Abstract
Background
Injection of a clot into the internal carotid artery is an experimental model of ischemic stroke that is considered to closely mimic embolic stroke in humans. In this model, the common carotid artery typically remains temporarily occluded to permit time for stabilization of the clot in the middle cerebral artery. However, the associated lengthening of the anesthesia duration could affect arterial blood pressure and stroke outcome.
New method
We refined the model by examining how increasing isoflurane anesthesia duration from 30 to 60 min after clot embolization affects mortality, infarct volume, edema, blood-brain barrier permeability, and the 8-h post-ischemic time course of blood pressure, which has not been reported previously in this model.
Results
We found that arterial pressure increased after discontinuing anesthesia in both embolized groups and that the increase was greater than in the corresponding non-embolized sham-operated rats. At 24 h, the group with 60-min post-ischemia anesthesia exhibited greater brain water content and a greater ipsilateral-to-contralateral ratio of extravasated Evans blue dye. Mortality was greater in the 60-min group, but infarct volume among survivors was not different from that in the 30-min anesthesia group.
Comparison with existing methods
This study refines the embolic stroke model by demonstrating the importance of minimizing the duration of anesthesia after embolization.
Conclusions
These data indicate that early discontinuation of isoflurane anesthesia after clot embolization permits an earlier hypertensive response that limits edema formation and mortality without significantly affecting infarct volume in survivors, thereby decreasing the required number of animals.
Keywords: animal model of stroke, embolic stroke, isoflurane anesthesia, middle cerebral artery occlusion, rat
1. Introduction
Injection of a blood clot into the internal carotid artery (ICA) of rodents has become increasingly used as a preclinical translation model to mimic cardioembolic stroke (Herson and Traystman, 2014). The technique typically involves temporary occlusion of the common carotid artery (CCA) after clot injection to permit intracranial stabilization of the clot position (Zhang et al., 1997a). However, extending the duration of CCA occlusion also extends the duration of anesthesia which, in turn, may influence hemodynamics. Clinically, arterial blood pressure often increases during the acute phase of ischemic stroke onset. Depending on the patency of collateral vessels, penumbral blood flow may be increased by the acute hypertensive response. With temporary mechanical arterial occlusion, increases in arterial blood pressure can partially restore cerebral blood flow (Drummond et al., 1989) and decrease infarct volume (Chileuitt et al., 1996), whereas decreases in arterial pressure decrease cerebral blood flow in the ischemic territory (Cole et al., 1990b). In contrast to the effects of temporary occlusion, the effect of blood pressure response has not been as well studied in the clot embolization model. Because the level of arterial blood pressure in experimental stroke is also influenced by the depth of anesthesia, the duration of anesthesia may influence outcome in the embolic stroke model. In support of this possibility, others have reported a 30% mortality and a 47% coefficient of variation in 24-h infarct volume among rats maintained on anesthesia for 2 h after embolization for the purpose of imaging (Beech et al., 2001).
The blood pressure response after anesthesia discontinuation has not been examined in the embolic clot model. We hypothesized that the association of an increased mortality with duration of anesthesia in the embolic clot model of stroke is related to anesthesia-induced low systemic arterial pressure during the early stage of cerebral ischemia. Therefore, the goals of this study were to investigate 1) the time course of change in mean arterial blood pressure (MABP) over an 8-h period after embolization, 2) 24-h mortality and cerebral infarction, 3) change in blood-brain barrier (BBB) permeability, and 4) brain edema formation after clot embolization with two different durations of post-stroke anesthesia. We compared groups exposed to an additional anesthesia duration of either 30 or 60 min after embolization, during which MABP was kept steady at baseline pre-stroke levels.
2. Materials and Methods
2.1. General preparation
All procedures on animals were approved by the Johns Hopkins University Animal Care and Use Committee and conformed to the NIH guidelines for the use of animals in research. A total of 202 male Wistar rats, weighing 400-450 g, were studied. Anesthesia was induced with 4% isoflurane in 35% oxygen-air mixture and maintained with 1.5–2.25% isoflurane. The right femoral artery was cannulated with a polyethylene-50 catheter and exteriorized through a swivel device for continuous monitoring of arterial blood pressure. For those rats in the study of BBB permeability, a femoral vein was also cannulated for injection of Evans blue dye. Rectal temperature was maintained at 37–38°C with a heating lamp.
2.2. Middle cerebral artery occlusion (MCAo)
The right MCA was occluded by placement of an embolus at its origin, according to the method by Zhang et al. (Zhang et al., 1997a) with modifications. Briefly a single intact, fibrin-rich, 24-h-old homologous clot (55 mm in length) was injected slowly via a polyethylene-8 catheter (ID, 0.203 mm; OD, 0.355 mm; ICA OD, 0.56 mm) placed in the right ICA with its tip 1–2 mm proximal to the origin of the MCA. The right and left CCAs were temporarily clipped during the injection to reduce blood flow (Chen et al., 1986) and thereby stabilize the position of the embolus. Twenty-five minutes after clot injection, the left CCA clip was removed, followed 3 minutes later by withdrawal of the polyethylene-8 catheter and release of the right CCA clip.
2.3. Assessment of BBB permeability
BBB permeability was assessed by the Evans blue extravasation method of Uyama et al. (Uyama et al., 1988). Briefly, a solution of 2% Evans blue in normal saline was infused intravenously at a dose of 3 ml/kg 4 h after MCAo. Two hours after the dye injection, rats were perfused intracardially with phosphate-buffered saline via the left ventricle under deep anesthesia. Perfusion continued until the fluid from the right atrium became colorless. Rats were then decapitated, and brains were quickly removed and divided into right (ischemic) and left (nonischemic) hemispheres. Each hemisphere was weighed and homogenized in 4 ml of 50% trichloroacetic acid solution. After centrifugation at 10,000g for 30 min, the supernatants were collected. The Evans blue concentration was determined with a spectrophotometer at 620 nm for absorbance against a standard curve. Evans blue extravasation was expressed as the ratio of absorbance intensity in the ischemic hemisphere to that in the nonischemic hemisphere (Evans blue extravasation index).
2.4. Assessment of brain edema
Eight hours after MCAo, rats were killed by decapitation under deep anesthesia. The brain was quickly removed and divided into right (ischemic) and left (nonischemic) hemispheres. The hemispheres were weighed immediately to obtain the wet weight (WW). Then the samples were dried at 110°C for 24 h to obtain their dry weight (DW). Water content was calculated as % H2O = 100 × (wet weight – dry weight/wet weight).
2.5. Study protocol
Rats subjected to MCAo were randomly assigned to have either 30 min (Group 1) or 60 min (Group 2) of anesthesia after MCAo. During this 30- or 60-min period, MABP was kept near baseline pre-stroke levels (95.4 ± 10.2 mmHg; ±SD) by adjusting inspired isoflurane concentration (from 1.73 ± 0.20% at baseline to 1.77 ± 0.17% at onset of MCAo). For rats in Group 1, anesthesia was terminated after establishment of carotid circulation and wound closure (1.81 ± 0.24% isoflurane before termination). For rats in Group 2, anesthesia was continued for an additional 30 min after establishment of carotid circulation (1.84 ± 0.24% isoflurane before termination). In some experiments sham surgery was performed with the corresponding additional 30 or 60 min of anesthesia. Sham surgery consisted of all surgical procedures except for injection of the blood clot.
Three sets of experiments were performed. In the first set, we assessed infarct volume and mortality 24 h after embolization in 70 rats from Group 1 and 70 rats from Group 2. We used the triphenyl tetrazolium staining method for infarct volume determination on 7 coronal sections of brain.
In the second set of experiments, we examined the time course of changes in MABP in 44 rats. Of these, 11 rats in Group 1 and 11 rats in Group 2 were subjected to MCAo and 8 rats in Group 1 and 8 rats in Group 2 were subjected to sham surgery. MABP was recorded every 10 min for the first 2 h, every 30 min for the next 2 h, and then hourly until 8 h after embolization. An additional group of 6 rats had only a short duration of anesthesia during which they underwent femoral artery cannulation for continuous measurement of MABP. These rats served as a naïve normal control group. At the end of the monitoring period, brain water content was measured in all 44 rats.
In the third set of experiments, we examined the integrity of the BBB in rats subjected to the two different anesthetic durations after sham surgery or MCAo (n = 6 per group). Tissue was harvested at 6 h after embolization (2 h after Evan’s blue dye injection) for assessment of BBB permeability.
2.6. Statistical analysis
Mortality between the two groups was compared with the χ2 test. Infarct volume in Group 1 and Group 2 was compared by the Mann-Whitney test and displayed as box-whisker plots. Two-way analysis of variance (ANOVA) with repeated measures was used to assess the effect of sham surgery and MCAo on MABP over time after anesthesia discontinuation. Multiple post hoc comparisons were made with the Holm-Sidak procedure. Other measurements in the sham and MCAo groups were compared by ANOVA and t-test. These data are displayed as means ± 95% confidence intervals. A significance level of 0.05 was used in all tests.
3. Results
3.1. Mortality
In Group 1, which had 30 min of post-embolization anesthesia, 16 of 70 rats died between 8 and 16 h after embolization (22.8% mortality), whereas in Group 2, which had 60 min of post-embolization anesthesia, 36 of 70 rats died (51.4% mortality). These mortality rates differed significantly.
3.2. Infarction
Blood clot injection led to infarctions in cerebral cortex and striatum (Figure 1). Infarct volumes did not differ between the surviving animals of Group 1 (n = 54) and those of Group 2 (n = 34) in the cortex (24.1 ± 21.6%; ± SD vs. 29.4 ± 26.0%), striatum (31.9 ± 26.4% vs. 37.5 ± 30.8%), or cerebral hemisphere (17.1 ± 17.2% vs. 21.8 ± 20.3%). Likewise, infarct volumes did not differ between the non-survivors of Group 1 (n = 16) and the non-survivors of Group 2 (n = 36) in the cortex (37.0 ± 19.3%; ± SD vs. 43.0 ± 22.1%), striatum (56.0 ± 17.5% vs. 49.5 ± 24.2%), or hemisphere (30.7 ± 15.6% vs. 34.7 ± 17.5%). However, the Mann-Whitney rank sum test indicated significant differences in infarct volume between the surviving and non-surviving rats within Group 1 in cortex (p = 0.023), striatum (p = 0.001), and hemisphere (p = 0.002) and within Group 2 in cortex (p = 0.02) and hemisphere (p = 0.003). We saw a trend toward difference between survivors and non-survivors in Group 2 for infarct volume in striatum (p = 0.08).
Figure 1.
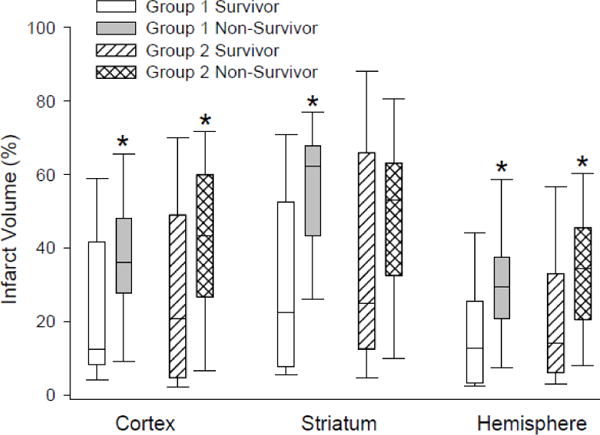
Box-whisker plots of infarct volume in survivors (n = 54) and non-survivors (n = 16) among Group 1 rats subjected to 30 min of anesthesia after embolization and in survivors (n = 34) and non-survivors (n = 36) among Group 2 rats subjected to 60 min of anesthesia after embolization. *p < 0.05 from corresponding survivor group by the Mann-Whitney rank sum test.
3.3. MABP time course
In the normal control group of rats with brief anesthesia for arterial catheterization, MABP returned to stable levels of 115–120 mmHg 30 min after termination of anesthesia and remained in this range throughout the 8-h study period (Figure 2). In both groups of sham-operated and MCAo rats, MABP increased significantly after termination of anesthesia and remained elevated throughout the 8-h observation period compared with that in the period just prior to anesthesia discontinuation. Beyond 30 min of recovery from anesthesia, MABP levels in both sham groups were indistinguishable from that in the normal control group. In contrast, termination of anesthesia in MCAo rats produced larger increases in MABP than in the corresponding sham rats. In the Group 1 embolized rats, MABP rebounded sharply to 140-150 mmHg within 20 min of anesthesia termination and remained significantly higher than that in control and sham-operated rats throughout the remaining study period. Importantly, the MABP at 40, 50, and 60 min after embolization in Group 1 (i.e., first 30 min of recovery from anesthesia) was significantly greater than that at the corresponding times in Group 2 when these rats remained anesthetized. Upon termination of anesthesia, MABP in the Group 2 embolized rats also rebounded, but the level of hypertension was significantly less than that of Group 1 rats through 110 min from the time of embolization (i.e., 40 min of recovery from anesthesia). Moreover, the MABP in the Group 2 embolized rats was no longer significantly greater than the values in the Group 2 sham groups beyond 1 h of recovery from anesthesia.
Figure 2.

Time course of mean arterial pressure over 480 min after clot embolization (time = 0 min) with continued anesthesia for an additional 30 min (A; n = 11) or 60 min (B; n = 11). Corresponding surgical sham groups received an additional 30 min (A; n = 8) or 60 min (B; n = 8) of anesthesia. Vertical lines indicate cessation of anesthesia for sham and clot embolization groups. An additional control group (n = 6) with a short duration of anesthesia ending at time = 0 min is also shown in A and B. Data are expressed as mean ± 95% confidence interval; *p < 0.05 from control group; †p <0.05 from corresponding anesthesia group after sham surgery; ‡p < 0.05 from 30 min anesthesia group at the same time after embolization.
3.4. BBB permeability
All rats that received Evans blue dye at 4 h after MCAo survived the additional 2 h before tissue measurements were made of the dye concentration. Evans blue concentration in the hemisphere ipsilateral to the embolization was greater than that in the contralateral hemisphere and greater than that in sham-operated rats (Figure 3). The concentration in the ipsilateral hemisphere did not differ significantly between Group 1 and Group 2. However, when normalized by the contralateral hemisphere concentration within the same rat, the ipsilateral/contralateral concentration ratio was significantly greater in Group 2 (4.15 ± 1.51; ± SD) than in Group 1 (2.22 ± 1.03; p<0.03).
Figure 3.
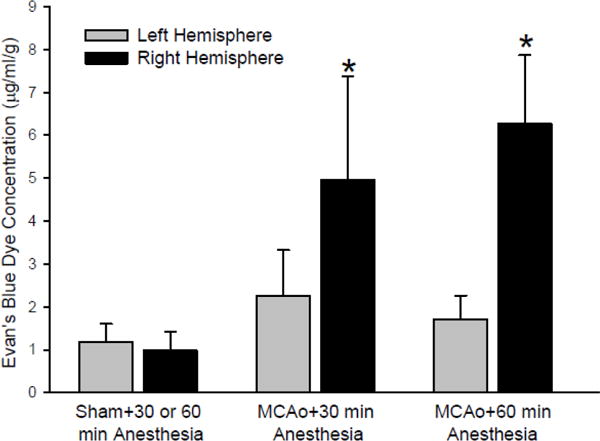
Evan’s blue dye concentration in cerebral hemispheres contralateral (left) and ipsilateral (right) to sham surgery or to clot embolization followed by 30 (n = 6) or 60 (n = 6) min of anesthesia. The dye was injected at 4 h after surgery/embolization, and tissue was analyzed at 6 h. Because there was no difference in sham groups exposed to 30 or 60 min of additional anesthesia, these groups were combined into a single sham group (n = 12) for statistical purposes. Data are expressed as mean ± 95% confidence interval; *p < 0.05 from sham.
3.5. Brain water content
In both sham-operated groups, brain water content in each hemisphere was similar to that in the normal control group (Figure 4). In Group 1 rats with MCAo, brain water content was moderately increased ipsilateral to the embolization (p<0.05). However in Group 2 rats with MCAo, the increase on the ipsilateral side was markedly enhanced compared to that in Group 1 rats (p<0.01; Figure 4).
Figure 4.

Water content of cerebral hemispheres contralateral (left) and ipsilateral (right) to sham surgery or clot embolization followed by an additional 30 or 60 min of anesthesia (sham surgery, n = 8 per group; embolization, n = 11 per group). Measurements were made at 8 h after surgery/embolization in the same rats that underwent blood pressure monitoring. A control group (n = 6) with a short duration of anesthesia is also shown. Data are expressed as mean ± 95% confidence interval; *p < 0.05 from corresponding anesthesia group after sham surgery; #p < 0.05 from 30 min anesthesia group after clot embolization.
4. Discussion
The present study shows that extending the anesthesia time after MCA clot embolization delays the period of ischemia-induced hypertension in the acute stage of stroke and that this delay is associated with greater mortality, edema formation, and BBB permeability normalized to the contralateral hemisphere. Furthermore, mortality was associated with larger infarcts. Thus, the extended duration of anesthesia can increase the incidence of malignant stroke. The most likely explanation is that delaying hypertension kept collateral blood flow low and recruited more rats into the malignant stroke phenotype than otherwise would have been if arterial pressure had been allowed to increase sooner.
Although many studies have investigated the pathophysiologic changes that occur after occlusion of the MCA in rat, all were performed under anesthesia and blood pressure was not always recorded (Beech et al., 2001; Busch et al., 1997; Henninger et al., 2006; Morris et al., 2003; Zhang et al., 1997a; Zhang et al., 1997b). To our knowledge, the time course of blood pressure changes after embolic MCAo and discontinuation of anesthesia in rats has not been previously reported. In rats with MCA embolization, we observed an elevation of blood pressure (reactive hypertension) after discontinuing anesthesia. This increase was greater than that seen after discontinuing anesthesia in the surgical sham-operated rats, suggesting an influence of ischemia and not simply the surgery. The magnitude of reactive hypertension was more marked after 30 min than after 60 min of post-embolization anesthesia. A similar observation has been reported when comparing the initial reactive hypertension after brief anesthesia versus 3 h of anesthesia following mechanical MCAo (Hashimoto et al., 2008).
The MABP recorded from normal awake rats in our study ranged between 112 and 124 mmHg, which is similar to the range of 111–129 mmHg reported in the literature (Hinojosa-Laborde et al., 1989; Sokrab and Johansson, 1989). MABP under a surgical plane of anesthesia (95–105 mmHg) was significantly lower than that in a normal awake state. Under normal circumstances, with an intact autoregulatory response, this level of blood pressure would be within the autoregulatory range and would not have a significant effect on cerebral blood flow. However, with the development of ischemia, the autoregulatory response is attenuated or lost, and cerebral blood flow in the ischemic area then depends on blood pressure (Dirnagl and Pulsinelli, 1990). Such a moderately low arterial pressure relative to awake values may not be adequate to provide sufficient blood flow to the ischemic penumbra that is not yet irreversibly injured between 30 and 60 min of embolization. Moreover, the reactive hypertension upon termination of anesthesia was greater in Group 1 than in Group 2. Our results suggest that this increase in arterial pressure at the early stage of cerebral ischemia may have limited malignant brain swelling.
The duration that the penumbra can remain reversible in focal ischemia is reported to be 4–8 h in the monkey (Crowell et al., 1981) but is thought to be only 2–3 h in the rat (Hashimoto et al., 2008; Memezawa et al., 1992). In the filament model of 60 min of MCAO and reperfusion, Zhu and Auer (Zhu and Auer, 1995) showed that very little cortical necrosis occurred in rat when MABP was maintained at 80 mmHg, whereas infarction progressively enlarged as MABP was maintained at progressively lower levels. Various laboratory investigations have shown that the infarction depends on the amount of blood flow during ischemia (Dirnagl et al., 1989; Jacewicz et al., 1992) and the duration of ischemia (Cole et al., 1990a; Miyabe et al., 1996). Penumbra blood flow through collateral channels can be increased by increasing the perfusion pressure (Aspey et al., 1987; Astrup et al., 1977). Induction of hypertension to improve local cerebral blood flow during ischemia has been studied primarily in the rat model of temporary mechanical MCAo. Pharmacologically increasing systemic blood pressure 40–60% above baseline pressure for 15 to 120 min either during ischemia or after reperfusion onset has been shown to decrease infarction. For example, Drummond et al. (Drummond et al., 1989) showed that 15 min of phenylephrine-induced hypertension immediately after permanent MCAo in rats acutely improved local cerebral blood flow and reduced the extent of brain regions in which regional blood flow was below levels that might result in neuronal death. In a short-term study, Aspey et al. (Aspey et al., 1987) showed that an increase in MABP over the first 2 h after permanent occlusion of the MCA prevented a 100% rise in hemispheric lactate in rats. Delayed institution of hypertension 60 min after MCAo was also effective at increasing cerebral blood flow in both ischemic core and penumbra (Shin et al., 2008). Our study differs from previous ones in that a clot was used to occlude the MCA and MABP was not raised pharmacologically, but simply by terminating anesthesia. The finding that early termination of anesthesia reduced the number of deaths by allowing blood pressure to rise is consistent with arterial hypertension increasing collateral blood flow.
Isoflurane anesthesia permits animals to regain consciousness and mobility quickly after ischemia induction and currently is the most commonly used anesthetic in rodent studies of stroke. It should be noted that isoflurane decreases cerebral energy metabolism and the number of peri-ischemic depolarizations (Zhao and Nowak, 2015), and it can exert direct protective effects during transient focal ischemia of 50–80-min duration compared to removal of isoflurane immediately after induction of MCAo (Sakai et al., 2007). However, our results in the embolic model of prolonged focal ischemia suggest that any benefit derived from extending the duration of isoflurane exposure by an additional 30 min is offset by the delay in post-anesthetic hypertension.
With the increased use of mechanical thrombectomy for clinical stroke, the question arises as to whether general anesthesia or conscious sedation should be used during the procedure. Two prospective trials failed to find a worse outcome with general anesthesia, as long as hypotension was prevented with pharmacological agents (Lowhagen Henden et al., 2017; Schonenberger et al., 2016). It should be noted that our experiments do not model the use of thrombectomy and that induction of general anesthesia in these clinical studies is typically delayed 3 h or longer from the onset of symptoms. Our study focused on the first hour of stroke when the MABP may play a crucial role in subsequent injury severity.
Postmortem analysis of infarct volume in our study indicated a greater infarction in non-survivors than in survivors. The smaller infarct size in the survivors most likely reflects a smaller volume-at-risk arising from a greater number and/or larger collateral arteries. Interestingly, the difference in infarct volume between survivors and non-survivors was independent of anesthesia duration. All non-survivors had a hemispheric infarct greater than 30% regardless of their anesthesia time. This value seems to reflect the fatal threshold in this model. Presumably, the non-survivors represent a subpopulation of rats with low collateral blood flow, analogous to the heterogeneity in collateral blood flow seen in clinical stroke.
It is interesting to note that cortical and striatal infarct volumes did not differ between survivors, regardless of whether they received 30 or 60 min of anesthesia after embolization. This result suggests that those that can survive the early brain swelling will develop infarcts independent of the additional 30 min of relative hypotension during the immediate period after embolization. Presumably, the prolonged ischemia associated with clot embolization eventually causes the infarct to expand into the penumbra in those that can survive the early brain swelling, resulting in an infarct size that is independent of the early MABP response. Others have shown that brief post-occlusion anesthesia resulted in smaller infarct volume compared to 3 h post-occlusion anesthesia when permanent occlusion was produced at a distal MCA site but not when produced at a proximal MCA site (Hashimoto et al., 2008). Because the clot produces proximal MCAo in our model, the lack of effect of 30 versus 60-min post-occlusion anesthesia in our model is consistent with this prior study of proximal MCAo.
A limitation of the clot model in the rat is that infarct volume had a high coefficient of variation, likely as a result of high variability in collateral blood flow in these animals. Such variability can be reduced by using specific strains of mice. We did not evaluate mice in this study because of the technical difficulty of monitoring arterial pressure for 8 h in awake mice.
In conclusion, our study shows that 24-h mortality is greatly reduced by simply shortening the anesthesia time to allow blood pressure to rise. The study further suggests that the edema formed within 8 h of embolization appears to depend on the systemic arterial blood pressure during the first hour after embolization. These results emphasize that arterial blood pressure is an important parameter to consider when designing an embolic stroke experiment.
Highlights.
Injection of a blood clot into the internal carotid artery more closely mimics clinical ischemic stroke than most other models, but the common carotid artery is usually occluded for 30–60 min to stabilize the clot while the animal remains anesthetized. Here, we refined the model by demonstrating a substantial benefit of limiting the post-embolization anesthesia to 30 minutes.
Blood pressure increased after anesthesia discontinuance and remained elevated over an 8-hour monitoring period compared to sham controls, but the increase was delayed and of smaller magnitude when anesthesia was sustained for 60 minutes.
Extending the post-embolization anesthesia to 60 minutes increased blood-brain barrier permeability, cerebral edema, and mortality.
Limiting the anesthesia duration to 30 minutes will reduce animal usage.
Acknowledgments
This work was supported by National Institutes of Health grants NS060703, NS038684, and HL139543 (to R. C. Koehler).
Abbreviations
- BBB
blood-brain barrier
- CCA
common carotid artery
- ICA
internal carotid artery
- MABP
mean arterial blood pressure
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
B. T. C. Chuang assisted in the performance of the experiments and data analysis. X. Liu performed the experiments with Evan’s blue dye, A. J. Lundberg assisted in the data analysis, T. J. K. Toung performed the experiments and drafted the manuscript, J. A. Ulatowski helped in the conceptual development of the experiments and in editing the manuscript. R. C. Koehler helped in the statistical analysis and writing the manuscript.
Competing Financial Interests
The authors declare that they have no conflict of interest.
References
- Aspey BS, Ehteshami S, Hurst CM, McCoy AL, Harrison MJ. The effect of increased blood pressure on hemispheric lactate and water content during acute cerebral ischaemia in the rat and gerbil. J Neurol Neurosurg Psychiatry. 1987;50:1493–8. doi: 10.1136/jnnp.50.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–7. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Beech JS, Williams SC, Campbell CA, Bath PM, Parsons AA, Hunter AJ, Menon DK. Further characterisation of a thromboembolic model of stroke in the rat. Brain Res. 2001;895:18–24. doi: 10.1016/s0006-8993(00)03331-x. [DOI] [PubMed] [Google Scholar]
- Busch E, Kruger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997;778:16–24. doi: 10.1016/s0006-8993(97)01008-1. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction 1. Stroke. 1986;17:738–43. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Chileuitt L, Leber K, McCalden T, Weinstein PR. Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats. Surg Neurol. 1996;46:229–34. doi: 10.1016/0090-3019(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Drummond JC, Ghazal EA, Shapiro HM. A reversible component of cerebral injury as identified by the histochemical stain 2,3,5-triphenyltetrazolium chloride (TTC) Acta Neuropathol. 1990a;80:152–5. doi: 10.1007/BF00308918. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Drummond JC, Shapiro HM, Zornow MH. Influence of hypotension and hypotensive technique on the area of profound reduction in cerebral blood flow during focal cerebral ischaemia in the rat. Br J Anaesth. 1990b;64:498–502. doi: 10.1093/bja/64.4.498. [DOI] [PubMed] [Google Scholar]
- Crowell RM, Marcoux FW, DeGirolami U. Variability and reversibility of focal cerebral ischemia in unanesthetized monkeys. Neurology. 1981;31:1295–302. doi: 10.1212/wnl.31.10.1295. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab. 1989;9:589–96. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cereb Blood Flow Metab. 1990;10:327–36. doi: 10.1038/jcbfm.1990.61. [DOI] [PubMed] [Google Scholar]
- Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke. 1989;20:1538–44. doi: 10.1161/01.str.20.11.1538. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Zhao L, Nowak TS., Jr Temporal thresholds for infarction and hypothermic protection in Long-Evans rats: factors affecting apparent ‘reperfusion injury’ after transient focal ischemia. Stroke. 2008;39:421–6. doi: 10.1161/STROKEAHA.107.495788. [DOI] [PubMed] [Google Scholar]
- Henninger N, Sicard KM, Schmidt KF, Bardutzky J, Fisher M. Comparison of ischemic lesion evolution in embolic versus mechanical middle cerebral artery occlusion in Sprague Dawley rats using diffusion and perfusion imaging. Stroke. 2006;37:1283–7. doi: 10.1161/01.STR.0000217223.72193.98. [DOI] [PubMed] [Google Scholar]
- Herson PS, Traystman RJ. Animal models of stroke: translational potential at present and in 2050. Future Neurol. 2014;9:541–51. doi: 10.2217/fnl.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Osborn JW, Jr, Cowley AW., Jr Hemodynamic effects of endothelin in conscious rats. Am J Physiol Heart Circ Physiol. 1989;256:H1742–6. doi: 10.1152/ajpheart.1989.256.6.H1742. [DOI] [PubMed] [Google Scholar]
- Jacewicz M, Tanabe J, Pulsinelli WA. The CBF threshold and dynamics for focal cerebral infarction in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12:359–70. doi: 10.1038/jcbfm.1992.53. [DOI] [PubMed] [Google Scholar]
- Lowhagen Henden P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, Dunker D, Schnabel K, Wikholm G, Hellstrom M, Ricksten SE. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: The AnStroke Trial (Anesthesia during Stroke) Stroke. 2017;48:1601–7. doi: 10.1161/STROKEAHA.117.016554. [DOI] [PubMed] [Google Scholar]
- Memezawa H, Smith ML, Siesjo BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke. 1992;23:552–9. doi: 10.1161/01.str.23.4.552. [DOI] [PubMed] [Google Scholar]
- Miyabe M, Mori S, van Zijl PC, Kirsch JR, Eleff SM, Koehler RC, Traystman RJ. Correlation of the average water diffusion constant with cerebral blood flow and ischemic damage after transient middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab. 1996;16:881–91. doi: 10.1097/00004647-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Morris DC, Yeich T, Khalighi MM, Soltanian-Zadeh H, Zhang ZG, Chopp M. Microvascular structure after embolic focal cerebral ischemia in the rat. Brain Res. 2003;972:31–7. doi: 10.1016/s0006-8993(03)02433-8. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–9. doi: 10.1097/00000542-200701000-00017. discussion 8-10. [DOI] [PubMed] [Google Scholar]
- Schonenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, Ringleb PA, Kieser M, Mohlenbruch MA, Bosel J. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: A randomized clinical trial. JAMA. 2016;316:1986–96. doi: 10.1001/jama.2016.16623. [DOI] [PubMed] [Google Scholar]
- Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–55. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokrab TE, Johansson BB. Regional cerebral blood flow in acute hypertension induced by adrenaline, noradrenaline and phenylephrine in the conscious rat. Acta Physiol Scand. 1989;137:101–6. doi: 10.1111/j.1748-1716.1989.tb08725.x. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–4. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997a;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997b;17:123–35. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Zhao L, Nowak TS., Jr Preconditioning cortical lesions reduce the incidence of peri-infarct depolarizations during focal ischemia in the Spontaneously Hypertensive Rat: interaction with prior anesthesia and the impact of hyperglycemia. J Cereb Blood Flow Metab. 2015;35:1181–90. doi: 10.1038/jcbfm.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Auer RN. Graded hypotension and MCA occlusion duration: effect in transient focal ischemia. J Cereb Blood Flow Metab. 1995;15:980–8. doi: 10.1038/jcbfm.1995.124. [DOI] [PubMed] [Google Scholar]


