Abstract
The silkworm Bombyx mori is an important lepidopteran model insect in which many kinds of natural mutants have been identified. However, molecular mechanisms of most of these mutants remain to be explored. Here we report the identification of a gene Bm‐app is responsible for the silkworm minute wing (mw) mutation which exhibits exceedingly small wings during pupal and adult stages. Compared with the wild type silkworm, relative messenger RNA expression of Bm‐app is significantly decreased in the u11 mutant strain which shows mw phenotype. A 10 bp insertion in the putative promoter region of the Bm‐app gene in mw mutant strain was identified and the dual luciferase assay revealed that this insertion decreased Bm‐app promoter activity. Furthermore, clustered regularly interspaced short palindromic repeats/RNA‐guided Cas9 nucleases‐mediated depletion of the Bm‐app induced similar wing defects which appeared in the mw mutant, demonstrating that Bm‐app controls wing development in B. mori. Bm‐app encodes a palmitoyltransferase and is responsible for the palmitoylation of selected cytoplasmic proteins, indicating that it is required for cell mitosis and growth during wing development. We also discuss the possibility that Bm‐app regulates wing development through the Hippo signaling pathway in B. mori.
Keywords: Bombyx mori, CRISPR/Cas9, minute wing, palmitoyltransferase, promoter
Introduction
Insects are the only winged invertebrates among arthropods and their flight ability is a key factor underlying their expansion, invasion, proliferation and reproduction (Lewin, 1985; Engel & Grimaldi, 2004). The wing has become an important model system in evolutionary biology, ecology and physiology (Zera & Denno, 1997; McCulloch et al., 2009). The silkworm Bombyx mori is an important lepidopteran model organism and more than 400 silkworm mutants have been identified in nature (Goldsmith et al., 2005). Among these mutants, multiple studies have been performed to investigate B. mori wing‐related mutations since they are ideal genetic materials for exploring the molecular basis of insect wing development. The wing‐related mutation in B. mori shows high diversity such as crayfish pupa (cf) (Tong et al., 2015). wingless locus flugellos (fl) (Fujiwara & Hojyo, 1997), winglet (rw) and minute wing (mw) (Goldsmith et al., 2005). The molecular mechanisms underlying fl (Fujiwara & Hojyo, 1997; Matsunaga & Fujiwara, 2002) and non‐lepis wing (nlw) (Zhou et al., 2004, 2006) mutations have been reported. However, most of these mutations remain to be functionally analyzed and little is known about the molecular mechanism generating these diversities.
Silkworm mw mutants show small and curly wings during pupal and adult stages compared with wild type animals (Fig. 1). In the previous study, we constructed a simple linkage map of mw in B. mori (Ma et al., 2013). However, it remains to be clarified which gene causes mw phenotype due to difficulty in positional cloning and lack of genetic tools. Recently, genome‐editing tools such as ZFN (zinc‐finger nucleases), TALEN (transcription‐activator like effector nucleases) and the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/RNA‐guided Cas9 nucleases) systems have been applied in functional gene analysis in different organisms (Hruscha et al., 2013; Wang et al., 2013). In B. mori, these techniques have also been successfully established (Wang et al., 2013; Li et al., 2015; Xu et al., 2017; Zeng et al., 2017), providing effective loss‐of‐function toolkits to explore the mechanisms for silkworm natural mutants such as mw.
Figure 1.
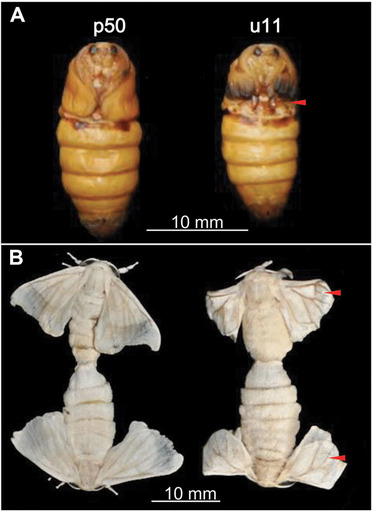
Phenotypes of wild‐type (p50) and mw mutant (u11) strains. Pupal and moth stages of the wild‐type (A) and mw mutant (B) are shown. Red arrows indicate smaller and slightly curled wings in u11 mutant moths.
In the current study, we describe identification of a palmitoyltransferase Approximated gene Bm‐app that is responsible for mw phenotype. Positional cloning revealed that Bm‐app locates in a 275 kb region on chromosome 22 which contains 15 mw candidate genes including Bm‐app. We generated somatic mutagenesis targeting Bm‐app by direct injection of Cas9 messenger RNA (mRNA) and sequence‐specific single‐guide RNAs (sgRNAs), resulting in the shrinkage of wings during the pupal and adult stages which are similar to mw natural mutants of the u11 strain. Further analysis reveals that a 10 bp insertion in the promoter region of Bm‐app gene resulted in low level expression. These findings reveal that Bm‐app regulates wing development in the silkworm.
Materials and methods
Silkworm strains
A bivoltine silkworm strain, u11, which shows minute wing (mw) phenotype, was used as the natural mutant strain. Another bivoltine silkworm strain, p50, which shows normal wings, was used as wild type animals for the back‐crossing strategy. A multivoltine silkworm strain, Nistari, was used in generating CRISPR/Cas9‐mediated mutagenesis and subsequent experiments. All silkworm strains were preserved in the Sericultural Research Institute, Chinese Academy of Agricultural Sciences, and larvae were fed with fresh mulberry leaves at 25 °C under standard conditions (Tan et al., 2005).
The BmN cell line originating from the B. mori ovary was used (Pan et al., 2010). The cells were maintained in TC‐100 medium (AppliChem, Gatersleben, Germany) containing 10% fetal bovine serum in 25 cm2 Petri dishes at 27 °C. The mammalian HEK293T cell line was maintained at 37 °C under 5% CO2 in Dulbecco's modified Eagle's medium high glucose medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco).
Positional cloning of mw candidate genes
Since mw is a recessive single locus, crosses based on a single‐pair backcross model were designed (Yutaka et al., 2005). The u11 mutant strain was crossed with the wild‐type p50 strain to prepare reciprocal populations. The resulting female F1 and male F1 crosses were backcrossed with mw to generate female backcross populations BC1F and male backcross populations BC1M. Due to the lack of crossing over between chromosomes Z and W of silkworm females, reciprocal backcrossed BC1F progeny were used for linkage analysis and mapping of the Bm‐mw gene in chromosome 22 using backcrossed BC1M. The sequence‐tagged site (STS) molecular marker linkage was used to make the linkage map. Linkage maps were generated by MAPMAKER 3.0 using the Kosambi function, as described elsewhere (Lander et al., 1987).
RNA isolation and cDNA synthesis
Total RNA was isolated from the wing disc on the 6th day of the 5th larval instar (wandering stage) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and subsequently was treated with DNase I (Invitrogen) to remove genomic DNA. To synthesise cDNA, 1 μg of total RNA was used with the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania).
Quantitative real‐time PCR (qPCR) analysis
qPCR was performed to analyze the relative mRNA expression levels of selected genes. cDNA synthesized from total RNA isolated from the wing disc at the wandering stage was used as the template. The PCR conditions were as follows: initial incubation at 95 °C for 1 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The cycling conditions were as follows: initial incubation at 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. The primers that were used in qPCR to investigate the mRNA expression levels of the genes of interest are listed in Supplementary Material Table S1. Another primer pair, RP49‐F and RP49‐R (Table S1), which amplifies a 136 bp fragment from the B. mori ribosomal protein 49, was used as an internal control (Tan et al., 2013). The data shown are mean ± SEM (n = 3). The asterisks indicate the significant differences with a two‐tailed t‐test: * P < 0.05; ** P < 0.01; *** P < 0.0001.
Preparation and microinjection of Cas9/sgRNA
The Cas9‐sgRNA system was used to target the candidate gene Bm‐app. Site one (S1) and site two (S2) were identified by screening the genomic sequence of Bm‐app mw following the 5ʹ‐GG‐N18‐NGG‐3ʹ rule (Hruscha, 2013; Hwang, 2013). The sgRNAs were prepared using a MAXIscripR T7 kit (Ambion, Austin, TX, USA), according to the manufacturer's instructions. Cas9 mRNA was synthesized using a mMESSAGE mMACHINER T7 kit (Ambion), according to the manufacturer's instructions, as previously reported (Wang et al., 2013).
Fertilized eggs were prepared as previously described (Zhang et al., 2015) and micro‐injected within 3 h after oviposition. A mixture solution of Cas9 mRNA (300 ng/mL), sgRNA‐1 (150 ng/mL) and sgRNA‐2 (150 ng/mL) were injected into 480 embryos. Injection with an equal amount of phosphate‐buffered saline (PBS) was performed as a control. The injected eggs were incubated at 25 °C in a humidified chamber for 10–12 days until larval hatching (Tan et al., 2013).
Genomic DNA extraction and mutagenesis analysis
Genomic PCR, followed by sequencing, was carried out to identify the Bm‐app mutant alleles induced by Cas9/sgRNA injection. Genomic DNA was extracted from the heterozygote with the DNA extraction buffer (1:1:2:2.5 ratio of 10% sodium dodecyl sulfate to 5 mol/L NaCl to 100 mmol/L ethylenediaminetetraacetic acid to 500 mmol/L Tris‐HCl, pH = 8), incubated with proteinase K and purified via standard phenol : chloroform extraction and isopropanol precipitation extraction, followed by RNaseA treatment. The PCR conditions were as follows: 98 °C for 2 min, 35 cycles of 94 °C for 10 s, 55 °C for 30 s, and 72 °C for 40 s, followed by a final extension period of 72 °C for 10 min. The PCR products were cloned into pJET1.2 vectors (Fermentas) and directly sequenced. The primer sequences are listed Table S1.
Plasmid construction and cell transfection
A plasmid PXL‐IE1DsRed2‐EGFP was used to construct PXL‐IE1DsRed2‐u11‐EGFP (PXL‐u11‐EGFP) or PXL‐IE1DsRed2‐p50‐EGFP (PXL‐p50‐EGFP) plasmid as previously described (Xu et al., 2014). In the PXL‐u11‐EGFP plasmid, the enhanced green fluorescent protein (EGFP) reporter gene was driven by a putative promoter sequence of Bm‐app cloned from the u11 strain. A control plasmid PXL‐p50‐EGFP, was also constructed in which the EGFP reporter gene was driven by a putative promoter sequence of Bm‐app cloned from the p50 strain. Additionally, there was a red fluorescent protein DsRed2 expression cassette driven by the IE1 promoter in three vectors as the selecting marker. The putative promoter sequence was PCR amplified and introduced into KpnI and ApaI restriction sites of PXLBacII‐IE1DsRed2‐EGFP to generate PXL‐u11‐EGFP or PXL‐p50‐EGFP, respectively.
Two hundred nanograms of each plasmid were transfected into BmN cells in a 24‐well cell culture plate in duplicate using lipofectamine reagent (Invitrogen), following the manufacturer's instruction. EGFP fluorescence was investigated 72 h after transfection under a fluorescence stereomicroscope (Nikon AZ100, Tokyo, Japan).
Dual luciferase reporter (DLR) assay
For high transfection efficiency and low background expression of Bm‐app, the mammalian HEK293T cell line was used for the DLR assay. Different genomic fragments containing potential promoter sequences of 2 kb, 1.5 kb, 1 kb and 0.5 kb, were cloned and inserted into the pGL3‐promoter plasmid separately (Promega, Madison, WI, USA) between the firefly luciferase open reading frame (ORF) and SV40 poly(A). The HEK293T cells were transfected with 5 ng of the pGL3 reporter plasmid, 5 ng of the pRL‐TK control plasmid mixed with 0.5 µL Lipofectamine 2000 Transfection Reagent (Gibco) in each well of a 96‐well plate. Equal pGL3‐Basic plasmid and pRL‐TK control plasmid transfected as negative control. The DLR Assay (Promega) was performed according to the manufacturer's protocol 48 h after transfection. The experiments were performed in triplicate with three technical repeats. The mean of the relative luciferase expression ratio (firefly luciferase/ renilla luciferase) of the control was set to 1.
Results
Identification of the mw candidate gene
In the previous study, we constructed a genetic linkage map between Bm‐mw gene and STS markers in B. mori (Ma et al., 2013). However, it remains to be clarified which gene causes the mw phenotype. On this basis, we used five STS markers (T2238, T2210, T2269, T2298 and T2272) which were searched at various positions along the nucleotide sequence of the scaffold nscaf3056 on chromosome 22 (Fig. 2A). PCR‐based DNA fragment amplification and the restriction fragment length polymorphism method were used to map the mw locus to the genomic DNA sequence and revealed a polymorphism between u11 and p50 strains. The genome distance was 275 kb between T2272 which was the closest marker to mw and the end of the chromosome. Since no other STS markers were found in this region, the mw locus was finally mapped to the 275 kb region between marker T2272 and the end of nscaf3056 (Fig. 2). Within this region, 15 candidate genes were identified according to the SilkDB database (Xia et al., 2004) (Fig. 2 and Table S2). We then performed qPCR analysis to investigate their relative mRNA expressions in the larval wing disc of the p50 strain (Fig. 3). Eight genes (C17, C21, C23, C24, C26, C27, C51 and C52) showed low or no signals in the wing disc and thus could be excluded (Fig. 3A). For the rest of the seven genes (C18, C19, C20, C22, C25, C53 and C54) which showed relatively high mRNA expressions in the wing disc (Fig. 3A), we further compared their relative mRNA expressions in the wing disc between p50 and u11 strains. As a result, only C54 showed significant difference and the mRNA expression level of C54 was much lower in the u11 strain compared with the p50 strain (Fig. 3B). These data suggested that C54 was the most possible candidate gene for mw phenotype.
Figure 2.
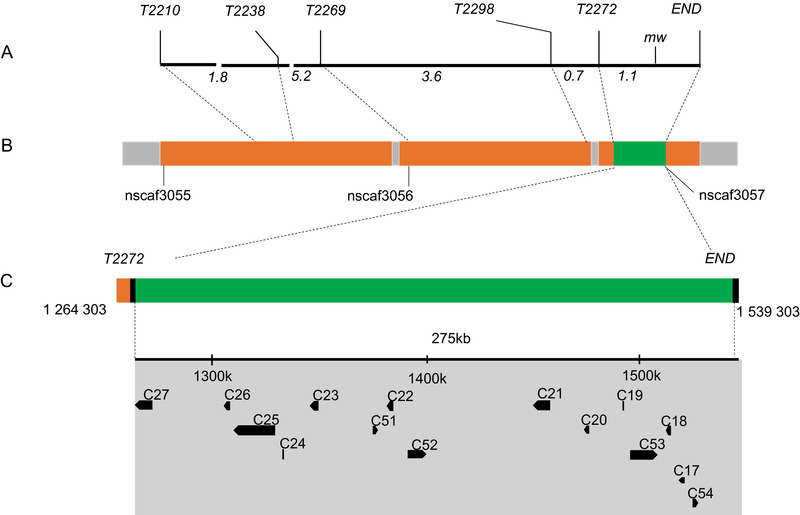
Bm‐mw mapping on Bombyx mori chromosome 22 and identification of candidate genes. (A) A linkage map based on BC1M genotyping was constructed for group 22. Five sequence‐tagged site (STS) markers and the Bm‐mw locus are shown above and distances between loci (cM) are shown below. (B) Scaffold map of chromosome 22, based on data from the SilkDB database. Orange boxes represent the assembled scaffolds, the names of which are given beneath. The STS markers used for genotyping are localized on the scaffold positions, as indicated by the dotted lines. The green box shows the candidate region linked to the mw locus. The enlarged candidate region is shown beneath along with the position on nscaf3056 of T2272 and terminal, which fix the candidate region. (C) Above is shown the coordinates of nscaf3056. The gray area represents the candidate region, which includes 15 predicted genes according to SilkDB. The directions of the transcription of these genes are represented by arrowheads. For convenience, they are named C17 to C27, C51 to C54 based on their positions. Detailed information for these 15 genes is given in Table S2 in the supplementary material.
Figure 3.
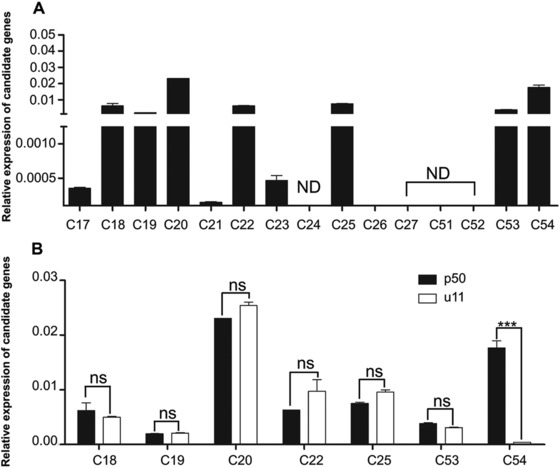
Quantitative real‐time polymerase chain reaction analysis of the minute wing candidate genes. (A) Expression patterns of 15 candidate genes in the wing disc of wandering stage larvae. (B) Expression of seven genes between p50 and u11 strains. The asterisks (***) indicate significant differences (P < 0.0001) compared with the relevant control with a two‐tailed t‐test. Error bars depict ± SEM. ND, not determined. NS, no significance.
CRISPR/Cas9‐mediated mutagenesis of C54 (Bm‐app)
To further verify whether C54 was responsible for the mw phenotype, CRISPR/Cas9‐mediated, targeted mutagenesis analysis was performed. The candidate gene C54 is designated as Bm‐app which encodes a palmitoyltransferase Approximated gene. Genomic structure of Bm‐app showed it has two exons with one intron (Fig. 4A) and encodes a protein of 110 amino acid residues and spanned approximately 2.8 kb on chromosome 22. Screening of the genomic sequence of Bm‐app according to the GGN18NGG rule identified two sgRNA target sites of site 1 (S1) and site 2 (S2) (Fig. 4A). These two sites located on exon 1 and intron 1, respectively, and the fragment spanning the two sites was 1486 bp in length (Fig. 4A).
Figure 4.
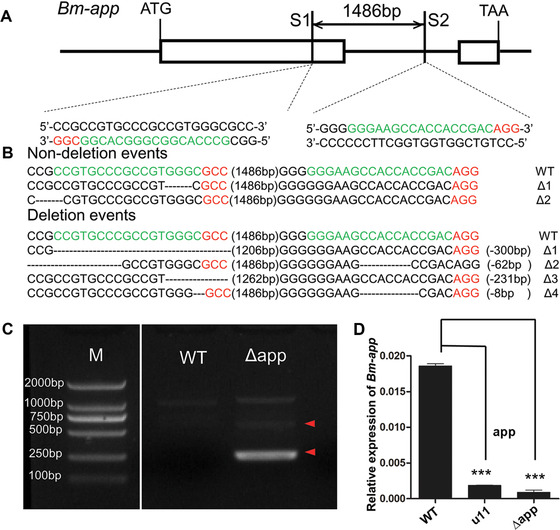
Schematic diagram of single‐guide RNA (sgRNA) targeting sites. (A) Schematic diagram of sgRNA‐targeting sites. The boxes indicate the two exons of Bm‐app, and the black line represents the gene locus. The sgRNA targeting sites, S1 and S2, are located on the sense strand of exon‐1 and the sense strand of intron, respectively. The protospacer adjacent motif (PAM) sequence is shown in red. (B) Various types of mutagenesis induced by Cas9/sgRNA injection. The numbers in brackets in the middle of each sequence refer to the 1486 bp‐long interspace fragment that was found between the S1 and S2 sites. The red sequence indicates the PAM sequence. (C) Genomic polymerase chain reaction analyses revealed deletion mutation events in the mutants. The red arrowhead indicates the deleted region. (D) The transcript level of Bm‐app is down‐regulated significantly in Δapp animals. The asterisks (***) indicate the significant differences (P < 0.0001) compared with the relevant control with a two‐tailed t‐test. Error bars depict ± SEM.
The sgRNAs that were synthesized in vitro were mixed with Cas9 mRNA and injected into preblastoderm embryos of the Nistari strain. In total, 480 eggs were injected with the Cas9/sgRNA mixture at a concentration of 300 ng/µL Cas9 mRNA and 300 ng/µL of each sgRNA. Among injected embryos, 71.3% (n = 342) hatched and 283 individuals survived to adult stages. Of the surviving moths, 86.6% (n = 245) showed defects in wings. In contrast, 480 eggs were injected with PBS and 78.1% (n = 375) hatched, 312 individuals survived to adult stages and no wing defects appeared in moths (Fig. 5). The majority of the mutant pupae exhibited minute wings not covering the meta‐thorax and moths exhibited malformed, curly wings, and the forewings did not cover the hind wings (Fig. 5). To confirm that the wing developmental defects described above were due to genomic mutagenesis induced by Cas9/sgRNA injection, genomic DNA was extracted from five randomly selected moths with mw phenotype. The fragments spanning one or both target sites were then PCR‐amplified and sequenced. PCR‐based analysis revealed mixed bands appeared in mutants (Fig. 4C) and both insertion and deletion mutations were detected (Fig. 4B). The transcript level of Bm‐app was also down‐regulated significantly in mutants (Fig. 4D). These data proved successful targeted mutagenesis occurred at the Bm‐app locus.
Figure 5.
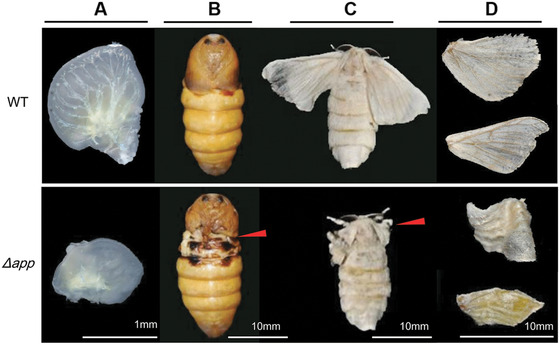
Mutants with abnormal wings induced by Cas9/single‐guide RNA injection. The mutants showed small and curly wing discs during the larval stage (A) and small wings during pupal (B) and adult stages (C, D) The red arrows indicated abnormal position.
Identification and validation of Bm‐app cis‐regulatory elements in the u11 mutants
No difference was detected in coding sequence of Bm‐app between p50 and u11 strains. However, relative mRNA expression of Bm‐app showed significant down‐regulation in the u11 strain (Fig. 3B), indicating that cis‐regulatory elements might be responsible for Bm‐app down‐regulation. We thus separately PCR‐amplified 2 kb putative promoter sequences of Bm‐app in p50 and u11 strains. Promoter prediction (http://www.fruitfly.org/seq_tools/promoter.html) revealed that there were six putative promoter sequences with score cutoff of 0.80 in the p50 strain (Supplementary sequence). The sequence in the u11 strain was identical, except there was a 10 bp insertion (ACATAGTAGT) which was located in one of the putative promoter sequences (Supplementary sequence).
To verify if this insertion was responsible for Bm‐app promoter activities, these 2 kb putative promoter sequences of Bm‐app in p50 and u11 strains were sub‐cloned into PXL‐p50‐EGFP or PXL‐u11‐EGFP plasmid separately to perform cell transfection by using the BmN cell lines. The results showed that the promoter sequence in PXL‐p50‐EGFP could drive EGFP expression while that in PXL‐u11‐EGFP did not (Fig. 6A). Further, different lengths of 0.5 kb, 1 kb, 1.5 kb and 2 kb upstream sequences were PCR‐amplified from p50 and u11 strains separately and sub‐cloned into pGL3‐promoter plasmid to perform DLR Assay in HK293T cell lines (Fig. 6). The result showed that there was significant difference in DLR activities between p50 and u11 strains when 1 kb, 1.5 kb or 2 kb sequences were subjected (Fig. 6B). The DLR activities decreased significantly when 0.5 kb sequence was applied (Fig. 6B). These data revealed that 10 bp insertion in u11 strain significantly decreased promoter activity, suggesting that it contributed to mw phenotype.
Figure 6.
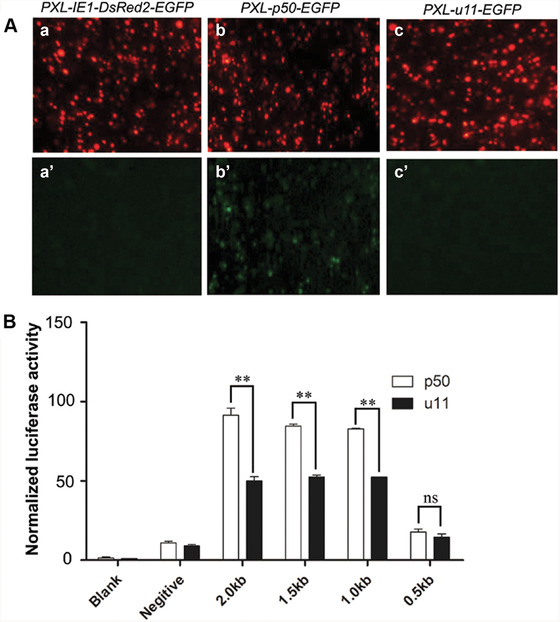
Promoter verification in BmN and HK293T cell lines. (A) Putative 2 kb promoter sequences of Bm‐app in p50 and u11 strains were sub‐cloned into PXL‐p50‐EGFP or PXL‐u11‐EGFP plasmid separately to perform cell transfection by using the BmN cell lines. PXL‐IE1Dsred2‐EGFP was used as a control. All three transfected plasmids could drive DsRed2 expression (a–c) while only PXL‐p50‐EGFP could drive enhanced green fluorescent protein (EGFP) expression (a′–c′). (B) Bm‐app promoter activity was analyzed in p50 and u11stains by measuring the Firefly/Renilla ratio. Blank, non‐transfected cells; negative, pGL3‐Basic‐transfected cells; tested, cells transfected with Bm‐app promoter‐2.0 kb (2.0 kb), Bm‐app promoter‐1.5kb (1.5 kb), Bm‐app promoter‐1.0kb (1.0 kb) and Bm‐app promoter‐0.5kb (0.5 kb) in p50 and u11 stains. All data are representative of three independent experiments and expressed as the mean ± SEM. The asterisks (**) indicate significant differences (** P < 0.01) compared with the p50 stain.
Transcription levels of genes in the Hippo pathway affected by Bm‐app down‐regulation
In Drosophila, App can bind both Dachsous (Ds) and Fat (Ft), the important components of the Hippo pathway (Mao et al., 2006; Zhang et al., 2016). App promotes the activity of Ds by simultaneously repressing Ft via post‐translational modification and recruiting Ds to the apical junctional region, thereby promoting tissue growth (Cho et al., 2006; Feng et al., 2007). We thus performed qPCR analysis to investigate transcriptional levels of some pivotal genes in the Hippo signaling pathway in the wing disc at the wandering‐stage, including the Hippo upstream inputs Ds and Ft, the Hippo kinase cassette genes Hippo (Hpo) (Pan et al., 2007), Warts (Wts) (Justice et al., 1995) and Mob‐as‐tumor‐suppressor (Mats) (Lai et al., 2005), and the Hippo downstream transcriptional regulators Yorkie (Yki) (Huang et al., 2005), Four‐jointed (Fj) (Ishikawa et al., 2008), and Wingless (Wg) (Ko et al., 2016). The results revealed that transcriptional expression levels of Ds and Ft did not change in mutants, while Hippo, Warts and Mats were significantly up‐regulated and Yki, Fj and Wg were down‐regulated (Fig. 7), suggesting that Bm‐app depletion resulted in disturbance of the Hippo signaling pathway in B. mori.
Figure 7.
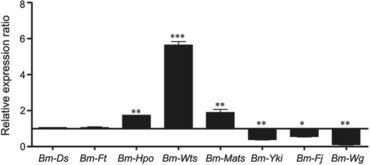
Bm‐app mutagenesis affected genes expression of the Hippo pathway in Bombyx mori. Relative messenger RNA expressions of genes in the Hippo signalling pathway were determined by quantitative real‐time polymerase chain reaction in wild‐type (WT) and Δapp silkworms. The asterisks (* or ** or ***) indicate significant differences (* P < 0.05; ** P < 0.01; *** P < 0.001) compared with the WT. Error bars depict ± SEM.
Discussion
In the current study, we identified that the silkworm mw phenotype in the u11 strain was attributed to down‐regulation of Bm‐app gene, caused by 10 bp insertion existing in the putative promoter region of Bm‐app. Furthermore, we confirmed this result by using the CRISPR/Cas9‐mediated targeted mutagenesis system. Microinjection of Cas9 mRNA and gene‐specific sgRNA elicited the mw phenotype in the Nistari moth with crooked small wings which were similar to that of the u11 mutant. Bm‐app is a homologous gene of palmitoyltransferase in Drosophila, which may regulate expression of related genes in the Hippo pathway to regulate the development of the wing disc.
Palmitoyltransferases are negative regulators of fat signaling in growth control (Linder & Deschenes, 2007; Feng & Irvine, 2009; Matis & Axelrod, 2013) and are characterized by the presence of a 50‐residue‐long domain called the DHHC domain (Politis et al., 2005; Drisdel et al., 2006), which in most but not all cases is also cysteine‐rich and gets its name from a highly conserved DHHC signature tetrapeptide (Asp‐His‐His‐Cys) (Nadolski & Linder, 2007; Fukata et al., 2016). In Drosophila, App encodes a four‐pass transmembrane protein containing a DHHC cysteine‐rich domain (Fukata & Fukata, 2010) and the catalytic domain in proteins function as palmitoyltransferase (Matakatsu & Blair, 2008). We performed qPCR analysis to investigate Bm‐app gene expression pattern in the wandering stage which is the important stage for wing disc development (Fig. S1). In this expression, Bm‐App had extensive expression in tissues. App binds both Ds and Ft and promotes the activity of Ds by simultaneously repressing Ft via post‐translational modification and recruiting Ds to the apical junctional region, thereby promoting tissue growth, including wing development (Cho et al., 2006; Matakatsu et al., 2017). Signaling via the large protocadherin Ft, regulated in part by its binding partner Ds and the Golgi‐resident kinase Four‐jointed (Fj), is required for a variety of developmental functions (Bennett & Harvey, 2006; Silva et al., 2006; Willecke et al., 2006).
Ds and Ft are important components of the Hippo signaling pathway which is a key regulator of organ size and is highly conserved in different organisms (Justice et al., 1995; Xu et al., 1995; Harvey et al., 2013). In Drosophila, the existence of the Hippo pathway coordinates multiple physiological processes, including developmental patterning, proliferation, apoptosis and cell growth, to regulate overall organ size (Boggiano & Fehon, 2012; Enderle & McNeill, 2013; Hariharan, 2015). In B. mori, all key components of the Hippo pathway exist (Liu et al., 2016; Zeng et al., 2017) and genes involved in the Hippo pathway are particularly enriched in several mitotic tissues, including the ovary, testis and wing disc in B. mori (Zeng et al., 2017; Cao et al., 2018). In the current study, although expressions of Ds and Ft did not show significant alteration, the Hippo kinase cassette genes Hippo, Warts and Mats, which play a part for inhibiting excessive cell proliferation, were significantly up‐regulated. In contrast, the Hippo downstream transcriptional regulators Yki, Fj and Wg, which are responsible for cell proliferation and anti‐apoptotic genes, were significantly down‐regulated. These genes involved in the Hippo pathway were affected after Bm‐app depletion, suggesting that Bm‐app may affect the Hippo pathway to regulate wing development in B. mori. We speculate that the down‐regulation of the Hippo pathway leads to minute wings (Zhang et al., 2017), which provides new insights into the role of Hippo pathway regulation in the silkworm. Furthermore, using the Cas9/sgRNA‐mediated mutagenesis provides a promising approach to explore molecular mechanisms of natural mutants in the future.
Supporting information
Table S1. Primers used in this work.
Table S2. Summary of the 15 mw candidate genes in B. mori.
Supplementary sequences: putative promoter sequences of Bm‐app.
Fig. S1. The expression pattern of Bm‐App in wandering stage. Epi, epidermis; MG, midgut; FB, fat body; ASG, anterior silk gland; MSG, middle silk gland; PSG, posterior silk gland; TE, testes; OV, ovary; MT, Malpighian tubule; WD, wing disc.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (31572320), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_1827) and Anhui Academy of Agricultural Sciences (16A0618).
*The copyright for this article was changed on June 4, 2020 after original online publication.
References
- Bennett, F.C. and Harvey, K.F. (2006) Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Current Biology, 16, 2101–2110. [DOI] [PubMed] [Google Scholar]
- Boggiano, J.C. and Fehon, R.G. (2012) Growth control by committee: Intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Developmental Cell, 22,695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, G.L. , Gong, Y.C. , Hu, X.L. , Zhu, M. , Liang, Z. , Huang, L.X . et al (2018) Identification of tarsal‐less peptides from the silkworm Bombyx mori . Applied Microbiology and Biotechnology, 102, 1809–1822. [DOI] [PubMed] [Google Scholar]
- Cho, E. , Feng, Y. , Rauskolb, C. , Maitra, S. , Fehon, R. and Irvine, K.D. (2006) Delineation of a Fat tumor suppressor pathway. Nature Genetics, 38, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Drisdel, R.C. , Alexander, J.K. , Sayeed, A. and Green, W.N. (2006) Assays of protein palmitoylation. Methods, 40,127–134. [DOI] [PubMed] [Google Scholar]
- Enderle, L. and McNeill, H. (2013) Hippo gains weight: Added insights and complexity to pathway control. Science Signaling, 296, 6–7. [DOI] [PubMed] [Google Scholar]
- Engel, M.S. and Grimaldi, D.A. (2004) New light shed on the oldest insect. Nature, 427, 627–630. [DOI] [PubMed] [Google Scholar]
- Feng, Y. and Irvine, K.D. (2007) Fat and Expanded act in parallel to regulate growth through Warts . Proceedings of the National Academy of Sciences USA, 104, 20362–20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. and Irvine, K.D. (2009) Processing and phosphorylation of the Fat receptor. Proceedings of the National Academy of Sciences USA, 106, 11989–11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, H. and Hojyo, T. (1997) Developmental profiles of wing imaginal discs of flügellos(fl), a wingless mutant of the silkworm, Bombyx mori . Development Genes and Evolution, 207, 12–18. [DOI] [PubMed] [Google Scholar]
- Fukata, Y. and Fukata, M. (2010) Protein palmitoylation in neuronal development and synaptic plasticity. Nature Reviews Neuroscience, 11, 161–175. [DOI] [PubMed] [Google Scholar]
- Fukata, Y. , Murakami, T. , Yokoi, N. and Fukata, M. (2016) Local palmitoylation cycles and specialized membrane domain organization. Current Topics in Membranes, 77, 97–141. [DOI] [PubMed] [Google Scholar]
- Goldsmith, M.R. , Shimada, T. and Abe, H. (2005) The genetics and genomics of the silkworm, Bombyx mori . Annual Review of Entomology, 50, 71–100. [DOI] [PubMed] [Google Scholar]
- Harvey, K.F. , Zhang, X. and Thomas, D.M. (2013) The Hippo pathway and human cancer. Nature Reviews Cancer, 13, 246–257. [DOI] [PubMed] [Google Scholar]
- Hariharan, I.K. (2015) Organ size control: Lessons from Drosophila . Developmental Cell, 34, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruscha, A. , Krawitz, P. , Rechenberg, A. , Heinrich, V. , Hecht, J. , Haass, C . et al (2013) Efficient CRISPR/Cas9 genome editing with low off‐target effects in zebrafish. Development, 140, 4982–4987. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Wu, S. , Barrera, J. , Matthews, K. and Pan, D. (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP . Cell, 122, 421–434. [DOI] [PubMed] [Google Scholar]
- Hwang, W.Y. , Fu, Y. , Reyon, D. , Maeder, M.L. , Tsai, S.Q. , Sander, J.D . et al (2013) Efficient genome editing in zebrafish using a CRISPR‐Cas system. Nature Biotechnology, 31, 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, H.O. , Takeuchi, H. , Haltiwanger, R.S. and Irvine, K.D. (2008) Four jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science, 321, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, R.W. , Ou, Z. , Woods, D.F. , Noll, M. and Bryant, P.J. (1995) The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and prolifexiration. Genes Development, 9, 534–546. [DOI] [PubMed] [Google Scholar]
- Ko, C. , Kim, Y.G. , Le, T.P. and Choi, K.W. (2016) Twinstar/cofilin is required for regulation of epithelial integrity and tissue growth in Drosophila . Oncogene, 35, 5144–5154. [DOI] [PubMed] [Google Scholar]
- Kursawe, J. , Baker, R.E. and Fletcher, A.G. (2018) Approximate Bayesian computation reveals the importance of repeated measurements for parameterising cell‐based models of growing tissues. Journal of Theoretical Biology, 443, 66–81. [DOI] [PubMed] [Google Scholar]
- Lai, ZC. , Wei, X. , Shimizu, T. , Ramos, E. and Rohrbaugh, M. (2005) Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell, 120, 675–885. [DOI] [PubMed] [Google Scholar]
- Lander, E.S. , Green, P. , Abrahamson, J. , Barlow, A. , Daly, M.J. , Lincoin, S.E . et al (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics, 1, 174–181. [DOI] [PubMed] [Google Scholar]
- Lewin, R. (1985) On the origin of insect wings: Experimental data on thermoregulation and aerodynamics give the first quantitative test of a popular hypothesis for the evolution of flight in insects. Science, 230, 428–429. [DOI] [PubMed] [Google Scholar]
- Linder, M.E. and Deschenes, R.J. (2007) Palmitoylation: policing protein stability and traffic. Nature Reviews of Molecular Cell Biology, 8, 74–84. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Zhang, P. , Song, H.S. , Qi, H.S. , Wei, Z.J. , Zhang, G . et al (2016) Yorkie facilitates organ growth and metamorphosis in Bombyx . International Journal of Biological Science, 12, 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z.Q. , You, L. , Zeng, B.S. , Ling, L. , Xu, J. , Chen, X . et al (2015) Ectopic expression of ecdysone oxidase impairs tissue degeneration in Bombyx mori . Proceedings of the Royal Society B: Biological Sciences, 282, 3238–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y. , Rauskolb, C. , Cho, E. , Hu, W.L. , Hayter, H. , Minihan, G . et al (2006) Dachs: An unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila . Development, 133, 2539–2551. [DOI] [PubMed] [Google Scholar]
- Matakatsu, H. and Blair, S.S. (2008) The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Current Biology, 18, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu, H. , Blair, S.S. and Fehon, R.G. (2017) The palmitoyltransferase Approximated promotes growth via the Hippo pathway by palmitoylation of Fat. Journal of Cell Biology, 216, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis, M. and Axelrod, J.D. (2013) Regulation of PCP by the Fat signaling pathway. Genes Development, 27, 2207–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, T.M. and Fujiwara, H. (2002) Identification and characterization of genes abnormally expressed in wing‐deficient mutant (flügellos) of the silkworm, Bombyx mori . Insect Biochemistry and Molecular Biology, 32, 691–699. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Liu, X.J. , Yi, X.L. , Hou, C.X. , Li, B. and Li, M.W. (2013) Mapping analysis of minute wing gene (mw) in silkworm, Bombyx mori by using STS markers. Science of Sericulture, 39, 1104–1107. (in Chinese with English abstract) [Google Scholar]
- McCulloch, G.A. , Wallis, G.P. and Waters, J.M. (2009) Do insects lose flight before they lose their wings? Population genetic structure in subalpine stoneflies. Molecular Ecology, 18, 4073–4087. [DOI] [PubMed] [Google Scholar]
- Nadolski, M.J. and Linder, M.E. (2007) Protein lipidation. FEBS Journal, 274, 5202–5210. [DOI] [PubMed] [Google Scholar]
- Pan, D. (2007) Hippo signaling in organ size control. Genes Development, 21, 886–897. [DOI] [PubMed] [Google Scholar]
- Pan, M.H. , Cai, X.J. , Liu, M. , Lv, J. , Tang, H. , Tan, J . et al (2010) Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori . Tissue Cell, 42, 42–46. [DOI] [PubMed] [Google Scholar]
- Politis, E.G. , Roth, A.F. and Davis, N.G. (2005) Transmembrane topology of the protein palmitoyl transferase Akr1. Journal of Biological Chemistry, 280, 10156–10163. [DOI] [PubMed] [Google Scholar]
- Silva, E. , Tsatskis, Y. , Gardano, L. , Tapon, N. and McNeill, H. (2006) The tumor‐suppressor gene fat controls tissue growth upstream of expanded in the Hippo signaling pathway. Current Biology, 16, 2081–2089. [DOI] [PubMed] [Google Scholar]
- Takanari, U. , Hideki, Y. , Hiromu, K. and Momoko, Y. (2017) Control of tissue size and development by a regulatory element in the yorkie 3’UTR. American Journal of Cancer Research, 27, 673–687. [PMC free article] [PubMed] [Google Scholar]
- Tan, A.J. , Fu, G.L. , Jin, L. , Guo, Q.H. , Li, Z.Q. , Niu, B.L . et al (2013) Transgene‐based, female‐specific lethality system for genetic sexing of the silkworm, Bombyx mori . Proceedings of the National Academy of Sciences USA, 110, 6766–6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, A.J. , Tanaka, H. , Tamura, T. and Shiotsuki, T. (2005) Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proceedings of the National Academy of Sciences USA, 102, 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, X.L. , He, S.Z. , Chen, J. , Hu, H. , Xiang, Z.H. , Lu, C . et al (2015) A novel laminin β gene BmLanB1‐w regulates wing‐specific cell adhesion in silkworm, Bombyx mori . Scientific Reports, 10, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Yang, H. , Shivalila, C.S. , Dawlaty, M.M. , Cheng, A.W. , Zhang, F . et al (2013) One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell, 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.Q. , Li, Z.Q. , Xu, J. , Zeng, B.S. , Ling, L. , You, L . et al (2013) The CRISPR/Cas system mediates efficient genome engineering in Bombyx mori . Cell Research, 23, 1414–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke, M. , Hamaratoglu, F. , Kango‐Singh, M. , Udan, R. , Chen, C.L. , Tao, C . et al (2006) The Fat cadherin acts through the Hippo tumor‐suppressor pathway to regulate tissue size. Current Biology, 16, 2090–2100. [DOI] [PubMed] [Google Scholar]
- Xia, Q.Y. , Zhou, Z. , Lu, C. , Cheng, D. , Dai, F. , Li, B . et al (2004) A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science, 306, 1937–1940. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Chen, S.Q. , Zeng, B.S. , James, A.A. , Tan, A.J. and Huang, Y.P. (2017) Bombyx mori P‐element somatic inhibitor (BmPSI) is a key auxiliary factor for silkworm male sex determination. PLoS Genetics, 13, e1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Wang, Y.Q. , Li, Z.Q. , Ling, L. , Zeng, B.S , You, L . et al (2014) Functional characterization of the vitellogenin promoter in the silkworm, Bombyx mori . Insect Molecular Biology, 23, 550–557. [DOI] [PubMed] [Google Scholar]
- Xu, T. , Wang, W.Y. , Zhang, S. , Stewart, R.A. and Yu, W. (1995) Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development, 121, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Yutaka, B. , Kohji, Y. , Marian, R.G. , Hiroshi, F. (2005) Integration of molecular and classical linkage groups of the silkworm, Bombyx mori (n = 28). Genome, 48, 626–629. [DOI] [PubMed] [Google Scholar]
- Zeng, B.S. , Huang, Y.P. , Xu, J. , Shiotsuki, T. , Bai, H. , Palli, S.R . et al (2017) The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori . Journal of Biological Chemistry, 292, 11659–11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, W.H. , Liu, R.P. , Zhang, T.Y. , Zuo, W.D. , Ou, Y. , Tang, Y.Y . et al (2018) BmYki is transcribed into four functional splicing isoforms in the silk glands of the silkworm Bombyx mori . Gene, 646, 39–46. [DOI] [PubMed] [Google Scholar]
- Zeng, W.H. , Wang, R. , Zhang, T. , Gong, C. , Zuo, W. , Liu, R . et al (2017) Cloning and expression analysis of BmYki gene in silkworm, Bombyx mori . PLoS ONE, 12, e0182690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera, A.J. and Denno, R.F. (1997) Physiology and ecology of dispersal polymorphism in insects. Annual Review of Entomology, 42, 207–230. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Danso, B. , Wu, C. , Liu, N. , Li, J. , Qin, S . et al (2017) Comparative transcriptomes analysis of the wing disc between two silkworm strains with different size of wings. PLoS ONE, 12, e0179560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.F. , Wang, X. , Matakatsu, H. , Fehon, R. and Blair, S. (2016) The novel SH3 domain protein Dlish/CG10933 mediates fat signaling in Drosophila by binding and regulating Dachs. eLife, 5, e16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.J. , Aslam, A. , Liu, X.J. , Li, M.W. , Huang, Y.P. and Tan, A.J. (2015) Functional analysis of Bombyx Wnt1 during embryogenesis using the CRISPR/Cas9 system. Journal of Insect Physiology, 79, 73–79. [DOI] [PubMed] [Google Scholar]
- Zhou, Q.X. , Tang, S.M. , Chen, Y. , Yi, Y.Z. , Zhang, Z.F. and Shen, G.F. (2004) A scaleless wings mutant associated with tracheal system developmental deficiency in wing discs in the silkworm, Bombyx mori . International Journal of Developmental Biology, 48, 1113–1117. [DOI] [PubMed] [Google Scholar]
- Zhou, Q.X. , Li, Y.N. , Shen, X.J. , Yi, Y.Z. , Zhang, Y.Z. and Zhang, Z.F. (2006) The scaleless wings mutant in Bombyx mori is associated with a lack of scale precursor cell differentiation followed by excessive apoptosis. Development Genes and Evolution, 216, 721–726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this work.
Table S2. Summary of the 15 mw candidate genes in B. mori.
Supplementary sequences: putative promoter sequences of Bm‐app.
Fig. S1. The expression pattern of Bm‐App in wandering stage. Epi, epidermis; MG, midgut; FB, fat body; ASG, anterior silk gland; MSG, middle silk gland; PSG, posterior silk gland; TE, testes; OV, ovary; MT, Malpighian tubule; WD, wing disc.


