Abstract
Background
To assess the extent to which multiple gestations mediate risk of pregnancy complications from fertility treatment and to address possible confounding by the underlying infertility.
Methods
From the nearly 1.8 million pregnancies recorded in the Swedish Medical Birth Register between 1996 and 2013, we selected the 9.9% (N = 174 067) that occurred to couples with known trouble conceiving (clinical infertility). Fertility treatment was identified from self-reports, medical records and procedural information from fertility clinics. We used logistic regression to estimate odds ratios (ORs) and their 95% confidence intervals (CIs), and decomposed the total effect into direct and mediated effects to estimate the proportion mediated by multiple gestations.
Results
Compared with pregnancies achieved without any assistance, those having received some treatment had higher odds of all studied complications except gestational diabetes. Associations with placenta previa (OR 2.17, 95% CI 1.95–2.40) and placental abruption (OR 1.77, 95% CI 1.54–2.03) were almost entirely independent of multiple gestations. In contrast, the majority of the associations with preterm birth (OR 1.69, 95% CI 1.62–1.77), caesarean delivery (RR 1.15, 95% CI 1.13–1.17) and pre-eclampsia (OR 1.17, 95% CI 1.11–1.22) were mediated by multiple gestations (87%, 62% and 91% of the effect mediated, respectively). Both direct and mediated pathways contributed to the remaining positive associations with chorioamnionitis, labour induction and postpartum haemorrhage. Results were similar when considering primi- and multi-parous women separately, and after restriction to assisted reproductive technologies only.
Conclusion
Multiple gestations are responsible for a large proportion of pregnancy complications associated with fertility treatment, suggesting that interventions to restrict the occurrence of multiples could reduce excess risk of pre-eclampsia, preterm birth and caesarean delivery after fertility treatment. However, the elevated risk of serious placental complications after fertility treatment appears to be largely independent of multiple gestations.
Keywords: fertility treatment, infertility, pregnancy complications, ART
Key Messages
Multiple gestations are responsible for a majority of the increased risk of pre-eclampsia, preterm birth and caesarean delivery following fertility treatment, but nearly none of the risk of placenta previa.
For assisted reproductive techniques specifically (and before single-embryo-transfer implementation), excess risks were even more pronounced, with the same patterns of mediation.
Interventional strategies to curb the occurrence of multiples from assisted reproductive techniques could be expected to mitigate the excess risk of several adverse pregnancy outcomes, but not serious placental complications.
Introduction
While undoubtedly of great benefit for couples with trouble conceiving, the safety of fertility treatments [including assisted reproductive techniques (ART), ovarian stimulation, surgery, etc.] is not yet fully understood. Many have reported associations with adverse birth outcomes for the offspring (e.g. preterm birth and low birth weight)1–5 and women using fertility treatments have been found at higher risk of complications in pregnancy (such as gestational diabetes, pre-eclampsia, placental pathology, caesarean delivery and postpartum haemorrhage).3–14
Studies into the safety of fertility treatments are however challenged by the need to disentangle (i) the role of the underlying infertility,15 (ii) the effects of fertility treatments and (iii) the role of multiple gestations.16 The first is an issue of confounding, in that the indication for treatment (infertility) may share common causes with adverse outcomes. Whereas many studies make some adjustment for maternal characteristics, few have accounted for the underlying infertility directly (e.g. by restricting to infertile couples, defined by the World Health Organization as ‘those failing to achieve pregnancy in 1 year’).17 The second and the third are questions of mediation, whereby complications could be due to multiple gestations (mediator) occurring more frequently after fertility treatment (exposure). For example, the higher incidence of pre-eclampsia in pregnancies resulting from fertility treatment has been suggested to be largely explained by the higher frequency of multiple gestations.18 To understand whether there is an effect of fertility treatment independent of multiple gestations, this has most commonly been addressed by assessing the influence of treatment in singletons and multiples separately. This approach is problematic and can induce bias if attention is not paid to potential common causes of the multiple gestations mediator and the pregnancy complications outcome, and it does not provide insight into how much of the effect is through the different pathways. Assessing the influence of intervention to reduce multiple gestations (through embryo-transfer policy and less aggressive ovulation-stimulation protocols) is of potential interest for both clinical practice and public health.
This study aims to (i) assess the overall association between exposure to fertility treatment and pregnancy outcomes independent of underlying infertility (total effect), (ii) quantify the extent associations are mediated by multiple gestation (mediated effect) and (iii) estimate the influence on pregnancy complications if the number of multiple gestations was decreased to baseline levels (direct effect).
The 2003 implementation of single-embryo-transfer policy in Sweden also allowed us to relate our analysis to direct observation of an intervention that curbs the occurrence of multiple gestations following ART, by contrasting risks of pregnancy complications before and after the intervention.
Materials and Methods
Data sources and study population
The cohort for this study was identified from the Medical Birth Register (MBR), which contains all births in Sweden since 1973, with stillbirth included if occurring after 22 completed weeks (and before July 2008 if occurring after 28 completed weeks). When compared with vital statistics, the register consistently covers 96–99% of all births in any given year, and includes electronic records of prospectively collected information at the antenatal visits, the delivery and paediatric examination of the newborn, with quality evaluated at several time points.19 Women are routinely enrolled in antenatal care, and 90% have their first visit during the first trimester. At the first visit, the midwife performs a standardized interview to record information about the mother’s weight and height, socio-demographic information (e.g. age and cohabitation status), tobacco smoking, chronic conditions and reproductive history (previous pregnancies and births, and last menstrual period). In 1995, questions were introduced to also capture involuntary childlessness by recording the time to pregnancy in years (if more than 1 year) and potential treatment for infertility (such as ART, ovarian stimulation, surgery or ‘other’). Before the establishment of a National Quality Register for Assisted Reproduction (Q-IVF) in 2007, the MBR also retrieved information from all clinics that provide ART concerning type: intra-cytoplasmic sperm injection (ICSI) or in-vitro fertilization (IVF), and embryo-transfer details such as date, number, fresh or frozen. Finally, the MBR also includes data from standardized charts used at the time of delivery and at discharge, and all relevant diagnoses are recorded using the WHO’s International Classification of Diseases (ICD).20
Linkage to the National Patient Register (NPR; with all inpatient care since 1987 and outpatient specialist care since 2001) allowed complementary identification of maternal chronic conditions, including potential infertility diagnosis or fertility-treatment procedures (generally made in the outpatient setting). The Education Register also provided information on the highest level of attained education. Permission for the study was obtained from the Regional Ethical Review board in Stockholm, Sweden (DNR2013–1849-31/2).
The study base was the 1 761 290 recorded pregnancies from 1 January 1996 to 31 December 2013. Nearly 10% of these (N = 174 067) occurred despite trouble conceiving identified mainly from antenatal self-report of involuntary childlessness for more than 1 year, but also from recording of infertility diagnosis or any record of use of fertility treatment. Fertility-treatment information was combined from three principally different sources: self-reports (MBR), coding in medical records (MBR or NPR) and procedural reports from fertility clinics (first to MBR and later to Q-IVF). Pregnancy outcomes were identified in records from delivery and discharge, having been set either through checkbox indicators (multiple gestation) or physician diagnosis (gestational diabetes, pre-eclampsia, chorioamnionitis, placenta previa, placental abruption and postpartum haemorrhage) or both (labour induction and caesarean delivery). Preterm birth was defined as gestational age at birth less than 37 weeks, with the majority of pregnancy-dating based on ultrasonogram.21
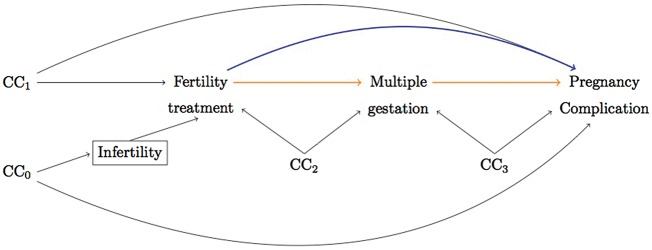
The hypothesized relationships between fertility treatment, multiple gestation and pregnancy complications, including all potential common causes, are given in Figure 1. Many factors that could lead to infertility, e.g. maternal age and chronic conditions, may also increase the risk of adverse outcomes and, since infertility is the indication for treatment, these common causes (CC0) will give rise to a non-causal association between treatment and outcome. This confounding influence can be controlled for if the path through infertility is blocked (box), e.g. by restricting to infertile couples only. Women treated for infertility may, however, still differ from the non-treated if e.g. women with more determination/resources are more likely to receive treatment (and more or less likely to experience complications in pregnancy; CC1). In addition, both exposure and outcome may share common causes with the mediator, since other known risk factors for multiple gestation, i.e. advancing maternal age, parity, height and body mass index (BMI),22 could influence the probability of both treatment and complications (CC2 & 3).
Figure 1.
Directed acyclic graph to illustrate a possible structural relationship between fertility treatment, multiple gestation and pregnancy complications. CC denotes all common causes (confounding factors) of any pair of variables in the graph, measured and unmeasured. Adjusting for infertility (box) blocks the non-causal pathway between fertility treatment and pregnancy complications through potential common causes of infertility and pregnancy complications. The mediated effect is represented by the pathway from fertility treatments to pregnancy complications that goes through multiple gestations and the direct effect is the pathway straight to pregnancy complications.
Statistical analysis
To quantify how much of the influence of fertility treatment on pregnancy complications is independent vs mediated by multiple gestations, the total effect was decomposed into direct and mediated effects (Figure 1). The approach, which builds on a counterfactual framework, has been extensively described23–25 and applied25,26 previously. A direct effect will represent the influence of fertility treatment that is independent of multiple gestations (e.g. if embryo transfer in ART affects placentation). Briefly, this is obtained by evaluating the effect of fertility treatment with the prevalence of multiples set to the level that would have naturally occurred in the absence of treatment (natural direct effect). A mediated effect will represent the influence of fertility treatment that can be explained by its influence on multiple gestations (e.g. if higher prevalence of multiples results in more preterm births among treated pregnancies). Briefly, this is obtained by comparing the outcomes under treatment when setting the prevalence of multiples to what it would have been in the presence, vs the absence, of treatment (natural indirect effect). The total effect decomposes into the product of the direct and mediated effect odds ratios (ORs). An advantage of these natural effects is that they can be validly estimated even if the effect of treatment differs in singleton and multiples (effect modification). We also estimated the proportion mediated on the risk difference scale,27,28 to give an indication of the extent of mediation, where 100% reflects all of the total effect being mediated (no direct effect) and 0% reflects when there is no mediation (all direct effect).
To allow critical adjustment for all potential common causes, effects are estimated in a logistic regression setting and thus expressed as ORs with 95% confidence intervals (CIs), except when outcomes are common and log-binomial or robust Poisson should be used (to estimate relative risks). The main model predicts the outcome according to exposure, mediator and confounders, allowing for interaction between exposure and mediator. Effects are conditional on confounders, and obtained from combining the estimates from this outcome regression and another regression of the mediator on the exposure and confounders. Briefly, this is obtained by integrating the average outcome conditional on the exposure, mediator and confounders over the distribution of the mediator conditional on exposure being present vs absent. Detailed information for each effect can be found in the documentation of the SAS macro %mediation.29 All analyses were run in SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Direct and indirect effect estimates require that baseline covariates control for exposure-outcome-, mediator-outcome- and exposure-mediator-confounding. Striving to cover all possible confounders (CC0 to 3 in Figure 1), the models included fixed maternal characteristics such as country of origin, civil status, highest attained level of education and chronic health conditions (diabetes mellitus, hypertension, renal disease, fibroids, endometriosis and polycystic ovarian syndrome). Pregnancy-specific characteristics included maternal age, parity, birth year, early pregnancy BMI and smoking, and known diagnosis of infertility (possibly indicating that cause of infertility was known). Analyses were performed in the 155 659 women with information on all covariates (89%). The selection steps from study base to complete case sample are summarized in a flowchart (Supplementary Figure 1, available as Supplementary data at IJE online).
Of the covariates with missing data (BMI, civil status, education and smoking), early pregnancy BMI was not only the main source (8.4%), but also at most suspicion to be missing not at random. We therefore evaluated its influence on the main analysis first as a covariate and then as a source of missing data. We also used sensitivity analysis for unmeasured confounding to assess the robustness of effects to violations of the assumptions.30 To explore potential effect modification by parity (with multiple birth considered a single parous experience), all analyses were also performed in primi- and multiparous women separately.
Lastly, the implementation of single-embryo-transfer policy in Sweden provided an opportunity to observe the effects of an intervention that reduces the number of multiple gestations following ART. To compare the time before and after the intervention, we plotted risk of pregnancy complications over time and assessed the total, direct and mediated effects of ART treatments specifically in the 1996–2003 and 2004–13 birth cohorts separately.
Results
Twins accounted for 97% of the multiple gestations in the study population (N = 260 were triplets). Maternal characteristics of all pregnancies with documented trouble conceiving are presented in Table 1. To assist the evaluation of possible common causes (illustrated in Figure 1), we show the distributions of measured covariates for pregnancies conceived with and without the assistance of fertility treatment (48% and 52%, respectively) and for multiple gestations in each of these respective groups. We describe here some of the more notable differences. Fertility treatment occurred in women who were on average 1 year older, and was more likely among women who were primiparous, highly educated, non-smokers and born in Scandinavia compared with the women who did not undergo any treatment. Treated women also had more than 2-fold higher prevalence of polycystic ovarian syndrome (5.3% compared with 2.5% in non-treated women) and endometriosis (5.8% compared with 2.7%), respectively. When achieved without fertility treatment, multiple gestations appeared more frequent in women of higher age, education, parity and with polycystic ovarian syndrome.
Table 1.
Maternal characteristics of pregnancies to infertile couples in Sweden, 1996–2013
| Mother’s characteristics |
N (%) |
||||
|---|---|---|---|---|---|
| Pregnancies conceived without fertility treatment |
Pregnancies conceived following fertility treatment |
||||
| All | Multiples | All | Multiples | ||
| N = 90 096 | N = 1083 | N = 83 971 | N = 7075 | ||
| Age at birth (years) | 31.9 ± 0.02a | 32.4 ± 0.15a | 33.0 ± 0.02a | 33.3 ± 0.06a | |
| Early pregnancy BMI (kg/m2) | 25.2 ± 0.02a | 25.2 ± 0.02a | 25.5 ± 0.17a | 24.7 ± 0.02a | |
| Height (cm) | 166.4 ± 0.02a | 166.6 ± 0.19a | 167.0 ± 0.02a | 167.3 ± 0.08a | |
| Civil status | Living with father | 85 072 (96.9) | 975 (95.5) | 78 264 (97.1) | 6482 (97.7) |
| Living alone/unknown | 2736 (3.1) | 46 (5) | 2360 (2.9) | 152 (2.3) | |
| Origin | Scandinavian | 73 822 (81.9) | 892 (82.4) | 71 685 (85.4) | 6120 (86.5) |
| Non-Scandinavian | 16 267 (18.1) | 191 (17.6) | 12 280 (14.6) | 955 (13.5) | |
| Education | 9 years compulsory | 7367 (8.2) | 79 (7.3) | 4164 (5.0) | 329 (4.7) |
| Upper secondary, <3 years | 15 358 (17.1) | 192 (17.8) | 11 910 (14.2) | 1221 (17.3) | |
| Upper secondary, 3 years | 22 911 (25.6) | 239 (22.2) | 18 643 (22.3) | 1552 (22.0) | |
| University | 44 052 (49.1) | 569 (52.7) | 49 013 (58.5) | 3956 (56.1) | |
| Tobacco smoking | No | 79 611 (90.9) | 919 (90.5) | 76 893 (95.5) | 6316 (96.0) |
| <10 cigarettes/day | 5820 (6.7) | 67 (6.6) | 2757 (3.4) | 189 (2.9) | |
| >10 cigarettes/day | 2116 (2.4) | 29 (2.9) | 858 (1.1) | 74 (1.1) | |
| Diabetes | 902 (1.00) | 15 (1.39) | 747 (0.89) | 43 (0.61) | |
| Hypertension | 1130 (1.25) | 11 (1.02) | 1005 (1.20) | 71 (1.00) | |
| Renal disease | 749 (0.83) | 6 (0.55) | 605 (0.72) | 43 (0.61) | |
| Fibroids | 1334 (1.48) | 21 (1.94) | 1807 (2.15) | 121 (1.71) | |
| PCOS | 2289 (2.54) | 40 (3.69) | 4459 (5.31) | 313 (4.42) | |
| Endometriosis | 2443 (2.71) | 37 (3.42) | 4835 (5.76) | 487 (6.88) | |
| Parity | Primiparous | 51 850 (57.6) | 550 (50.8) | 55 143 (65.7) | 4560 (64.5) |
| Multiparous | 38 246 (42.5) | 533 (49.2) | 28 828 (34.3) | 2515 (35.6) | |
| Birth year | 1996–2000 | 16 768 (18.6) | 240 (22.2) | 15 863 (18.9) | 2161 (30.5) |
| 2001–04 | 18 868 (20.9) | 225 (20.8) | 15 394 (18.3) | 1799 (25.4) | |
| 2005–08 | 22 635 (25.1) | 261 (24.1) | 20 210 (24.1) | 1243 (17.6) | |
| 2009–13 | 31 825 (35.3) | 357 (33.0) | 32 504 (38.7) | 1872 (26.5) | |
Data are means ± standard error. Some frequencies may not add up to 100% due to missing data (overall <5% for civil status, education and smoking, respectively).
Next we evaluated the role of multiple gestations with respect to pregnancy outcomes. Table 2 shows how, in addition to preterm birth, complications in pregnancy (pre-eclampsia, chorioamnionitis and placental abruption) and delivery (labour induction, caesarean delivery and postpartum haemorrhage) occurred more frequently in pregnancies with multiples—also after adjustment for potential differences in preconception characteristics. Most of these associations were prominent in both treated and non-treated women, with no difference in risk of placenta previa between singleton and multiple pregnancies following fertility assistance a notable exception. Also, for chorioamnionitis, the excess risk from having multiples appeared smaller among the pregnancies achieved without any fertility treatment (Table 2).
Table 2.
Risk of pregnancy outcomes due to multiples among infertile couples in Sweden, 1996–2013
|
N (%) |
aAdjusted odds ratio (95% CI) |
||||
|---|---|---|---|---|---|
| All | Multiples | All | No fertility treatment | Fertility treatment | |
| Outcome in pregnancy | N = 155 659 | N = 6900 | N = 155 659 | N = 81 169 | N = 74 490 |
| Gestational diabetes | 3427 (2.0) | 160 (2.0) | 1.10 (0.92, 1.32) | 1.20 (0.78, 1.84) | 1.07 (0.87, 1.31) |
| Pre-eclampsia | 9956 (5.7) | 1293 (15.8) | 3.51 (3.27, 3.77) | 3.62 (3.00, 4.37) | 3.51 (3.24, 3.81) |
| Chorioamnionitis | 701 (0.4) | 92 (1.1) | 2.97 (2.33, 3.80) | 2.05 (0.90, 4.63) | 2.87 (2.19, 3.74) |
| Placenta previa | 2041 (1.2) | 118 (1.4) | 1.22 (0.99, 1.49) | 1.37 (0.71, 2.67) | 0.95 (0.76, 1.18) |
| Placental abruption | 1109 (0.6) | 140 (1.7) | 2.76 (2.25, 3.37) | 2.34 (1.24, 4.41) | 2.32 (1.87, 2.89) |
| Preterm birth | 16 019 (9.2) | 3880 (47.6) | 11.6 (11.0, 12.2) | 12.7 (11.2, 14.5) | 10.9 (10.3, 11.6) |
| Labour inductionb | 31 073 (17.9) | 1942 (23.8) | 1.39 (1.33, 1.46) | 1.53 (1.37, 1.71) | 1.34 (1.27, 1.40) |
| Caesarean deliveryb | 43 671 (25.1) | 4874 (59.7) | 2.51 (2.43, 2.59) | 2.42 (2.27, 2.57) | 2.45 (2.39, 2.51) |
| Postpartum haemorrhage | 11 363 (6.5) | 892 (10.9) | 1.85 (1.71, 2.00) | 1.88 (1.52, 2.33) | 1.74 (1.60, 1.90) |
Adjusting for birth year and mother’s preconception characteristics (age at birth, education, civil status, country of origin, smoking, early pregnancy BMI, chronic morbidity and parity). CI, confidence interval.
Relative risks estimated with Poisson regression (robust standard errors).
Having established that multiple gestations were both more common following fertility treatment and independently associated with adverse pregnancy outcome (Table 2), we assessed their role in the association between fertility treatment and pregnancy complications. The full decomposition of the total effect into direct and mediated effects is presented in Table 3, along with indications of the proportion mediated (noting that, due to random variability, or if direct and mediated effects are in opposite directions, this may be estimated outside the expected range of 0–100%, respectively).28 Compared with unassisted pregnancies to infertile couples, those assisted by fertility treatment had higher odds of all pregnancy complications except gestational diabetes (overall prevalence 1.9%, OR 1.03, 95% CI 0.96–1.12). Positive associations with placenta previa (1.2%, OR 2.17, 95% CI 1.95–2.40) and placental abruption (0.6%, OR 1.77, 95% CI 1.54–2.03) were almost entirely independent of multiple gestations (little to no mediated effect and consequentially a very small proportion mediated). In contrast, multiple gestations appeared to mediate the majority (91%, 87% and 62%, respectively) of the association with pre-eclampsia (5.7%, OR 1.17, 95% CI 1.11–1.22), preterm birth (8.6%, OR 1.69, 95% CI 1.62–1.77) and caesarean delivery (24.8%, RR 1.15, 95% CI 1.13–1.17). Both direct and mediated pathways contributed to the remaining positive associations with chorioamnionitis, labour induction and postpartum haemorrhage (proportions mediated 35%, 20% and 26% respectively; Table 3).
Table 3.
Comparing assisted to non-assisted pregnancies in couples with known trouble conceiving: effect of any fertility treatment on pregnancy outcomes overall (total), through multiple gestations (mediated) and independent of multiple gestations (direct)
| Fertility treatment |
|||||
|---|---|---|---|---|---|
| Outcome in pregnancy | N (%) | Total effect OR | Direct effect OR | Mediated OR | % Mediateda |
| Gestational diabetes | 3042 (1.9) | 1.03 (0.96, 1.12) | 1.03 (0.95, 1.12) | 1.00 (0.99, 1.02) | – |
| Pre-eclampsia | 8819 (5.7) | 1.17 (1.11, 1.22) | 1.02 (0.97, 1.06) | 1.15 (1.13, 1.17) | 91 |
| Preterm birth | 13 798 (8.9) | 1.69 (1.62, 1.77) | 1.09 (1.04, 1.13) | 1.55 (1.52, 1.59) | 87 |
| Caesarean deliveryb | 38 580 (24.8) | 1.15 (1.13, 1.17) | 1.06 (1.04, 1.08) | 1.09 (1.08, 1.09) | 62 |
| Placenta previa | 1803 (1.2) | 2.17 (1.95, 2.40) | 2.17 (1.95, 2.41) | 1.00 (0.98, 1.01) | 0 |
| Placental abruption | 945 (0.6) | 1.77 (1.54, 2.03) | 1.64 (1.42, 1.89) | 1.08 (1.05, 1.11) | 17 |
| Chorioamnionitis | 610 (0.4) | 1.40 (1.18, 1.66) | 1.26 (1.06, 1.50) | 1.11 (1.07, 1.16) | 35 |
| Labour inductionb | 27 902 (17.9) | 1.11 (1.09, 1.14) | 1.09 (1.07, 1.11) | 1.02 (1.02, 1.02) | 20 |
| Postpartum haemorrhage | 10 120 (6.5) | 1.20 (1.15, 1.25) | 1.15 (1.10, 1.20) | 1.05 (1.04, 1.05) | 26 |
Proportion mediated = DE*(ME-1)/(DE*ME-1), where DE is direct effect and ME is mediated effect. Note that when all or none of the effect is mediated (= no statistically significant direct or mediated effect, respectively), the percent mediated may be estimated outside of 100% or 0% due to random variability.
Relative risks estimated with Poisson regression.
Complete case analysis is N = 155 659; N (%) is number and prevalence of outcome, OR is odds ratio (95% confidence interval). Models are adjusted for birth year and mother’s preconception characteristics (age at birth, education, civil status, country of origin, smoking, early pregnancy BMI, chronic morbidity and parity).
Finding that adjustment for BMI had a negligible influence on the results, we redid the main analysis without consideration of BMI (reintroducing those with missing BMI). Reassuringly, the main effects in this less restricted sample (including 95% of the study population) were nearly identical to those noted in the original complete case sample (including 89%). Further sensitivity analysis regarding the role of unmeasured confounding showed that, for preterm birth and caesarean delivery, for example, it would require a confounder related to the outcome and multiple gestations mediator by risk ratios of approximately 2-fold each (above and beyond all of the measured confounders combined) to explain away the effect. Effect decomposition in primiparous and multiparous pregnancies separately showed a few smaller differences in magnitudes of effects, whereas the main pattern of mediation remained in both groups (Supplementary Table 1, available as Supplementary data at IJE online).
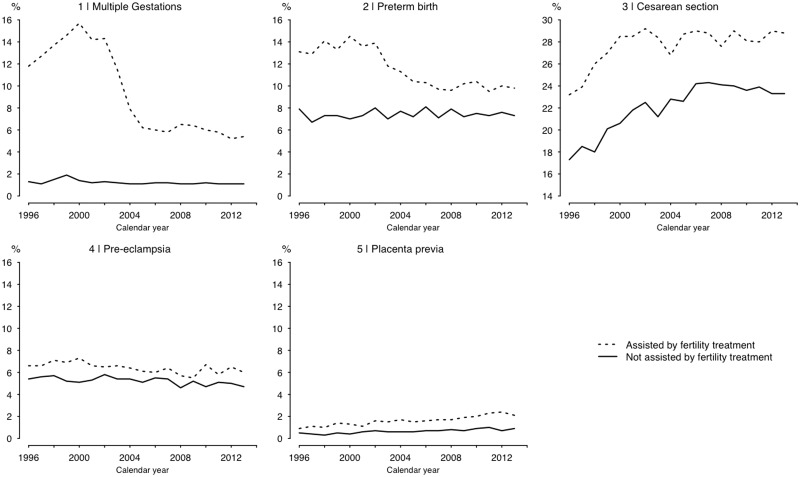
Next we related our findings to the influence of the 2003 single-embryo-transfer recommendation in Sweden, plotting the prevalence of select pregnancy complications over time in the study cohort (Figure 2). Multiple gestations dropped markedly in the group assisted by fertility treatment (on average 13% before 2004 and 6% after) while remaining stable in the non-assisted group (∼1.5%). Preterm births followed a similar pattern, whereas, for caesarean delivery, a general trend of increasing prevalence in the first part of the study period came to a halt in 2003 among the assisted (compared with 2006 in the non-assisted). For pre-eclampsia, the higher prevalence in the assisted group appeared to be nearing that of the non-assisted over time, though this was disrupted by an uptick in more recent years. Trends for placenta previa showed no clear relation to the decline in multiple gestations (Figure 2). Lastly, we also compared the total, direct and mediated effects of treatment before and after the policy recommendation. Considering only the treatments subject to change (ART; 49 332 of the 74 490 treated pregnancies), in births prior to the intervention (1996–2003) associations were overall more pronounced, with same patterns of mediation as in our main analysis (Table 4). Whereas ART remained associated with all noted complications also after the intervention (2004–13), the excess risk dropped for the complications that had been found largely mediated by multiple gestations (pre-eclampsia, preterm birth and caesarean; Table 4). In contrast, the excess risk of placenta previa was largely unchanged.
Figure 2.
Plot of pregnancy complications to couples with known trouble conceiving in the study period 1996–2013.
Table 4.
Comparing assisted to non-assisted pregnancies in couples with known trouble conceiving: effect of ART (IVF and ICSI) on pregnancy outcomes overall (total), through multiple gestations (mediated) and independent of multiple gestations (direct) before and after single-embryo-transfer policy recommendation
| Outcome in pregnancy | Assisted reproductive technologies |
||||
|---|---|---|---|---|---|
| 1996–2003 | N (%) | Total effect OR | Direct effect OR | Mediated OR | % Mediateda |
| Gestational diabetes | 645 (1.63) | 0.97 (0.81, 1.16) | 0.95 (0.79, 1.16) | 1.01 (0.95, 1.08) | – |
| Pre-eclampsia | 2387 (6.03) | 1.31 (1.15, 1.50) | 0.98 (0.88, 1.09) | 1.34 (1.19, 1.50) | 100 |
| Preterm birth | 3935 (9.94) | 2.81 (2.29, 3.45) | 1.24 (1.14, 1.35) | 2.26 (1.83, 2.80) | 87 |
| Cesarean deliveryb | 9256 (23.4) | 1.39 (1.33, 1.44) | 1.11 (1.06, 1.16) | 1.24 (1.22, 1.27) | 70 |
| Placenta previa | 350 (0.88) | 2.88 (2.28, 3.64) | 2.85 (2.24, 3.62) | 1.01 (0.95, 1.08) | 2 |
| Placental abruption | 285 (0.72) | 2.61 (2.02, 3.37) | 2.31 (1.76, 3.03) | 1.13 (1.02, 1.25) | 18 |
| Chorioamnionitis | 168 (0.42) | 1.69 (1.20, 2.38) | 1.36 (0.95, 1.95) | 1.25 (1.05, 1.47) | 48 |
| Labor inductionb | 6138 (15.5) | 1.18 (1.12, 1.26) | 1.10 (1.04, 1.17) | 1.07 (1.04, 1.11) | 44 |
| Postpartum hemorrhage | 2485 (6.28) | 1.34 (1.21, 1.47) | 1.22 (1.10, 1.34) | 1.10 (1.04, 1.16) | 35 |
|
2004–2013 | |||||
| Gestational diabetes | 1883 (2.07) | 1.03 (0.92, 1.14) | 1.03 (0.92, 1.14) | 1.00 (0.98, 1.02) | – |
| Pre-eclampsia | 5070 (5.58) | 1.25 (1.17, 1.33) | 1.07 (1.00, 1.15) | 1.16 (1.14, 1.19) | 70 |
| Preterm birth | 7755 (8.53) | 1.66 (1.56, 1.77) | 1.12 (1.06, 1.18) | 1.48 (1.43, 1.53) | 82 |
| Cesarean deliveryb | 23601 (26.0) | 1.14 (1.11, 1.17) | 1.07 (1.04, 1.10) | 1.07 (1.06, 1.07) | 51 |
| Placenta previa | 1276 (1.40) | 2.66 (2.34, 3.02) | 2.69 (2.37, 3.06) | 0.99 (0.98, 1.00) | 0 |
| Placental abruption | 501 (0.55) | 1.72 (1.42, 2.09) | 1.62 (1.33, 1.97) | 1.06 (1.03, 1.11) | 15 |
| Chorioamnionitis | 361 (0.40) | 1.61 (1.28, 2.03) | 1.49 (1.18, 1.89) | 1.08 (1.03, 1.13) | 20 |
| Labor inductionb | 17556 (19.3) | 1.15 (1.12, 1.18) | 1.14 (1.11, 1.18) | 1.01 (1.00, 1.01) | 5 |
| Postpartum hemorrhage | 6165 (6.78) | 1.31 (1.24, 1.38) | 1.26 (1.19, 1.34) | 1.03 (1.02, 1.05) | 14 |
Proportion mediated = DE*(ME-1)/(DE*ME-1) where DE is direct effect and ME is mediated effect. Note that when all or none of the effect is mediated (= no statistically significant direct or mediated effect respectively) the percent mediated may be estimated outside of 100 or 0% due to random variability.
Relative risks estimated with Poisson regression.
Complete case analysis in N= 39 583 (1996–2003) and N=90 918 (2004–13) respectively. N (%) is number and prevalence of outcome, OR is Odds Ratio (95% Confidence Interval).
Models are adjusted for birth year and mother's preconception characteristics (age at birth, education, civil status, country of origin, smoking, early pregnancy BMI, chronic morbidity and parity).
Discussion
This large nationwide population study confirms findings of increased pregnancy complications following fertility treatment in a comparison restricted to infertile couples. Effect decomposition further suggested that multiple gestations mediated most of the associations with pre-eclampsia, preterm birth and caesarean delivery. Interestingly, the fertility-treatment association to other complications of placentation (i.e. placenta previa and placental abruption) appeared largely independent of multiple gestations. Both direct and mediated pathways contributed to the remaining positive associations with labour induction and postpartum haemorrhage, indicating that a higher prevalence of multiples can only partially explain the excess risk of these complications following fertility treatment.
Previously published evidence linking fertility treatments to placental complications appears strongest for placenta previa,8–14,31 placental abruption8,10–14 and pre-eclampsia.8–11 Our findings indicate that multiple gestations are responsible for the vast majority of a relatively modest increased risk of pre-eclampsia following fertility treatment. Tracing the effects of Sweden’s single-embryo-transfer recommendation, which greatly reduced the number of multiples from ART in the second half of the study period, there were also some indications that the excess risk of pre-eclampsia after treatment was decreasing. A corresponding drop for placenta previa was not seen, and should not be expected based on the present study showing the risk to be independent of multiple gestations. When considering ART only (pre-intervention), our effect size for placenta previa (OR 2.9, 95% CI 2.3–3.6) was similar to previous reports for IVF in singletons with the ability to account for either years of involuntary childlessness (OR 3.4, 95% CI 2.7–4.2)13 or all maternal fixed characteristics (OR 2.9, 95% CI 1.4–6.1).31 A putative explanation involves epigenetic processes, which may be vital for the behaviour of trophoblast invasion,32 and may be disrupted during artificial conception and embryo cultivation. This possible mechanism is indirectly supported by finding higher risk of placenta previa after blastocyst compared with non-blastocyst transfer (since blastocysts are exposed to longer embryo culture time).33
Failure to replicate a previously reported increased risk of gestational diabetes in treated women7,9,10 could be a result of the present study’s restriction to pregnancies that were all preceded by trouble conceiving. Some of the underlying reasons behind such challenges may also be risk factors for gestational diabetes (e.g. increasing maternal age and BMI, PCOS, etc.)34,35 and failure to account for such common causes (CC0 in Figure 1) may explain the findings of some previous studies. Indeed, in the only previous study to account for years of involuntary childlessness, IVF was not found to be associated with gestational diabetes.13
The present study also replicates previously reported increased risks of preterm birth and delivery complications following exposure to fertility treatment.1–5 Multiple gestations were found responsible for a large majority of the association with preterm birth and caesarean delivery. For both complications, we also observed that risks among treated were greatly reduced following the reduction of multiple gestations from national recommendations to limit the number of embryos transferred. Increased risk of caesarean delivery among women treated for infertility3–10 has been speculated to reflect physician and patient choice rather than some biological effect,10 presumably on account of greater anxiousness among women who had trouble conceiving.36 Although restricted to those with infertility, this study still found fertility treatment associated with higher odds of both labour induction and caesarean delivery, and multiple gestations did not account for all of the risk. Findings of a moderately increased risk of postpartum haemorrhage following ART appear similar to a few previous studies comparing women undergoing IVF to the population8,14 and not only a result of multiple gestations (as both direct and mediated pathways contributed to the association). Finally, our findings suggest that the risk increase of chorioamnionitis in multiple gestations was higher after fertility treatment (than in pregnancies achieved without treatment). A possible explanation could be closer examination of pregnancies resulting from fertility treatment, adding to the already higher degree of monitoring of women carrying multiples. Without much support in the previous literature, however, this finding warrants replication.
The present study includes pregnancies to infertile couples in the Swedish MBR during a 17-year period. Despite the multiple sources of data, we may not have captured all pregnancies achieved after trouble conceiving but, since the risk of false positives should be low, we still achieve the objective to compare individuals at risk of treatment. By combining data from three different sources, we identified nearly half as exposed to some type of fertility treatment, which appears consistent with findings across countries worldwide.37 Variation in ART practice between countries is surely expected but may more likely concern access to and indications for ART rather than the medical procedures themselves (or their effect on pregnancy complications and the mediating role of multiple gestations). In Sweden, treatment is made available and free of cost to all infertile women with a few clinical restrictions (mainly concerning age and BMI). We were able to adjust for several factors that may have further influenced the likelihood of treatment (e.g. civil status, education and a known diagnosis of infertility). As noted earlier, effect decomposition requires additional control for common causes of the exposure and mediator, and mediator and outcome. Being able to adjust for all known and measurable risk factors for multiple gestation, we hope to have largely addressed this, and our sensitivity analysis further indicated that the main results of the paper were robust to a moderate violation of our assumption of no unmeasured confounding. There could be some concern that complications are more likely detected or recorded in women exposed to fertility treatment, as in those with multiple gestation. This may be mitigated by the finding that the perhaps most likely such diagnosis—gestational diabetes—was not more common in treated pregnancies, nor pregnancies with multiples (among infertile). Finally, if early fetal loss is greater in multiples, the requirement of survival up to when the outcomes under study typically occur could have led to differential selection. For this to cause bias, the resulting fetal resilience would have to affect the risk of complications that appear predominantly influenced by maternal or placental factors. This study showed multiples at increased risk of nearly all outcomes studied, so the selection of early pregnancy survivors would, if anything, have made these risks an underestimation. For this to have influenced our main comparison of treated and non-treated pregnancies further, the potential resilience of multiples would have to also be influenced by treatment status.
In this direct evaluation of the role of multiple gestation in mediating potential risk of pregnancy complications following fertility treatment, using a large nationwide sample and restricting analysis to women with known trouble conceiving, findings indicate that interventions to reduce the occurrence of multiples would reduce the risk of delivery complications and diminish the excess risk of pre-eclampsia and preterm birth following fertility treatment. Finding increased risks of serious placental complications largely independent of multiple gestations demonstrates that pregnancies achieved after fertility treatment may be considered high-risk pregnancies irrespective of the number of fetuses.
Supplementary Material
Funding
This work was supported by grants from the Swedish Council for Working Life and Social Research (Forte; DNR 2011–1477), the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no. 340–2013-5867 and NIH Grant ES017876.
Conflict of interest: None to declare.
References
- 1. Okun N, Sierra S.. Pregnancy outcomes after assisted human reproduction. JOGC 2014;36:64–83. [DOI] [PubMed] [Google Scholar]
- 2. Pinborg A, Wennerholm UB, Romundstad LB. et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- 3. Helmerhorst FM, Perquin DA, Donker D, Keirse MJ.. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ 2004;328:261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDonald S, Murphy K, Beyene J, Ohlsson A.. Perinatal outcomes of in vitro fertilization twins: a systematic review and meta-analyses. Am J Obstet Gynecol 2005;193:141–52. [DOI] [PubMed] [Google Scholar]
- 5. McDonald SD, Murphy K, Beyene J, Ohlsson A.. Perinatel outcomes of singleton pregnancies achieved by in vitro fertilization: a systematic review and meta-analysis. JOGC 2005;27:449–59. [DOI] [PubMed] [Google Scholar]
- 6. Reddy UM, Wapner RJ, Rebar RW, Tasca RJ.. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol 2007;109:967–77. [DOI] [PubMed] [Google Scholar]
- 7. Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A.. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- 8. Kallen B, Finnstrom O, Nygren KG, Otterblad Olausson P, Wennerholm UB.. In vitro fertilisation in Sweden: obstetric characteristics, maternal morbidity and mortality. Br J Obstet Gynecol 2005;112:1529–35. [DOI] [PubMed] [Google Scholar]
- 9. Jackson RA, Gibson KA, Wu YW, Croughan MS.. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004;103:551–63. [DOI] [PubMed] [Google Scholar]
- 10. Shevell T, Malone FD, Vidaver J. et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol 2005;106:1039–45. [DOI] [PubMed] [Google Scholar]
- 11. Schieve LA, Cohen B, Nannini A. et al. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern Child Health J 2007;11:517–25. [DOI] [PubMed] [Google Scholar]
- 12. Welmerink DB, Voigt LF, Daling JR, Mueller BA.. Infertility treatment use in relation to selected adverse birth outcomes. Fertil Steril 2010;94:2580–86. [DOI] [PubMed] [Google Scholar]
- 13. Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C.. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod 2011;26:442–50. [DOI] [PubMed] [Google Scholar]
- 14. Healy DL, Breheny S, Halliday J. et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod 2010;25:265–74. [DOI] [PubMed] [Google Scholar]
- 15. Baird DD, Wilcox AJ, Kramer MS.. Why might infertile couples have problem pregnancies? Lancet 1999;353:1724–25. [DOI] [PubMed] [Google Scholar]
- 16. ESHRE. Multiple gestation pregnancy. The ESHRE Capri Workshop Group. Hum Reprod 2000;15:1856–64. [PubMed] [Google Scholar]
- 17. Zegers-Hochschild F, Adamson GD, de Mouzon J. et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod 2009;24:2683–87. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez-Diaz S, Werler MM, Mitchell AA.. Gestational hypertension in pregnancies supported by infertility treatments: role of infertility, treatments, and multiple gestations. Fertil Steril 2007;88:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centre for Epidemiology. The Medical Birth Register – A Summary of Content and Quality. Stockholm: National Board of Health and Welfare, 2003.
- 20. WHO. International Classification of Diseases. Geneva: World Health Organization, 1992. [Google Scholar]
- 21. Hagenfeldt K, Alton V, Axelsson O. et al. Routine Ultrasound Examination during Pregnancy—Summary in English. Stockholm: The Swedish Council on Health Technology Assessment (SBU; ), 1998. [Google Scholar]
- 22. Hoekstra C, Zhao ZZ, Lambalk CB. et al. Dizygotic twinning. Hum Reprod Update 2008;14:37–47. [DOI] [PubMed] [Google Scholar]
- 23. Robins JM, Greenland S.. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992;3:143–55. [DOI] [PubMed] [Google Scholar]
- 24. Pearl J. Direct and indirect effects Proceedings of the Seventeenth Conference on the Uncertainty and Artificial Intelligence. San Fransisco: Morgan Kaufman, 2001, pp. 411–20. [Google Scholar]
- 25. Ananth CV, VanderWeele TJ.. Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects. Am J Epidemiol 2011;174:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vanderweele TJ, Hernandez-Diaz S.. Is there a direct effect of pre-eclampsia on cerebral palsy not through preterm birth? Paediatr Perinat Epidemiol 2011;25:111–15. [DOI] [PubMed] [Google Scholar]
- 27. Vanderweele T. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press, 2015. [Google Scholar]
- 28. Vanderweele TJ, Vansteelandt S.. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010;172:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valeri L, Vanderweele TJ.. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding P, Vanderweele TJ.. Sharp sensitivity bounds for mediation under unmeasured mediator-outcome confounding. Biometrika 2016;103:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ.. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod 2006;21:2353–58. [DOI] [PubMed] [Google Scholar]
- 32. Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL.. Epigenetics and the placenta. Hum Reprod Update 2011;17:397–417. [DOI] [PubMed] [Google Scholar]
- 33. Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C.. Factors affecting obstetric outcome of singletons born after IVF. Hum Reprod 2011;26:2878–86. [DOI] [PubMed] [Google Scholar]
- 34. Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ.. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab 2014;99:E447–52. [DOI] [PubMed] [Google Scholar]
- 35. Makgoba M, Savvidou MD, Steer PJ.. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. Br J Obstet Gynecol 2012;119:276–82. [DOI] [PubMed] [Google Scholar]
- 36. McMahon CA, Ungerer JA, Beaurepaire J, Tennant C, Saunders D.. Anxiety during pregnancy and fetal attachment after in-vitro fertilization conception. Hum Reprod 1997;12:176–82. [DOI] [PubMed] [Google Scholar]
- 37. Boivin J, Bunting L, Collins JA, Nygren KG.. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.