et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 2017;33:2160–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs I, Singh E, Sewell KL et al. Patient attitudes and understanding about biosimilars: An international cross‐sectional survey. Patient Prefer Adherence 2016;10:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Generic Drug Regulators Programme (IGDRP) [Web site]. Available at https://www.igdrp.com/. Accessed October 26, 2017.
- 24.Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on Similar Biological Medicinal Products London, U.K.: European Medicines Agency; October 30, 2005. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf. Accessed October 26, 2017.
- 25.European Medicines Agency. European public assessment reports. European Medicines Agency Web site. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed February 7, 2018.
- 26.Therapeutic Goods Administration, Department of Health, Australian Government. Biosimilar Medicines Regulation Version 2.1. Canberra, Australia: Department of Health, Australian Government; February 2018. Available at https://www.tga.gov.au/sites/default/files/biosimilar-medicines-regulation.pdf. Accessed February 28, 2018.
- 27.Health Canada. Guidance Document: Information and Submission Requirements for Biosimilar Biologic Drugs Ottawa, Ontario, Canada: Health Canada; November 14, 2016. Available at http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/brgtherap/applic-demande/guides/seb-pbu/seb-pbu-2016-eng.pdf. Accessed October 25, 2017.
- 28.Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Guideline for the Quality, Safety, and Efficacy Assurance of Follow‐On Biologics Tokyo, Japan: Ministry of Health, Labour and Welfare; March 4, 2009; provisional translation published April 19, 2013. Available at https://www.pmda.go.jp/files/000153851.pdf. Accessed June 20, 2017.
- 29.Ministry of Food and Drug Safety, Republic of Korea. Guidelines on the Evaluation of Biosimilar Products English version, Revision 1. Cheongwon‐gun, Chungcheongbuk‐do, Republic of Korea: Ministry of Food and Drug Safety; October 2015. Available at http://www.mfds.go.kr/eng/eng/index.do?nMenuCode=166&searchKeyCode=171&page=4&mode=view&boardSeq=70199. Accessed October 26, 2017.
- 30. Schiestl M. A biosimilar industry view on the implementation of the WHO guidelines on evaluating similar biotherapeutic products. Biologicals 2011;39:297–299. [DOI] [PubMed] [Google Scholar]
- 31.Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology‐Derived Proteins as Active Substance: Non‐Clinical and Clinical Issues London, U.K.: European Medicines Agency; December 18, 2014. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed June 16, 2017.
- 32. Weise M, Bielsky MC, De Smet K et al. Biosimilars: What clinicians should know. Blood 2012;120:5111–5117. [DOI] [PubMed] [Google Scholar]
- 33.Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Monoclonal Antibodies ‐ Non‐Clinical and Clinical Issues London, U.K.: European Medicines Agency; May 30, 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf. Accessed June 20, 2017.
- 34. Ho RJ. Midyear commentary on trends in drug delivery and clinical translational medicine: Growth in biosimilar (complex injectable drug formulation) products within evolving collaborative regulatory interagency (FDA, FTC, and DOJ) practices and enforcement. J Pharmaceut Sci 2017;106:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs I, Petersel D, Shane LG et al. Monoclonal antibody and fusion protein biosimilars across therapeutic areas: A systematic review of published evidence. BioDrugs 2016;30:489–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacobs I, Petersel D, Isakov L et al. Biosimilars for the treatment of chronic inflammatory diseases: A systematic review of published evidence. BioDrugs 2016;30:525–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacobs I, Ewesuedo R, Lula S et al. Biosimilars for the treatment of cancer: a systematic review of published evidence. BioDrugs 2017;31:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celltrion. Truxima, the first biosimilar mAb in oncology, granted EU marketing authorisation [press release]. February 22, 2017. Available at https://www.celltrion.com/en/pr/reportDetail.do?seq=409. Accessed November 9, 2017.
- 39.Sandoz. Sandoz receives approval in Europe for Rixathon (biosimilar rituximab) to treat blood cancers and immunological diseases [press release]. June 19, 2017. Available at https://www.sandoz.com/news/media-releases/sandoz-receives-approval-europe-rixathonr-biosimilar-rituximab-treat-blood. Accessed October 26, 2017.
- 40.Amgen. FDA approves Amgen and Allergan's MVASI (bevacizumab‐awwb) for the treatment of five types of cancer [press release]. September 14, 2017. Available at http://www.amgen.com/media/news-releases/2017/09/fda-approves-amgen-and-allergans-mvasi-bevacizumabawwb-for-the-treatment-of-five-types-of-cancer/. Accessed October 26, 2017.
- 41.Amgen. European Commission approves Amgen and Allergan's MVASI (biosimilar bevacizumab) for the treatment of certain types of cancer [press release]. January 18, 2018. Available at http://www.amgen.com/media/news-releases/2018/01/european-commission-approves-amgen-and-allergans-mvasi-biosimilar-bevacizumab-for-the-treatment-of-certain-types-of-cancer/. Accessed February 1, 2018.
- 42.Samsung Bioepis. Samsung Bioepis receives regulatory approval for Europe's first trastuzumab biosimilar, ONTRUZANT [press release]. November 20, 2017. Available at http://www.samsungbioepis.com/en/newsroom/detail/Samsung-Bioepis-Receives-Regulatory-Approval.html. Accessed November 21, 2017.
- 43.Celltrion Inc. Celltrion receives EU approval for trastuzumab biosimilar [press release]. February 14, 2018. Available at http://www.celltrion.com/en/pr/reportDetail.do?seq=476. Accessed February 20, 2018.
- 44.Mylan. U.S. FDA approves Mylan and Biocon's Ogivri, the first biosimilar for trastuzumab, for the treatment of HER2‐positive breast and gastric cancers [press release]. December 1, 2017. Available at http://newsroom.mylan.com/2017-12-01-U-S-FDA-Approves-Mylan-and-Biocons-Ogivri-TM-the-First-Biosimilar-for-Trastuzumab-for-the-Treatment-of-HER2-Positive-Breast-and-Gastric-Cancers. Accessed December 6, 2017.
- 45.Mylan. Biocon and Mylan's biosimilar trastuzumab receives approval from ANVISA, Brazil through their partner Libbs [press release]. December 29, 2017. Available at http://newsroom.mylan.com/2017-12-29-Biocon-and-Mylans-Biosimilar-Trastuzumab-Receives-Approval-from-ANVISA-Brazil-Through-their-Partner-Libbs. Accessed February 20, 2018.
- 46.Celltrion. [Brochure.] June 30, 2017. Available at https://www.celltrion.com/en/pr/brochure.do. Accessed October 26, 2017.
- 47.Celltrion. Celltrion applied for approval of anticancer drug biosimilar ‘Herzuma’ in Japan [press release]. April 12, 2017. Available at http://celltrion.com/en/pr/reportDetail.do?seq=422. Accessed February 20, 2018.
- 48.Pipeline. Samsung Bioepis Web site. December 2017. Available at http://www.samsungbioepis.com/en/pipeline/. Accessed February 20, 2018.
- 49. Tabernero J, Vyas M, Giuliani R et al. Biosimilars: A position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open 2016;1:e000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Baelen M, Dylst P, Lopes Pereira C et al. Fighting counterfeit medicines in Europe: The effect on access to medicines. Med Access Point Care 2017;1:e49–e54. [Google Scholar]
- 51.IMS Institute for Healthcare Informatics. Medicines Use and Spending in the U.S.: A Review of 2015 and Outlook to 2020. Parsippany, NJ: IMS Institute for Healthcare Informatics; April 2016. Available at https://morningconsult.com/wp-content/uploads/2016/04/IMS-Institute-US-Drug-Spending-2015.pdf. Accessed October 26, 2017. [Google Scholar]
- 52.IMS Institute for Healthcare Informatics. Delivering on the Potential of Biosimilar Medicines: The Role of Functioning Competitive Markets. Parsippany, NJ: IMS Institute for Healthcare Informatics; March 2016. Available at http://www.medicinesforeurope.com/wp-content/uploads/2016/03/IMS-Institute-Biosimilar-Report-March-2016-FINAL.pdf. Accessed December 12, 2017. [Google Scholar]
- 53.World Health Organization. WHO to begin pilot prequalification of biosimilars for cancer treatment [press release]. May 4, 2017. Available at http://www.who.int/mediacentre/news/releases/2017/pilot-prequalification-biosimilars/en/. Accessed October 26, 2017.
- 54. Araújo FC, Gonçalves J, Fonseca JE. Pharmacoeconomics of biosimilars: What is there to gain from them? Curr Rheumatol Rep 2016;18:50. [DOI] [PubMed] [Google Scholar]
- 55. Rémuzat C, Dorey J, Cristeau O et al. Key drivers for market penetration of biosimilars in Europe. J Mark Access Health Policy 2017;5:1272308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic dseases. JAMA 2017;317:2163–2164. [DOI] [PubMed] [Google Scholar]
- 57. Jørgensen KK, Olsen IC, Goll GL et al. Switching from originator infliximab to biosimilar CT‐P13 compared with maintained treatment with originator infliximab (NOR‐SWITCH): A 52‐week, randomised, double‐blind, non‐inferiority trial. Lancet 2017;389:2304–2316. [DOI] [PubMed] [Google Scholar]
- 58. Dranitsaris G, Jacobs I, Kirchhoff C et al. Drug tendering: Drug supply and shortage implications for the uptake of biosimilars. Clinicoecon Outcomes Res 2017;9:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mestre‐Ferrandiz J, Towse A, Berdud M. Biosimilars: How can payers get long‐term savings? Pharmacoeconomics 2016;34:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chapman K, Adjei A, Baldrick P et al. Waiving in vivo studies for monoclonal antibody biosimilar development: national and global challenges. MAbs 2016;8:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pineda C, Castañeda Hernández G, Jacobs IA et al. Assessing the immunogenicity of biopharmaceuticals. BioDrugs 2016;30:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Declerck P, Danesi R, Petersel D et al. The language of biosimilars: Clarification, definitions, and regulatory aspects. Drugs 2017;77:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ebbers HC, Chamberlain P. Controversies in establishing biosimilarity: Extrapolation of indications and global labeling practices. BioDrugs 2016;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stergiopoulos S, Getz K. Evaluating AE reporting of two off‐patent biologics to inform future biosimilar naming and reporting practices. Drug Saf 2015;38:687–692. [DOI] [PubMed] [Google Scholar]
- 65.U.S. Food and Drug Administration. Nonproprietary Naming of Biological Products: Guidance for Industry Silver Spring, MD: U.S. Food and Drug Administration; January 2017. Available at http://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf. Accessed October 26, 2017.
- 66. Stevenson JG, Popovian R, Jacobs I et al. Biosimilars: Practical considerations for pharmacists. Ann Pharmacother 2017;51:590–602. [DOI] [PubMed] [Google Scholar]
- 67. Tomaszewski D. Biosimilar naming conventions: Pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm 2016;22:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mysler E, Pineda C, Horiuchi T et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int 2016;36:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Health Canada. Release of the revised guidance document: Information and Submission Requirements for Biosimilar Biologic Drugs [press release]. December 2, 2016. Available at http://www.hc-sc.gc.ca/dhp-mps/brgtherap/activit/announce-annonce/notice-avis_biosimilars-biosimilaires-eng.php. Accessed October 26, 2017.
- 70.European Medicines Agency; European Commission. Biosimilars in the EU: Information Guide for Healthcare Professionals 2017. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf. Accessed October 26, 2017.
- 71.European Medicines Agency. QRD General Principles Regarding the SmPC Information for a Generic/Hybrid/Biosimilar Product London, U.K.: European Medicines Agency; May 3, 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/05/WC500127589.pdf. Accessed October 26, 2017.
- 72.U.S. Food and Drug Administration. Labeling for Biosimilar Products. Guidance for Industry. Draft Guidance Silver Spring, MD: U.S. Food and Drug Administration; March 2016. Available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM493439.pdf. Accessed October 26, 2017.
- 73. Kurki P, van Aerts L, Wolff‐Holz E et al. Interchangeability of biosimilars: A European perspective. BioDrugs 2017;31:83–91. [DOI] [PubMed] [Google Scholar]
- 74.European Commission. What You Need to Know About Biosimilar Medicinal Products: Process on Corporate Responsibility in the Field of Pharmaceuticals Access to Medicines in Europe. A Consensus Information Document Brussels, Belgium: European Commission; 2013. Available at http://www.medicinesforeurope.com/wp-content/uploads/2016/03/biosimilars_report_en.pdf. Accessed June 20, 2017.
- 75.U.S. Food and Drug Administration. Considerations in Demonstrating Interchangeability with a Reference Product: Guidance for industry. Draft Guidance Silver Spring, MD: U.S. Food and Drug Administration; January 2017. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM537135.pdf. Accessed June 20, 2017.
- 76. Mielke J, Jilma B, Koenig F et al. Clinical trials for authorized biosimilars in the European Union: A systematic review. Br J Clin Pharmacol 2016;82:1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Welch AR. IQPC biosimilars event emphasizes need for regulatory alignment. Clinical Leader Web site. Available at https://www.clinicalleader.com/doc/iqpc-biosimilars-event-emphasizes-need-for-regulatory-alignment-0001. February 27, 2017. Accessed October 26, 2017.
- 78. Kay J, Isaacs JD. Clinical trials of biosimilars should become more similar. Ann Rheum Dis 2017;76:4–6. [DOI] [PubMed] [Google Scholar]
- 79.International Federation of Pharmaceutical Manufacturers & Associations. Policy Statement: Non‐Comparable Biotherapeutic Products Geneva, Switzerland: International Federation of Pharmaceutical Manufacturers & Associations; July 24, 2014. Available at https://www.ifpma.org/wp-content/uploads/2016/02/Non-comparable_Biotherapeutic_Products__English__02.pdf. Accessed October 24, 2017.
- 80. Álvarez AA, Mysler E, Ruiz de Castilla EM et al. Recommendations for the regulation of biosimilars and their implementation in Latin America. GaBI J 2014;3:143–148. [Google Scholar]
- 81.World Health Organization. Regulatory assessment of approved rDNA‐derived biotherapeutics. Annex 3 of WHO Technical Report Series, No. 999 2016. Available at http://www.who.int/biologicals/areas/biological_therapeutics/Annex_3_Regulatory_assessment_of_approved_rDNA-derived_biotherapeutics.pdf?ua=1. Accessed October 25, 2017.
- 82.Aquino JT. Biosimilar applicants must anticipate regulatory differences among countries. Bloomberg Web site. Available at http://www.bna.com/biosimilar-applicants-anticipate-n57982082038/. October 31, 2016. Accessed June 20, 2017.
- 83.International Pharmaceutical Regulators Forum. 2016 Work Plan: IPRF Biosimilar Working Group International Pharmaceutical Regulators Forum; June 12, 2016. Available at https://www.i-p-r-f.org/files/3314/6709/6204/03_IPRF_Biosimilar_WG_Work_Plan_2016_adopted.pdf. Accessed June 20, 2017.
- 84.International Pharmaceutical Regulators Forum. IPRF Biosimilars Working Group Reflection Paper on Extrapolation of Indications in Authorization of Biosimilar Products International Pharmaceutical Regulators Forum; June 16, 2017. Available at https://www.i-p-r-f.org/index.php/en/news/biosimiar-reflection-paper/. Accessed February 1, 2018.
- 85.Public Assessment Summary Information for Biosimilar (PASIB) ‐ Final documents after consultation. International Pharmaceutical Regulators Forum Web site. Available at: https://www.i-p-r-f.org/index.php/en/news/pasib-final/. August 18, 2016. Accessed February 6, 2018.
- 86.Welch AR. FDA stresses scientific alignment, not harmonization, for global biosimilars. Biosimilar Development Web site. Available at https://www.biosimilardevelopment.com/doc/fda-preaches-scientific-alignment-not-harmonization-for-global-biosimilars-0001. December 5, 2016. Accessed October 26, 2017.
- 87.Cluster activities. European Medicines Agency Web site. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/partners_and_networks/general/general_content_000655.jsp&mid=WC0b01ac0580c4d400. Accessed February 1, 2018.
- 88. Webster CJ, Woollett GR. A ‘global reference’ comparator for biosimilar development. BioDrugs 2017;31:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peyrin‐Biroulet L, Lönnfors S, Roblin X et al. Patient perspectives on biosimilars: A survey by the European Federation of Crohn's and Ulcerative Colitis Associations. J Crohns Colitis 2017;11:128–133. [DOI] [PubMed] [Google Scholar]
- 90. Wilkins AR, Venkat MV, Brown AS et al. Patient perspectives on biosimilar insulin. J Diabetes Sci Technol 2014;8:23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zelenetz AD, Ahmed I, Braud EL et al. NCCN biosimilars white paper: Regulatory, scientific, and patient safety perspectives. J Natl Compr Canc Netw 2011;9(suppl 4):S1–S22. [DOI] [PubMed] [Google Scholar]
- 92. Hemmington A, Dalbeth N, Jarrett P et al. Medical specialists’ attitudes to prescribing biosimilars. Pharmacoepidemiol Drug Saf 2017;26:570–577. [DOI] [PubMed] [Google Scholar]
- 93. Beck M, Michel B, Rybarczyk‐Vigouret MC et al. Rheumatologists’ perceptions of biosimilar medicines prescription: Findings from a French web‐based survey. BioDrugs 2016;30:585–592. [DOI] [PubMed] [Google Scholar]
- 94. Beck M, Michel B, Rybarczyk‐Vigouret MC et al. Knowledge, behaviors and practices of community and hospital pharmacists towards biosimilar medicines: Results of a French web‐based survey. MAbs 2017;9:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.European Medicines Agency. New guide on biosimilar medicines for healthcare professionals [press release]. May 5, 2017. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2017/05/news_detail_002739.jsp&mid=WC0b01ac058004d5c1. Accessed December 4, 2017.
- 96. Gottlieb S, Christl L. FDA taking new steps to better inform physicians about biosimilars through education about these potentially cost‐saving options [press release]. October 23, 2017. Available at https://blogs.fda.gov/fdavoice/index.php/2017/10/fda-taking-new-steps-to-better-inform-physicians-about-biosimilars-through-education-about-these-potentially-cost-saving-options/. Accessed December 4, 2017.
- 97.Association for Accessible Medicines. Trust, education critical to ensure patient access to biosimilar, FDA says at inaugural Biosimilars Council Conference [press release]. September 7, 2016. Available at http://www.gphaonline.org/gpha-media/press/trust-education-critical-to-ensure-patient-access-to-biosimilars-fda-says-at-inaugural-biosimilars-council-conference/. Accessed October 26, 2017.
- 98. Danese S, Gomollon F; Governing Board and Operational Board of ECCO. ECCO position statement: The use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis 2013;7:586–589. [DOI] [PubMed] [Google Scholar]
- 99. Radin M, Sciascia S, Roccatello D et al. Infliximab biosimilars in the treatment of inflammatory bowel diseases: A systematic review. BioDrugs 2017;31:37–49. [DOI] [PubMed] [Google Scholar]
- 100.British Society of Gastroenterology. BSG Guidance on the Use of Biosimilar Infliximab CT‐P13 in Inflammatory Bowel Disease London, U.K.: British Society of Gastroenterology; February 2017. Available at https://www.bsg.org.uk/asset/82EA3731-D395-4F9C-8D92B579225115F7. Accessed December 12, 2017.
- 101. Danese S, Bonovas S, Peyrin‐Biroulet L. Biosimilars in IBD: From theory to practice. Nat Rev Gastroenterol Hepatol 2017;14:22–31. [DOI] [PubMed] [Google Scholar]
- 102.Huge discount on biosimilar infliximab in Norway. GaBI Online Web site. Available at http://www.gabionline.net/Biosimilars/General/Huge-discount-on-biosimilar-infliximab-in-Norway. March 13, 2015. Accessed February 1, 2018.
- 103.Development of biosimilars. GaBI Online Web site. Available at http://www.gabionline.net/Biosimilars/Research/Development-of-biosimilars. July 1, 2011. Accessed February 1, 2018.
- 104. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits 2013;6:469–478. [PMC free article] [PubMed] [Google Scholar]
- 105.IMS Health. Shaping the Biosimilars Opportunity: A Global Perspective on the Evolving Biosimilars Landscape. London, U.K.: IMS Health; December 2011. Available at https://weinberggroup.com/pdfs/Shaping_the_biosimiliars_opportunity_A_global_perspective_on_the_evolving_biosimiliars_landscape.pdf. Accessed February 1, 2018.
- 106. Mulcahy AW, Hlavka JP, Case SR. Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Santa Monica, CA: RAND Co; rporation; 2017. Available at https://www.rand.org/pubs/perspectives/PE264.html. Accessed February 1, 2018. [PMC free article] [PubMed] [Google Scholar]
- 107. Moon S. Powerful ideas for global access to medicines. N Engl J Med 2017;376:505–507. [DOI] [PubMed] [Google Scholar]
- 108. Cherny NI, Sullivan R, Torode J et al. ESMO International Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in countries outside of Europe. Ann Oncol 2017;28:2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mackey TK, Liang BA. Promoting access to biosimilars: A public‐private partnership model for biosimilar development in underserved populations. GaBI J 2012;1:84–88. [Google Scholar]
- 110. Stevens H, Huys I. Innovative approaches to increase access to medicines in developing countries. Front Med (Lausanne) 2017;4:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hospira Pty Ltd. Inflectra product information. September 16, 2015. Available at https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2017-PI-01185-1&d=2017030716114622483. Accessed October 25, 2017.
- 112.Summary basis of decision (SBD) for Inflectra. Health Canada Web site. Available at https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00253. October 13, 2016. Accessed October 25, 2017.
- 113.Summary basis of decision (SBD) for Remsima. Health Canada Web site. Available at https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00330. October 13, 2016. Accessed October 25, 2017.
- 114.Celltrion Healthcare Co. Ltd., Hospira Healthcare Corporation. Product monograph. Remsima (infliximab). August 5, 2016. Available at https://pdf.hres.ca/dpd_pm/00036007.PDF. Accessed October 26, 2017.
- 115.Celltrion Healthcare Co. Ltd., Hospira Healthcare Corporation. Product monograph. Inflectra (infliximab). June 10, 2016. Available at https://pdf.hres.ca/dpd_pm/00035250.PDF. Accessed October 26, 2017.
- 116.Regulatory decision summary for Inflectra. Health Canada Web site. Available at https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00124. October 13, 2016. Accessed October 25, 2017.
- 117.Regulatory decision summary for Remsima. Health Canada Web site. Available at https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00177. October 13, 2016. Accessed October 25, 2017.
- 118.Committee for Medicinal Products for Human Use, European Medicines Agency. Assessment Report: Inflectra London, U.K.: European Medicines Agency; June 27, 2013. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf. Accessed October 25, 2017.
- 119.Committee for Medicinal Products for Human Use, European Medicines Agency. Assessment Report: Remsima June 27, 2013. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf. Accessed October 26, 2017.
- 120.Hospira UK Limited. Summary of product characteristics: Inflectra. August 14, 2017. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002778/WC500151489.pdf. Accessed October 26, 2017.
- 121.Celltrion Healthcare Hungary Kft. Summary of product characteristics: Remsima. July 4, 2017. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002576/WC500150871.pdf. Accessed October 26, 2017.
- 122. Takeuchi T, Yamanaka H, Tanaka Y et al. Evaluation of the pharmacokinetic equivalence and 54‐week efficacy and safety of CT‐P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2015;25:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Arato T. Japanese regulation of biosimilar products: Past experience and current challenges. Br J Clin Pharmacol 2016;82:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kishioka Y. Regulatory framework for biotherapeutic products including similar biotherapeutic products. March 2015. Available at https://www.pmda.go.jp/files/000204341.pdf. Accessed March 5, 2017.
- 125.Nippon Kayaku Co. Ltd. Product information for infliximab BS for IV infusion 100 mg NK [Japanese]. March 2017. Available at https://mink.nipponkayaku.co.jp/product/di/te_file/sedi_infi_te.pdf. Accessed June 19, 2017.
- 126. Park W, Lee SJ, Yun J et al. Comparison of the pharmacokinetics and safety of three formulations of infliximab (CT‐P13, EU‐approved reference infliximab and the US‐licensed reference infliximab) in healthy subjects: A randomized, double‐blind, three‐arm, parallel‐group, single‐dose, phase I study. Expert Rev Clin Immunol 2015;11(suppl 1):S25–S31. [DOI] [PubMed] [Google Scholar]
- 127.Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Summary review: BLA 125544 for CT‐P13, a proposed biosimilar to Remicade (infliximab). 2016. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/125544Orig1s000SumR.pdf. Accessed October 26, 2017.
- 128.U.S. Food and Drug Administration. Inflectra prescribing information. April 2016. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125544s000lbl.pdf. Accessed June 20, 2017.