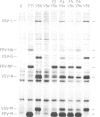
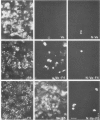
Abstract
We have used filter-grown Madin-Darby canine kidney (MDCK) cells to explore the mechanism by which influenza virus facilitates secondary virus infection. Vesicular stomatitis virus (VSV) and Semliki Forest virus (SFV) infect only through the basolateral surface of these polarized epithelial cells and not through the apical surface. Prior infection with influenza virus rendered the cell susceptible to infection by VSV or SFV through either surface. The presence of both a permissive and a restrictive surface for virus entry in the same cell allowed us to determine how the influenza infection enhanced the subsequent infection of a second virus. Biochemical and morphological evidence showed that influenza haemagglutinin on the apical surface serves as a receptor for the superinfecting virus by binding to its sialic acid-bearing envelope proteins. Influenza virus also facilitates secondary virus infection in non-epithelial cells; baby hamster kidney cells (BHK-21), which are normally resistant to infection by the coronavirus (mouse hepatitis virus MHV-A59), could be infected via the haemagglutinin-sialic acid interaction. Facilitation of secondary virus infection requires only the sialic acid-binding properties of the haemagglutinin since the uncleaved haemagglutinin could also mediate virus entry.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcarova-Ständer J., Pfeiffer S. E., Fuller S. D., Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984 Nov;3(11):2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Pearse B. M. Coated pits in action. Cell. 1984 Aug;38(1):3–4. doi: 10.1016/0092-8674(84)90519-1. [DOI] [PubMed] [Google Scholar]
- Cantell K., Valle M. The ability of Sendai virus to overcome cellular resistance to vesicular stomatitis virus. II. The possible role of interferon. Ann Med Exp Biol Fenn. 1965;43(2):61–64. [PubMed] [Google Scholar]
- Compans R. W. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol. 1974 Nov;14(5):1307–1309. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROTHINGHAM T. E. FURTHER OBSERVATIONS ON CELL CULTURES INFECTED CONCURRENTLY WITH MUMPS AND SINDBIS VIRUSES. J Immunol. 1965 Apr;94:521–529. [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- GINDER D. R., FRIEDEWALD W. F. Effect of Semliki Forest virus on rabbit fibroma. Proc Soc Exp Biol Med. 1951 Jun;77(2):272–276. doi: 10.3181/00379727-77-18747. [DOI] [PubMed] [Google Scholar]
- HERMODSSON S. Inhibition of interferon by an infection with parainfluenza virus type 3 (PIV-3). Virology. 1963 Jun;20:333–343. doi: 10.1016/0042-6822(63)90123-5. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Boothroyd B., Gregory H. Early events following the binding of epidermal growth factor to surface receptors on ovarian granulosa cells. Eur J Cell Biol. 1981 Jun;24(2):259–265. [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Inhibition of vesicular stomatitis virus infection by spike glycoprotein. Evidence for an intracellular, G protein-requiring step. J Cell Biol. 1980 Feb;84(2):430–437. doi: 10.1083/jcb.84.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Effect of persistent fibroma virus infection on susceptibility of cells to other viruses. J Virol. 1970 Feb;5(2):199–204. doi: 10.1128/jvi.5.2.199-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. II. Immunological quantitation. J Cell Biol. 1983 Sep;97(3):638–643. doi: 10.1083/jcb.97.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. C., Simmons N. L. Demonstration of protein asymmetries in the plasma membrane of cultured renal (MDCK) epithelial cells by lactoperoxidase-mediated iodination. FEBS Lett. 1979 Sep 15;105(2):201–204. doi: 10.1016/0014-5793(79)80611-0. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Rodriguez-Boulan E., Sabatini D. D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol. 1984 Apr;98(4):1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Tsuchiya Y., Tagaya I. Enhanced or inhibited plaque formation of superinfecting viruses in Yaba virus-infected cells. J Gen Virol. 1970 Apr;7(1):71–73. doi: 10.1099/0022-1317-7-1-71. [DOI] [PubMed] [Google Scholar]
- Valle M., Cantell K. The ability of Sendai virus to overcome cellular resistance to vesicular stomatitis virus. I. General characteristics of the system. Ann Med Exp Biol Fenn. 1965;43(2):57–60. [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Závada J. The pseudotypic paradox. J Gen Virol. 1982 Nov;63(Pt 1):15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. An efficient method for introducing defined lipids into the plasma membrane of mammalian cells. J Cell Biol. 1983 Nov;97(5 Pt 1):1365–1374. doi: 10.1083/jcb.97.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]