Abstract
Objective:
To determine the safety and diagnostic accuracy of renal tumour biopsies in a defined population of small renal masses (SRMs) only <4 cm using 3 × 2 table, intention to diagnose approach. 3 × 2 table approach examines indeterminate results as a separate category rather than pushing these through traditional 2 × 2 table (four-cell matrix) approach.
Methods:
A highly sensitive search was performed in the Cochrane Library, Database of Abstracts of Reviews of Effects; MEDLINE and MEDLINE in Process, EMBASE and conference proceedings (1966–2016) for the acquisition of data on the diagnostic accuracy and complications of RTB in patients with SRM <4 cm. Methodological quality and risk of bias was assessed using QUADAS-2. Test characteristics were calculated using conventional 2 × 2 contingency table analysis excluding non-diagnostic biopsies, and an intention-to-diagnose approach with a 3 × 2 table for pooled estimates of the sensitivity and specificity.
Results:
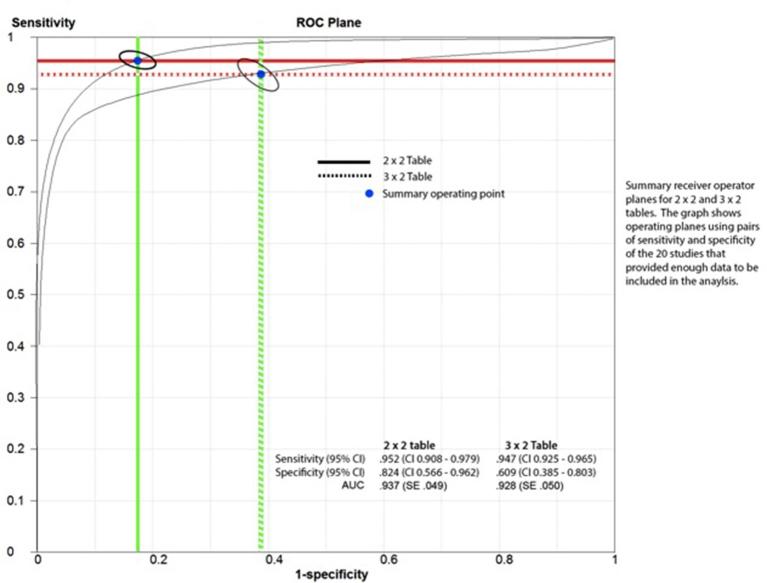
A total of 20 studies were included with a total sample size of 974. The pooled estimates for sensitivity and specificity of RTB based upon univariate analysis using 2 × 2 table observed sensitivity 0.952 [confidence interval (CI) 0.908–0.979] and specificity 0.824 (CI 0.566–0.962). Using the 3 × 2 table and intention-to-diagnose principle, sensitivity 0.947 (CI 0.925–0.965) and specificity 0.609 (CI 0.385–0.803) decreased.
Conclusion:
RTB in SRMs (<4 cm) is associated with a high diagnostic sensitivity but poor specificity when non-diagnostic results are included by a 3 × 2 table for analysis (intention to diagnose approach). Risk of non-diagnostic results and poor quality of research need addressing through future studies, preferably by a well-designed prospective study appropriately powered for diagnostic accuracy using valid reference standards.
Advances in knowledge:
A comprehensive synthesis of literature on image-guided biopsies in SRMs using a different methodology and study design.
Introduction
Surgical series have demonstrated that 20–30% of small renal masses (SRMs) are benign upon final pathology assessment after excision,1 and as a consequence it is ever pressing to obtain histological evidence to avoid over and unnecessary treatment. The role of renal tumour biopsy (RTB) has been acknowledged recently, but there many areas which remain poorly understood including its role in active surveillance of SRM <4 cm as a pre-defined patient group, and especially the handling of indeterminate results in evaluating the diagnostic accuracy of this technique.
Recent systematic review and meta-analyses2,3 aimed to assess the diagnostic performance and safety of renal biopsy are both fraught with many methodological limitations. The studies included a large number of original papers which biopsied renal masses >4 cm with the largest biopsied mass of 32 cm.4–7 In a sensitivity analysis limited to studies reporting on SRMs, (<4 cm), Marconi et al2 failed to mention whether non-diagnostic results were treated as negative or were excluded from analysis in the included studies of their reported systematic review. Therefore, the generalizability of these findings to patients with SRMs less than 4 cm is limited and clinical challenge in decision-making for indeterminate results remains a core issue in contemporary urological practice. It could be argued, that a larger biopsy target will improve sensitivity and accuracy outcomes because evidence suggests that tumour size plays a pivotal role, and small tumours are pushed by the biopsy needle instead of penetration to obtain adequate tissue.8,9 With this in mind, a contrary view can put forward that that larger tumours are often necrotic and this could diminish accuracy, however no evidence has been provided to support this assertion in the previous reviews in this area.4–7
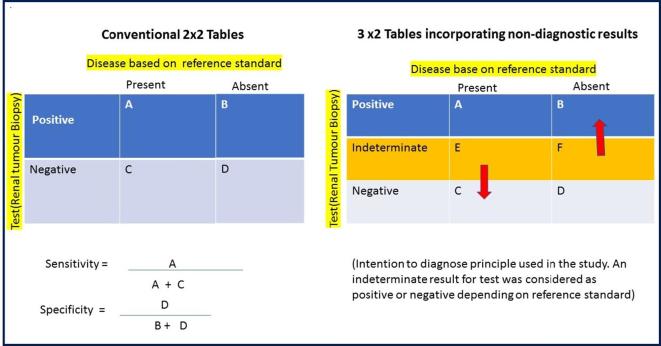
Simel et al10 described a 3 × 2 contingency table approach to deal with non-diagnostic results (non-positive and non-negative)—a common scenario in RTB during clinical decision-making. The traditional approach has been to report outcomes of RTB using 2 × 2 tables (four-cell matrix) and making a number of assumptions such as treating non-diagnostic results as negative, excluding them from the analysis or treating these as positive. These approaches have the potential of leading to spurious diagnostic accuracy outcome for diagnostic tests, both sensitivity and specificity.11 Figure 1 summarises the intention to diagnose principle used in this study. We considered, if the reference standard (histopathology of excised mass) proved to be positive for cancer, then an indeterminate RTB result was false-negative (FN) and, in contrast, if the reference standard showed no cancer (benign), then the indeterminate result was considered false-positive (FP). In other words if an indeterminate test missed a true-positive (TP), it was considered as FP and if it missed a true-negative (TN), it was considered as FP. This is similar to the approach used by Schuetz et al11 in a meta-analysis of coronary CT angiography.
Figure 1.
Explanation of 3 × 2 table analysis for the non-diagnostic results.
The primary objective of the study was to determine the diagnostic accuracy of percutaneous image-guided renal biopsy for detecting renal malignancy in individuals with only small (<4 cm), solid and enhancing renal masses employing the 3 × 2 table approach to minimize overestimation of diagnostic accuracy as described in the previous studies. The secondary objectives were to: (1) determine the rate of complications of the biopsy procedure such as post-procedural bleeding, infection (local or systemic), arteriovenous fistula formation, renal loss or seeding, and (2) establish the accuracy of the biopsy procedure to determine cancer grade and (3) establish the accuracy of biopsy procedure to determine pathological type of renal cell carcinoma (e.g. papillary, clear cell carcinoma).
Methods and Materials
Types of studies
All observational studies reporting on image-guided biopsy in SRMs (<4 cm) were included. Studies with sufficient data to produce 2 × 2 and 3 × 2 contingency tables were included in the meta-analysis. Studies were excluded reporting on ex vivo kidney biopsies, non-image guided biopsies of renal masses such as those with the endoscopic (or laparoscopic) approach and those conducted on animals.
Participants
Safety and diagnostic accuracy of image guided biopsies was assessed in patients with SRMs in adults with small (<4 cm) solid renal mass and signs of contrast enhancement (CT, MRI). Studies were excluded with participants with known metastatic disease, either from renal cell carcinoma or other primary (e.g. breast cancer) cancers, and lesions >4 cm.
Index tests
Image-guided biopsy (obtaining a tissue sample using a needle under imaging guidance) in a renal mass which included renal core biopsies. Studies with indeterminate biopsy were also evaluated for patient outcomes. Inconclusive results were handled as a separate category as uninterpretable, intermediate and indeterminate. We also analysed these results as test–test strategy underpinned by clinical practice of repeating the index test in cases of inconclusive results.
Target conditions
Pathology-confirmed renal cell carcinoma.
Reference standards
We regarded histopathology of resection specimen (nephrectomy or partial nephrectomy) as the reference standard. In those studies where histopathology of the resected tissue was not available, we utilized long-term follow-up information (3 years) as an indirect assessment of the presence of malignant renal mass. The development of metastatic disease that is clinically determined to be of renal origin or the delayed surgical removal of the mass with confirmed malignancy was interpreted as evidence of a FN biopsy; the remainder were treated as TN. We excluded studies with no histopathological confirmation through subsequent resection of target condition or those with shorter follow up of less than 3 years. Growth in size alone was not used as a reference standard as benign lesions are known to exhibit growth upon serial imaging.
Search methods for identification of studies
Electronic searches
We performed an extensive electronic search to identify reports of relevant published and ongoing studies as well as grey literature, and recent meeting abstracts. A highly sensitive search strategy was developed using both appropriate subject headings and text word terms that reflect the clinical condition, interventional procedure (renal mass biopsy and subsequent management) and study designs that are within the scope of this project. Our search strategy is provided in Supplemental Table 1. This strategy was tested against a list of references to verify that these relevant records were found. The following databases were consulted: the Cochrane Library (Wiley) including the Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE); MEDLINE and MEDLINE in Process (Ovid SP) (from 1948 onwards); EMBASE (Ovid) including conference proceedings (from 1947 onwards); BIOSIS Citation Index (from 1985 onwards); and Web of Science including the ISI Science Citation Index and Index to Conference proceedings (http://ipscience. thomsonreuters.com) (from 1900 onwards). We applied no methodological filter to minimize any risk of missing relevant studies.12
Searching other resources
We searched for ongoing studies at ClinicalTrials.gov. We also searched for conference abstracts via the conference proceedings sections in the Web of Science and EMBASE searches. The research team screened the diagnostic database Medion as well as the Aggressive Research Intelligence Facility databases (http://www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx). An internet search included the websites of the American Urological Association (http://www.auanet.org/) and European Association of Urology (http://uroweb.org/) from the year 2010 onwards as well as manufacturers of biopsy equipment.
Data collection and analysis
Selection of studies
All observational studies reporting on image -guided biopsy in SRMs and with data sufficient to populate 2 × 2 and 3 × 2 tables for diagnostic accuracy assessment were included. Two review authors (CP, JG) screened titles and abstracts independently and in duplicate. All disagreements were resolved by discussion or by involving a third review author (GN) as an arbiter. A pre-defined electronic spreadsheet was used to assess and document studies for inclusion and exclusion according to the aforementioned selection criteria (criteria for considering studies for this review).
Data extraction and management
Four review authors (JG, CP, CSB, AA) independently performed data extraction of full-text papers using a pre-defined electronic spreadsheet. A fifth review author (GN) independently verified all the extracted data. Any discrepancy was resolved by discussions. Where necessary, we contacted study authors to obtain raw data. We used Cochrane statistical software13 for further analysis. The reviewers abstracted information from all the included studies. The extracted information included: (a) the distribution of diagnoses in groups of patients with malignant and benign diseases; (b) the size of the SRMs; (c) the location of the lesions (upper, mid- or lower polar); peripheral or central in relation to renal parenchyma (d) the type of needle; (e) the type of radiological guidance; (f) whether a uropathologist was consulted during or prior to the procedure; (g) the reference standard utilised by the investigators (h) the type and number of complications (pneumothorax and bleeding) associated with the biopsy; and (i) the final results related to the accuracy of the test.
Assessment of methodological quality
Study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)−2 quality checklist.14–16 Four review authors (JG, CP, CSB, AA) independently scored each item as “yes”, “no” or “unclear”. All quality appraisal and any discrepancies were resolved by discussion with the fifth author (GN). Briefly, the included studies were assessed in four main key domains: (1) patient selection; (2) index test; (3) reference standard; (4) flow and timing. We evaluated study validity by systematically taking into account each of the potential sources of biases: work-up bias, by excluding patients from the analysis because they were not submitted to the reference standard procedure; review bias which is introduced when the test result was not verified by the reference standard procedure; and the test review bias which is introduced when the observers are aware of either the clinical condition of the patient or the final diagnosis.
Statistical analysis and data synthesis
The index test used in this review was to provide a binary outcome (presence of or absence of malignant condition). A univariate random-effects model was employed to obtain summary estimates of the sensitivity and specificity of the test.17,18 Review Manager software19 was for primary analyses and Meta-DiSc (http://www.hrc.es/investigacion/metadisc_en.htm), a publicly available software program for the diagnostic accuracy of tests and for secondary analyses.20 We calculated summary diagnostic performance values including 95% confidence intervals (CIs) from standard data of a 2 × 2 table (after excluding indeterminate results) or the 3 × 2 table, including indeterminate results either in the “FN” or the “FP” cell of a 2 × 2 table according to the results of the reference standard (intention-to-diagnose principle). Where possible, the quality items relating to spectrum of patients, technical problems in the conduction of index testing using different imaging modalities leading to verification and detection biases, and other domains in the QUADAS-2 tool were considered as potential covariates for sensitivity analysis.12,14,15
Assessment of reporting bias
The funnel plot was applied to determine the possibility of reporting bias and small study effects using plots of log diagnostic odds ratio vs 1/ESS1/2, where ESS is “effective sample size” defined as (4 n + n2)/(n1 + n2); moreover a test for asymmetry was assessed using regression and rank correlation tests.21 However, testing for reporting bias and small study effects may not be especially useful in the context of studies of diagnostic tests.22
Results
Results of the search
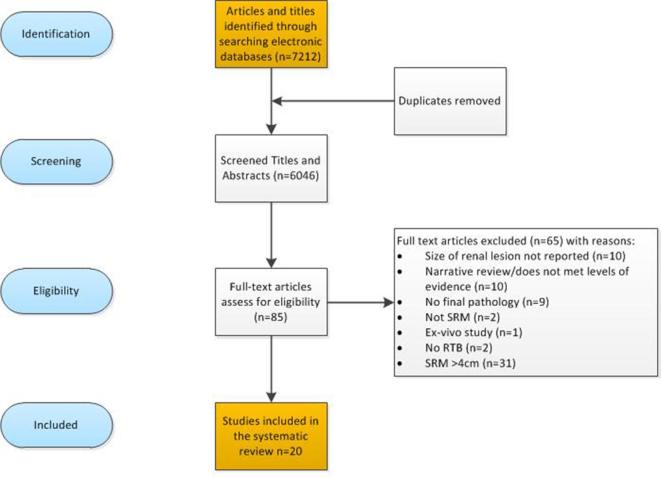
A total of 7212 titles and abstracts were identified by the literature search. Of the 85 full-text publications, 65 were excluded for the following reasons: renal lesions >4 cm;23–31 no final histopathology as reference standard;9,32–40 no description of tumour size,41,42 RTB not performed,43,44 not SRM’s, and45–54 descriptive narrative papers and one ex-vivo study55 (Figure 1 for PRISMA). This list is available from the authors on request. The remaining 20 publications were reviewed in full: two prospective longitudinal studies and eighteen retrospective studies (Table 1). The included studies were carried out in a number of international centres, mainly located in European countries or in the USA (16/20; 80%). The data collection ranged between 1982 and 2013, mainly retrospective in design (19/20; 90%. The sample sizes ranged from 5 to 529, with a total sample size of 974 of participants that had final pathology available as a reference standard.
Table 1.
Characteristics of included studies
| Author and year | Study design and level of evidence | Country and time period | Characteristics (n, gender, age, co-morbidities, performance status, BMI) | Tumour size (mean, SD, min and max) | Needle size | Guidance ultrasound scan, CT or MRI | Number of passes | Indeterminate biopsy n (%) | Complications |
| Abe and Saitoh (1992) | Retrospective C1 |
Japan (June 1982–May 1990) | n36. Gender: 23 males, 13 females; Age 39 to 78 years. No further clinical/demographic data. Duration of follow-up not reported. |
n16 <4 cm, n20 >4 cm. Reference standard: n16 |
14G Tru-Cut biopsy needle, 15G Sure-Cut biopsy needle and 18G biopsy needle used (depending on patients) | Not clearly reported, ultrasound scan imaging mentioned. | Not reported. | n3 indeterminate (1 angiomyolipomasarcoma, 1 metastatic disease primary unknown, and 1 no information of outcome or follow up of patient) |
No complications. Seeding suspected in 1 patients for recurrence reported following 2 years and 6 months post RTB |
| Halverson et al (2013) | Retrospective C1 |
USA (1999–2011) | n151. Age 59 (SD 14, range 22 to 84) years. No further clinical/demographic data provided. Duration between RTB and surgery 48 days (IQR 29–68) |
Mean mass 2.8 (SD 0.8, 1.0 to 4 cm) Reference standard: n133 |
17-gauge introducer and an 18 gauge spring-loaded gun | CT or ultrasoundscan | ≤2 cores | n14 indeterminate (outcomes of patients not reported) |
Major complications < 1% |
| Wang et al (2009) | Retrospective C1 |
USA (1999–2006) | n110. Age 60.4 (SD 15.4, 28 to 91) years; n68 male, n38 female. Ethnicity: White n92, Africa-American n6, Asian n3, other n5. No further clinical/demographic data reported. 106 RTB <4 cm. Median f/up 1.1 years (0 to 4.7 years) |
Mean mass 2.7 (0.5 to 4) cm Reference standard: n36 |
17-gauge introducer and an 18 gauge spring-loaded gun | CT n66 (60%) /ultrasoundscan n44 (40%) | ≤2 cores. Median cores 4. | n10 indeterminate (n2 observed for 1 and 2 years with no change in mass size, n2 died due to unrelated causes, n1 chemo for TCC. n2 underwent surgical excision revealing chromophobe RCC and papillary RCC. n1 cryotherapy, n1 RFA, n1 surveillance benign angiomyolipoma) |
n1 hypotensive during procedure and needed IV fluid resuscitation. n2 haematoma n5 flank pain or wound infection |
| Richard et al (2015) | Retrospective C1 |
Canada (January 2001–December 2013) | n529. Age 64 years. Sex: male n319 (60.3%), female n210 (39.7%); BMI: 27.3 (24.3–31.1) Duration of follow up not reported. |
2.5 (1.8–3.2) cm Reference standard: n162 |
17-gauge coaxial sheath and an 18-gauge core needle. | ultrasoundscan n388 (73.4%)/CT n115 (21.7%), unknown n26 (4.9%) | 1–2 n100 (18.9%), 3–4 n211 (39.9%),≥5 n203 (38.4%), unknown n15 (2.8%) | n4 indeterminate (all underwent surgery and all demonstrated malignancy) |
n42 (8.5%). 75% perirenal haematomas, all classified as Clavien-Dindo Grade 1. Follow-up of 28 months not seeding reported. |
| Kroeze et al (2012) | Retrospective C1 |
Netherlands (August 2009–September 2010) | n13. Median age 74 (37.9 to 80.4) years. n9 solid masses, n2 bosniak III and n2 Bosnial IV. No further clinical demographic data reported. f/up 16 (6.4–19.8) months |
Median tumour size 2.6 (range 1.0 to 14) cm. n9 <4 cm (of which 5 SRM, 4 cystic), n4 >4 cm Reference standard: n9 |
17-gauge guiding | Real-time 3D fluoroscopy— CT | 2 (1–4) | n3 indeterminate (all confirmed malignancy) |
No complication during intervention. (Within 30 days, n1 experienced Grade 2 Femoropopliteal bypass occlusion due to stop of anticoagulants before biopsy) |
| Park et al (2013) | Retrospective C1 |
Korea (June 2004–May 2011) | n58. Age 56.8 (24 to 79) years. Male n33, female n25. BMI 17.7–31.1 (23.5, SD 3.1). No further demographic data reported. Duration of follow up not reported. |
1.2–3.9 (2.4, SD 0.7) cm Reference standard: n14 |
18-gauge 15 cm automatic core biopsy system | Ultrasoundscan | mean 3.5, median 4, min 1 max 6 | n11 indeterminate (5 AS, 2 surgery [ confirmed malignancy], 2 RFA, lost to follow-up 1, 1 re-biopsy, [had surgery confirmed malignancy]) |
n12 minor (9 perirenal haematoma, n3 pain). |
| Li et al (2012) | Retrospective C1 |
France (Jan 2004–Dec 2009). | n90. Sex: male n45, female 35. Age: mean 64.8 (27–88) Duration of follow up not reported. |
FNA (n32, size 2.80 ± 0.18 cm), CB (n30, size 2.85 ± 0.19 cm). FNA + CB (n28, size 2.83 ± 0.16 cm) Reference standard: n59 |
Standard 18-gauge needle attached to 20 ml syringe. 18G | States usually CT guided, does not specify variation in imaging modality. | Not reported. | n2 indeterminate (underwent surgery and confirmed malignancy) |
Not reported. |
| Leveridge et al (2011) | Retrospective C1 |
Canada (January 2000–December 2009) | n294. Age 25.7–89.5, median 64. Duration of follow up 25 months. |
Median tumour size 2.5 cm (range: 0.6–4.0 cm). Reference standard: n77 |
17-gauge guiding cannula and an 18-gauge core needle. Core biopsies used in all cases. | US guided: 184 cases. CT guided: 60 cases. Both were used in 91 cases. | “Multiple” passes. | n58 indeterminate (32 AS, 9 re-biopsy, 17 RFA, 5 surgery,[confirmed malignancy] 1 lost to follow-up) |
29 patients experienced complication, 22—small or moderate haematoma post-procedure or moderate bleeding through the coaxial sheath. 2 patients—small, asymptomatic pneumothoraces, 1 patient—post-biopsy syncope, 3 patients—gross haematuria (1 required admission for urinary retention due to clots). Complication data unavailable in 67 cases |
| Shah et al (2005) | Retrospective C1 |
USA (1999–2005) | n110. Further information not available. Duration of follow up not reported. |
2.9 cm mean Reference standard: n16 |
18-Gauge biopsy needle gun | Not reported | 4.5 cores (range 1–10 cores) | 14 inadequate biopsies (outcomes not reported following advised re-biopsy) |
Not reported |
| Salem et al (2012) | Retrospective C1 |
USA (10 year study period—dates not stated) | 145 patients (99 Male, 46 Female). Mean age was 67.2 ± 11.6 years. Duration of follow up not reported. |
Mean mass size 2.4 ± 2.1 cm) Reference standard: n14 |
16–20-gauge cutting Trucut or Temno needle | CT guided in all lesions | 1–3 passes | 19 (9 had repeat biopsy -->3 clear cell, 2 papillary, 1 unclassified, 5 RFA, and 5 AS). | Minor complications in 3 patients (2.1%)—2 developed sub capsular haematoma and 1 developed flank ecchymosis. No major complications |
| Millet et al (2012) | Retrospective C1 |
France, 2006–2011 | n187 (n60 treated by surgery, 30 male and 30 females, median age 60 years, range 20 to 85 years) Duration of follow up not reported. |
Median size 3 cm (range 0.9 to 4). Reference standard: n73 |
17 gauge | 8-multidetector CT scanner and CT fluoroscopic guidance | 2–5 cores | Not reported | No complications |
| Walton et al (2012) | Retrospective C1 |
UK, 1999–2009 | 71 (54 males, 17 females), age ranged 61–76. Duration of follow up not reported. |
≤4=25 patients (35.2%)>4=25 patients (35.2%) Reference standard: n36 |
18 gauge | Ultrasound in 69 (97.2%) patients and CT scan in 2 (2.8) patients | 1–2 cores | n6 indeterminate (outcomes not reported) |
Haematuria n = 1 (1.4%). Transfusion requirement n = 1 (1.4%) |
| Eshed et al (2004) | Retrospective C1 |
Israel, 1996–2001 | 23 patients (age range 36–89 years, mean 61; 15 males and eight females) Duration of follow up not reported. |
>3 cm = 13≤3 cm=6 Reference standard: n18 |
18-gauge | CT Scan | Not reported | n1 non-diagnostic in obese patient | 1 suspected bowel perforation |
| Granon et al (2014) | Retrospective C1 |
France, 2010–2013 | 26 patients (15 males and 11 females) with a mean age of 68 years (range 23–89 years). Duration of follow up median 18 months. |
Mean size of tumour was 3.6 cm (range 0.6–9 cm). (There were 17 masses measured <4 cm and 9 masses measured >4 cm) Reference standard: n26 |
16 gauge | MRI | 2 cores | Not reported | No complication |
| Hu et al (2015) | Retrospective C1 |
USA, 2008–2011 | 269 patients (74 females and 195 males), age ranged from 18 to 92 years with a median age of 66 years. Duration of follow up not reported. |
Tumour size ranged between 0.5 to 24 cm with an average of 3.4 cm and a median of 2.6 cm 178 < 4 cm Reference standard: n55 |
Not reported | Not reported | Not reported | n3 indeterminate (follow-up outcomes not fully reported) |
No complication |
| Menhadji (2013) | Prospective B3 |
USA, May–Dec 2012 | n7, 5M and 2F, age 54–79, (mean 68.2) ASA - III (5), II (2), indication cancer (6), renal disease (1) BMI—not reported Duration of follow up not reported. |
Right (2), Left (4). Mean size 2.55 (2.0–2.8 cm) Reference standard: n1 |
18 gauge, 1.5-inch biopsy needle gun, the device was 13.8 cm | Ultrasoundscan | Number of passes not reported. Mean biopsy cores 4.1 (3–6) | n1 indeterminate (outcome RFA) |
No complications |
| Neuzillet (2004) | Retrospective C1 |
France, June 1995–March 2003 | n88, age 21–88 (mean 61.32, median 64) Duration of follow-up not reported. |
2.8 cm (0.2–4 cm) Reference standard: n61 |
18 gauge allowing 1.7 × 0.1 cm core | Helical CT under LA, prone position | 2 cores | n3 indeterminate (2 radical nephrectomy RCC, 1 lost to follow-up) |
No complications and no tumour seeding reported. |
| Chyhrai (2010) | Prospective B3 |
Germany, July 2004–February 2006 Mean follow-up 2 years |
n25 Indication = non-cystic homogenous <4 cm SRM, old and multiple morbid patient. Mean age 63 ± 7.7 Mean follow up 2 years |
2.5 cm (1.5 to 4 cm) Reference standard: n18 |
18 gauge, 1.7 × 0.1 cm specimen | Ultrasoundscan, repeat ultrasoundscan 2 h and next day post-RTB | 3 cores | n2 indeterminate (1 surgery confirmed RCC, and 1 died before pre-operatively of myocardial infarction) |
Bleeding 1 |
| Volpe (2008) | Retrospective C1 |
Canada, Jan 2000–May 2007 | 91 patients. 100 bx, age 60 years (25-89) Duration of follow up not reported. |
<4 cm Reference standard: n21 |
18 for bx, FNA = 22 gauge | 44 ultrasoundscan + CT, 45 ultrasoundscan, 11 CT | 2 cores | n8, (5 AS, 2 RFA, 1 partial nephrectomy—benign) | 1 syncope. 1 flank pain |
| Menogue (2012) | Retrospective C1 |
Australia, 1998–2009 | 250 patients, mean age 64, (range 22–88) years Duration of follow up not reported. |
2.5 (0.9–4 cm), 268 bx Reference standard: n129 |
18 G | Ultrasoundscan or CT | mean 2.7 cores (1-10) | n9 indeterminate (underwent surgery 8 malignant and 1 benign) |
1 blood transfusion and 1 haematoma |
AS, active surveillance; BMI, body mass index; FNA, fine needle aspiration; IQR, interquartile range; LA, local anesthetic; RCC, renal cell carcinoma; RFA, radiofrequency ablation; RTB, renal tumour biopsy; SRM, small renal mass; TCC, transitional cell cCarcinoma.
Operating characteristic of renal tumour biopsy in SRMs
Across all the included studies (Table 1) data was tabulated into TP, TN, FN, and FP, At the individual patient level, indeterminate biopsy results were tabulated as positive and negative (Table 2). Due to the small sample sizes of the available data as a reference standard it was not possible to conduct individual analysis of each of the primary studies.
Table 2.
Analysed studies with recalculated 3 × 2 tables including indeterminate results
| 2 × 2 table | Indeterminate at patient level | ||||||
| Author and year | Handling of indeterminate biopsies at patient level | True-positive | False-positive | False-negative | True-negative | Indeterminate positive | Indeterminate negative |
| Abe and Saitoh (1992) | 3 indeterminate patients excluded | 8 | 0 | 1 | 7 | – | – |
| Halverson et al (2013) | 14 indeterminate patients excluded | 122 | 3 | 2 | 6 | – | – |
| Wang et al (2009) | 10 indeterminate patients, of which 2 patients considered negative | 34 | 0 | 0 | 0 | 2 | 0 |
| Richard et al (2015) | 4 indeterminate patients considered negative | 155 | 3 | 0 | 0 | 4 | 0 |
| Kroeze et al (2012) | 3 indeterminate patients considered negative | 6 | 0 | 0 | 0 | 3 | 0 |
| Park et al (2013) | 11 indeterminate patients, of which 3 considered negative | 11 | 0 | 0 | 0 | 3 | 0 |
| Li et al (2012) | 2 indeterminate patients considered negative | 57 | 0 | 0 | 0 | 2 | 0 |
| Leveridge et al (2011) | 58 indeterminate patients, of which 5 considered negative | 70 | 2 | 0 | 0 | 5 | 0 |
| Shah et al (2005) | 14 indeterminate patients excluded | 15 | 0 | 1 | 0 | – | – |
| Salem et al (2012) | 19 indeterminate patients excluded | 14 | 0 | 0 | 0 | – | – |
| Millet et al (2012) | Not reported | 73 | 0 | 0 | 0 | – | – |
| Walton et al (2012) | 6 indeterminate patients excluded | 30 | 0 | 5 | 1 | – | – |
| Eshed et al (2004) | 1 indeterminate patient excluded | 14 | 0 | 4 | 0 | – | – |
| Granon et al (2014) | Not reported | 25 | 0 | 1 | 0 | – | – |
| Hu et al (2015) | 3 indeterminate excluded | 55 | 0 | 0 | 0 | – | – |
| Menhadji et al (2013) | 1 indeterminate patient excluded | 1 | 0 | 0 | 0 | – | – |
| Neuzillet et al (2004) | 3 indeterminate patients, 1 excluded, and 2 considered negative | 56 | 0 | 3 | 0 | 2 | 0 |
| Chyhrai et al (2010) | 2 indeterminate patients, 1 excluded and 1 considered negative | 14 | 0 | 3 | 0 | 1 | 0 |
| Volpe et al (2008) | 8 indeterminate patients, 7 patients excluded, and 1 patient considered positive | 20 | 0 | 0 | 0 | 0 | 1 |
| Menogue et al (2012) | 9 indeterminate, 8 considered negative and 1 patient considered positive | 117 | 0 | 3 | 0 | 8 | 1 |
| Total | 171 | 897 | 8 | 23 | 14 | 30 | 2 |
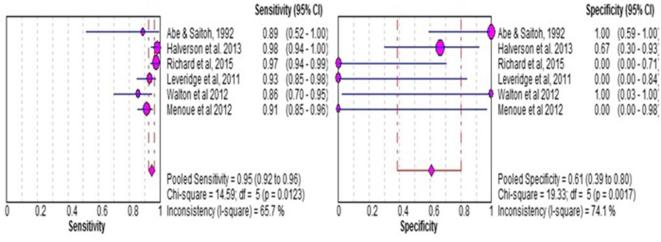
Overall pooled sensitivity (0.952, CI 0.908–0.979) and specificity (0.824, CI 0.566–0.962) were considered to be at a satisfactory diagnostic rate when using a 2 × 2 table contingency. The pooled estimates for sensitivity and specificity of core biopsy based upon bivariate analysis significantly decreased for the 3 × 2 table contingency at a value of 0.945 (CI 0.920–0.960) and 0.609 (CI 0.397–0.800), p < 0.001, respectively (Figure 2). The median prevalence of non-diagnostic core biopsy was three patients [interquartile range (IQR 2–12)] across the patient series, and two studies did not report indeterminate biopsy numbers.56,57 There were 171 patients with indeterminate results (171/974; 17.5%). The effects of different ways of handling indeterminate biopsies results upon pooled diagnostic accuracy values are depicted in Figure 3.
Figure 2.
Flow of the diagnostic review.
Figure 3.
Diagnostic accuracy of image guided biopsies in small renal masses (<4 cm). CI, confidence interval.
There is very limited available data on the accuracy of the subtype and Fuhrman grade of RTB when compared to final surgical histopathology. Four studies reported58–61 grade concordance ranging from 90 to 98%, with a substantial agreement κ of 0.69 (substantial agreement), however, tumour grade accuracy assignment of SRM <4 cm was more challenging. Fuhrman grade was accurately assigned in 50/72 (69%) when compared to nephrectomy specimens, upgraded in 17 (25%) and downgraded in 5 (7%).60 Data identifying tumours were graded erroneously as low (I or II) or high (III or IV), with accuracy for grade evaluation 69.8% (44/63).61 Similar rates reported elsewhere 11/21 (52.3%).58 Other series reported robust grade assignment with RTB at 93%.56
Complications
All the included studies reported on the prevalence of complications post-RTB, with the exception of a few.8,56,62–64 Four studies reported no complications57,61,65,66 with no seeding at mean follow up 18 months,67 and longer at 28 months (IQR 11–53) or upon final histopathology.1,63,65,68 One reported suspected seeding69 over the course of follow up (2 years and 6 months) for a liposarcoma. Across the included studies, three patients (3/974; 0.3%) required blood transfusions58,60 because of post-operative bleeding, one patient was admitted due to gross haematuria and urinary clots,59 and one patient required percutaneous angioembolization,57 though all recovered without sequelae. In another patient, a 2 cm intrarenal haematoma was mistaken for a tumour and excised at laparoscopic partial nephrectomy and subsequently, patient required to undergo a radical nephrectomy to remove renal (clear) cell carcinoma.60 Minor complications included hypotension59 pain64,67,70 wound infection perirenal haematomas1,64,65 and small pneumothorax1,59 all managed conservatively. Minor complication rates ranged from 2.1%,65 8.5% 10.4%, to 20%.64 No mortality was reported across all studies related to RTB.
Risk of bias and quality assessment
The methodology quality assessed by the QUADAS-2 tool across the 20 included studies is summarised in Table 3. Overall, there was a high risk of bias across all the included studies. The majority of the included studies were retrospective 19/20; 90%), and none of the included studies featured sample size calculations when estimating power for diagnostic accuracy in RTB. Most sample sizes were small, with a lack of clinical and demographic information to sufficiently characterise the patient population. Moreover, most studies did not detail the follow-up duration of the included participants over time, with the exception of,66,67,70,76 with the longest follow-up of just over 2 years. Only one study reported the time interval of RTB to the date of surgery72 (48 days, IQR 29–68), and the remaining studies were at risk of flow and timing bias. None of the included studies described the learning curve of the interventional radiologist or pathologist, which could inevitably influence RTB outcomes. 37.8% (974/2573) participants in the included studies had reference standards reported. However, more than half the participants (1546/2573; 62.2%) did not have reference standards listed; raising concerns of verification-bias as pathology of the resected SRMs was not available (opting for cryotherapy, RFA and AS), therefore the FP and FN rates remain unknown. Hence, these patients were excluded in the pooled sensitivity and specificity analysis. Finally, none of the included studies reported blinding of the outcome assessor/s.
Table 3.
Risk of bias summary
| Study | Risk of bias | Applicability concerns | |||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Abe and Saitoh71 |  |
 |
 |
 |
 |
 |
 |
| Neuzillet et al61 |  |
 |
 |
 |
 |
 |
 |
| Eshed et al8 |  |
 |
 |
 |
 |
 |
 |
| Shah et al63 |  |
 |
 |
 |
 |
 |
 |
| Li et al65 |  |
 |
 |
 |
 |
 |
 |
| Volpe et al72 |  |
 |
 |
 |
 |
 |
 |
| Wang et al69 |  |
 |
 |
 |
 |
 |
 |
| Chyhrai et al73 |  |
 |
 |
 |
 |
 |
 |
| Leveridge et al59 |  |
 |
 |
 |
 |
 |
 |
| Kroeze et al74 |  |
 |
 |
 |
 |
 |
 |
| Menogue et al60 |  |
 |
 |
 |
 |
 |
 |
| Salem et al75 |  |
 |
 |
 |
 |
 |
 |
| Millet et al56 |  |
 |
 |
 |
 |
 |
 |
| Walton et al58 |  |
 |
 |
 |
 |
 |
 |
| Halverson et al73 |  |
 |
 |
 |
 |
 |
 |
| Park et al71 |  |
 |
 |
 |
 |
 |
 |
| Garnon et al69 |  |
 |
 |
 |
 |
 |
 |
| Richard et al70 |  |
 |
 |
 |
 |
 |
 |
| Hu et al64 |  |
 |
 |
 |
 |
 |
 |
| Menhadji et al66 |  |
 |
 |
 |
 |
 |
 |
Imaging modalities, needle gauges and number of biopsies
CT imaging modality and ultrasound scan was the most commonly reported approach to RTB,1,58–60,62,69,74 real-time 3D fluoroscopy CT56,76 and MRI.67 The majority of studies used a 18G needle, with the biggest size report as 14G.73 The number of cores ranged from 1 to 5 across of a number of studies.60,63,72,74,77 Based on the small sample sizes, there was no usable data to conduct sensitivity analysis to explore the impact of procedural approaches on diagnostic yield. The procedural time and cost-consequence were not addressed in any of the included studies.
Discussion
We set out to affirm the significance of the diagnostic accuracy of RTB in SRMs (<4 cm), specifically to address the existing bias and methodological problems of handling indeterminate results.2 In a well-defined patient population of SRMs; RTB exhibited a high overall diagnostic rate (95%). However, specificity of the intervention remains low (61%) when we applied the intention-to-diagnose principle using a 3 × 2 table approach. From a clinical perspective, by transforming the 2 × 2 table into a 3 × 2 table, we reported all results accordingly and hence outcomes are fully transparent and summarised. This approach significantly altered diagnostic accuracy of RTB particularly in terms of specificity of the results. This may help in clinical decision-making and exploring the true clinical potential of RTB. Low rates of true negative (specificity) may raise a possibility of missing a malignant tumour using RTB which clinically is not acceptable as this provides false reassurance. Moreover, it limits choices of interventions and scope of discussion/options with patients diagnosed with incidental SRMs specifically for active surveillance. A strength of the current study was the definition of TP and TN because RTB’s were only compared to final surgical specimen histopathology, and the intention-to-diagnosis principle using 3 × 2 table analysis (Figure 1). A summary of including indeterminate results as FPs or FNs according to reference standard offers transparent evidence for potential clinical use of the RTB test most adequately.
While it is clear that there is no agreed upon policy in the literature regarding how to handle indeterminate results of RTB, our review showed that 17.5% participants were reported to have indeterminate outcomes of first RTB attempts in 18 studies. Again, there was lack of clarity as to how these results were handled at the analysis level. Moreover, only 10 (10/20; 50%) of the included studies provided enough data to calculate alternative the 3 × 2 tables. None of the included studies used 3 × 2 table approach to analyse the outcomes of RTB.
Although overall, the diagnostic accuracy of RTB, RCC subtypes appeared to be reliable, the agreement between tumour grade at biopsy and upon the final surgical specimen had a much poorer performance, and this has been reported even in a meta-analysis with much larger tumour >4 cm targets.2 Grade of tumour, a surrogate marker for tumour aggressiveness, is an independent and powerful prognostic predictor of cancer-specific outcomes.77 Poor performance of RTB in our study may be based on variations in the level of expertise of the reporting pathologist, quality of biopsy material and operator of the RTB procedure.56 In the included studies, very little or no information was detailed on these parameters. It is a common clinical observation that there is a learning curve for this intervention, which again was not estimated in any of the included studies. We pooled data from studies with available reference standard, however across the majority of patients in the included studies, they did not have their masses surgically removed and therefore, “definitively” determining the relationship between indeterminate or negative biopsy and the presence of cancer was impossible for these individuals. This summary of evidence should be considered while applying RTB in active surveillance of SRMs.
In the present study, we were unable to perform sensitivity analyses on the performance of RTB for the following factors: location of the tumour (anterior/posterior); lesion’s echogenicity/enhancement; amount of adipose tissue; skin to tumour depth; expertise of radiologist/pathologist; imaging modality; number of passes and needle gauge; small sample size and lack of usable data. However, intuitively the diagnostic rate should increase with the number of biopsy cores. Across the vast majority of studies “multiple passes” were performed to achieve a histological diagnosis. However, this may increase the risk of complications, but evidence does not clearly delineate the number of passes required or indeed the optimum technique or imaging modality. Multivariate analyses from previous studies have suggested factors that can predict diagnosis of SRM biopsy include tumour type, the presence of a cystic lesion, odds ratio of 13.9 (95% CI = 3.78–50.7; p < 0.0001) and tumour size, and for every 1 cm increase in diameter the odds ratio for diagnostic biopsy was 3.11 (95% CI = 1.54–6.28, p = 0.002).59
Unfortunately, the methodological quality of the included studies was poor, and the level of evidence underscores that despite many years of experience in RTB, there is a pressing need for a multicentre international collaboration to prospectively examine the RTB in SRM <4 cm. There were a number of patients who underwent AS and ablative therapies whereby, ultimately the prevalence of FP, FN will remain unknown. With a very limited follow-up, the median was reported at 25 months. Moreover, the majority were at risk of flow and timing bias, with only one study describing the duration between RTB procedure and surgery. Finally, studies were at risk of selection bias and through the use of different reference standards (differential-verification bias) including clinical and demographic differences in patients, clinical follow-up schedules, and RTB protocol-based approaches. Despite these limitations, we followed a robust, transparent methodology for reproducibility with strict study selection to address the role of RTB in SRMs, specifically <4 cm. We acknowledge that our findings are constrained because of the methodological limitations of the included studies. However, this systematic review has enabled a broad summary of the evidence, which facilitated refinement of future research directions and clinical implications.
Implication for clinical practice and future research
There are a number of uncertainties in the body of evidence of research to guide clinical practice:
There are a number of studies focusing on outcome of intervention; mostly retrospective in design without prior protocols, power calculation and independent data assessors. Guidelines for clinical practice are mainly based on a large number of studies with risk of biases. Future improved designed prospective studies with better executions are necessary.
. None of the studies in the review defined accuracy of needle biopsies in relation to size of the target condition. The larger the mean size of the lesions, the more accurate the test is likely to be because large lesions tend to be easily accessible.
Inconsistencies in “reference standard”, duration of follow-up, number of cores needed for good quality tissue, and experience of interventional radiologist and pathologist exist in the present literature. This needs rectification in future research.
Future research should also include data on patient experience, health economics and patients/public engagement. Data on these outcomes was lacking in the primary studies included in this review.
Role of emerging ancillary methods to improve diagnostic yield of biopsies such as novel immunohistochemistry, cytogenetic and molecular markers as highlighted in some of the included studies require assessment.30,62 Such novel approaches might aid and improve the biopsy accuracy and subtype identification for predicting disease-free survival. Extracting RNA and performing polymerase chain reaction has been shown to considerably increase the diagnostic accuracy of renal biopsy in defining histological subtypes.73 Moreover, similar results have been observed when fluorescence in situ hybridization analysis was added to standard assessment.74 Further studies have shown that integrating cytogenetic information to existing prognostic factors could lead to a nomogram having accuracy of 0.89 for predicting disease-specific survival.75 If further confirmed, RTB might be used to guide more personalised management in the future.
Conclusions
RTB in SRMs (<4 cm) is associated with a high diagnostic sensitivity but poor specificity (TN). Following intention-to-diagnose principles, all results are transparent and provided heightened caution in the diagnostic performance of RTB. High risk of biases, risk of false negative and poor quality of research need addressing through future research efforts, preferably through well-designed prospective studies appropriately powered for diagnostic accuracy using valid reference standards.
Figure 4.
Receiver operating characteristic (ROC) analysis of the results.
Contributor Information
Catherine Paterson, Email: c.paterson@nhs.net.
Joseph Ghaemi, Email: j.ghaemi@nhs.net.
Abduelmenem Alashkham, Email: a.alashkham@nhs.net.
Chandra Shekhar Biyani, Email: s.biyani@nhs.net.
Bernadette Coles, Email: b.cole@nhs.net.
Lee Baker, Email: l.baker@dundee.ac.uk.
Magdalena Szewczyk-Bieda, Email: m.Szewczyk-Bieda@nhs.net.
Ghulam Nabi, Email: g.nabi@dundee.ac.uk.
REFERENCES
- 1.Volpe A, Terrone C, Scarpa RM. The current role of percutaneous needle biopsies of renal tumours. Arch Ital Urol Androl 2009; 81: 107–12. [PubMed] [Google Scholar]
- 2.Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 2016; 69: 660–73. doi: 10.1016/j.eururo.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 3.He Q, Wang H, Kenyon J, Liu G, Yang L, Tian J, et al. Accuracy of Percutaneous Core Biopsy in the Diagnosis of Small Renal Masses (≤ 4.0 cm): A Meta-analysis. Int Braz J Urol 2015; 41: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel EJ, Culp SH, Matin SF, Tamboli P, Wallace MJ, Jonasch E, et al. Percutaneous biopsy of primary tumor in metastatic renal cell carcinoma to predict high risk pathological features: comparison with nephrectomy assessment. J Urol 2010; 184: 1877–81. doi: 10.1016/j.juro.2010.06.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology 2010; 76: 610–3. doi: 10.1016/j.urology.2009.09.095 [DOI] [PubMed] [Google Scholar]
- 6.Lebret T, Poulain JE, Molinie V, Herve JM, Denoux Y, Guth A, et al. Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 2007; 1781184–8. doi: 10.1016/j.juro.2007.05.155 [DOI] [PubMed] [Google Scholar]
- 7.Maturen KE, Nghiem HV, Caoili EM, Higgins EG, Wolf JS, Wood DP. Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol 2007; 188: 563–70. doi: 10.2214/AJR.06.0220 [DOI] [PubMed] [Google Scholar]
- 8.Eshed I, Elias S, Sidi AA. Diagnostic value of CT-guided biopsy of indeterminate renal masses. Clin Radiol 2004; 59: 262–7. doi: 10.1016/j.crad.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 9.Hara I, Miyake H, Hara S, Arakawa S, Hanioka K, Kamidono S. Role of percutaneous image-guided biopsy in the evaluation of renal masses. Urol Int 2001; 67: 199–202. doi: 10.1159/000050987 [DOI] [PubMed] [Google Scholar]
- 10.Simel DL, Feussner JR, DeLong ER, Matchar DB, Intermediate MDB. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making 1987; 7: 107–14. doi: 10.1177/0272989X8700700208 [DOI] [PubMed] [Google Scholar]
- 11.Schuetz GM, Schlattmann P, Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ 2012; 345: e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011; 64: 602–7. doi: 10.1016/j.jclinepi.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Manager R. Copenhagen: The Nordic Cochrane Centre. The Cochranen Collaboration 2014. [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: 25. doi: 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingworth W, Medina LS, Lenkinski RE, Shibata DK, Bernal B, Zurakowski D, et al. Interrater reliability in assessing quality of diagnostic accuracy studies using the QUADAS tool. A preliminary assessment. Acad Radiol 2006; 13: 803–10. doi: 10.1016/j.acra.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58: 982–90. doi: 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 18.Van Houwelingen HC, Zwinderman KH, Stijnen T. A bivariate approach to meta-analysis. Stat Med 1993; 12: 2273–84. [DOI] [PubMed] [Google Scholar]
- 19.Cochrane Rev Man: Cochrane. 2014. Available from: http://tech.cochrane.org/revman.
- 20.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6: 31. doi: 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58: 882–93. doi: 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 22.van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol 2014; 14: 70. doi: 10.1186/1471-2288-14-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieper CC, Fischer S, Strunk H, Meyer C, Thomas D, Willinek WA, et al. Percutaneous CT-guided radiofrequency ablation of solitary small renal masses: a single center experience. Rofo 2015; 187: 577–83. doi: 10.1055/s-0034-1399340 [DOI] [PubMed] [Google Scholar]
- 24.Prasad N, Kumar S, Manjunath R, Bhadauria D, Kaul A, Sharma RK, et al. Real-time ultrasound-guided percutaneous renal biopsy with needle guide by nephrologists decreases post-biopsy complications. Clin Kidney J 2015; 8: 151-6. doi: 10.1093/ckj/sfv012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubomirova M, Krasteva R, Bogov B. Local reduction of renal blood flow after kidney biopsy. C R Acad Bulg Sci 2015; 69: 231–6. [Google Scholar]
- 26.Atri M, Tabatabaeifar L, Jang HJ, Finelli A, Moshonov H, Jewett M. Accuracy of contrast-enhanced US for differentiating benign from malignant solid small renal masses. Radiology 2015; 276: 900–8. doi: 10.1148/radiol.2015140907 [DOI] [PubMed] [Google Scholar]
- 27.Zardawi IM. Renal fine needle aspiration cytology. Acta Cytol 1999; 43: 184–90. doi: 10.1159/000330974 [DOI] [PubMed] [Google Scholar]
- 28.Prince J, Bultman E, Hinshaw L, Drewry A, Blute M, Best S, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol 2015; 193: 1899–904. doi: 10.1016/j.juro.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal SK. Cytohistological study of clear cell carcinoma of kidney with special reference to nuclear grading and tumor size. Diagn Cytopathol 2012; 40: 1083–7. doi: 10.1002/dc.21740 [DOI] [PubMed] [Google Scholar]
- 30.Li G, Cuilleron M, Cottier M, Gentil-Perret A, Lambert C, Genin C, et al. The use of MN/CA9 gene expression in identifying malignant solid renal tumors. Eur Urol 2006; 49: 401–5. doi: 10.1016/j.eururo.2005.10.025 [DOI] [PubMed] [Google Scholar]
- 31.Kümmerlin IP, Smedts F, ten Kate FJ, Horn T, Algaba F, Trias I, et al. Cytological punctures in the diagnosis of renal tumours: a study on accuracy and reproducibility. Eur Urol 2009; 55: 187–98. doi: 10.1016/j.eururo.2008.04.072 [DOI] [PubMed] [Google Scholar]
- 32.Torp-Pedersen S, Juul N, Larsen T, Karstrup S, Sehested M, Glenthøj A. US-guided fine needle biopsy of solid renal masses-comparison of histology and cytology. Scand J Urol Nephrol Suppl 1991; 137: 41–3. [PubMed] [Google Scholar]
- 33.Richter F, Kasabian NG, Irwin RJ, Watson RA, Lang EK. Accuracy of diagnosis by guided biopsy of renal mass lesions classified indeterminate by imaging studies. Urology 2000; 55: 348–52. [DOI] [PubMed] [Google Scholar]
- 34.Caoili EM, Bude RO, Higgins EJ, Hoff DL, Nghiem HV. Evaluation of sonographically guided percutaneous core biopsy of renal masses. AJR Am J Roentgenol 2002; 179: 373–8. doi: 10.2214/ajr.179.2.1790373 [DOI] [PubMed] [Google Scholar]
- 35.Truong LD, Todd TD, Dhurandhar B, Ramzy I. Fine-needle aspiration of renal masses in adults: analysis of results and diagnostic problems in 108 cases. Diagn Cytopathol 1999; 20: 339–49. [DOI] [PubMed] [Google Scholar]
- 36.Bishop JA, Hosler GA, Kulesza P, Erozan YS, Ali SZ. Fine-needle aspiration of renal cell carcinoma: is accurate Fuhrman grading possible on cytologic material? Diagn Cytopathol 2011; 39: 168–71. doi: 10.1002/dc.21352 [DOI] [PubMed] [Google Scholar]
- 37.Renshaw AA, Lee KR, Madge R, Granter SR. Accuracy of fine needle aspiration in distinguishing subtypes of renal cell carcinoma. Acta Cytol 1997; 41: 987–94. doi: 10.1159/000332777 [DOI] [PubMed] [Google Scholar]
- 38.Somani BK, Nabi G, Thorpe P, N'Dow J, Swami S, McClinton S, et al. Image-guided biopsy-diagnosed renal cell carcinoma: critical appraisal of technique and long-term follow-up. Eur Urol 2007; 51: 1289–95discussion 96-7. doi: 10.1016/j.eururo.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 39.Karp W, Ekelund L. Ultrasound, angiography and fine needle aspiration biopsy in diagnosis of renal neoplasms. Acta Radiol Diagn 1979; 20: 649–59. [DOI] [PubMed] [Google Scholar]
- 40.Juul N, Torp-Pedersen S, Holm HH. Ultrasonically guided fine needle aspiration biopsy of retroperitoneal mass lesions. Br J Radiol 1984; 57: 43–6. doi: 10.1259/0007-1285-57-673-43 [DOI] [PubMed] [Google Scholar]
- 41.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003; 170:2217–20. doi: 10.1097/01.ju.0000095475.12515.5e [DOI] [PubMed] [Google Scholar]
- 42.Chen F, Huhdanpaa H, Desai B, Hwang D, Cen S, Sherrod A, et al. Whole lesion quantitative CT evaluation of renal cell carcinoma: differentiation of clear cell from papillary renal cell carcinoma. Springerplus 2015; 4: 66. doi: 10.1186/s40064-015-0823-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang EK, Macchia RJ, Gayle B, Richter F, Watson RA, Thomas R, et al. CT-guided biopsy of indeterminate renal cystic masses (Bosniak 3 and 2F): accuracy and impact on clinical management. Eur Radiol 2002; 12: 2518–24. doi: 10.1007/s00330-001-1292-z [DOI] [PubMed] [Google Scholar]
- 44.Harisinghani MG, Maher MM, Gervais DA, McGovern F, Hahn P, Jhaveri K, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging-guided biopsy precede surgery? AJR Am J Roentgenol 2003; 180: 755–8. doi: 10.2214/ajr.180.3.1800755 [DOI] [PubMed] [Google Scholar]
- 45.Kennedy SA, Milovanovic L, Midia M. Major bleeding after percutaneous image-guided biopsies: frequency, predictors, and periprocedural management. Semin Intervent Radiol 2015; 32: 26–33. doi: 10.1055/s-0034-1396961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopes RI, Moura RN, Artifon E. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of kidney lesions: a review. World J Gastrointest Endosc 2015; 7: 253–7. doi: 10.4253/wjge.v7.i3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Poppel H, Becker F, Cadeddu JA, Gill IS, Janetschek G, Jewett MA, et al. Treatment of localised renal cell carcinoma. Eur Urol 2011; 60: 662–72. doi: 10.1016/j.eururo.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 48.Borghesi M, Brunocilla E, Volpe A, Dababneh H, Pultrone CV, Vagnoni V, et al. Active surveillance for clinically localized renal tumors: an updated review of current indications and clinical outcomes. Int J Urol 2015; 22: 432–8. doi: 10.1111/iju.12734 [DOI] [PubMed] [Google Scholar]
- 49.Kao JS, Behr S, Westphalen AC, Zagoria RJ. Renal mass biopsy in the era of surgical alternatives. Current Radiology Reports 2015; 3: 8. doi: 10.1007/s40134-015-0102-3 [DOI] [Google Scholar]
- 50.Lechevallier E. Core biopsy of solid renal masses under CT guidance. Eur Urol Supp 2007; 6: 540–3. [Google Scholar]
- 51.Leão RR, Richard PO, Jewett MA. Indications for biopsy and the current status of focal therapy for renal tumours. Transl Androl Urol 2015; 4: 283–93. doi: 10.3978/j.issn.2223-4683.2015.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol 2014; 31: 20–6. doi: 10.1055/s-0033-1363839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alasker A, Williams SK, Ghavamian R. Small renal mass: to treat or not to treat. Curr Urol Rep 2013; 14: 13–18. doi: 10.1007/s11934-012-0296-3 [DOI] [PubMed] [Google Scholar]
- 54.Remzi M, Marberger M. Renal tumor biopsies for evaluation of small renal tumors: why, in whom, and how? Eur Urol 2009; 55: 359–67. doi: 10.1016/j.eururo.2008.09.053 [DOI] [PubMed] [Google Scholar]
- 55.Rogers CG, Ditlev JA, Tan MH, Sugimura J, Qian CN, Cooper J, et al. Microarray gene expression profiling using core biopsies of renal neoplasia. Am J Transl Res 2009; 1: 55–61. [PMC free article] [PubMed] [Google Scholar]
- 56.Millet I, Curros F, Serre I, Taourel P, Thuret R. Can renal biopsy accurately predict histological subtype and Fuhrman grade of renal cell carcinoma? J Urol 2012; 188: 1690–4. doi: 10.1016/j.juro.2012.07.038 [DOI] [PubMed] [Google Scholar]
- 57.Garnon J, Schlier A, Buy X, Tsoumakidou G, de Mathelin M, Breton E, et al. Evaluation of percutaneous biopsies of renal masses under MRI-guidance: a retrospective study about 26 cases. Eur Radiol 2015; 25: 617–23. doi: 10.1007/s00330-014-3449-6 [DOI] [PubMed] [Google Scholar]
- 58.Walton TJ, Amery C, Moore D, Mayer NJ, Rajesh A, Kockelbergh RC. Utility of renal mass biopsy in a UK tertiary referral centre. Br J Med Surg Urol 2012; 5: 216–23. doi: 10.1016/j.bjmsu.2011.10.002 [DOI] [Google Scholar]
- 59.Leveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shiff DA, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 2011; 60: 578–84. doi: 10.1016/j.eururo.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 60.Menogue SR, O'Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int 2013; 111: E146–51. doi: 10.1111/j.1464-410X.2012.11384.x [DOI] [PubMed] [Google Scholar]
- 61.Neuzillet Y, Lechevallier E, Andre M, Daniel L, Coulange C. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol 2004; 171: 1802–5. doi: 10.1097/01.ju.0000120147.51090.2b [DOI] [PubMed] [Google Scholar]
- 62.Chyhrai A, Sanjmyatav J, Gajda M, Reichelt O, Wunderlich H, Steiner T, et al. Multi-colour FISH on preoperative renal tumour biopsies to confirm the diagnosis of uncertain renal masses. World J Urol 2010; 28: 269–74. doi: 10.1007/s00345-010-0551-5 [DOI] [PubMed] [Google Scholar]
- 63.Kroeze SG, Huisman M, Verkooijen HM, van Diest PJ, Ruud Bosch JL, van den Bosch MA. Real-time 3D fluoroscopy-guided large core needle biopsy of renal masses: a critical early evaluation according to the IDEAL recommendations. Cardiovasc Intervent Radiol 2012; 35: 680–5. doi: 10.1007/s00270-011-0237-4 [DOI] [PubMed] [Google Scholar]
- 64.Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 2013; 189: 441–6. doi: 10.1016/j.juro.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 65.Volpe A, Kachura JR, Evans AJ, Gharajeh A, Saravanan A, Saravanan A, et al. Techniques, Safety and Accuracy of Sampling of Renal Tumors by Fine Needle Aspiration and Core Biopsy. J Urol 2007; 178: 379–86. [DOI] [PubMed] [Google Scholar]
- 66.Shah RB, Bakshi N, Hafez KS, Wood DP, Kunju LP. Image-guided biopsy in the evaluation of renal mass lesions in contemporary urological practice: indications, adequacy, clinical impact, and limitations of the pathological diagnosis. Hum Pathol 2005; 36: 1309–15. [DOI] [PubMed] [Google Scholar]
- 67.Li G, Cuilleron M, Zhao A, Obadia F, Mouracade P, Tostain J, et al. Combination of core biopsy and fine-needle aspiration increases diagnostic rate for small solid renal tumors. Anticancer Res 2012; 32: 3463–6. [PubMed] [Google Scholar]
- 68.Wang R, Wolf JS, Wood DP, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology 2009; 73: 586–90discussion 90-1. doi: 10.1016/j.urology.2008.08.519 [DOI] [PubMed] [Google Scholar]
- 69.Garnon J, Schlier A, Buy X, Tsoumakidou G, de Mathelin M, Breton E, et al. Evaluation of percutaneous biopsies of renal masses under MRI-guidance: a retrospective study about 26 cases. Eur Radiol 2015; 25: 617–23. doi: 10.1007/s00330-014-3449-6 [DOI] [PubMed] [Google Scholar]
- 70.Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol 2015; 68: 1007–13. doi: 10.1016/j.eururo.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 71.Hu R, Montemayor-Garcia C, Das K. Role of percutaneous needle core biopsy in diagnosis and clinical management of renal masses. Hum Pathol 2015; 46: 570–6. [DOI] [PubMed] [Google Scholar]
- 72.Volpe A, Finelli A, Gill IS, Jewett MA, Martignoni G, Polascik TJ, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol 2012; 62: 491–504. doi: 10.1016/j.eururo.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 73.Abe M, Saitoh M. Selective renal tumour biopsy under ultrasonic guidance. Br J Urol 1992; 70: 7–11. [DOI] [PubMed] [Google Scholar]
- 74.Park SY, Park BK, Kim CK, Kwon GY. Ultrasound-guided core biopsy of small renal masses: diagnostic rate and limitations. J Vasc Interv Radiol 2013; 24: 90–6. doi: 10.1016/j.jvir.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 75.Menhadji AD, Nguyen V, Okhunov Z, Bucur P, Chu WH, Cho J, et al. Technique for office-based, ultrasonography-guided percutaneous biopsy of renal cortical neoplasms using a novel transducer for facilitated ultrasound targeting. BJU Int 2016; 117: 948–53. [DOI] [PubMed] [Google Scholar]
- 76.El-Mokadem I, Lim A, Kidd T, Garret K, Pratt N, Batty D, et al. Microsatellite alteration and immunohistochemical expression profile of chromosome 9p21 in patients with sporadic renal cell carcinoma following surgical resection. BMC Cancer 2016; 16: 546. doi: 10.1186/s12885-016-2514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–63. [DOI] [PubMed] [Google Scholar]
- 78.Klatte T, Rao PN, de Martino M, LaRochelle J, Shuch B, Zomorodian N, et al. Cytogenetic profile predicts prognosis of patients with clear cell renal cell carcinoma. J Clin Oncol 2009; 27: 746–53. doi: 10.1200/JCO.2007.15.8345 [DOI] [PubMed] [Google Scholar]
- 79.Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 2013; 189: 441–6. doi: 10.1016/j.juro.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 80.Salem S, Ponsky LE, Abouassaly R, Cherullo EE, Isariyawongse JP, Maclennan GT, et al. Image-guided biopsy of small renal masses in the era of ablative therapies. Int J Urol 2013; 20: 580–4. [DOI] [PubMed] [Google Scholar]