Abstract
Background
Appendiceal neoplasms are rare and generally determined in appendectomy specimens for acute appendicitis. Depending on a tumor’s histopathology and size, appendectomy or right hemicolectomy are the surgical treatment options. Adenocarcinomas, mucinous neoplasms, goblet cell carcinoids and neuroendocrine tumors are the types of the primary appendiceal neoplasm histopathology. In this study, we aimed to determine the incidence of appendiceal neoplasms in an acute appendicitis cohort. Also, histopathological distributions, demographic data, preoperative radiological diagnosis, and intraoperative findings were revealed for analysis, retrospectively.
Material/Methods
Between October 2011 and September 2017, 3554 appendectomies were performed for acute appendicitis in Bezmialem University Hospital, Istanbul Turkey. The medical records of these consecutive 3554 patients were evaluated retrospectively. After the histopathological analysis of the appendectomy specimens, a total of 28 patients were detected as having appendiceal neoplasm including appendiceal adenocarcinoma, low grade mucinous neoplasia, and appendiceal neuroendocrine tumors.
Results
Appendiceal neoplasms were determined in 28 out of 3554 acute appendicitis patients with an incidence of 0.78%. According to the histopathological types, 3 of the cases (10.7%) were appendiceal adenocarcinoma, 8 of the cases (28.5%) were low grade mucinous neoplasia, and 17 of the cases (60.8%) were neuroendocrine tumors. The overall incidence of the appendiceal neuroendocrine tumors was 0.48%.
Conclusions
The information obtained from our study suggests that pathological examination of the specimen may not be necessary if there is no doubt according to preoperative radiological diagnosis and/or intraoperative findings of the surgeon.
MeSH Keywords: Appendiceal Neoplasms, Appendicitis, Neuroendocrine Tumors
Background
Appendiceal neoplasms are rare and generally determined in appendectomy specimens for acute appendicitis [1]. Appendiceal neoplasms are very rarely diagnosed before or during surgery. Although appendectomy for acute appendicitis is generally sufficient treatment for most of these neoplasia [2], surgeons do not many options without definitive pathological results. Depending on a tumor’s histopathology and size, appendectomy or right hemicolectomy are the surgical treatment options. Adenocarcinomas, mucinous neoplasms, goblet cell carcinoid and neuroendocrine tumors are the types of the primary appendiceal neoplasm histopathology [3]. Neuroendocrine tumors are the most common type of these neoplasms [2,4]. Surgical resections are the main treatment options due to the tumor size, because of limited systemic therapies [5]. Only appendectomy is adequate treatment for neuroendocrine tumors <1 cm; right hemicolectomy is recommended for >2 cm tumors. Treatment of 1–2 cm tumors is still controversial [6]. Patients with tumors 1–2 cm in size with positive resection margins or with deep mesoappendiceal invasion, a higher proliferation rate (Ki-67 labelling index >2%), and/or angioinvasion, as well as all patients with tumors exceeding 2 cm, should receive an oncological RH within 3 months after appendectomy [6].
In this study, we aimed to determine the incidence of appendiceal neoplasms in an acute appendicitis cohort. Also, histopathological distributions, demographic data, preoperative radiological diagnosis, and intraoperative findings were revealed for analysis, retrospectively.
Material and Methods
Between October 2011 and September 2017, 3554 appendectomies were performed for acute appendicitis in Bezmialem University Hospital, Istanbul Turkey. The medical records of these consecutive 3554 patients were evaluated retrospectively.
After the histopathological analysis of the appendectomy specimens, a total of 28 patients were identified as having appendiceal neoplasm including appendiceal adenocarcinoma, low grade mucinous neoplasia, and appendiceal neuroendocrine tumors. Nine simple mucocele cases (retention cysts caused by appendicolith, endometriosis, inflammatory conditions without mucosal hyperplasia) were excluded from this neoplastic group.
A retrospective database including gender, age, preoperative radiological diagnosis, intraoperative surgical findings, histopathological type of the tumors, tumor grades, and the diameters of the tumors were obtained.
All acute appendicitis cases were managed for wound healing the 1st week after the surgery, with the final pathology reports on the 15th day after surgery. If a premalignant lesion was detected in pathology report, the patient was called for follow-up on the 1st month and 6th month after surgery. Total colonoscopy examination was performed on appendiceal neoplasia cases for suspicious synchronous colorectal cancers. All appendiceal adenocarcinoma cases were included in the colorectal cancer follow-up program of the hospital.
Results
Demographics
There were 3554 appendectomies performed for acute appendicitis between October 2011 and September 2017 in Bezmialem Vakif University Hospital, Istanbul Turkey. Of the patients with acute appendicitis, 1282 patients (36.1%) were female and 2272 patients (63.9%) were male. The median age was 33 years (15–91 years). Appendiceal neoplasms were determined in 28 out of 3554 acute appendicitis patients with an incidence of 0.78%. Gender distribution of these 28 patients was 10 females and 18 males. The median age of this group was 44.5 years (17–89 years). Ten of 28 patients (35.7%) were older than 50 years of age. The median symptom time was 2 days (1–10 days). Appendiceal neoplasms were diagnosed in 1.4% of the male acute appendicitis patients and 0.44% of the female acute appendicitis patients. The demographic data and distribution of the cases based on year are shown in Figure 1.
Figure 1.
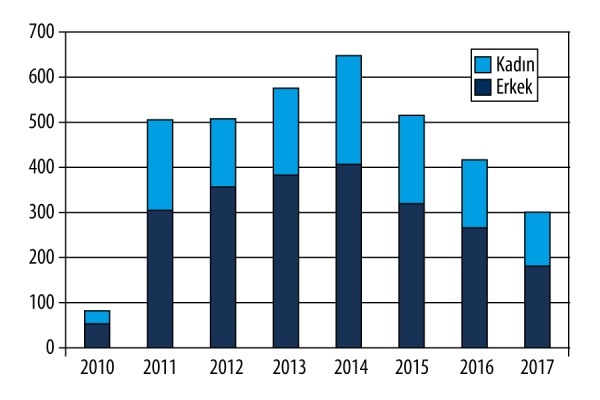
Demographic distrubition of the acute appendicitis cases.
According to the histopathological types, 3 of the cases (10.7%) were appendiceal adenocarcinoma, 8 of the cases (28.5%) were low grade mucinous neoplasia and 17 of the cases (60.8%) were neuroendocrine tumors. The overall incidence of the appendiceal neuroendocrine tumors was 0.48%. These findings are summarized in Table 1.
Table 1.
Distrubition of the appendiceal neoplasia.
| Cases (n) | % | Incidence % | |
|---|---|---|---|
| Adenocarcinoma | 3 | 10.7 | 0.08 |
| Low grade mucinous neoplasia | 8 | 28.5 | 0.22 |
| Neuroendocrine tumor | 17 | 60.8 | 0.48 |
Preoperative diagnosis
Thirteen patients were diagnosed by ultrasound, the others were by abdominal computed tomography (CT). All patients were diagnosed with acute appendicitis except for 5 patients. For 1 of these 5 patients, the CT was reported as normal due to the presence of the non-inflamed appendix vermiformis with a diameter of 8 mm. This patient’s final pathological diagnosis was neuroendocrine tumor. For another of the 5 patients, cystic masses caused by perforated appendicitis with diameter 45 mm was determined in CT. This patient’s final pathological diagnosis was adenocarcinoma. For 2 of the 5 patients, cystic masses with diameters of 20 mm and 30 mm were reported as mucocele. The final diagnosis of the patient with the 20 mm cystic mass on CT scan was adenocarcinoma and the patient with the 30 mm cystic mass was low grade mucinous neoplasia. There was 1 perforated acute appendicitis case on CT scan among these 5 patients without preoperative diagnosis of acute appendicitis only. The final pathological result of this patient was also low grade mucinous neoplasia. The mean radiological diameter of the appendiceal tumors of these 5 patients was 11 mm (range 8–45 mm). Any neoplastic appendicitis was not diagnosed correctly in preoperative period.
Intraoperative findings
Appendectomy was performed for all cases. Three patients had laparoscopic surgery, whereas all the other patients had conventional surgery. Fifteen of 28 patients were staged as catarrhal, 3 as gangrenous, 7 as perforated, 1 as plastron appendicitis, and 2 as suspicious mucinous masses by the attending surgeons. Final pathology of the case with the intraoperative diagnosis of plastron appendicitis was adenocarcinoma. Adenocarcinoma and mucinous neoplasia were determined in 2 intraoperative suspicious mucinous masses. Only 2 out of 3 adenocarcinoma cases (7.1%) required a second surgical procedure, as an oncological procedure.
Pathological results
In 17 of the 28 patients, the final pathological diagnosis was G1 well-differentiated neuroendocrine tumor according to the World Health Organization (WHO) 2010 recommendation [7]. Two patients were diagnosed as primary appendiceal adenocarcinoma, with pathological stages pT3NxM1b and pTisNxM0 according to TNM 8th edition [8]; 1 patient had adenocarcinoma overlapping with a mucinous neoplasia, which was pT1Nx with positive distal margin and 8 patients had low grade mucinous neoplasia according to PSOGI 2016 [9]. All pathological findings and surgical treatment modalities are summarized in Table 2.
Table 2.
Pathological findings and surgical treatment modalities.
| Pathological findings | Surgical procedure | 2. step surgery |
|---|---|---|
| Adenocarcinoma 3 cases | ||
| Case 1 pT3NxM1b | Appendectomy + segmentary ileum resection | Right hemicolectomy and peritonectomy |
| Case 2 pTisNxMo | Appendectomy | – |
| Case 3 pT1NxMo | Appendectomy | Right hemicolectomy |
|
| ||
| Neuroendocrine tumors | ||
| 17 cases G1 stage | Appendectomy | – |
|
| ||
| Low grade mucinous neoplasia | ||
| 8 cases | Appendectomy | – |
Appendiceal adenocarcinomas
Three of the 3554 acute appendicitis cases were diagnosed as adenocarcinoma of the appendix vermiformis with an incidence of 0.08%. Male to female ratio was 2: 1. One of these 3 cases was the patient with the 45 mm cystic mass on CT scan and intraoperative diagnosis of plastron appendicitis was T3 adenocarcinoma with a positive surgical margin. This patient had a right hemicolectomy and peritonectomy, received FOLFOX chemotherapy, and was alive at the postoperative 4th month.
Another of the adenocarcinoma cases was diagnosed preoperatively as perforated appendicitis with abscess formation, and a mucinous mass was found intraoperatively. The final pathological analysis revealed that the tumor was developed in association with mucinous cystic neoplasia. The patient was operated on via the right hemicolectomy.
The other patient with the diagnosis of adenocarcinoma was diagnosed as acute appendicitis from a preoperative CT scan, and gangrenous stage appendicitis was determined intraoperatively. The final pathological analysis showed that the tumor invaded into the lamina propria (Tis) with clear surgical margins. The patient has been followed postoperative for 19 months without any surgical or oncological treatment.
Mucinous neoplasia
Six cases were diagnosed as acute appendicitis by CT scan and staged as catarrhal appendicitis intraoperatively, the final pathological diagnosis was low grade mucinous neoplasia. Another 2 cases were diagnosed as perforated appendicitis and a 20 mm suspicious mucinous mass in CT scan. Surgical margins were clear for all cases. The patients did not receive any other treatment modality.
Neuroendocrine tumors
Seventeen specimens were reported as neuroendocrine tumor. All of them were grade 1 according to the WHO 2010 classification. The median tumor diameter was 6 mm (2–19 mm). In only 1 case was the tumor diameter longer than 15 mm. The Ki-67 index ratios 1–2% and the mitosis counts of all cases were between 0 and 2 in high power field (HPF). All cases were diagnosed as acute appendicitis preoperatively and there were not any pathological signs on CT scan. The distribution of the intraoperative staging was 11 catarrhal cases 2 gangrenous cases, and 4 cases of perforated appendicitis. There were not any suspicious findings determined intraoperatively for neuroendocrine appendiceal tumors. There were not any pathological signs in control colonoscopies of appendiceal neuroendocrine tumors. All cases were disease free at the 6th postoperative month.
The radiological diagnosis, intraoperative findings, and final pathological results of the cases are summarized in Table 3.
Table 3.
Radiological diagnosis and intraoperative findings of the appendiceal neoplasia.
| Radiological diagnosis | Intraoperative findings | |||
|---|---|---|---|---|
| Adenocarcinoma | Cyctic mass | (n: 1) | Plastrone | (n: 1) |
| Mucocele | (n: 1) | Mucocele | (n: 1) | |
| Acute appendicitis | (n: 1) | Gangrenous | (n: 1) | |
|
| ||||
| Low grade mucinous neoplasia | Mucocele | (n: 1) | Mucocele | (n: 1) |
| Perforated | (n: 1) | Perforated | (n: 2) | |
| Acute appendicitis | (n: 6) | Catarrhal | (n: 5) | |
|
| ||||
| Neuroendocrine tumor | Acute appendicitis | (n: 16) | Gangrenous | (n: 2) |
| Perforated | (n: 4) | Normal appendix | (n: 1) | |
| Catarrhal | (n: 11) | |||
Discussion
Appendiceal tumors are rarely seen entities [2]. Collins reported that appendiceal tumors were noted in between 0.9% and 1.4% of 280 000 appendectomies performed in USA [10]. However, it is difficult to study these tumors because of their low incidence (4). Treatment modalities could be further developed using a large series from institutions or population-based studies [2,5,6,11].
This study cohort could be considered as a large volume sample size with a similar incidence rate of appendiceal tumors in accordance with previous studies [2,12–14]. The proportion of appendiceal tumors that have been reported in the previous studies are shown in Table 4.
Table 4.
Proportion of the appendiceal tumors that have been reported in the previous studies.
| Lead Author | Number of patients enrolled | Number | Years | Incidence % |
|---|---|---|---|---|
| Connor | 7970 | 74 | 1979–1994 | 0.9 |
| Bucher | 2500 | 43 | 1991–2001 | 1.7 |
| Egin | 3769 | 10 | 2006–2012 | 0.26 |
| Lee | 3744 | 28 | 2000–2005 | 0.7 |
| Current series | 3554 | 28 | 2011–2017 | 0.78 |
Neuroendocrine tumors are the most common type of these tumors [2,4]. Only appendectomy is adequate treatment for neuroendocrine tumors if they are <1 cm, and right hemicolectomy is recommended for >2 cm tumors. However, optimal treatment of 1–2 cm tumors is still controversial [6]. In our case series, appendectomy was regarded as an adequate treatment for neuroendocrine tumors. Raoof et al. [4] reported that the probability of nodal metastases was 2.7% in tumors ≤1.0 cm, 31.0% in tumors with a diameter of 1.1 to 2.0 cm, and 64.0% in tumors >2.0 cm. They suspected that lymph node count was a prognostic factor for well-differentiated neuroendocrine tumors [4]. In our study, all neuroendocrine tumors were smaller than 15 mm except for 1 which was 19 mm; the Ki-67 index ratios were 1–2% and mitosis counts were between 0–2 in HPF. Therefore, we did not recommend wider resection for grade 1 neuroendocrine tumors.
Surgeons, in general, may not notice appendiceal tumors, especially neuroendocrine tumors; and preoperative radiologic examination may not be effective in identifying them [15]. Some authors recommend frozen section if there is doubt [16]. However, generally this is not possible because appendiceal tumors usually present as acute appendicitis [17]. Therefore, if there is not any pathological sign from radiological diagnosis or during surgery, we recommend that appendectomy is sufficient, and patients do not need additional treatment.
Egin et al. published their case series which included 3769 consecutive emergency appendectomies; they observed 10 carcinoid tumors of the appendix vermiformis [14]. No adjuvant treatment was applied. They presented 37.9 months of follow-up without any disease or symptoms. Although the current study’s follow-up period was shorter, no additional treatment was deemed necessary.
Colorectal cancers may be associated with appendiceal neuroendocrine tumors. Bucher et al. reported that 14% of appendiceal neoplasm had synchronous colon cancers [12]. In our current study, we did not find any associated cancers.
There is no doubt that appendiceal adenocarcinomas may require further surgical or oncological treatment. Withfield et al. [17] suggested that surgeons should be attentive to older patients with simple appendicitis and longer symptom duration, as well as when a mass presents [17]. Approximately a quarter of the current study tumor group was older than 50 years of age and their symptomatology was limited to a few days. We think that imaging and operative findings are also important, in addition to the pathology result, when making the decision regarding appendiceal tumors.
Conclusions
The information obtained from our study, suggests that pathological examination of the specimen may not be necessary if there is no doubt based on preoperative radiological diagnosis and/or intraoperative findings of the surgeon. For a catarrhal, gangrenous, or perforated appendicitis without any pathologic signs on CT scan or at operation, at worst the histopathological examination probably would be a low grade neuroendocrine tumor. The patient would not require further treatments. On the other hand, if there is a discordance in clinical presentation, radiological diagnosis, and intraoperative findings, pathological examination would be very important to determine the prognosis of the patient.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Turaga KK, Pappas SG, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol. 2012;19(5):1379–85. doi: 10.1245/s10434-012-2238-1. [DOI] [PubMed] [Google Scholar]
- 2.Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: Retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41(1):75–80. doi: 10.1007/BF02236899. [DOI] [PubMed] [Google Scholar]
- 3.Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40:14–26. doi: 10.1097/PAS.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 4.Raoof M, Dumitra S, O’Leary MP, et al. Mesenteric lymphadenectomy in well differentiated appendiceal neuroendocrine tumors. Dis Colon Rectum. 2017;60(7):674–81. doi: 10.1097/DCR.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 5.Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–22. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pape UF, Niederle B, Costa F, et al. Vienna Consensus Conferenceparticipants. ENETS consensus guidelines for neuroendocrine neoplasms of the appendix (excluding goblet cell carcinomas) Neuroendocrinology. 2016;103:144–52. doi: 10.1159/000443165. [DOI] [PubMed] [Google Scholar]
- 7.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):3–7. doi: 10.1007/s10555-011-9292-1. [DOI] [PubMed] [Google Scholar]
- 8.UICC TNM Classification of Malignant Tumours – 8th edition. Dec, 2016. [Google Scholar]
- 9.Carr NJ, Cecil TD, Mohamed F, et al. A Consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the Peritoneal Surface Oncology Group International (PSOGI) modified delphi process. Am J Surg Pathol. 2016;40(1):14–26. doi: 10.1097/PAS.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 10.Collins DC. 71,000 human appendix specimens. A final report, summarizing forty years’ study. Am J Proctol. 1963;14:265–81. [PubMed] [Google Scholar]
- 11.Landry CS, Woodall C, Scoggins CR, et al. Analysis of 900 appendiceal carcinoid tumors for a proposed predictive staging system. Arch Surg. 2008;143:664–70. doi: 10.1001/archsurg.143.7.664. [DOI] [PubMed] [Google Scholar]
- 12.Bucher P, Mathe Z, Demirag A, Morel P. Appendix tumors in the era of laparoscopic appendectomy. Surg Endosc. 2004;18(7):1063–66. doi: 10.1007/s00464-003-9255-x. [DOI] [PubMed] [Google Scholar]
- 13.Egin S, Hot S, Yesiltas M, et al. Apendiks’in Karsinoid tümörü: 3769 Ardışık Acil Apendektomi. Okmeydanı Tıp Dergisi. 2014;30(3):135–38. [in Turkish] [Google Scholar]
- 14.Lee WS, Choi ST, Lee JN, et al. A retrospective clinicopathological analysis of appendiceal tumors from 3,744 appendectomies: A single-institution study. Int J Colorectal Dis. 2011;26(5):617–21. doi: 10.1007/s00384-010-1124-1. [DOI] [PubMed] [Google Scholar]
- 15.Deans GT, Spence RA. Neoplastic lesions of the appendix. Br J Surg. 1995;82:299–306. doi: 10.1002/bjs.1800820306. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge RH, Alexander JW. Primary appendiceal malignancies: Rare but important. Surgery. 1992;111(3):244–50. [PubMed] [Google Scholar]
- 17.Whitfield CG, Amin SN, Garner JP. Surgical management of primary appendiceal malignancy. Colorectal Dis. 2012;14(12):1507–11. doi: 10.1111/j.1463-1318.2012.03052.x. [DOI] [PubMed] [Google Scholar]


