Abstract
For modelling sexually transmitted infections, duration of partnerships can strongly influence the transmission dynamics of the infection. If partnerships are monogamous, pairs of susceptible individuals are protected from becoming infected, while pairs of infected individuals delay onward transmission of the infection as long as they persist. In addition, for curable infections re-infection from an infected partner may occur. Furthermore, interventions based on contact tracing rely on the possibility of identifying and treating partners of infected individuals. To reflect these features in a mathematical model, pair formation models were introduced to mathematical epidemiology in the 1980's. They have since been developed into a widely used tool in modelling sexually transmitted infections and the impact of interventions. Here we give a basic introduction to the concepts of pair formation models for a susceptible-infected-susceptible (SIS) epidemic. We review some results and applications of pair formation models mainly in the context of chlamydia infection.
Keywords: Pair formation, Mathematical model, Partnership duration, Sexually transmitted infections, Basic reproduction number
1. Introduction
In classical epidemic models, essential underlying assumptions are that contacts are instantaneous and every contact is with another individual of the population (Diekmann, Heesterbeek, & Britton, 2012). For many infectious diseases these assumptions are reasonable and lead to good results. For example, for modelling the spread of influenza or measles, taking instantaneous contacts into account has resulted in valid descriptions of transmission dynamics and these models have been used successfully to assess the impact of vaccination (Anderson & May 1991). For modelling dynamics of sexually transmitted infections (STI) the situation is different, as these assumptions may not always be valid. If individuals of a population form long lasting partnerships and have repeated contacts with the same individual over a long time period, this influences the transmission risk of an infection that spreads via those contacts. If partnerships are monogamous, individuals in a pair of two susceptibles are protected from becoming infected as long as the partnership lasts. A partnership has to dissolve and a new one be formed before transmission to another person can occur. Also, if a person who is in a partnership with an infectious partner recovers, he or she may acquire a re-infection from his/her infected partner. Such effects are especially influential for infections with relatively long infectious periods and for populations where individuals have few but long lasting partnerships. If duration of infection and duration of partnerships are of the same order of magnitude, partnership dynamics interacts with disease transmission and determines the potential of the disease to establish itself in a population.
To deal with such dynamic properties of sexually transmitted infections, pair formation models were first introduced into the field of infectious disease modelling by Dietz and Hadeler (Dietz & Hadeler, 1988). They modified models from mathematical demography to include transmission of infection in an age-structured two-sex population. Simplified versions of this pair formation epidemic model were later formulated by Kretzschmar and Dietz (Kretzschmar & Dietz, 1998) and compared with models, which do not take partnership duration into account. Since then, pair formation models have been used in many variants, have been implemented into simulation models for STI (Kretzschmar et al., 1996, Low et al., 2007, Turner et al., 2006), and have been applied to analyse the impact of various types of interventions (Heijne et al., 2011, Powers et al., 2011). Pair formation models in structured populations have been analysed mathematically by Hadeler et al. (Hadeler, 2012, Hadeler et al., 1988). More recently, extensions to the pair formation approach which are capable of describing also concurrent partnerships have been derived and analysed (Leung et al., 2012, Leung et al., 2015).
Here we review the approach of pair formation models with the aim of giving an easy introduction into the main ideas and related literature for readers, who want to obtain a quick overview. We introduce and explain assumptions and structure of simple pair formation models. We formulate models for one-sex populations for reasons of simplicity, but all models can easily be extended to two-sex populations. We give some examples for the application of pair formation models, where we focus mainly on curable sexually transmitted infections (STI) with chlamydia trachomatis (chlamydia) as an example.
2. Modelling partnership dynamics
We start with introducing a model for the partnership dynamics without infection. We assume that the population is subdivided into singles (denoted by ) and pairs of individuals (denoted by ). The total population size is given by , because a pair consists of 2 individuals. Singles form new pairs with a rate ρ and pairs separate with rate σ. New individuals enter the population with a constant recruitment rate B and leave the population (by death or ceasing sexual activity) with rate μ. We further assume that and for all t. The pair formation process is then described by the system of differential equations
This system of equations has a unique equilibrium given by
In equilibrium we have . Therefore, a fraction is single. The fraction of people in a partnership is given by . In the following we will always assume that the pair formation and separation process is at equilibrium, i.e. that there is a constant proportion of singles and paired individuals in the population.
The fractions of singles and paired individuals can be used to estimate ρ and σ from sexual behaviour data. For example, if we observe that survey participants report 1.5 new partners per year and 70% of participant report that they are in a partnership, we can set
If we assume for simplicity that , we get and . Note that these estimates were derived under the assumption that there are no concurrent partnerships.
We can also use the equations to derive some other relevant quantities describing the typical life course of an individual. The fraction of the population that is in a partnership
also describes the expected fraction of his lifetime that an individual is in a partnership. The mean duration of partnerships is given by
Using that the expected lifetime of an individual is given by , we can now compute the expected number M of lifetime partners of an individual as
3. Sexually transmitted infections in a model with pairs
We now want to include transmission of infection into the model. We assume that the infection can be described as an infection, i.e. after recovery from infection an individual is immediately susceptible again. This assumption is reasonable for sexually transmitted infections like chlamydia and gonorrhoea, where there is hardly any immunity and repeated infections are common. Furthermore, we assume that an infection does not lead to disease related mortality. Individuals enter the population as susceptibles, and transmission can only take place in a pair of a susceptible and an infected individual (Fig. 1). For simplicity, we assume that there is no difference between men and women. We denote by the populations of susceptible () and infected () singles. Similarly, by we denote pairs, where can be 0 or 1 depending on whether paired individuals are susceptible or infected. We get the system of equations
| (1) |
where β denotes the transmission rate in discordant pairs, (i.e. in ), and γ is the natural clearance rate. Note that β is composed of a rate of having sexual intercourse during a partnership and a transmission probability per act. The per partnership transmission probability can be computed from β if the partnership duration is known. If partnership duration is long, the per partnership transmission probability can be high even for a low per act probability.
Fig. 1.
Flow scheme for the SIS pair formation model.
Using the assumption that the pair formation process is at equilibrium, we get and . The system (1) can then be reduced to a 3-dimensional system
We can write the prevalence as .
4. The basic reproduction number and other thresholds
Some ingredients for computing the basic reproduction number for the above model can be computed easily. The expected time an individual stays in state is given by
The probability b that the infected partner infects the susceptible partner in is
The expected time an individual stays in state is given by
The probability that a pair of two infected persons dissolves before one of them recovers, i.e. of moving directly into the single state from , is
while the probability of first moving to and then to the single compartment is
For an infection without recovery, i.e. the case , the basic reproduction number is now given by the product of
-
•
the probability of moving into the single state after being infected
-
•
the number of partners in the remaining life time M
-
•
the probability of infecting a susceptible partner b
We get
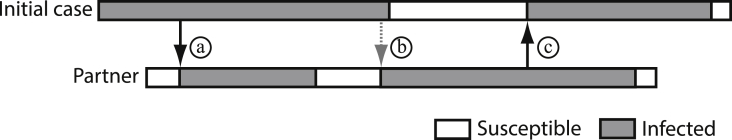
If the situation is more complicated, because an infected individual can clear his infection even while still in a partnership. But then he/she can get reinfected, if his/her partner is infected. The infection can bounce back and forth between partners and in this way the infectious period is effectively prolonged (Fig. 2). How should re-infections within a partnership be counted for computation of ? Are we interested in the number of secondary infections that one infected individual produces or in the number of newly infected persons? This question was discussed in Heijne, Herzog, Althaus, Low, and Kretzschmar (2013).
Fig. 2.
Illustration of infections that can go back and forth between persons. In this example the initial case (top row) transmitted the infection twice to the partner (a and b). The case reproduction number in this example would be 1 (since the initial case only infected one person), but the basic reproduction number would be 2 (since the partner became infected twice). Black arrows denote initial infection and grey dashed arrows re-infections.
The authors introduced two other concepts, which they termed the case reproduction number () and the partnership reproduction number (). The case reproduction number is defined as the average number of secondary cases, i.e. other individuals, a primary case will infect during his or her infectious period (Table 1). For this number, a secondary infection of the same partner, who has cleared an earlier infection, does not count and therefore is always lower (or equal) than (Fig. 2). The average number of secondary partnerships consisting of two infected individuals one typical infected individual will produce during his or her infectious lifetime is denoted as partnership reproduction number (). In Heijne, Herzog, Althaus, Low et al. (2013) it is shown that, for a curable STI, the average number of secondary cases an infected individual infects does not always equal the average number of secondary infections because of re-infection within partnerships. Values of and approach for long durations of immunity after natural clearance or if partnership durations are very short. There is a partnership duration that maximizes the three reproduction numbers. and are not threshold values in the sense of , but they describe important components of case reproduction. An -infection in a population with pairs can be endemic, even if , i.e. even if one case produces on average less than one new cases during the average infectious period. The reason is that the infectious period is in effect prolonged by possible reinfections within pairs. The basic reproduction number takes that into account, whereas just counts the number of secondary cases produced by one infected individual. If there is no re-infection within partnerships, then . The case reproduction number is important in relation with data from contact tracing studies, which are sometime used for estimating (Potterat et al., 1999), but reflect . In a contact tracing study one measures the number of secondary cases generated by one index case, but there is no information about re-infection within those partnerships. Therefore, an estimate based on contact tracing data will always underestimate the true .
Table 1.
Definitions modified from Heijne, Herzog, Althaus, Low et al. (2013).
| Term | Definition |
|---|---|
| Average number of secondary infected individuals (cases) one typical infected individual will produce during his or her infectious period starting in a partnership with two infected individuals in a totally susceptible population | |
| Average number of secondary partnerships consisting of two infected individuals one typical infected individual will produce during his or her infectious lifetime starting in a partnership with two infected individuals in a totally susceptible population | |
| Average number of secondary infections one typical infected individual will produce during his or her infectious period starting in a partnership with two infected individuals in a totally susceptible population |
5. Types of partnerships
Data from sexual behaviour surveys show that there are large differences between individuals in their number of reported partnerships. First, in most surveys the majority of respondents reports very few partners in the last year, usually one, while a small proportion reports having had 2 or more partners. Men usually report on average more partners than women, but also a larger proportion reports zero partners in the last year (Fig. 3). Different explanations for this observation have been put forward, the most accepted one is that women tend to underreport their numbers of partners while men tend to overreport (Bell et al., 2010, Brewer et al., 2000, Morris, 1993). But also the age distribution of respondents may play a role and differences between men and women in the timing of periods of low and high sexual risk behavior during the life courses of men and women (sexual careers).
Fig. 3.
A typical distribution of numbers of partners in the last year reported in a heterosexual population. Most respondents report 0–1 partners, a small fraction report more than five partners. Based on data from Johnson et al. (2001).
There are also large differences between partnership durations ranging from very short one night stands to long-lasting steady partnerships with durations of many years (Foxman et al., 2006, Nelson et al., 2010). It is interesting to observe that survival of long-lasting partnerships can be well described by an exponential distribution (Nelson et al., 2010). Therefore, using a linear combination of two exponential distributions for survival of partnerships results in a flexible but parsimonious description of distributions observed in surveys.
Finally, also the gaps between partnerships play a role in transmission dynamics if transmission can only take place within partnerships (Chen, Ghani, & Edmunds, 2008). In Chen and Ghani (2010) an important finding was that core groups of high risk maybe defined by short gaps between successive partnerships. Empirical data for the distribution of gap length was also provided by Foxman et al. (2006).
This leads us to introduce pair formation models with two types of pairs, which are different in their durations determined by the separation rates σ. In particular, we define partnerships with a low dissolution rate , which we call steady partnerships, and those with high dissolution rate , which are called casual. At the moment of partnership formation a certain fraction f of partnerships are steady, the remaining fraction are casual. We describe the pair formation process by the following system of equations, where P now denotes the number of steady pairs, and Q the number of casual pairs.
As before we can determine the steady state and find
In steady state the ratio of steady to casual partnerships is then given by
This relationship shows that even if the fraction of newly formed steady partnerships is small, due to their long duration the prevalence of steady partnerships can be high. In other words, in a survey asking about the duration of ongoing partnerships there is a bias towards long duration partnerships. This can be seen very well in the data presented in Foxman et al. (2006), where survival of ongoing relationships is much higher than those which have already ended. As above we can describe an SIS infection on a population with two types of partnerships. To account for the possibility that transmission rates may differ in different types of partnerships, we distinguish between for steady pairs and for casual pairs. We get the equations
| (2) |
| (3) |
Under the assumption that the pair formation process is at equilibrium, it is possible to reduce this system to a 5-dimensional system in the variables , , , , and . The prevalence of infection is given by
| (4) |
In Kretzschmar, Jager, Reinking, Van Zessen, and Brouwers (1994) it was shown that as computed from the Jacobian matrix at the disease-free equilibrium of the 5-dimensional system is given by
| (5) |
In this formulation of the contributions of the two types of partnerships can be quantified. So if, for example, steady partnerships (defined by long average duration) in cannot sustain the infection in the population, we could determine at which value of the transmission rate in casual partnerships the infection could establish itself. In general, we find that for infectious with long duration of infection the distinction between long and short term partnerships is not so important for determining the threshold. If however, duration of the infection is short, casual partnerships are more important in keeping up transmission of infection. In other words, the “core group” concept is more important for STIs with shorter infectious periods.
6. Applications of pair formation models in the context of chlamydia control
An important area of application of pair formation models is to assess the impact of population based interventions on the prevalence of chlamydia infections. Chlamydia is the most commonly reported sexually transmitted infection in many developed countries (European Centre for Disease Prevention and Control (ECDC), 2013). A chlamydia infection has an average infectious period of around one year (Althaus et al., 2010, Price et al., 2013). Most chlamydia infections occur asymptomatically, and when untreated, chlamydia can ascend to the upper genital tract and might cause pelvic inflammatory disease (PID) and infertility in women (Holmes, 2008). When detected, chlamydia can be treated by antibiotics. Repeated infections after treatment are common for chlamydia (Batteiger et al., 2010). These repeated infections can come from a re-infection by an untreated infected partner in the existing partnership, from a new partner, from a treatment failure (Kong et al., 2014) or from auto-inoculation from an infection at another anatomic location within the same individual (Heijne et al., 2016).
Since pair formation models take partnership durations explicitly into account and allow for re-infections to happen within partnerships, they are very powerful tools for estimating the impact of intervention measures to control chlamydia transmission. There are several different intervention measures to control chlamydia transmission. The aim of the interventions is to prevent onward transmission and reduce complications. First, asymptomatically infected individuals need to be detected through testing (screening), which shortens the duration of infection and reduces further transmission (Fig. 4). After detection, sexual partners of infected individuals can be informed of their exposure and be treated (partner notification). Since repeated infections are common, people can be offered a repeated test after treatment. There are several countries that implemented guidelines regarding screening, partner notification and repeated testing (Low et al., 2012). These guidelines differ greatly because it is very difficult and costly to perform randomised controlled trials to determine the optimal intervention measures. Mathematical modelling can overcome this limitation.
Fig. 4.
This figure illustrates different intervention measures for controlling chlamydia transmission. First, asymptomatically infected individuals are actively found and treated through screening (either when they are single or in a pair). When tested positive for chlamydia, partner notification can be initiated and positive partners can also be treated. When partner notification is unsuccessful, the index case can become re-infected from her infected partner. For illustration purposes, we assumed that treatment is 100% effective.
Using pair formation models, the effect of different partner notification strategies (in terms of number of partners per index case or the time window in which partners should be notified) was determined (Althaus, Heijne, Herzog, Roellin, & Low, 2012). It was shown that the prevalence of infection was highest in the most recent partners, especially in the last three partners from the preceding 18 months. However, on the population level, notifying the current partner gave the biggest reduction in chlamydia prevalence, and notifying more previous partners did not reduce the population prevalence much further. This suggested that priority should be given to the most recent partner and that going back more partners only has an impact on the individual level.
Regarding repeated testing, a pair formation model showed that the period of highest risk of a repeated infection in women was between two and five months after treatment, indicating that repeated testing can be done most effectively in this time window (Heijne, Herzog, Althaus, Tao et al., 2013). When comparing the impact of notifying the current partner and repeated testing 3 months after treatment on reducing population prevalence (using a fixed screening uptake) it was shown that partner notification was more effective in reducing population prevalence compared to repeated testing. This can be explained by the fact that partner notification not only prevents re-infection, but also onward transmission once the partnership dissolves. Both interventions combined gave the largest reduction in chlamydia prevalence although this reduction was relatively small compared to the reduction in prevalence by testing alone (Heijne, Herzog, Althaus, Tao et al., 2013).
As a last example of the application of pair formation models in enhancing our understanding of chlamydia transmission and control, the pair formation model framework was extended incorporating two anatomical infection locations in women: the urogenital and anorectal locations (Heijne et al., 2016). This allowed not only re-infection to happen from partners of infected individuals, but also from one anatomical location to the other through auto-inoculation. The model estimated that auto-inoculation contributed substantially to keeping chlamydia endemic.
7. Comparisons of pair formation models with instantaneous contact models
Several studies have compared the estimated impact of interventions between pair formation models and instantaneous contact model (Althaus et al., 2012a, Heijne et al., 2011, Lloyd-Smith et al., 2004, Ong et al., 2012, Van de Velde et al., 2010). Lloyd-Smith et al. (2004) showed that generally speaking, instantaneous contact models overestimate endemic prevalence as compared to pair formation models. Only if pair formation and separation dynamics are fast compared to transmission dynamics, are frequency-dependent instantaneous contact models a good approximation for the full pair formation transmission model. Similarly, instantaneous contact models overestimate the impact of interventions on reducing population prevalence compared to pair formation models. For example, regarding chlamydia screening, a pair formation model estimated a weaker impact of screening when compared to the instantaneous contact model (Althaus et al., 2012a, Heijne et al., 2011). This is because in a pair formation model, re-infection of the current partner after screening and treatment is possible, making the impact on reducing prevalence less pronounced. For HPV vaccination, similar results were found for short durations of vaccine protection (Van de Velde et al., 2010). However, for vaccines with high efficacy providing lifelong protection the results were reversed: a pair formation model showed larger reductions in HPV population prevalence compared to its instantaneous counterpart. Last, a theoretical analyses comparing the two models for a range of parameters reflecting several sexually transmitted infections, it was shown that instantaneous contact models predicted higher critical levels of condom use required to prevent transmission for gonorrhoea and chlamydia, but similar levels for HIV (Ong et al., 2012).
In Lloyd-Smith et al. (2004) sexually transmitted infections were classified according to their time-scales of transmission, and it was shown that instantaneous contact models can well approximate prevalence and epidemic growth rates for long lasting chronic infectious such as HIV, but not for -infections with a faster transmission dynamics such as bacterial STI. The differences between the pair formation models and instantaneous contacts models can be explained by two mechanisms, namely that in pair formation models people in pairs have repeated contacts with the same individual, allowing for reinfection within pairs to occur which effectively increases the duration of infectious periods. Second, susceptible individuals who are single or in a pair with another susceptible are temporarily protected from getting infected.
8. Extension to concurrent partnerships
A serious shortcoming of pair formation models is that they cannot take overlap between partnerships, or concurrency, into account. If individuals can have more than one partnership with a duration , connected chains of individual can occur, which cannot be described easily with systems of differential equations. There have been some attempts to extend the pair formation approach at least to some simple situations. An early approach in that direction was published by Dietz and Tudor (1992), who considered a model where one of the two partners in a pair could have another partnership with a third individual. Another approach to include concurrent partnerships in a simple way was used in Xiridou et al., 2003, Xiridou et al., 2004, where individuals could form steady partnerships described as pairs, and have instantaneous contacts modelled by a mass action term next to their steady partner. The rates of having instantaneous contacts could differ between singles and individuals in a pair. The model was used to capture the effect of high infectivity in the short primary phase of HIV infection and was applied to model a population of MSM in Amsterdam, The Netherlands. Short concurrent partnerships during a primary HIV infection can lead to rapid transmission in a population where steady partnerships alone would keep the impact of primary infection limited. The model structure was used and modified by Powers et al. (2011), who extended the framework to a population structured by age, sex and activity levels and parameterized it for a population in Malawi.
If the aim is to model concurrent partnerships with no restrictions on partnership duration and numbers of concurrent partners, one needs to deal with networks in the sense that the contact structure contains larger connected components. This generates dependencies between partners and partners of partners that cannot easily be described by deterministic models. A first step in defining such models for a relatively simple situation was made by Miller and colleagues (Miller and Volz, 2013, Miller et al., 2012) and by Leung and colleagues (Leung et al., 2012, Leung et al., 2015).
Another approach was taken by Bauch and Rand, 2000, Eames and Keeling, 2002, Ferguson and Garnett, 2000, who introduced the pair-approximation approach. In these type of models a full dynamic network is described in terms of the dynamics of pairs, and additional assumptions on correlations in triples. In this way, a closed system of ordinary differential equations can be derived in terms of numbers of pairs and triples of various types. The pair approximation approach was applied to analyse impact of interventions for Chlamydia infections (Clarke, White, & Turner, 2012). While pair formation models as introduced in section 3 are an exact representation of the SIS epidemic, approximations of a network or a model with concurrency cannot handle SIS epidemics exactly. Keeling, House, Cooper, and Pellis (2016) compared SIS epidemics on pair approximation models and certain full networks and found that including information on numbers of re-infections can improve the approximation. In a comparison of network models, pair approximation models and instantaneous contact models Keeling and Rohani (2008) concluded that general epidemics on networks spread slower than in randomly mixing populations.
9. Pair formation models in individual based simulations
When modelling the impact of population based screening for chlamydia, the population needs to be stratified by age and sex. Furthermore, it is useful to distinguish between symptomatic and asymptomatic infections, as data may be available for the former but not for the latter. Also, treatment of symptomatic cases, who seek health care, may shorten the average duration of infection as compared with asymptomatic undiagnosed infections. Taking all these population stratifications into account, quickly renders the deterministic pair formation model formulation very complex and confusing. Therefore, a number of individual based stochastic models have been developed which also take long term partnerships into account. These models have the additional advantage that it is easy to also allow concurrent partnerships to occur. The individual based model (Kretzschmar et al., 1996, Kretzschmar et al., 2001) was a direct translation of the pair formation model introduced above into a stochastic simulation (Kretzschmar, 1995). Other similar models were developed by Turner and colleagues (Turner et al., 2006) and Low and colleagues (Low et al., 2007). Systematic comparisons of those models were conducted in Althaus et al., 2012b, Kretzschmar et al., 2009.
10. Discussion
Taking partnership duration explicitly into account in modelling sexually transmitted infections influences the transmission dynamics and the estimated impact of intervention measures on reducing transmission. Having a long term monogamous partnership with a non-infected partner protects an individual from becoming infected. On the other hand, a long term monogamous partnership of two infected individuals in essence traps the pathogen in the partnership and prevents its further transmission. Therefore, it is clear that partnership duration can have various effects. If an infection clears and re-infection within partnerships is possible, a partnership can in essence prolong the duration of the infectious period by infection being repeatedly passed back and forth between partners. On the other hand, if partnerships last too long, the pathogen has too few opportunities to be passed on to other individuals in order to establish itself in the population. Similarly, if partnership duration is too short, and gaps between partnerships are long, effective transmission cannot take place. There is an optimal partnership duration which maximizes the basic reproduction number given all other parameters remain constant. If the infectivity changes with time since infection, partnership duration can become even more influential, as in the case of HIV. A highly infectious primary phase can have little effect on transmission if partnerships are long and monogamous, but can cause a large proportion of onward transmission if partnership duration is in the order of the duration of the primary infection stage (Leung & Kretzschmar, 2015).
Pair formation models can capture the complex dynamics of partnership duration and infection duration. There are also two major limitations to the use of pair formation models. One is that adding any structure to a pair formation model leads to a quickly increasing number of differential equations needed to describe the dynamics, because pairs of every combination of characteristics need to be described explicitly. The other limitation is that concurrent partnerships can only be added as instantaneous contacts. However, that may already be a good description when modelling populations where concurrency of steady partnerships is not that common (Althaus, Heijne et al., 2012). For situations where long term concurrent partnerships are important, dynamic partnerships have been incorporated into simulation models, for example to study the impact of concurrent partnerships on HIV transmission.
Pair formation models have proven very useful in modelling transmission of curable STI's and interventions. They have been used extensively to model chlamydia infections in heterosexual populations, where partnership duration can be in the order of duration of an untreated chlamydia infection. Due to the often asymptomatic nature of a chlamydia infection, much attention has been given to the possible impact of population based screening interventions. Furthermore, contact tracing is an important tool for case finding. Both re-infection within partnerships and repeated infection following cure occur regularly in young heterosexual populations. Pair formation models are capable of incorporating all those features into a relatively simple, deterministic framework, which can be readily analysed numerically.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- Althaus C.L., Heijne J.C., Herzog S.A., Roellin A., Low N. Individual and population level effects of partner notification for chlamydia trachomatis. PLoS One. 2012;7(12):e51438. doi: 10.1371/journal.pone.0051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus C.L., Heijne J.C., Roellin A., Low N. Transmission dynamics of chlamydia trachomatis affect the impact of screening programmes. Epidemics. 2010;2(3):123–131. doi: 10.1016/j.epidem.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Althaus C.L., Turner K.M.E., Schmid B.V., Heijne J.C.M., Kretzschmar M., Low N. Transmission of Chlamydia trachomatis through sexual partnerships: A comparison between three individual-based models and empirical data. Journal of the Royal Society Interface. 2012;9:136–146. doi: 10.1098/rsif.2011.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Oxford University Press; Oxford: 1991. Infectious diseases of humans. [Google Scholar]
- Batteiger B.E., Tu W., Ofner S., Van Der Pol B., Stothard D.R., Orr D.P. Repeated chlamydia trachomatis genital infections in adolescent women. The Journal of Infectious Diseases. 2010;201(1):42–51. doi: 10.1086/648734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch C., Rand D. A moment closure model for sexually transmitted disease transmission through a concurrent partnership network. Proceedings of the Royal Society of London B: Biological Sciences. 2000;267(1456):2019–2027. doi: 10.1098/rspb.2000.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.L., van Roode T., Dickson N.P., Jiang Z.J., Paul C. Consistency and reliability of self-reported lifetime number of heterosexual partners by gender and age in a cohort study. Sexually Transmitted Diseases. 2010;37(7):425–431. doi: 10.1097/OLQ.0b013e3181d13ed8. [DOI] [PubMed] [Google Scholar]
- Brewer D.D., Potterat J.J., Garrett S.B., Muth S.Q., Roberts J.M.J., Kasprzyk D. Prostitution and the sex discrepancy in reported number of sexual partners. Proceedings of the National Academy of Sciences U.S.A. 2000;97(22):12385–12388. doi: 10.1073/pnas.210392097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.I., Ghani A.C. Populations and partnerships: Insights from metapopulation and pair models into the epidemiology of gonorrhoea and other sexually transmitted infections. Sexually Transmitted Infections. 2010;86(6):433–439. doi: 10.1136/sti.2009.040238. [DOI] [PubMed] [Google Scholar]
- Chen M., Ghani A., Edmunds J. Mind the gap: The role of time between sex with two consecutive partners on the transmission dynamics of gonorrhea. Sexually Transmitted Diseases. 2008;35:435–444. doi: 10.1097/OLQ.0b013e3181612d33. [DOI] [PubMed] [Google Scholar]
- Clarke J., White K., Turner K. Exploring short-term responses to changes in the control strategy for chlamydia trachomatis. Computational and Mathematical Methods in Medicine. 2012;2012 doi: 10.1155/2012/803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O., Heesterbeek J.A.P., Britton T. Princeton University Press; 2012. Mathematical tools for understanding infectious disease dynamics, princeton series in theoretical and computational biology. [Google Scholar]
- Dietz K., Hadeler K.P. Epidemiological models for sexually transmitted diseases. Journal of Mathematical Biology. 1988;26(1):1–25. doi: 10.1007/BF00280169. [DOI] [PubMed] [Google Scholar]
- Dietz K., Tudor D. Triangles in heterosexual hiv transmission. In: Jewell N., Dietz K., Farewell V., editors. AIDS epidemiology: methodological issues. Birkhauser; Boston: 1992. p. 143. [Google Scholar]
- Eames K.T., Keeling M.J. Modeling dynamic and network heterogeneities in the spread of sexually transmitted diseases. Proceedings of the National Academy of Sciences. 2002;99(20):13330–13335. doi: 10.1073/pnas.202244299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2013. Annual epidemiological report 2013. reporting on 2011 surveillance data and 2012 epidemic intelligence data. 2013. ecdc: Stockholm., Tech. rep. [Google Scholar]
- Ferguson N.M., Garnett G.P. More realistic models of sexually transmitted disease transmission dynamics: Sexual partnership networks, pair models, and moment closure. Sexually Transmitted Diseases. 2000;27(10):600–609. doi: 10.1097/00007435-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Foxman B., Newman M., Percha B., Holmes K.K., Aral S.O. Measures of sexual partnerships: Lengths, gaps, overlaps, and sexually transmitted infection. Sexually Transmitted Diseases. 2006;33(4):209–214. doi: 10.1097/01.olq.0000191318.95873.8a. [DOI] [PubMed] [Google Scholar]
- Hadeler K.P. Pair formation. Journal of Mathematical Biology. 2012;64(4):613–645. doi: 10.1007/s00285-011-0454-0. [DOI] [PubMed] [Google Scholar]
- Hadeler K.P., Waldstätter R., Wörz-Busekros A. Models for pair formation in bisexual populations. Journal of Mathematical Biology. 1988;26(6):635–649. doi: 10.1007/BF00276145. [DOI] [PubMed] [Google Scholar]
- Heijne J.C., Althaus C.L., Herzog S.A., Kretzschmar M.E., Low N. The role of reinfection and partner notification in the efficacy of chlamydia screening programs. Journal of Infectious Diseases. 2011;203(3):372–377. doi: 10.1093/infdis/jiq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijne J.C., Herzog S.A., Althaus C.L., Low N., Kretzschmar M.E. Case and partnership reproduction numbers for a curable sexually transmitted infection. Journal of Theoretical Biology. 2013;331:38–47. doi: 10.1016/j.jtbi.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Heijne J.C., Herzog S.A., Althaus C.L., Tao G., Kent C.K., Low N. Insights into the timing of repeated testing after treatment for chlamydia trachomatis: Data and modelling study. Sexually Transmitted Infections. 2013;89(1):57–62. doi: 10.1136/sextrans-2011-050468. [DOI] [PubMed] [Google Scholar]
- Heijne J.C., van Liere G.A., Hoebe C.J., Bogaards J.A., van Benthem B.H., Dukers-Muijrers N.H. What explains anorectal chlamydia infection in women? implications of a mathematical model for test and treatment strategies. Sexually Transmitted Infections. 2016;93(4):270–275. doi: 10.1136/sextrans-2016-052786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.K. 4th ed. McGraw-Hill professional, McGraw-Hill Education; 2008. Sexually transmitted diseases. [Google Scholar]
- Johnson A.M., Mercer C.H., Erens B., Copas A.J., McManus S., Wellings K. Sexual behaviour in britain: Partnerships, practices, and hiv risk behaviours. Lancet. 2001;358(9296):1835–1842. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- Keeling M.J., House T., Cooper A.J., Pellis L. Systematic approximations to susceptible-infectious-susceptible dynamics on networks. PLOS Computational Biology. 2016;12(12):e1005296. doi: 10.1371/journal.pcbi.1005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M.J., Rohani P. Princeton University Press; Princeton and Oxford: 2008. Modeling infectious diseases in humans and animals. [Google Scholar]
- Kong F.Y., Tabrizi S.N., Law M., Vodstrcil L.A., Chen M., Fairley C.K. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: A meta-analysis of randomized controlled trials. Clinical Infectious Diseases. 2014;59(2):193–205. doi: 10.1093/cid/ciu220. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M.E. Deterministic and stochastic pair formation models for the spread of sexually transmitted diseases. Journal of Biological Systems. 1995;3(03):789–801. [Google Scholar]
- Kretzschmar M.E., Dietz K. The effect of pair formation and variable infectivity on the spread of an infection without recovery. Mathematical Biosciences. 1998;148(1):83–113. doi: 10.1016/s0025-5564(97)10008-6. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M.E., Jager J.C., Reinking D.P., Van Zessen G., Brouwers H. The basic reproduction ratio for a sexually transmitted disease in a pair formation model with two types of pairs. Mathematical Biosciences. 1994;124(2):181–205. doi: 10.1016/0025-5564(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M.E., Turner K.M.E., Barton P.M., Edmunds W.J., Low N. Predicting the population impact of chlamydia screening programmes: Comparative mathematical modelling study. Sexually Transmitted Infections. 2009;85(5):359–366. doi: 10.1136/sti.2009.036251. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M.E., van Duynhoven Y.T., Severijnen A.J. Modeling prevention strategies for gonorrhea and chlamydia using stochastic network simulations. American Journal of Epidemiology. 1996;144(3):306–317. doi: 10.1093/oxfordjournals.aje.a008926. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M.E., Welte R., van den Hoek A., Postma M.J. Comparative model-based analysis of screening programs for chlamydia trachomatis infections. American Journal of Epidemiology. 2001;153(1):90–101. doi: 10.1093/aje/153.1.90. [DOI] [PubMed] [Google Scholar]
- Leung K.Y., Kretzschmar M.E. Concurrency can drive an hiv epidemic by moving R0 across the epidemic threshold. Aids. 2015;29(9):1097–1103. doi: 10.1097/QAD.0000000000000676. [DOI] [PubMed] [Google Scholar]
- Leung K.Y., Kretzschmar M.E., Diekmann O. Dynamic concurrent partnership networks incorporating demography. Theoretical Population Biology. 2012;82(3):229–239. doi: 10.1016/j.tpb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Leung K.Y., Kretzschmar M.E., Diekmann O. Si infection on a dynamic partnership network: Characterization of r0. Journal of Mathematical Biology. 2015;71(1):1–56. doi: 10.1007/s00285-014-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., Getz W.M., Westerhoff H.V. Frequency–dependent incidence in models of sexually transmitted diseases: Portrayal of pair–based transmission and effects of illness on contact behaviour. Proceedings of the Royal Society of London B: Biological Sciences. 2004;271(1539):625–634. doi: 10.1098/rspb.2003.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low N., Cassell J.A., Spencer B., Bender N., Hilber A.M., van Bergen J. Chlamydia control activities in europe: Cross-sectional survey. The European Journal of Public Health. 2012;22(4):556–561. doi: 10.1093/eurpub/ckr046. [DOI] [PubMed] [Google Scholar]
- Low N., McCarthy A., Macleod J., Salisbury C., Campbell R., Roberts T.E. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technology Assessment (Winchester, England) 2007;11(8) doi: 10.3310/hta11080. (iii–iv) [DOI] [PubMed] [Google Scholar]
- Miller J.C., Slim A.C., Volz E.M. Edge-based compartmental modelling for infectious disease spread. Journal of the Royal Society Interface. 2012;9(70):890–906. doi: 10.1098/rsif.2011.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.C., Volz E.M. Model hierarchies in edge-based compartmental modeling for infectious disease spread. Journal of Mathematical Biology. 2013;67(4):869–899. doi: 10.1007/s00285-012-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. Telling tails explain the discrepancy in sexual partner reports. Nature. 1993;365(6445):437–440. doi: 10.1038/365437a0. [DOI] [PubMed] [Google Scholar]
- Nelson S.J., Hughes J.P., Foxman B., Aral S.O., Holmes K.K., White P.J. Age-and gender-specific estimates of partnership formation and dissolution rates in the seattle sex survey. Annals of Epidemiology. 2010;20(4):308–317. doi: 10.1016/j.annepidem.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J.B., Fu X., Lee G.K., Chen M.I. Comparability of results from pair and classical model formulations for different sexually transmitted infections. PLoS One. 2012;7(6):e39575. doi: 10.1371/journal.pone.0039575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterat J.J., Zimmerman-Rogers H., Muth S.Q., Rothenberg R.B., Green D.L., Taylor J.E. Chlamydia transmission: Concurrency, reproduction number, and the epidemic trajectory. American Journal of Epidemiology. 1999;150:1331–1339. doi: 10.1093/oxfordjournals.aje.a009965. [DOI] [PubMed] [Google Scholar]
- Powers K.A., Ghani A.C., Miller W.C., Hoffman I.F., Pettifor A.E., Kamanga G. The role of acute and early HIV infection and implication for transmission prevention strategies in Lilongwe, Malawi: A modeling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.J., Ades A.E., De Angelis D., Welton N.J., Macleod J., Soldan K. Mixture-of-exponentials models to explain heterogeneity in studies of the duration of chlamydia trachomatis infection. Statistics in Medicine. 2013;32(9):1547–1560. doi: 10.1002/sim.5603. [DOI] [PubMed] [Google Scholar]
- Turner K.M.E., Adams E.J., Gay N., Ghani A.C., Mercer C., Edmunds W.J. Developing a realistic sexual network model of chlamydia transmission in britain. Theoretical Biology and Medical Modelling. 2006;3(1):3. doi: 10.1186/1742-4682-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde N., Brisson M., Boily M.C. Understanding differences in predictions of hpv vaccine effectiveness: A comparative model-based analysis. Vaccine. 2010;28(33):5473–5484. doi: 10.1016/j.vaccine.2010.05.056. [DOI] [PubMed] [Google Scholar]
- Xiridou M., Geskus R., Wit J.d., Coutinho R.A., Kretzschmar M.E. The contribution of steady and casual partnerships to the incidence of hiv infection among homosexual men in amsterdam. AIDS. 2003;17:1029–1038. doi: 10.1097/00002030-200305020-00012. [DOI] [PubMed] [Google Scholar]
- Xiridou M., Geskus R., Wit J.d., Coutinho R.A., Kretzschmar M.E. Primary hiv infection as source of hiv transmission within steady and casual partnerships among homosexual men. AIDS. 2004;18:1311–1320. doi: 10.1097/00002030-200406180-00010. [DOI] [PubMed] [Google Scholar]