Abstract
We have cloned a full-length cDNA coding for human alcohol dehydrogenase (ADH; alcohol:NAD+ oxidoreductase, EC 1.1.1.1) from a human liver cDNA library constructed in phage lambda gt11. The library was screened by using a rabbit antibody against human ADH as a first probe, by the modified method of Young and Davis [Young, R. A. & Davis, R. W. (1983) Proc. Natl. Acad. Sci. USA 80, 1194-1198]. Mixed 14-mer synthetic oligonucleotides encoding Asp-Asp-His-Val-Val and Gln-Cys-Gly-Lys-Cys were used as a second probe. These amino acid sequences are considered to be common in all three subunits (alpha, beta, and gamma) controlled by the ADH1, ADH2, and ADH3 loci. Ten lambda gt11 recombinants of 35 positive plaques obtained by antibody screening contained inserted cDNAs of 1.5-2.4 kilobase pairs and were found to exhibit positive signals by hybridization with synthetic probes. One of them, with an inserted cDNA of 1631 base pairs, contained a sequence that encodes 374 amino acid residues of the human beta 1 subunit, a chain initiation codon, a chain termination codon, and additional 3' and 5' untranslated regions. A complete amino acid sequence of the human beta 1 subunit was deduced from the cDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Duester G., Hatfield G. W., Bühler R., Hempel J., Jörnvall H., Smith M. Molecular cloning and characterization of a cDNA for the beta subunit of human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4055–4059. doi: 10.1073/pnas.81.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Goedde H. W., Harada S., Agarwal D. P. Racial differences in alcohol sensitivity: a new hypothesis. Hum Genet. 1979 Oct 2;51(3):331–334. doi: 10.1007/BF00283404. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Hempel J., Vallee B. L., Bosron W. F., Li T. K. Human liver alcohol dehydrogenase: amino acid substitution in the beta 2 beta 2 Oriental isozyme explains functional properties, establishes an active site structure, and parallels mutational exchanges in the yeast enzyme. Proc Natl Acad Sci U S A. 1984 May;81(10):3024–3028. doi: 10.1073/pnas.81.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Sep;16(1):25–40. doi: 10.1111/j.1432-1033.1970.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky R. D., Troy F. A. Possible DNA-RNA tumor virus interaction in human lymphomas: expression of retroviral proteins in Ramos lymphoma lines is enhanced after conversion with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jan;81(1):33–37. doi: 10.1073/pnas.81.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Alcohol dehydrogenase isozymes in adult human stomach and liver: evidence for activity of the ADH 3 locus. Ann Hum Genet. 1972 Mar;35(3):243–253. doi: 10.1111/j.1469-1809.1957.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Studies on the properties of the human alcohol dehydrogenase isozymes determined by the different loci ADH1, ADH2, ADH3. Ann Hum Genet. 1973 Jul;37(1):49–67. doi: 10.1111/j.1469-1809.1973.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Studies on the subunit structure and molecular size of the human alcohol dehydrogenase isozymes determined by the different loci, ADH1, ADH2, and ADH3. Ann Hum Genet. 1973 Apr;36(4):401–414. doi: 10.1111/j.1469-1809.1973.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Chen S. H., Fukui M. Liver alcohol dehydrogenase in Japanese: high population frequency of atypical form and its possible role in alcohol sensitivity. Am J Hum Genet. 1975 Nov;27(6):789–796. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy F. A., Fenyö E. M., Klein G. Moloney leukemia virus-induced cell surface antigen: detection and characterization in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5270–5274. doi: 10.1073/pnas.74.12.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Huang I. Y., Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A. 1984 Jan;81(1):258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Impraim C. C., Huang I. Y. Enzymatic and structural differences between usual and atypical human liver alcohol dehydrogenases. J Biol Chem. 1981 Dec 10;256(23):12430–12436. [PubMed] [Google Scholar]
- Yoshida A., Wang G., Davé V. Determination of genotypes of human aldehyde dehydrogenase ALDH2 locus. Am J Hum Genet. 1983 Nov;35(6):1107–1116. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
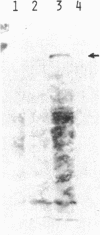
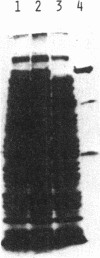
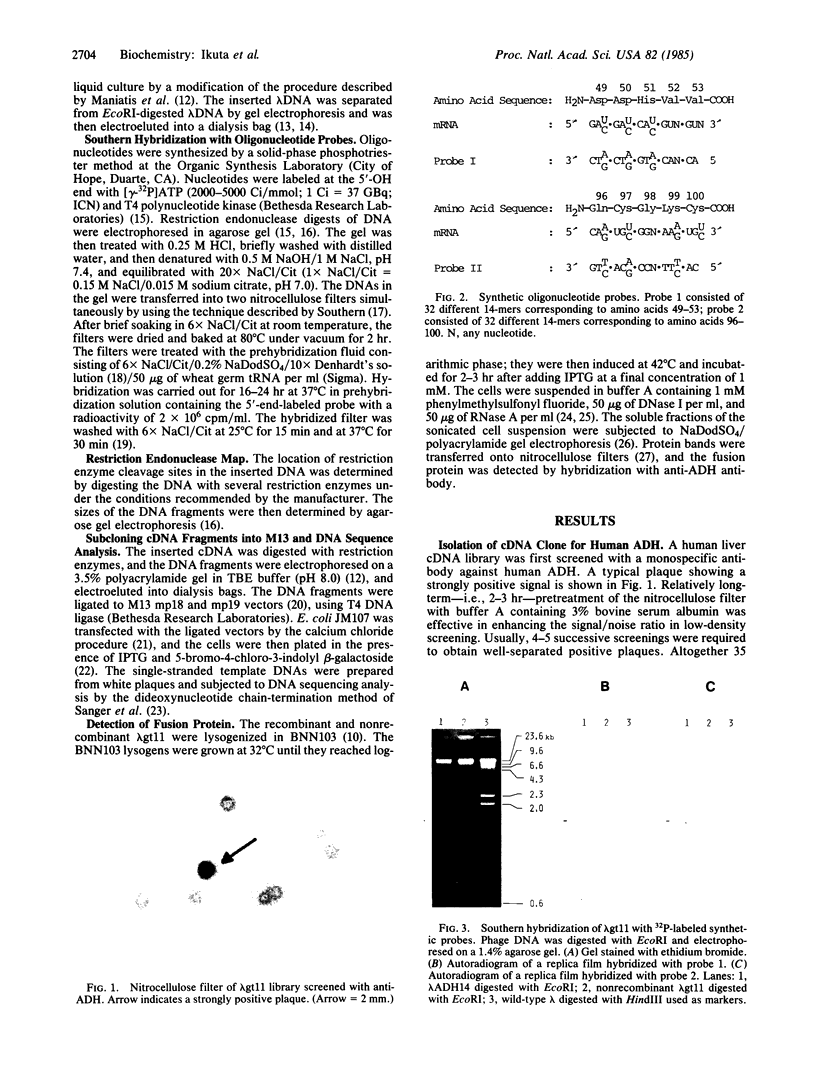
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]