Abstract
Background: Among women in China, gynecological cancers are the second most common cancers after breast cancer. Cancer-related cognitive impairment (CRCI) has emerged as a significant problem affecting gynecological cancer survivors. While acupuncture has been used in different aspects of cancer care, the possible positive effects of acupuncture on cognitive impairment have received little attention. This study hypothesized that patients would demonstrate lower neurocognitive performance and lower structural connectivity compared to healthy controls. This pilot study also hypothesized that acupuncture may potentially be effective in treating CRCI of cancer patients by increasing brain structural connectivity and integrity. Methods: This prospective cohort study consisted of 3 stages: the first stage included a group of gynecological cancer patients and a group of age-matched healthy controls. This baseline stage used a core set of neurocognitive tests to screen patients with cognitive impairment and used a multimodal approach of brain magnetic resonance imaging (MRI) to explore the possible neurobiological mechanism of cognitive impairment in cancer patients, comparing the results with a group of noncancer controls. The second stage involved assigning CRCI patients into the acupuncture intervention group, while patients without CRCI were assigned into the cancer control group. The third stage was a postintervention assessment of neurocognitive function by the same set of neurocognitive tests at baseline. To explore the possible neurobiological basis of acupuncture for treating CRCI, this study also used a multimodal MRI approach to assess changes in brain structural connectivity, and neurochemical properties in patients at pre- and postacupuncture intervention. Results: This study found that the prevalence of cognitive impairment in Chinese gynecological cancer patients at diagnosis was 26.67%. When investigating the microstructural white matter in the brain, diffusion tensor imaging data in this study indicated that premorbid cognitive functioning (before clinical manifestations become evident) has already existed, as the global and local connectome properties in the entire patient group were lower than in the healthy control group. Using magnetic resonance spectroscopy, this study indicated there was a significant reduction of relative concentration of NAA (N-acetyl aspartate) in the left hippocampus, comparing these results with healthy controls. Regarding the effects of acupuncture on reducing CRCI, patients in the acupuncture group reported better neurocognitive test performance after matching for age, menopausal status, cancer stage, and chemotherapy regimen dosage. On a microstructural level, acupuncture’s ability to reduce CRCI may be attributed to a reduction in demyelination and an enhancement of the neuronal viability of white matter in the hippocampus. Conclusion: This pilot study indicates that acupuncture is a promising intervention in treating CRCI in gynecological cancer patients undergoing chemotherapy; however, it requires evaluation in larger randomized controlled studies to definitively assess its benefit. By using a multimodal imaging approach, this pilot study also provides novel insights into the neurobiological basis of cognitive impairment on the human brain that has been induced by cancer and/or its treatment.
Keywords: cancer-related cognitive impairment, acupuncture, neurobiological mechanism, gynecological cancer, Chinese women
Introduction
Among women in China, gynecological cancers as a group of cervical, uterine, ovarian, vaginal, and vulvar cancers are the second most common cancers after breast cancer.1 Because of medical technology advancements, bringing more potentially curative treatments such as surgery, radiotherapy, chemotherapy, and targeted therapies,2 the current 5-year relative survival rate of patients with gynecological cancer ranges from 46% to 82%.3 As more patients with gynecological cancer are living longer after curative treatment, long-term or late effects of cancer and its treatment are becoming more common in cancer survivors.4 One such long-term and late effect is neurocognitive change, which has emerged as a significant problem affecting gynecological cancer survivors.5,6
Gynecological cancer and its treatment can have neurotoxic effects which are associated with brain injury, resulting in cognitive impairment.5 Cancer-related cognitive impairment (CRCI) is often described colloquially as chemo brain or chemofog.7,8 CRCI has the potential to significantly affect social and occupational functioning, interfering with the ability to carry out normal daily activities, all of which in turn contribute to lower quality of life in cancer survivors.5,9 The domain of cognitive impairment may affect memory, concentration, information processing speed, and executive function.10 These types of cognitive impairment could exert a significant impact on social and occupational functioning, interfering with the ability to carry out normal daily activities, all of which in turn contributes to lower quality of life for cancer survivors.5,9
Advanced neuroimaging studies in cancer patients provide a better understanding of CRCI, and there is accumulating evidence to support the assertion that CRCI is a pathophysiologic process.5,11,12 In recent years, diffusion tensor imaging (DTI) has been able to characterize water diffusion and microstructure in biological tissues, especially for white matter integrity and diffusivity.13,14 Using DTI could also identify degradation of neural structures, and determine whether axonal death and/or deterioration of the myelin sheath are involved.14 While DTI is becoming a promising technique to assess whether cancer and its therapy-induced subtle white matter changes could explain CRCI in cancer patients,15 DTI could not provide information about the underlying biological mechanism of neural degeneration.14 Magnetic resonance spectroscopy (MRS) is an imaging technique that can provide further insight regarding the biochemical properties of the brain, and whether white matter changes represent inflammation or axonal death by detecting changes in brain metabolites.9,13,14,16 As axonal death is irreversible, it may be unlikely that cognitive impairment in cancer survivors can be restored.14 Thus, there is a need for intervention strategies in preventing and managing CRCI.
There are limited pharmacological treatment approaches for the management of CRCI, and it is noted that pharmacological treatments often have side effects.11,17 A large body of evidence confirms that acupuncture is effective in reducing anticancer treatment–caused side effects, including pain, nausea, hot flashes, fatigue, anxiety, depression, and sleep disturbances.18,19 A number of other studies have shown the effectiveness of acupuncture therapy in improving the cognitive function of patients with cancer.19-21 The reduced severity of cognitive symptoms is associated with neuroimaging improvement in brain regions relevant to learning and memory processes.13-15 In various animal studies, acupuncture has also been shown to ameliorate cognitive impairment.22 While acupuncture has been a part of traditional Chinese medicine for thousands of years and has been used in different aspects of cancer care, the possible positive effects of acupuncture on cognitive impairment have received little attention. Hence, we hypothesized that patients would demonstrate lower neurocognitive performance and lower structural connectivity compared with healthy controls. We also hypothesized that acupuncture may potentially be effective in treating CRCI of cancer patients by increasing brain structural connectivity and integrity.
Aims
This pilot study aimed to assess cognitive outcomes of gynecological cancer patients compared to healthy controls, and to examine the possible effects of acupuncture on patient cognitive outcomes, as well as acupuncture’s possible underlying neurobiological mechanisms of mitigating cognitive impairment in cancer patients.
Methods
This pilot, prospective cohort study was conducted to assess cancer patients’ neurocognition, brain structural connectivity, and neurochemical properties at pre- and postacupuncture intervention.
Subjects and Study Procedure
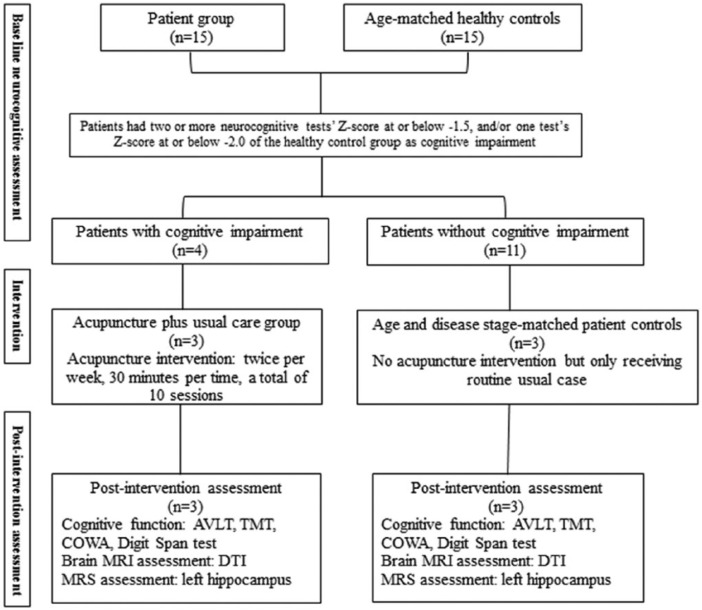
All subjects were recruited in the Unit of Gynecological Oncology at a general teaching hospital. Ethical approval was obtained from the ethics committees at both The Hong Kong Polytechnic University and The Third Affiliated Hospital of Guangzhou Medical University. Subjects were Chinese females aged 18 to 65 years; with a primary diagnosis of stage I-III gynecological cancer; and who were ready for adjuvant chemotherapy after surgical treatment. Exclusion criteria were women with a previous history of cancer (not a primary diagnosis of cancer), and/or who were in a terminal stage of cancer, and/or had a severe needle phobia. Inclusion criteria for healthy controls included women within 1 year of age and same menopausal status as the patient group. Exclusion criteria for both patient and healthy control groups included potential psychiatric disorders, such as depression and anxiety, a history of any neurological condition, traumatic brain injury, intellectual disability, and the use of psychotropic medication. The entire study procedure is shown in Figure 1.
Figure 1.
Study procedure.1
Acupuncture Interventions
Patients with cognitive impairment at the time of diagnosis were invited to receive manual acupuncture, which was provided by a single acupuncturist trained in traditional Chinese medicine. Sterile, disposable, stainless steel needles (0.25 mm in diameter and 40 mm in length, Huanqiu brand, made in China) were inserted at the following forehead acupuncture points: EX-HN1 (left and right, anterior and posterior Sishencong), EX-HN3 (Yintang), EX-HN5 (bilateral Taiyang), GB8 (bilateral Shuaigu), GB15 (Toulinqi), GB20 (Fengchi), GV20 (Baihui), and ST8 (bilateral Touwei), unilaterally or bilaterally, depending on each woman’s traditional diagnosis (constitution) as determined by the acupuncturist. All selected acupuncture points were related to cognitive function.23 The duration of needling was 30 minutes, and the frequency of interventions was 2 times per week. As the total number of chemotherapy cycles for gynecological cancer patients ranges from 4 to 6 cycles, so the average total number of interventions was 10 sessions per patient. The depth of needling varied between 25 and 40 mm, depending on the individual point.
Neurocognitive Function Assessment
As suggested by Joly et al,10 the most common domains of cognitive impairment in cancer survivors include learning and memory, information processing speed, and executive function. The International Cognition and Cancer Task Force (ICCTF) recommends that the following measures (at minimum) be included in assessing cognitive function in cancer patients: the Hopkins Verbal Learning Test–Revised (HVLT-R), the Trail Making Test (TMT), and the Controlled Oral Word Association Test (COWA).24 This study administered the Chinese version of the Auditory Verbal Learning Test–Revised version (AVLT-R) to measure the domains of learning and memory25; the TMT-A, to measure information processing speed, and the TMT-B, to measure executive function.26 According to Zeng et al,27 attention, working memory, and language/verbal comprehension problems were the most common cognitive complaints among Chinese cancer patients. This study also included the WAIS-III (Wechsler Adult Intelligence Scale–III) Digit Span test for measuring attention and working memory,28 and the COWA for assessing the verbal fluency and language comprehension of Chinese gynecological cancer patients.26
MRI and MRS Data Acquisition
The MRI data were acquired using a Philips 3T Achieva MRI/MRS scanner with an 8-channel head coil. Neurocognition evaluation and MRI scans took place on the same day. DTI and MRS were used to investigate changes in subjects’ brain structural connectivity, and changes in brain metabolites, respectively. DTI, high-resolution structural T1-weighted brain scans were obtained using single-shot echo-planar imaging (EPI) (acquisition matrix = 128 × 128; TE (echo time) = minimum; TR (repetition time) = 16 000 ms; field of view = 256 mm × 256 mm; slice thickness/gap = 2.0 mm/0 mm; scanning time = 6 minutes 56 seconds) with 32 distributed isotropic orientations for the diffusion-sensitizing gradients at a b-value of 1000 s/mm2 and a b-value of 0. T1-weighted imaging was achieved for morphometric (gray matter volume, cortical thickness, and surface area) analysis using 3-dimensional fast spoiled-gradient recalled acquisition in steady state (3D-FSPGR) in 166 coronal slices (acquisition matrix = 128 × 128; TE = 3.9 ms; TR = 9.6 ms; field of view = 256 mm × 256 mm; slice thickness/gap = 2 mm/0 mm; scanning time approximately 7 minutes).
As the hippocampus is an important brain structure due to its well-known function in the maintenance of memory, especially on the left side of the brain,29 1H-MRS data were located in the region of the left hippocampus. Single voxel proton MRS was acquired in the left hippocampus to assess the neurochemical properties of white matter. The region of interest is 2.5 × 1 × 1 cm3, and voxels contained the head, body, and tail of the hippocampus. Fully automated PRESS (point-resolved spectroscopy), including global shimming (TR/TE = 2000/35 ms, number of signal averages [NSA] = 16) was acquired in the red box area of the left hippocampus (Figure 2).
Figure 2.
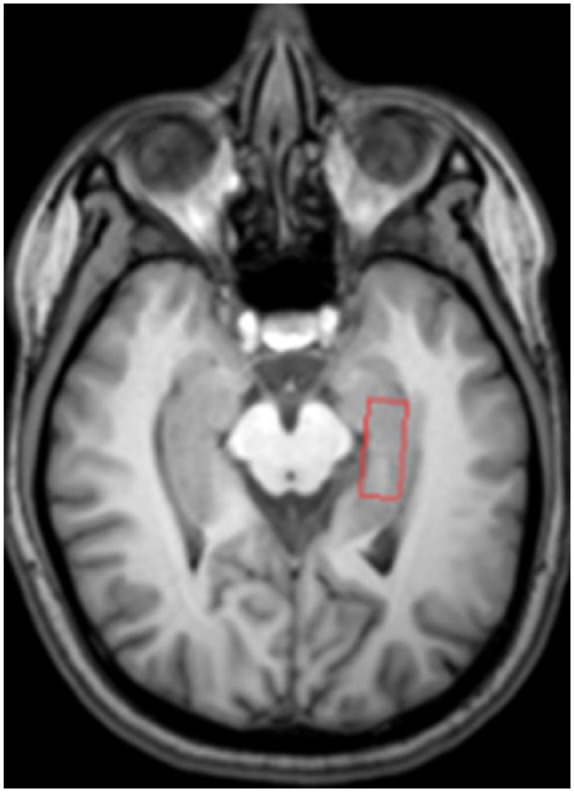
Left hippocampus volume of interest (VOI).
MRI and MRS Data Processing and Analyses
The DTI images were preprocessed using PANDA: a pipeline toolbox for analyzing brain diffusion images (https://www.nitrc.org/projects/panda/). Each individual’s DTI data set was registered to the same individual’s high-resolution structural image and then into the standard Montreal Neurological Institute (MNI) space using affine transformations. Fractional anisotropy (FA) images were created from the preprocessed DTI data of all subjects. All FA images were then non-linearly aligned to a common space. The mean FA image was used to represent the center of all tracts common to the group. Then, all subjects’ aligned FA data were projected onto the skeleton, and the resulting data were subjected to voxel wise cross-subject statistics. Whole brain tractography was then performed in the patient’s native space for each subject at each time point using a deterministic streamlined approach,30,31 in which fiber pathways were reconstructed by following the main diffusion tensor direction as indicated by the principal eigenvector, until an FA value of 0.20 or lower was reached, or until an angular turn of 45 degrees or more was made.30,31 The DTI data were used to construct the large-scale connectivity of the brain network and to assess network outcome measures using PANDA. MRS data were analyzed using MRS software integrated into the MR scanner. The experimentally measured spectra included N-acetyl aspartate (NAA), creatine (Cr), and choline (Cho). Metabolites were expressed in relative concentrations. The ratios of NAA/Cr, NAA/Cho, Cho/Cr, and Cho/NAA were automatically determined by this integrated software.
Statistical Analysis
Preliminary descriptive statistics and correlation analyses were conducted using SPSS for Windows (version 21; IBM SPSS Statistics, Armonk, NY, USA). The threshold for statistical significance was set at P < .05. Descriptive statistics are presented as mean, standard deviation (SD), and range. Cancer patients were rated as cognitively impaired “if two or more neurocognitive tests (AVLT, TMT, COWA and Digit Span test) had a Z-score at or below −1.5, and/or one test had a Z score at or below −2.0 of the healthy control group.”24 Transformation of Z scores was computed as subjects’ raw score minus the mean group score and divided by standard deviation. Correlations of neurocognitive outcomes with brain structural connectivity and neurochemical properties were made using Pearson correlation coefficients.
Results
Research Participant Characteristics
A total 18 potentially eligible women with gynecological cancer were approached, with 15 agreeing to take part. Three patients were refused, as they felt the MRI scans and neurocognition assessment would be too burdensome. There were 15 healthy control subjects who were matched in terms of age and menopausal status. The mean age of healthy controls was 49.6 years (range 29-59 years). All healthy controls were employed at the time of assessment. Detailed information on the characteristics of all research participants is shown in Table 1. In the patient group, more than half of all subjects (n = 8, 53.3%) had been diagnosed with cervical cancer. All patients had taken chemotherapy as an adjuvant cancer treatment (Table 1).
Table 1.
Demographic and Clinical Characteristics of Subjects.
| Variables | Cancer Patients (n = 15), n (%) | Healthy Controls (n = 15), n (%) |
|---|---|---|
| Age, years mean ± SD (range) | 49.33 ± 9.14 (28-60) | 49.60 ± 8.27 (29-59) |
| Highest education | ||
| Primary school or below | 12 (80.0) | 14 (93.3) |
| High school | 2 (13.3) | 1 (6.7) |
| University and above | 1 (6.7) | |
| Employment status | ||
| Employed | 2 (13.3) | 15 (100) |
| Unemployed | 13 (86.7) | |
| Marital status | ||
| Never married | 1 (13.3) | 1 (6.7) |
| Married | 14 (93.3) | 13 (86.7) |
| Divorced | 1 (6.7) | |
| Menopausal status | ||
| Premenopausal | 8 (53.3) | 8 (53.3) |
| Perimenopausal | 1 (6.7) | 1 (6.7) |
| Postmenopausal | 6 (40.0) | 6 (40.0) |
| Cancer type | ||
| Cervical cancer | 8 (53.3) | |
| Ovarian cancer | 1 (6.7) | |
| Uterine cancer | 6 (40.0) | |
| Disease stage | ||
| Early stage (stage I-IIa) | 9 (60.0) | |
| Middle stage (stage IIb-IIIa) | 3 (20.0) | |
| Advanced stage (stage IIIb) | 3 (20.0) | |
| Treatment type | ||
| Surgery + chemotherapy | 13 (86.7) | |
| Surgery + chemotherapy + radiation | 2 (13.3) | |
Neurocognitive Function of Cancer Patients Compared With Healthy Controls
All research participants in this study were right-handed. Four out of 15 patients could be categorized as cognitively impaired, with a CRCI rate of 26.67%. From Table 2, mean neurocognitive test scores in the patient group were lower than in the healthy control group, especially in the domain of working memory and verbal memory scores.
Table 2.
Mean Scores of Objective Cognitive Tests at Baseline.
| Variables | Cancer Patients (n = 15), Mean (SD) | Healthy Controls (n = 15), Mean (SD) |
|---|---|---|
| Attention and working memory | ||
| Digit span forward | 6.23 (2.73) | 7.46 (1.99) |
| Digit span backward | 2.26 (1.27) | 2.92 (2.20) |
| Verbal memory | ||
| AVLT immediate recall | 11.60 (4.76) | 13.33 (3.65) |
| AVLT delayed recall | 3.86 (2.38) | 4.46 (2.29) |
| AVLT recognition | 9.53 (2.61) | 10.73 (0.96) |
| Psychomotor speed | ||
| TMT-A | 53.13 (25.48) | 58.80 (24.86) |
| Executive function | ||
| TMT-B | 72.33 (36.07) | 75.13 (29.55) |
| Language | ||
| COWA | 31.06 (6.48) | 32.93 (8.89) |
Abbreviations: AVLT, Auditory Verbal Learning Test; COWA, Controlled Oral Word Association; TMT, Trail Making Test.
DTI Data and Correlations With Neurocognitive Test Performance
As shown in Figure 3, both groups had a small-worldness index greater than 1 across network densities. According to Humphries and Gurney,32 a small-worldness index greater than 1 across network densities demonstrates as small-world connectome organization. But the patient group had a lower small-worldness index compared with healthy controls (Figure 3). In terms of regional connectome properties, this study calculated the mean node degree of structural connectivity. The patient group also demonstrated lower mean node degree across 90 brain regions, based on the template from the AAL (Automated Anatomical Labeling) atlas, than did the healthy control group (Figure 4). For correlations of DTI parameters with cognitive test performance, FA values in the patient group had positive significant correlations with AVLT-immediate scores (r = 0.654, P = .018) among 15 patients, although other DTI parameters of mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) had no significant correlations with neurocognitive test performance.
Figure 3.
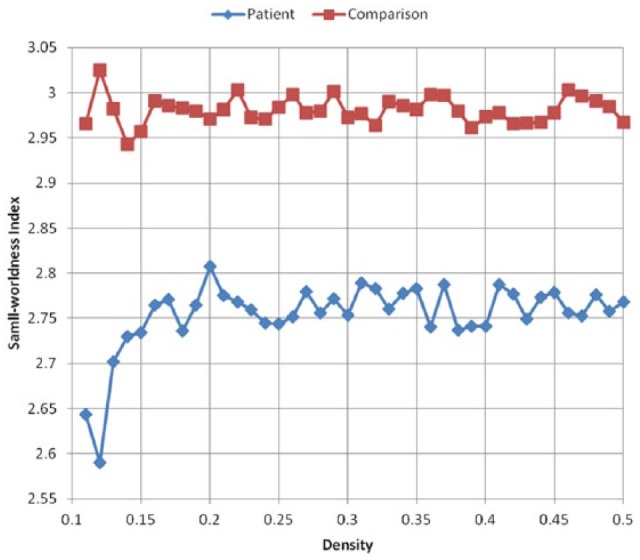
Global structural connectome properties (N = 30).
Figure 4.
Regional structural connectome properties (N = 30).
1H-MRS Data and Correlations With Neurocognitive Test Performance
From Table 2, memory scores in the patient group were significantly lower than in the healthy controls.33 While there were no significant differences in absolute concentrations of the main metabolites of NAA, Cho or Cr between groups, differences between the groups in metabolite ratios relative to NAA (NAA/Cr and NAA/Cho) were significantly lower for the patient group than for the healthy controls (Table 3). Only in the patient group (n = 15), there were significant positive correlations of NAA/Cr with total digit span test scores (r = 0.701, P = .005).
Table 3.
Comparison 1H-MRS of Parameters in the Left Hippocampus at Baseline.
| Intervention Group (n = 15) | Healthy Controls (n = 15) | |
|---|---|---|
| NAA/Cr | 1.42 (0.23) | 1.89 (0.12) |
| NAA/Cho | 1.28 (0.08) | 1.62 (0.19) |
| Cho/Cr | 0.96 (0.11) | 0.82 (0.07) |
| Cho/NAA | 0.71 (0.07) | 0.62 (0.24) |
Abbreviations: MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; Cr, creatine; Cho, choline.
Effects of Acupuncture on Neurocognitive Test Performance
During chemotherapy, 3 cancer patients received a total of 10 sessions of acupuncture interventions, respectively. Compared with age-matched cancer controls, patients with acupuncture interventions achieved a better neurocognitive test performance (Table 4). Differences between the groups in terms of DTI parameters are shown in Table 5. Within the left hippocampus, FA values decreased more in the cancer control group, and MD values increased more in the cancer control group (Table 5), indicating that acupuncture therapy may have positive effects in maintaining white matter integrity. As shown in Table 6, the changes in NAA/Cr and NAA/Cho ratios were found to have significantly decreased in the cancer control group, compared with the acupuncture intervention group.
Table 4.
Mean Scores of Objective Cognitive Tests at Postintervention.
| Variables | Intervention Group (n = 3), Mean (SD) | Cancer Controls (n = 3), Mean (SD) |
|---|---|---|
| Attention and working memory | ||
| Digit span forward | 6.76 (1.94) | 6.57 (2.87) |
| Digit span backward | 2.11 (1.41) | 1.85 (1.57) |
| Verbal memory | ||
| AVLT immediate recall | 16.65 (6.45) | 16.28 (3.65) |
| AVLT delayed recall | 5.86 (1.73) | 5.42 (3.64) |
| AVLT recognition | 10.53 (2.98) | 10.78 (1.96) |
| Psychomotor speed | ||
| TMT-A | 57.13 (27.48) | 53.80 (21.86) |
| Executive function | ||
| TMT-B | 75.33 (36.07) | 74.17 (29.55) |
| Language | ||
| COWA | 27.42 (6.89) | 26.76 (9.48) |
Abbreviations: AVLT, Auditory Verbal Learning Test; COWA, Controlled Oral Word Association; TMT, Trail Making Test.
Table 5.
Changes in DTI Parameters for White Matter in Left Hippocampus Between Pre- and Postintervention.
| Intervention Group (n = 3) | Cancer Controls (n = 3) | |||
|---|---|---|---|---|
| FA | 0.456 (0.012) | 0.454 (0.015) | 0.581 (0.036) | 0.572 (0.034) |
| MD (µm/s2) | 0.426 (0.040) | 0.433 (0.027) | 0.770 (0.018) | 0.785 (0.021) |
| AD (µm/s2) | 0.734 (0.023) | 0.751 (0.028) | 0.765 (0.034) | 0.745 (0.027) |
| RD (µm/s2) | 0.273 (0.049) | 0.274 (0.039) | 0.262 (0.034) | 0.274 (0.037) |
Abbreviations: DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity.
Table 6.
Changes of 1H-MRS of Parameters in the Left Hippocampus Between Pre- and Postintervention.
| Acupuncture Group (n = 3) | Cancer Controls (n = 3) | |||
|---|---|---|---|---|
| NAA/Cr | 1.39 (0.10) | 1.37 (0.10) | 1.41 (0.08) | 1.21 (0.01) |
| NAA/Cho | 1.35 (0.16) | 1.37 (0.17) | 1.45 (0.11) | 1.30 (0.07) |
| Cho/Cr | 0.98 (0.13) | 0.94 (0.05) | 1.02 (0.12) | 1.01 (0.17) |
| Cho/NAA | 0.75 (0.09) | 0.76 (0.15) | 0.69 (0.05) | 0.77 (0.04) |
Abbreviations: MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; Cr, creatine; Cho, choline.
Discussion
This pilot cohort study aimed to describe the prevalence of CRCI and explore the neurobiological mechanism of cognitive impairment in cancer patients on a microscopic level by using DTI and MRS. This study found that the prevalence of cognitive impairment in Chinese gynecological cancer patients at diagnosis was 26.67%, which was lower than the 40% reported in previous research.9 Based on the mean score of the neurocognitive tests, only working memory and immediate verbal memory scores in the patient group were statistically significantly lower than in the age-matched healthy controls. But when investigating the microstructural white matter in the brain, DTI data in this study indicated that premorbid cognitive functioning (before clinical manifestations became evident) already existed at cancer diagnosis, as the global and local connectome properties in the entire patient group were lower than in the healthy control group. “Group differences in nodal degree and global network efficiency of the brain can help identify specific brain regions that show altered integration within the network, which could help find specific neural circuits may be at high risk for loss of response plasticity.”34(p333)
Although one of the essential DTI parameters, FA, had a statistically significant association with immediate verbal memory score, this study did not find any significant correlation between global and local connectome properties, and neurocognitive test scores. Consistent with previous research, Bruno et al34 also found that breast cancer patients displayed alterations in global and regional network characteristics, but these network alterations had no significant correlation with cognitive performance. However, a cross-sectional study found that breast cancer survivors had reduced brain structural network efficiency, which was associated with a simulated neurodegeneration in these patients compared with healthy controls.35 Another cross-sectional study also indicated that poorer network organization was found to be associated with greater cognitive impairment.36 Furthermore, recent longitudinal research reported that decreased small-worldness and local efficiency was related to poorer overall cognitive performance across time in a group of male cancer patients.37 This study failed to find either small-worldness or nodal degree associated with neurocognitive test scores, which may be due to the small sample size, as supporting the optimal level of cognitive processes depends on an effective network organization and integration across brain regions.38
By using multimodal neuroimaging of MRS, this study investigated absolute and relative concentrations of NAA, Cr, and Cho in the left hippocampus. Although the absence of absolute concentrations of NAA, Cr, and Cho abnormalities in the patient group may be due to the mild degree of cognitive impairment in these patients before chemotherapy, the findings of the present study indicated a statistically significant reduction of NAA/Cr in the left hippocampus. As NAA is localized almost exclusively in neurons, the reduction in relative NAA in the left hippocampus suggests that axonal degeneration contributed to the observed diffusion abnormalities.13 In addition, this study found that the reduction of NAA/Cr was associated with lower mean digit span score (lower working memory functioning). Previous research also found that neurochemical properties were associated with cognitive deficits.13 Perhaps abnormalities in both the metabolic-level and network-level changes in the brain may appear before the alterations in clinical performance of a neurocognitive test.39 Thus, detecting alterations in structural connectivity networks and brain metabolic properties might provide a potential earlier biomarker of CRCI, which could be used for the relevant development of prevention strategies.
This is the first pilot study to investigate the effects of acupuncture in preventing and reducing cognitive impairment. Compared with the cancer control group, the results showed that acupuncture improved neurocognitive performance over the chemotherapy period. While patients in the acupuncture group reported a mild degree of cognitive impairment at baseline, after receiving 10 sessions of acupuncture treatment, patients in the intervention group had higher mean neurocognitive test scores than the cancer control group. As both groups were matched for age, menopause status, cancer stage, and dosage of chemotherapy regimen (all had a standard-dose regimen), better neurocognitive test performance in the intervention group may be due to the positive effects of acupuncture. Admittedly, this pilot cohort study had a small number of patients and the lack of any randomization, which may account for the possibility of residual confounding. Although patients in both groups after chemotherapy had impairment of white matter integrity (reduced FA values and increased MD values), changes in DTI parameters in the cancer control group were higher than in the acupuncture intervention group. Previous research has indicated that changes in FA and MD values could be due to demyelination.13 Preclinical research evidence specifically indicates that the possible mechanism of decreased white matter integrity may be attributed to incoherence of myelin basic protein fiber.40 Thus, findings of this pilot study suggest that acupuncture may mitigate cognitive impairment by reducing demyelination.
Additional positive effects of acupuncture on CRCI included a lower reduction in relative concentrations of NAA for patients in the acupuncture group. Previous research also indicated that the ratio of NAA/Cr was obtained by measuring the level of NAA and Cr to evaluate neuronal activity in the hippocampus.41 A review suggested that lower levels of NAA may reflect inefficient neuronal viability.42 Therefore, on a microstructural level, acupuncture preventing or reducing CRCI may be attributed to its reducing demyelination and enhancing neuronal viability of white matter in the hippocampus. Monitoring structural alterations of white matter connections and concentrations of NAA can be potential markers for acupuncture interventions for preventing or reducing CRCI in patients with gynecological cancer.
The strength of this study lies in the fact that this was a pilot cohort study exploring the effects of acupuncture on preventing cognitive impairment in cancer patients during chemotherapy. The study also examined the neurobiological mechanism of CRCI by multimodal MRI of structural brain connectivity and brain metabolite properties. The main study limitation was the small cohort size, although there were similar demographic characteristics between the intervention group and the control group. In addition, the intrinsic clinical differences between cancer patients (eg, types of cancer, disease stage) resulted in different chemotherapy regimens assigned to each patient. Therefore, future studies using a larger cohort size and including homogeneous cancer patients, preferably with identical chemotherapy regimens, should be conducted to replicate these study findings.
Conclusions
This pilot study indicates that acupuncture is a promising intervention in preventing CRCI in gynecological cancer patients undergoing chemotherapy. However, it requires evaluation in larger randomized controlled studies to definitively assess its benefit. By using a multimodality imaging approach, this pilot study also provides novel insights into the neurobiological basis of cognitive impairment on the human brain, induced by cancer and/or its treatment. Information from this study could potentially serve as a guide in future treatment and rehabilitation strategy development for this vulnerable population.
Acknowledgments
The authors thank all the subjects for participating in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Guangzhou Medical University Fund for Overseas Returners (No. 2013C57).
ORCID iD: Yingchun Zeng  https://orcid.org/0000-0001-9250-4086
https://orcid.org/0000-0001-9250-4086
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [DOI] [PubMed] [Google Scholar]
- 2. Lange M, Rigal O, Clarisse B, et al. Cognitive dysfunctions in elderly cancer patients: a new challenge for oncologists. Cancer Treat Rev. 2014;40:810-817. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 4. Treanor C, Donnelly M. Late effects of cancer and cancer treatment: a rapid review. J Community Support Oncol. 2014;12:137-148. [DOI] [PubMed] [Google Scholar]
- 5. Craig CD, Monk BJ, Farley JH, Chase DM. Cognitive impairment in gynecologic cancers: a systematic review of current approaches to diagnosis and treatment. Support Care Cancer. 2014;22:279-287. [DOI] [PubMed] [Google Scholar]
- 6. Faubion SS, MacLaughlin KL, Long ME, Pruthi S, Casey PM. Surveillance and care of the gynecologic cancer survivor. J Womens Health (Larchmt). 2015;24:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267-275. [DOI] [PubMed] [Google Scholar]
- 8. Hines S, Ramis MA, Pike S, Chang AM. The effectiveness of psychosocial interventions for cognitive dysfunction in cancer patients who have received chemotherapy: a systematic review. Worldviews Evid Based Nurs. 2014;11:187-193. [DOI] [PubMed] [Google Scholar]
- 9. Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joly F, Giffard B, Rigal O, et al, et al. Impact of cancer and its treatment on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force symposium and update since 2012. J Pain Symptom Manage. 2015;50:830-841. [DOI] [PubMed] [Google Scholar]
- 11. Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12:255-269. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich J, Prust M, Kaiser J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience. 2015;309:224-232. [DOI] [PubMed] [Google Scholar]
- 13. de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;30:3675-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson WL, Suls J. New approaches to understand cognitive changes associated with chemotherapy for non-central nervous system tumors. J Pain Symptom Manage. 2013;46:707-721. [DOI] [PubMed] [Google Scholar]
- 15. Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32:480-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kesler S, Hosseini SMH, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. King S, Green HJ. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol. 2015;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng YC, Luo TZ, Finnegan-John J, Cheng AS. Meta-analysis of randomized controlled trials of acupuncture for cancer-related fatigue. Integr Cancer Ther. 2014;13:193-200. [DOI] [PubMed] [Google Scholar]
- 19. Henneghan AM, Harrison T. Complementary and alternative medicine therapies as symptom management strategies for the late effects of breast cancer treatment. J Holist Nurs. 2015;33:84-97. [DOI] [PubMed] [Google Scholar]
- 20. Avisar A, River Y, Schiff E, Bar-Sela G, Steiner M, Ben-Arye E. Chemotherapy-related cognitive impairment: does integrating complementary medicine have something to add? Review of the literature. Breast Cancer Res Treat. 2012;136:1-7. [DOI] [PubMed] [Google Scholar]
- 21. Johnston MF, Yang C, Hui KK, Xiao B, Li XS, Rusiewicz A. Acupuncture for chemotherapy-associated cognitive dysfunction: a hypothesis-generating literature review to inform clinical advice. Integr Cancer Ther. 2007;6:36-41. [DOI] [PubMed] [Google Scholar]
- 22. Leung MCP, Yip KK, Ho YS, Siu FK, Li WC, Garner B. Mechanisms underlying the effect of acupuncture on cognitive improvement: a systematic review of animal studies. J Neuroimmune Pharmacol. 2014;9:492-507. [DOI] [PubMed] [Google Scholar]
- 23. Zhang ZJ, Wang XM, McAlonan GM. Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid Based Complement Alternat Med. 2012;2012:429412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703-708. [DOI] [PubMed] [Google Scholar]
- 25. Guo QH. Handbook of Neuropsychological Assessment. 2nd ed. Shanghai, China: Shanghai Science and Technology; 2016. [Google Scholar]
- 26. Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 27. Zeng YC, Cheng ASK, Liu XY, Chan CCH. Cognitive complaints and supportive care needs in Chinese cervical cancer survivors: a qualitative study. BMJ Open. 2017;7:e014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 29. Menning S, de Ruiter MB, Veltman DJ, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. Neuroimage Clin. 2015;7:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Irfanoglu MO, Walker L, Sarlls J, Marenco S, Pierpaoli C. Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. Neuroimage. 2012;61:275-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Humphries MD, Gurney K. Network “small-world-ness”: a quantitative method for determining canonical network equivalence. PLoS One. 2008;3:e0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richard GR, Titiz A, Tyler A, Holmes GL, Scott RC, Lenck-Santini PP. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus. 2013;23:1269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;36:2429-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kesler SR, Gugel M, Huston-Warren E, Watson C. Atypical structural connectome organization and cognitive impairment in young survivors of acute lymphoblastic leukemia. Brain Connect. 2016;6:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amidi A, Hosseini SMH, Leemans A, et al. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109:djx085. [DOI] [PubMed] [Google Scholar]
- 38. Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109-125. [DOI] [PubMed] [Google Scholar]
- 39. Mayeux R. Clinical practice. Early Alzheimer’s disease. N Engl J Med. 2010;362:2194-2201. [DOI] [PubMed] [Google Scholar]
- 40. Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One. 2016;11:e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang X, Wang C, Xia L, Zhu W, Zhao L, Zhu W. Volumetric MRI and 1H MRS study of hippocampus in unilateral MCAO patients: relationship between hippocampal secondary damage and cognitive disorder following stroke. Eur J Radiol. 2012;81:2788-2793. [DOI] [PubMed] [Google Scholar]
- 42. Yoon S, Lyoo IK, Renshaw PF. Application of magnetic resonance spectroscopic imaging to addiction research. In: MacKilop J. eds. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. Malden, MA: Wiley-Blackwell; 2013:707-750. [Google Scholar]