Abstract
Purpose
To evaluate the impact of molecular subtype on incidence and prognosis of brain metastasis from breast cancer.
Methods
The Surveillance, Epidemiology, and End Results (SEER) 18 registry was used to select breast cancer patients from 2010 to 2014. Molecular subtypes were classified as luminal A (hormone receptor [HR]+/human epidermal growth factor receptor 2 [HER2]-), luminal B (HR+/HER2+), HER2 (HR-/HER2+), or triple negative breast cancer (TNBC) (HR-/HER2-). The incidence and prognosis of brain metastasis was evaluated according to molecular subtype.
Results
Among the 206913 breast cancer patients, the HER2 subtype showed the highest incidence of brain metastasis (1.0%). HER2 and TNBC with multiple extracranial metastases (bone, liver, and lung) showed a high incidence of brain metastasis (28.0 and 30.8%, respectively). Median survival of luminal A, luminal B, HER2, and TNBC in brain metastasis was 12, 23, 10, and 6 months (p < 0.001), and in brain metastasis without visceral metastasis was 14, 34, 17, and 8 months (p < 0.001). On multivariate analysis, the order of subtype by favorable prognosis was luminal B, luminal A, HER2, and TNBC in all brain metastasis, while for brain metastasis patients without visceral metastasis, the order was luminal B, HER2, luminal A, and TNBC.
Conclusions
Molecular subtype and visceral metastasis should be considered for prediction of prognosis for patients with brain metastasis. The patients with HER2 and TNBC cancer subtypes having visceral metastasis, close surveillance could contribute to early detection of brain metastasis and may putatively lead to improved quality of life and survival.
Electronic supplementary material
The online version of this article (10.1007/s00432-018-2697-2) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Brain metastasis, Molecular subtype, Incidence, Prognosis
Introduction
Breast cancer is the most common female cancer and the second most frequent cancer in the world (Ferlay et al. 2015; Siegel et al. 2016). It ranks as the most common cause of female cancer death in less developed regions and the second cause of all cancer death in more developed regions (Ferlay et al. 2015). Brain metastasis in breast cancer has been considered the last stage of distant metastasis and has a very poor prognosis (Kodack et al. 2015). However, the development of hormone or targeted therapies for each tumor subtype has led to a significant increase in survival rate even for stage IV patients (Finn et al. 2015; Swain et al. 2015). Additionally, radiotherapy such as radiosurgery (Cho et al. 2015) or hippocampal sparing technique in whole brain radiotherapy (Gondi et al. 2014) provide effective intracranial disease control with minimized cognitive defects. Therefore, accurate estimation of prognosis in brain metastasis has become increasingly important in choosing an optimal treatment policy.
Molecular subtype has impacts on the incidence (Heitz et al. 2009; Hicks et al. 2006; Lim et al. 2017), prognosis (Sperduto et al. 2012), and treatment response of brain metastasis (Dawood et al. 2009). Human epidermal growth factor receptor 2 (HER2) (hormone receptor [HR] negative, and HER2 positive) and triple negative breast cancer (TNBC, HR negative, and HER2 negative) subtypes have higher incidence of brain metastasis compared to HR positive subtypes (Heitz et al. 2009; Hicks et al. 2006; Lim et al. 2017). The addition of trastuzumab to cytotoxic chemotherapy has improved outcomes for patients with HER2 positive breast cancer. However, increased survival coupled with limited blood–brain barrier (BBB) penetration of trastuzumab may contribute to the increased incidence of brain metastases (Swain et al. 2015).
Evaluation of brain metastasis according to molecular subtype may help identify high risk patients who need early surveillance and may also help determine prognosis for patients with brain metastases. The Breast Cancer Specific Graded Prognostic Assessment (BS-GPA) reflects the prognostic value of molecular subtype. In this assessment, the TNBC subtype has the poorest prognosis, followed by the luminal A (HR positive, HER2 negative), HER2, and luminal B (HR positive, HER2 positive) subtypes (Sperduto et al. 2011, 2012). The BS-GPA requires only three input values (age, the Karnofsky performance status scale [KPS], and molecular subtype) and is a simple and effective method for predicting prognosis in brain metastasis. However, in the KPS, the population enrolled to develop the BS-GPA showed a narrow spectrum of scale with 74% of patients scoring from 80 to 100 (Sperduto et al. 2012). Additionally, the prognostic value of visceral and non-visceral extracranial metastasis was not evaluated, and the burden of extracranial metastasis was not investigated according to molecular subtype.
Many studies demonstrate that HER2 and TNBC cancer subtypes are more likely to metastasize to visceral tissues rather than bone (Bartmann et al. 2017; Gerratana et al. 2015; Savci-Heijink et al. 2015), and visceral metastasis is associated with poor prognosis in breast cancer (Solomayer et al. 2000). Although half of the patients with brain metastasis die of intracranial disease progression, systemic failure is another major cause of death in patients with breast cancer (Lin et al. 2008). Therefore, prognostic evaluation according to each molecular subtype and stratification according to the sites and numbers of combined extracranial metastases may provide more information with which to predict prognosis and improve survival for breast cancer patients with brain metastasis.
Materials and methods
Study population
This study analyzed de-identified data obtained from the Surveillance, Epidemiology, and End Results (SEER) 18 registry. The SEER registry provided molecular subtype information for each patient. Specifically, the subtypes included hormone receptor (HR) positive and HER2 negative (luminal A), HR positive and HER2 positive (luminal B), HR negative and HER2 positive (HER2), and both HR and HER2 negative (triple negative breast cancer, [TNBC]). Data were also available regarding metastasis to brain, bone, liver, and lung at the time of the first diagnosis of breast cancer.
Selection criteria included the following: female breast cancer patients diagnosed from 2010 to 2014 who were 20 years old or older, and for whom information was available regarding molecular subtype and whether metastasis occurred to distant tissues (brain, bone, liver, or lung). Patients who were diagnosed via autopsy or death certificate were excluded from the study. Only patients with breast cancer which were either the first diagnosed cancer or the only cancer in life were included.
If 1% or greater cells were stained positive in ER and PR tests, the result was considered positive. If both an immunehistochemistry (IHC) and a gene-amplification test [fluorescence (FISH) or chromogenic in situ hybridizations (CISH)] were performed for HER2 test, the result of the gene-amplification test was reported.
Analysis of de-identified data from the SEER program was exempt from medical ethics review and no informed consent was required. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
The incidence of brain metastasis was compared between specific subgroups of breast cancer patients according to age, T and N stages, other distant metastasis, and cancer subtype using a Pearson’s Chi-square test.
To predict the probability of brain metastasis, a nomogram was generated. All patients were randomly and evenly divided into a training set and a validation set. Univariate and multivariate logistic regression analyses were performed. The Hosmer and Lemeshow test was used to evaluate the goodness of the logistic regression model. p value less than 0.05 indicated a good fit. Variables with p value less than 0.05 in the multivariate analysis and/or clinically important were selected to generate a nomogram.
Internal and external validations of the Nomogram were performed on the training set and the validation set, respectively. Internal validation was performed using a calibration plot to compare the actual probability and the predicted probability of brain metastasis, and using a receiver operating characteristic (ROC) curve to calculate the area under the curve (AUC). External validation was performed by measuring AUC. The AUC difference between the training set and the validation set was assessed using the DeLong method. The cut-off point of nomogram with the highest sensitivity and specificity was automatically obtained by R programming using the Youden’s method.
In stage IV cancer patients, median overall survival (OS) for each molecular subtype was calculated for every combination of metastatic sites (brain, bone, and visceral organ [lung or liver]) using Kaplan–Meier estimates and compared using log-rank tests.
In patients with brain metastasis, patient characteristics for each molecular subtype were compared using Pearson’s Chi-square tests. The variables compared in these tests were age, race, tumor grade, T and N stages, surgery, chemotherapy, radiotherapy, and distant metastasis (brain, bone, liver, and lung). In each subgroup of variables, median OS rate of each molecular subtype was calculated using Kaplan–Meier estimates, and a log-rank test was used to compare survival rates among molecular subtypes.
For each molecular subtype, univariate analysis was performed to evaluate prognostic power for OS rate. Multivariate analyses were performed incorporating variables that were statistically significant in univariate analyses (p < 0.05) and/or considered clinically significant. Covariate-adjusted Kaplan–Meier survival graphs stratified by molecular subtype were obtained after adjusting for variables that were statistically significant in multivariate analysis. All p values were two-sided, and p values less than 0.05 were considered statistically significant. Statistics were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), Stata/MP 14.2 (Stata Corp., College Station, Texas, USA), and the package of ‘rms’ and ‘pROC’ in R version 3.5.0 (http://www.r-project.org/).
Results
Incidence of brain metastasis in all breast cancer patients according to molecular subtype
The total numbers of patients comprising each molecular subtype group were the following: luminal A (HR+/HER2-), luminal B (HR+/HER2+), HER2 (HR-/HER2+), and TNBC (HR-/HER2-) were 149,861 (72.4%), 22,648 (10.9%), 9942 (4.8%), and 24,462 (11.8%), respectively. Incidence of brain metastasis according to molecular subtype in all breast cancer patients was 0.2, 0.6, 1.0, and 0.7%, respectively (Table 1). The HER2 and TNBC subtypes showed high rates of brain metastasis. In patients younger than 40, the HER2 subtype showed even higher incidence of brain metastasis (1.7%).
Table 1.
Incidence of brain metastasis in all breast cancer patients according to molecular subtypes
| Characteristics | Luminal A (HR+/HER2-) |
Luminal B (HR+/HER2+) |
HER2 (HR-/HER2+) |
TNBC (HR-/HER2-) |
P value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain | All | (%) | Brain | All | (%) | Brain | All | (%) | Brain | All | (%) | ||
| All | 339 | 149,861 | (0.2) | 132 | 22,648 | (0.6) | 104 | 9942 | (1.0) | 163 | 24,462 | (0.7) | < 0.001 |
| Age | |||||||||||||
| < 40 | 20 | 6285 | (0.3) | 8 | 2231 | (0.4) | 15 | 890 | (1.7) | 8 | 2333 | (0.3) | < 0.001 |
| 40–59 | 138 | 61,400 | (0.2) | 70 | 11,358 | (0.6) | 49 | 5101 | (1.0) | 75 | 11,418 | (0.7) | < 0.001 |
| ≥ 60 | 181 | 82,156 | (0.2) | 54 | 9059 | (0.6) | 40 | 3951 | (1.0) | 80 | 10,711 | (0.7) | < 0.001 |
| T stage | |||||||||||||
| ≤1 | 36 | 93,503 | (0.0) | 21 | 10,705 | (0.2) | 8 | 4102 | (0.2) | 18 | 10,074 | (0.2) | < 0.001 |
| 2 | 78 | 41,305 | (0.2) | 29 | 8259 | (0.4) | 22 | 3577 | (0.6) | 38 | 10,022 | (0.4) | < 0.001 |
| 3 | 41 | 8041 | (0.5) | 14 | 1724 | (0.8) | 12 | 998 | (1.2) | 25 | 2148 | (1.2) | 0.002 |
| 4 | 125 | 4451 | (2.8) | 47 | 1383 | (3.4) | 47 | 975 | (4.8) | 60 | 1673 | (3.6) | 0.012 |
| Unknown | 59 | 2502 | (2.4) | 21 | 577 | (3.6) | 15 | 290 | (5.2) | 22 | 545 | (4.0) | 0.008 |
| N stage | |||||||||||||
| 0 | 99 | 103,478 | (0.1) | 30 | 12,957 | (0.2) | 21 | 5181 | (0.4) | 27 | 15,336 | (0.2) | < 0.001 |
| 1 | 131 | 33,694 | (0.4) | 53 | 6771 | (0.8) | 38 | 3159 | (1.2) | 76 | 6138 | (1.2) | < 0.001 |
| 2 | 38 | 7398 | (0.5) | 19 | 1644 | (1.2) | 14 | 817 | (1.7) | 10 | 1515 | (0.7) | < 0.001 |
| 3 | 44 | 4128 | (1.1) | 14 | 1020 | (1.4) | 25 | 688 | (3.6) | 31 | 1226 | (2.5) | < 0.001 |
| Unknown | 27 | 1119 | (2.4) | 16 | 242 | (6.6) | 6 | 97 | (6.2) | 19 | 250 | (7.6) | < 0.001 |
| Category | |||||||||||||
| T1N0Bone(-)Visceral(-) | 5 | 76,979 | (0.0) | 0 | 8146 | (0.0) | 0 | 3015 | (0.0) | 0 | 8020 | (0.0) | 0.742 |
| T1N1 | 0 | 13,555 | (0.0) | 2 | 1909 | (0.1) | 1 | 746 | (0.1) | 3 | 1483 | (0.2) | < 0.001 |
| T1N2 | 0 | 1485 | (0.0) | 0 | 276 | (0.0) | 0 | 128 | (0.0) | 1 | 247 | (0.4) | 0.054 |
| T1N3 | 0 | 512 | (0.0) | 0 | 105 | (0.0) | 0 | 70 | (0.0) | 2 | 120 | (1.7) | 0.009 |
| T2N0 | 7 | 21,188 | (0.0) | 3 | 3718 | (0.1) | 1 | 1585 | (0.1) | 3 | 5902 | (0.1) | 0.584 |
| T2N1 | 5 | 13,462 | (0.0) | 2 | 2917 | (0.1) | 2 | 1215 | (0.2) | 5 | 2707 | (0.2) | < 0.001 |
| T2N2 | 2 | 3404 | (0.1) | 0 | 756 | (0.0) | 2 | 353 | (0.6) | 1 | 663 | (0.2) | 0.023 |
| T2N3 | 0 | 1545 | (0.0) | 1 | 360 | (0.3) | 0 | 211 | (0.0) | 2 | 411 | (0.5) | 0.056 |
| T3N0 | 0 | 2267 | (0.0) | 0 | 391 | (0.0) | 0 | 231 | (0.0) | 1 | 741 | (0.1) | 0.272 |
| T3N1 | 4 | 2771 | (0.1) | 1 | 668 | (0.1) | 0 | 405 | (0.0) | 4 | 741 | (0.5) | 0.126 |
| T3N2 | 0 | 1240 | (0.0) | 1 | 238 | (0.4) | 0 | 113 | (0.0) | 1 | 229 | (0.4) | 0.112 |
| T3N3 | 2 | 915 | (0.2) | 0 | 188 | (0.0) | 0 | 113 | (0.0) | 1 | 214 | (0.5) | 0.599 |
| T4N0 | 2 | 641 | (0.3) | 0 | 159 | (0.0) | 0 | 104 | (0.0) | 2 | 252 | (0.8) | 0.494 |
| T4N1 | 4 | 1180 | (0.3) | 1 | 398 | (0.3) | 3 | 303 | (1.0) | 2 | 481 | (0.4) | 0.425 |
| T4N2 | 3 | 562 | (0.5) | 2 | 162 | (1.2) | 0 | 110 | (0.0) | 2 | 224 | (0.9) | 0.601 |
| T4N3 | 2 | 452 | (0.4) | 2 | 163 | (1.2) | 6 | 136 | (4.4) | 7 | 236 | (3.0) | 0.006 |
| Other metastases | |||||||||||||
| Bone(−)Visceral(−) | 49 | 144,528 | (0.0) | 21 | 21,114 | (0.1) | 18 | 9133 | (0.2) | 47 | 23,282 | (0.2) | < 0.001 |
| Bone(+)Visceral(−) | 112 | 2893 | (3.9) | 41 | 585 | (7.0) | 15 | 174 | (8.6) | 16 | 327 | (4.9) | 0.001 |
| Bone(−)Visceral(+) | 32 | 847 | (3.8) | 16 | 417 | (3.8) | 24 | 354 | (6.8) | 43 | 525 | (8.2) | 0.001 |
| Bone(+)Visceral(+) | 146 | 1593 | (9.2) | 54 | 532 | (10.2) | 47 | 281 | (16.7) | 57 | 328 | (17.4) | < 0.001 |
| Bone(−)Lung(+)Liver(+) | 64 | 446 | (14.3) | 24 | 198 | (12.1) | 32 | 143 | (22.4) | 35 | 170 | (20.6) | 0.019 |
| Bone(+)Lung(+)Liver(+) | 57 | 291 | (19.6) | 19 | 144 | (13.2) | 26 | 93 | (28.0) | 28 | 91 | (30.8) | 0.001 |
HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer
†Pearson’s Chi-square test
High N and T stages also showed high incidence of brain metastasis. In subgroup analysis according to specific combination of T and N stages, TNBC showed a sporadic distribution regardless of T and N stages. And 4.4 and 3.0% of HER2 and TNBC subtype patients, respectively, who were also classified as T4N3 without bone and visceral metastasis (lung or liver), had brain metastasis.
The incidence of brain metastasis was analyzed according to no-cranial metastasis and molecular subtype. For the patients who had only bone metastasis without visceral metastases, the incidence of brain metastasis in HER2 positive subtypes (luminal B and HER2 subtypes) was 7.0 and 8.6%, respectively. For the patients who had visceral metastasis without bone metastasis, the HER2 and TNBC subtypes had higher rates of brain metastasis compared with luminal A and B subtypes. For the patients who had both bone and visceral metastases, the incidence of brain metastasis was prominent in the HER2 (16.7%) and TNBC (17.4%) subtypes. If patients had bone, lung, and liver metastases, the probability of brain metastasis in the HER2 and TNBC subtypes was approximately 30% (28.0 and 30.8%, respectively).
Nomogram for probability of brain metastasis
In the univariate logistic regression performed in the training set, the p values of all variables were less than 0.05, so all variables were incorporated into a multivariate logistic regression analysis. Multivariate analysis showed that the number of metastatic organs except brain, young age, high grade, high T, N categories, and HER2 or TNBC subtypes were associated with a high probability of brain metastasis (Table 2).
Table 2.
Multivariate logistic regression analysis predicting brain metastasis from breast cancer in all breast cancer patients
| Characteristics | OR | 95% CI | p value |
|---|---|---|---|
| Age (continuous) | 0.995 | (0.988–1.003) | 0.216 |
| Race | |||
| White | Reference | ||
| Black | 0.984 | (0.745–1.302) | 0.912 |
| Others | 0.996 | (0.685–1.450) | 0.985 |
| Unknown | 0.382 | (0.051–2.856) | 0.348 |
| Grade | |||
| 1 | Reference | ||
| 2 | 1.527 | (0.873–2.670) | 0.138 |
| ≥ 3 | 1.541 | (0.874–2.715) | 0.135 |
| Unknown | 2.428 | (1.352–4.361) | 0.003 |
| Histology | |||
| IDC | Reference | ||
| ILC | 0.676 | (0.416–1.097) | 0.113 |
| Others | 1.086 | (0.827–1.426) | 0.551 |
| T stage | |||
| ≤ 1 | Reference | ||
| 2 | 1.513 | (1.008–2.269) | 0.045 |
| 3 | 2.167 | (1.366–3.436) | 0.001 |
| 4 | 3.045 | (2.004–4.626) | < 0.001 |
| Unknown | 3.888 | (2.449–6.172) | < 0.001 |
| N stage | |||
| 0 | Reference | ||
| 1 | 1.085 | (0.808–1.458) | 0.587 |
| 2 | 1.211 | (0.804–1.823) | 0.360 |
| 3 | 1.564 | (1.082–2.261) | 0.017 |
| Unknown | 1.161 | (0.737–1.827) | 0.520 |
| Subtype | |||
| Luminal A | Reference | ||
| Luminal B | 1.255 | (0.929–1.695) | 0.139 |
| HER2+ | 1.916 | (1.381–2.659) | < 0.001 |
| TNBC | 1.749 | (1.291–2.368) | < 0.001 |
| No. of metastatic organs except brain | |||
| 0 | Reference | ||
| 1 | 35.551 | (25.242–50.070) | < 0.001 |
| 2 | 71.158 | (49.564–102.159) | < 0.001 |
| 3 | 150.858 | (100.693–226.014) | < 0.001 |
OR odds ratio, CI confidence interval, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer
The Hosmer and Lemeshow Goodness of Fit test: x2 = 26.236, df = 8, p value = 0.001
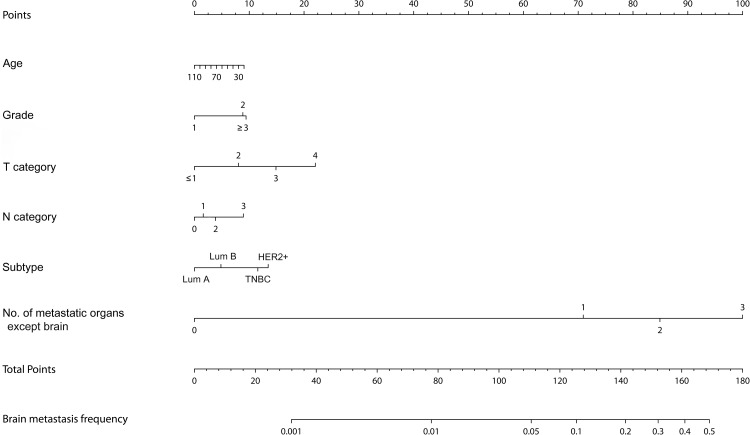
Based on this logistic regression, a nomogram for the probability of brain metastasis was generated (Fig. 1). Our nomogram showed a moderately reasonable calibration result (Supplementary Fig. 1) with high AUC values of more than 95% in both training and validation sets (Supplementary Fig. 2).
Fig. 1.
Nomogram predicting the probability of brain metastasis at the time of breast cancer diagnosis. Lum A, luminal A; Lum B, luminal B; HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer
According to the nomogram, if a patient is 50 years old, grade 3, T4N2 categories, HER2 + type, and has three metastatic organs except brain, the probability of brain metastasis is about 30% with a total score of 155 in the nomogram. On the other hand, the probability of brain metastasis was less than 5% in patients aged 70 years, grade 1, T1, N1, and luminal A type, even if there are three metastatic organs except brain.
Median survival of stage IV patients according to molecular subtype and brain metastasis
Median survival for stage IV patients was evaluated according to molecular subtype (Table 3). The median survival time of all stage IV patients was 29 months. The median survival of patients with brain metastasis was 11 months. Median survival time of the luminal A, B, HER2, and TNBC subtypes was 12 months, 23 months, 10 months, and 6 months, respectively, suggesting that the TNBC and HER2 subtypes had the poorest survival rates.
Table 3.
Median survival of stage IV patients according to molecular subtypes and brain metastasis
| Characteristics | All | Luminal A (HR+/HER2-) |
Luminal B (HR+/HER2+) |
HER2 (HR-/HER2+) |
TNBC (HR-/HER2-) |
p value† | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||
| Stage IV | 29 | (27.9–30.067) | 32 | (30.603–33.397) | 42 | (37.802–46.198) | 31 | (11.221–12.779) | 12 | (11.221–12.779) | < 0.001 |
| No brain metastasis | 30 | (28.8–31.162) | 33 | (31.510–34.490) | 44 | (39.933–48.067) | 36 | (29.730–42.270) | 12 | (11.193–12.807) | < 0.001 |
| Brain metastasis | 11 | (9.2–12.701) | 12 | (9.312–14.688) | 23 | (12.641–33.359) | 10 | (7.262–12.738) | 6 | (4.051–7.949) | < 0.001 |
| Multiple metastases | |||||||||||
| Brain(−)Bone(+)Visceral(−) | 39 | (36.910–41.090) | 39 | (36.834–41.166) | 48 | (40.087–55.913) | > 58 | NA | 13 | (11.454–14.546) | < 0.001 |
| Brain(−)Bone(−)Visceral(+) | 23 | (20.770–25.230) | 29 | (24.923–33.077) | 44 | (30.971–57.029) | 33 | (22.949–43.051) | 12 | (10.691–13.309) | < 0.001 |
| Brain(−)Bone(+)Visceral(+) | 21 | (19.417–22.583) | 23 | (20.973–25.027) | 32 | (27.659–36.341) | 22 | (17.253–26.747) | 8 | (6.448–9.552) | < 0.001 |
| Brain(+)Bone(−)Visceral(−) | 13 | (9.778–16.222) | 12 | (8.200–15.800) | 30 | (22.976–37.024) | 14 | (6.776–21.224) | 8 | (4.792–11.208) | 0.006 |
| Brain(+)Bone(+)Visceral(−) | 17 | (12.686–21.314) | 15 | (9.295–20.705) | 34 | (18.050–49.950) | 17 | NA | 9 | (1.538–16.462) | 0.011 |
| Brain(+)Bone(−)Visceral(+) | 7 | (4.152–9.848) | 6 | (0.000-12.146) | > 42 | NA | 6 | (2.082–9.918) | 6 | (1.228–10.772) | 0.014 |
| Brain(+)Bone(+)Visceral(+) | 8 | (6.200–9.800) | 8 | (3.491–12.509) | 12 | (5.102–18.898) | 6 | (1.771–10.229) | 5 | (3.015–6.985) | < 0.001 |
HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, CI confidence interval, NA not applicable
†Kaplan–Meier method and compared by log-rank test
HER2 positive (luminal B and HER2 subtypes) patients with bone metastasis had a median survival time of four or more years (48 and above 58 months, respectively), while TNBC subtype patients with bone metastasis had a median survival time of only 13 months.
The HER2 subtype had an excellent median survival rate if there was no brain metastasis. With brain metastasis, however, unlike the luminal B subtype, median survival time of HER2 subtype patients was significantly lower. When brain metastasis is combined with visceral metastasis, the median survival rate was extremely poor in all molecular subtypes, although the luminal B subtype showed the highest median survival among subtypes.
Brain metastasis cancer patient characteristics according to molecular subtype
Among HER2 subtype patients, 14.4% of patients were younger than 40, which was considerably higher than the other subtypes (5.9, 6.1, and 4.9% in luminal A, B, and TNBC subtypes, respectively) (Table 4). Only 42.5% of luminal A subtype patients received chemotherapy, implying chemotherapy-refractory characteristics and hormone therapy may be the reason for the relatively low rate of chemotherapy. TNBC subtype patients were treated with surgery more frequently (24.5%) than the other subtypes (13.6–15.9%). Approximately 70% of patients received radiotherapy.
Table 4.
Brain metastasis cancer patient characteristics according to molecular subtypes
| Characteristics | Luminal A (HR+/HER2-) n = 339 |
Luminal B (HR+/HER2+) v |
HER2 (HR-/HER2+) n = 104 |
TNBC (HR-/HER2-) n = 163 |
p value† | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | ||
| Age | |||||||||
| < 40 | 20 | (5.9) | 8 | (6.1) | 15 | (14.4) | 8 | (4.9) | 0.004 |
| 40–59 | 138 | (40.7) | 80 | (60.6) | 49 | (47.1) | 75 | (46.0) | |
| ≥ 60 | 181 | (53.4) | 54 | (40.9) | 40 | (38.5) | 80 | (49.1) | |
| Race | |||||||||
| White | 261 | (77.0) | 88 | (66.7) | 71 | (68.3) | 124 | (76.1) | 0.027 |
| Black | 57 | (16.8) | 29 | (22.0) | 19 | (18.3) | 33 | (20.2) | |
| Others | 21 | (6.2) | 14 | (10.6) | 14 | (13.5) | 6 | (3.7) | |
| Unknown | 0 | (0.0) | 1 | (0.8) | 0 | (0.0) | 0 | (0.0) | |
| Grade | |||||||||
| 1 | 24 | (7.1) | 2 | (1.5) | 0 | (0.0) | 3 | (1.8) | < 0.001 |
| 2 | 119 | (35.1) | 42 | (31.8) | 22 | (21.2) | 23 | (14.1) | |
| 3 | 109 | (32.2) | 58 | (43.9) | 57 | (54.8) | 102 | (62.6) | |
| 4 | 1 | (0.3) | 1 | (0.8) | 2 | (1.9) | 5 | (3.1) | |
| Unknown | 86 | (25.4) | 29 | (22.0) | 23 | (22.1) | 30 | (18.4) | |
| Histology | |||||||||
| IDC | 226 | (66.7) | 109 | (82.6) | 73 | (70.2) | 113 | (69.3) | < 0.001 |
| ILC | 34 | (10.0) | 4 | (3.0) | 2 | (1.9) | 3 | (1.8) | |
| Others | 79 | (23.3) | 19 | (14.4) | 29 | (27.9) | 47 | (28.8) | |
| T stage | |||||||||
| 0 | 8 | (2.4) | 4 | (3.0) | 3 | (2.9) | 3 | (1.8) | 0.833 |
| 1 | 28 | (8.3) | 17 | (12.9) | 5 | (4.8) | 15 | (9.2) | |
| 2 | 78 | (23.0) | 29 | (22.0) | 22 | (21.2) | 38 | (23.3) | |
| 3 | 41 | (12.1) | 14 | (10.6) | 12 | (11.5) | 25 | (15.3) | |
| 4 | 125 | (36.9) | 47 | (35.6) | 47 | (45.2) | 60 | (36.8) | |
| Unknown | 59 | (17.4) | 21 | (15.9) | 15 | (14.4) | 22 | (13.5) | |
| N stage | |||||||||
| 0 | 99 | (29.2) | 30 | (22.7) | 21 | (20.2) | 27 | (16.6) | 0.003 |
| 1 | 131 | (38.6) | 53 | (40.2) | 38 | (36.5) | 76 | (46.6) | |
| 2 | 38 | (11.2) | 19 | (14.4) | 14 | (13.5) | 10 | (6.1) | |
| 3 | 44 | (13.0) | 14 | (10.6) | 25 | (24.0) | 31 | (19.0) | |
| Unknown | 27 | (8.0) | 16 | (12.1) | 6 | (5.8) | 19 | (11.7) | |
| Surgery | |||||||||
| No | 285 | (84.1) | 110 | (83.3) | 87 | (83.7) | 122 | (74.8) | 0.005 |
| Yes | 54 | (15.9) | 18 | (13.6) | 15 | (14.4) | 40 | (24.5) | |
| Unknown | 0 | (0.0) | 4 | (3.0) | 2 | (1.9) | 1 | (0.6) | |
| Chemotherapy | |||||||||
| No/unknown | 195 | (57.5) | 42 | (31.8) | 23 | (22.1) | 48 | (29.4) | < 0.001 |
| Yes | 144 | (42.5) | 90 | (68.2) | 81 | (77.9) | 115 | (70.6) | |
| Radiotherapy | |||||||||
| No/unknown | 106 | (31.3) | 42 | (31.8) | 20 | (19.2) | 51 | (31.3) | 0.096 |
| Yes | 233 | (68.7) | 90 | (68.2) | 84 | (80.8) | 112 | (68.7) | |
| Bone metastasis | |||||||||
| No | 81 | (23.9) | 37 | (28.0) | 42 | (40.4) | 90 | (55.2) | < 0.001 |
| Yes | 258 | (76.1) | 95 | (72.0) | 62 | (59.6) | 73 | (44.8) | |
| Liver | |||||||||
| No | 236 | (69.6) | 87 | (65.9) | 56 | (53.8) | 115 | (70.6) | 0.017 |
| Yes | 103 | (30.4) | 45 | (34.1) | 48 | (46.2) | 48 | (29.4) | |
| Lung | |||||||||
| No | 200 | (59.0) | 83 | (62.9) | 49 | (47.1) | 76 | (46.6) | 0.005 |
| Yes | 139 | (41.0) | 49 | (37.1) | 55 | (52.9) | 87 | (53.4) | |
| Visceral | |||||||||
| No | 161 | (47.5) | 62 | (47.0) | 33 | (31.7) | 63 | (38.7) | 0.016 |
| Yes | 178 | (52.5) | 70 | (53.0) | 71 | (68.3) | 100 | (61.3) | |
| Visceral | |||||||||
| 1 Site (liver or lung) | 114 | (33.6) | 46 | (34.8) | 39 | (37.5) | 65 | (39.9) | 0.041 |
| 2 Sites (liver and lung) | 64 | (18.9) | 24 | (18.2) | 32 | (30.8) | 35 | (21.5) | |
HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma
†Pearson’s Chi-square test
Bone metastasis was prominent in the luminal A and B subtypes. The HER2 subtype showed a high rate of liver metastasis and the HER2 and TNBC subtypes showed a high rate of lung metastasis. The HER2 and TNBC subtypes had the highest rates of visceral metastasis (liver or lung). Among HER2 subtype patients, 30.8% of patients with brain metastasis had both liver and lung metastases, suggesting that HER2 subtype patients had a high burden of visceral metastases when being diagnosed with brain metastasis.
Median survival for brain metastasis cancer patients according to molecular subtype and patient characteristics
In the luminal A and B subtypes, patients less than 40 years of age showed high median survival rates (Table 5), whereas in the HER2 and TNBC subtypes, young age (less than 40 years) was not a favorable prognostic factor. TNBC patients aged 60 years or older had a median survival rate of only three months.
Table 5.
Median survival for brain metastasis cancer patients according to molecular subtypes and characteristics
| Characteristics | Luminal A (HR+/HER2-) n = 339 |
Luminal B (HR+/HER2+) n = 132 |
HER2 (HR-/HER2+) n = 104 |
TNBC (HR-/HER2-) n = 163 |
p value† | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||
| All | 12 | (9.312–14.688) | 23 | (12.641–33.359) | 10 | (7.262–12.738) | 6 | (4.051–7.949) | < 0.001 |
| Age | |||||||||
| < 40 | 35 | NA | Not reached | NA | 11 | (5.088–16.912) | 7 | (2.842–11.158) | 0.037 |
| 40–59 | 15 | (10.146–19.854) | 23 | (12.679–33.321) | 13 | (7.050–18.950) | 8 | (5.100–10.900) | 0.001 |
| ≥ 60 | 10 | (6.714–13.286) | 17 | (0.000–34.378) | 6 | (2.513–9.487) | 3 | (2.067–3.933) | < 0.001 |
| p value‡ | 0.280 | 0.336 | 0.403 | 0.010 | |||||
| Race | |||||||||
| White | 13 | (10.168–15.832) | 34 | (16.533–51.467) | 11 | (7.078–14.922) | 5 | (3.168–6.832) | < 0.001 |
| Black | 7 | (5.585–8.415) | 19 | (4.749–33.251) | 6 | (0.217–11.783) | 5 | (1.121–8.879) | 0.007 |
| Others | 20 | (11.163–28.837) | 8 | (0.000–32.001) | NA | NA | 10 | (2.799–17.201) | 0.842 |
| p value‡ | 0.188 | 0.493 | 0.047 | 0.230 | |||||
| Grade | |||||||||
| 1–2 | 12 | (7.700–16.300) | 29 | (10.721–47.279) | 8 | (1.567–14.433) | 4 | (1.509–6.491) | 0.001 |
| 3–4 | 10 | (5.336–14.664) | 29 | (11.672–46.328) | 9 | (5.327–12.673) | 7 | (4.855–9.145) | < 0.001 |
| p value‡ | 0.099 | 0.911 | 0.840 | 0.430 | |||||
| T stage | |||||||||
| 0–2 | 12 | (8.058–15.942) | 34 | (26.056–41.944) | 9 | (4.410–13.590) | 7 | (4.250–9.750) | < 0.001 |
| 3–4 | 13 | (7.694–18.306) | 21 | (5.193–36.807) | 11 | (7.407–14.593) | 6 | (3.758–8.242) | < 0.001 |
| p value‡ | 0.637 | 0.402 | 0.941 | 0.840 | |||||
| N stage | |||||||||
| 0–1 | 12 | (8.272–15.728) | 20 | (6.263–33.737) | 9 | (4.141–13.859) | 6 | (3.590–8.410) | < 0.001 |
| 2–3 | 13 | (8.861–17.139) | 34 | (25.868–42.132) | 14 | (7.559–20.441) | 5 | (2.534–7.466) | < 0.001 |
| p value‡ | 0.597 | 0.143 | 0.798 | 0.533 | |||||
| Surgery | |||||||||
| No | 12 | (8.704–15.296) | 17 | (11.262–22.738) | 8 | (4.597–11.403) | 4 | (2.663–5.337) | < 0.001 |
| Yes | 14 | (8.928–19.072) | NA | NA | 28 | (0.000-64.788) | 14 | (9.675–18.325) | 0.003 |
| p value‡ | 0.201 | 0.009 | 1.000 | < 0.001 | |||||
| Chemotherapy | |||||||||
| No/unknown | 8 | (3.906–12.094) | 4 | (0.000-8.337) | 2 | (0.318–3.682) | 1 | (0.387–1.613) | < 0.001 |
| Yes | 17 | (11.742–22.258) | 35 | NA | 14 | (8.893–19.107) | 8 | (6.507–9.493) | < 0.001 |
| p value‡ | 0.004 | < 0.001 | < 0.001 | < 0.001 | |||||
| Radiotherapy | |||||||||
| No/unknown | 7 | (0.000-14.130) | 15 | (0.587–29.413) | 2 | (0.205–3.795) | 3 | (0.364–5.636) | 0.008 |
| Yes | 13 | (10.394–15.606) | 29 | (14.802–43.198) | 11 | (7.320–14.680) | 6 | (4.223–7.777) | < 0.001 |
| p value‡ | 0.060 | 0.188 | 0.039 | 0.419 | |||||
| Bone metastasis | |||||||||
| No | 10 | (5.774–14.226) | 34 | (27.144–40.856) | 10 | (4.493–15.507) | 6 | (3.333–8.667) | < 0.001 |
| Yes | 13 | (10.109–15.891) | 19 | (10.944–27.056) | 10 | (5.360–14.640) | 5 | (3.026–6.974) | < 0.001 |
| p value‡ | 0.593 | 0.382 | 0.872 | 0.254 | |||||
| Liver metastasis | |||||||||
| No | 14 | (10.371–17.629) | 29 | (18.009–39.991) | 14 | (9.331–18.669) | 6 | (4.041–7.959) | < 0.001 |
| Yes | 7 | (1.649–12.351) | 15 | (5.920–24.080) | 6 | (1.790–10.210) | 3 | (0.000-6.845) | 0.001 |
| p value‡ | 0.008 | 0.428 | 0.007 | 0.046 | |||||
| Lung metastasis | |||||||||
| No | 13 | (10.265–15.735) | 34 | (21.213–46.787) | 14 | (6.063–21.937) | 8 | (5.088–10.912) | < 0.001 |
| Yes | 12 | (5.941–18.059) | 12 | (0.440–23.560) | 5 | (1.288–8.712) | 5 | (2.665–7.335) | < 0.001 |
| p value‡ | 0.375 | 0.135 | 0.001 | 0.150 | |||||
| Visceral metastasis | |||||||||
| No | 14 | (9.664–18.336) | 34 | (22.723–45.277) | 17 | (10.944–23.056) | 8 | (3.819–12.181) | < 0.001 |
| Yes | 8 | (3.604–12.396) | 15 | (5.717–24.283) | 6 | (2.848–9.152) | 5 | (2.689–7.311) | < 0.001 |
| p value‡ | 0.015 | 0.266 | 0.001 | 0.045 | |||||
| Visceral metastasis | |||||||||
| No | 14 | (9.664–18.336) | 34 | (22.723–45.277) | 17 | (10.944–23.056) | 8 | (3.819–12.181) | < 0.001 |
| One site (liver or lung) | 8 | (2.932–13.068) | 17 | (6.414–27.586) | 9 | (5.980–12.020) | 5 | (2.240–7.760) | < 0.001 |
| Two sites (liver and lung) | 12 | (3.922–20.078) | 7 | (2.724–11.276) | 5 | (1.831–8.169) | 3 | (1.312–4.688) | 0.033 |
| p value‡ | 0.051 | 0.280 | 0.001 | 0.099 | |||||
HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple negative breast cancer, CI confidence interval
†,‡Kaplan–Meier method and compared by log-rank test
T and N stages and tumor grade were not significant prognostic factors for brain metastasis patients, but surgery, chemotherapy, and radiotherapy increased median survival rates. However, this result may reflect some selection bias (patients whose life expectancy seemed to be long) as well as the effects of the treatments.
Bone metastasis was not a significant prognostic factor in any molecular subtype, whereas visceral metastasis was associated with decreased median survival in the luminal A, HER2, and TNBC subtypes. In the luminal B subtype, visceral metastasis may be associated with a decreased median survival time of 15 months versus 34 months with no visceral metastasis, but these values did not reach statistical significance (p = 0.266). The number of visceral metastases was also correlated with median survival. In the HER2 subtype, median survival for patients with no visceral metastasis, one metastasis, and two metastases was 17 months, 9 months, and 5 months, respectively (p = 0.001).
Univariate and multivariate analyses
Variables that were statistically significant in univariate analyses (p < 0.05) for OS in one or more molecular subtype and/or were considered clinically significant were selected and incorporated into a multivariate analysis. The variables included in this analysis were age, molecular subtype, extracranial metastases (bone, liver, lung), and treatment variables (surgery, chemotherapy, and radiotherapy) across all patients with brain metastasis.
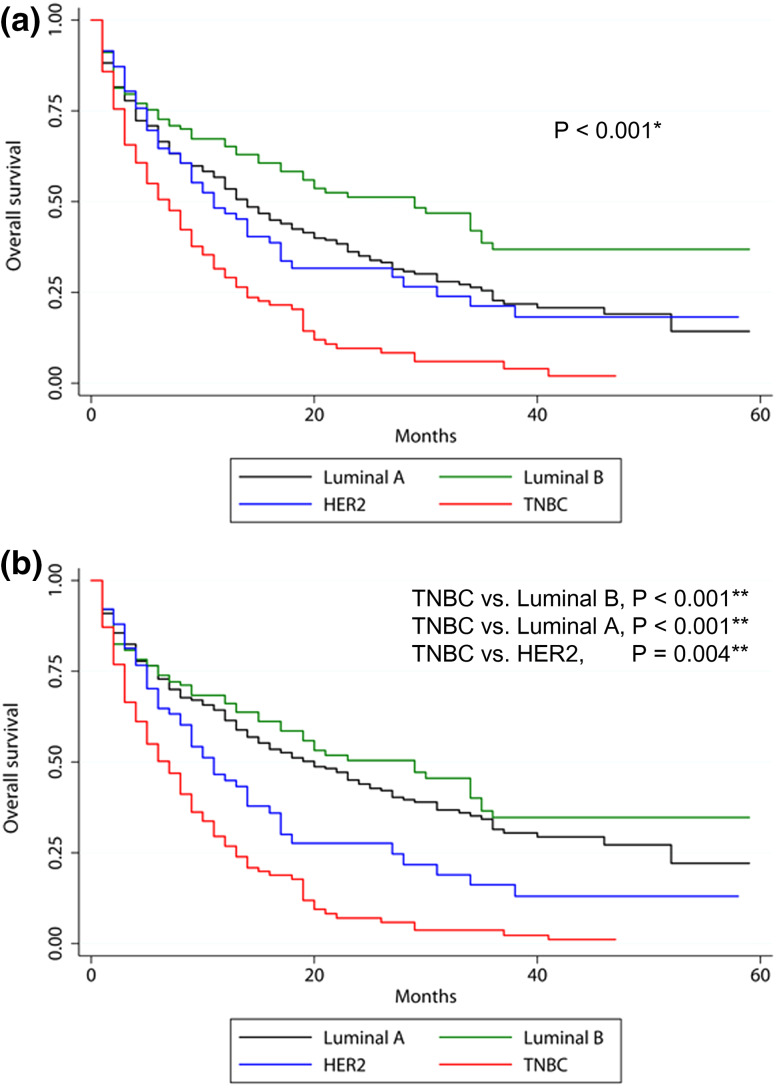
Molecular subtype was significantly correlated with OS. The most favorable subtype was the luminal subtype B, followed by the luminal A, HER2, and TNBC subtypes (hazard ratio [HR] of luminal B compared with TNBC, 0.352, 95% confidence interval, 0.258–0.480, p < 0.001; HR of luminal A, 0.408, 95% CI 0.319–0.522, p < 0.001; HR of HER2 subtype, 0.646, 95% CI 0.478–0.873, p = 0.004). Bone metastasis did not impact OS (p = 0.151), while liver and lung metastases significantly decreased OS (p < 0.001 and p = 0.029, respectively). Surgery and chemotherapy increased OS (p < 0.001 and p < 0.001, respectively) and radiotherapy appeared to be associated with increased survival rate, but failed to reach statistical significance (p = 0.075) (Table 6; Fig. 2).
Table 6.
Cox multivariate analysis for overall survival in brain metastasis from breast cancer
| Characteristics | HR | 95% CI | p value† |
|---|---|---|---|
| Age (continuous) | 1.236 | (1.021–1.495) | 0.029 |
| Subtype | |||
| TNBC | Reference | ||
| Luminal A | 0.408 | (0.319–0.522) | < 0.001 |
| Luminal B | 0.352 | (0.258–0.480) | < 0.001 |
| HER2 | 0.646 | (0.478–0.873) | 0.004 |
| Bone metastasis | |||
| No | Reference | ||
| Yes | 0.861 | (0.701–1.056) | 0.151 |
| Liver metastasis | |||
| No | Reference | ||
| Yes | 1.461 | (1.191–1.792) | < 0.001 |
| Lung metastasis | |||
| No | Reference | ||
| Yes | 1.236 | (1.021–1.495) | 0.029 |
| Surgery | |||
| No | Reference | ||
| Yes | 0.618 | (0.477-0.800) | < 0.001 |
| Unknown | 0.161 | (0.022–1.151) | 0.069 |
| Radiotherapy | |||
| No/unknown | Reference | ||
| Yes | 0.837 | (0.688–1.018) | 0.075 |
| Chemotherapy | |||
| No/unknown | Reference | ||
| Yes | 0.514 | (0.418–0.630) | < 0.001 |
HR hazard ratio, CI confidence interval, TNBC triple negative breast cancer, HER2 human epidermal growth factor receptor 2
†Cox multivariate regression analysis
Fig. 2.
Kaplan–Meier survival curves according to molecular subtype in all patients with brain metastasis from breast cancer. a Covariate-unadjusted and b covariate-adjusted Kaplan–Meier survival graphs stratified by molecular subtype after adjusting for age, surgery, chemotherapy, radiotherapy, bone, lung, and liver metastases. HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer. *Log-rank test, **Cox multivariate regression analysis
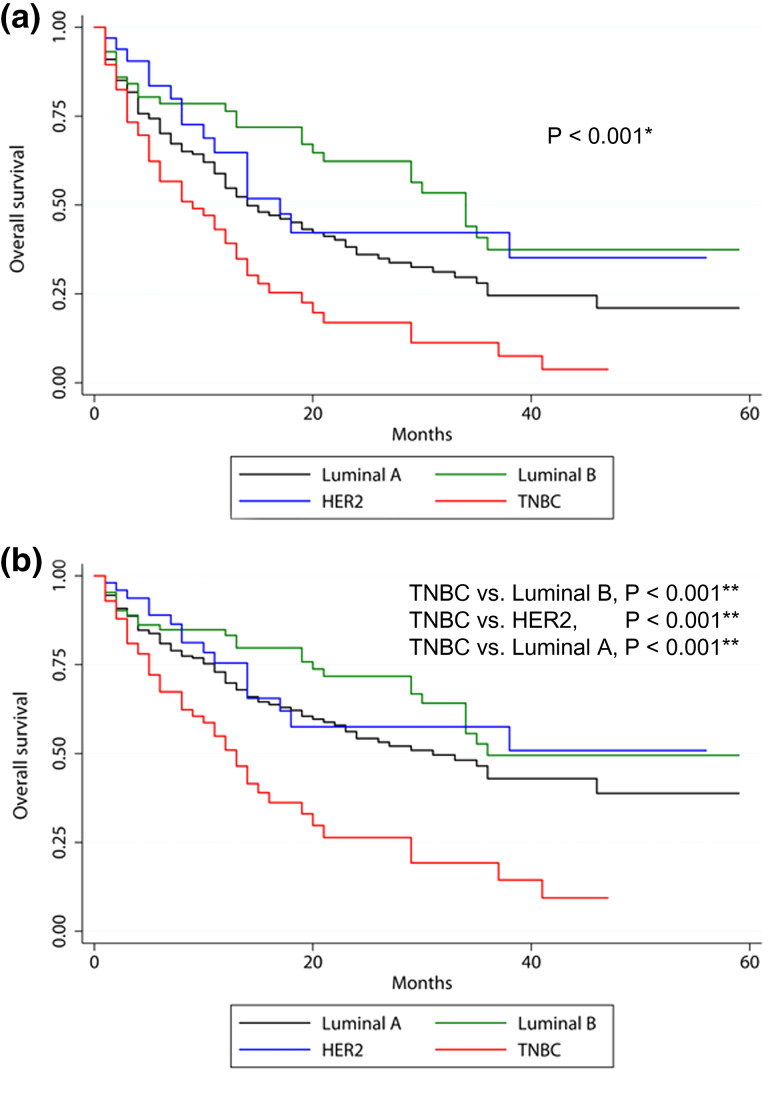
Multivariate analysis of OS in brain metastasis patients who had no visceral metastases was also performed. This analysis incorporated age, molecular subtype, bone metastasis, and treatment variables (surgery, chemotherapy, and radiotherapy). This analysis revealed that the subtype with the most favorable prognosis was the luminal B subtype, followed by the HER2, luminal A, and TNBC subtypes (HR of luminal B compared with TNBC, 0.310, 95% CI 0.190–0.504, p < 0.001; HR of HER2 subtype, 0.344, 95% CI 0.191–0.620, p < 0.001, HR of luminal A, 0.403, 95% CI 0.268–0.606, p < 0.001) (Table 7; Fig. 3).
Table 7.
Cox multivariate analysis for overall survival in brain metastasis from breast cancer without visceral metastasis
| Characteristics | HR | 95% CI | p value† |
|---|---|---|---|
| Age (continuous) | 1.015 | (1.002–1.028) | 0.022 |
| Subtype | |||
| TNBC | Reference | ||
| Luminal A | 0.403 | (0.268–0.606) | < 0.001 |
| Luminal B | 0.310 | (0.190–0.504) | < 0.001 |
| HER2 | 0.344 | (0.191–0.620) | < 0.001 |
| Bone metastasis | |||
| No | Reference | ||
| Yes | 0.865 | (0.635–1.178) | 0.357 |
| Surgery | |||
| No | Reference | ||
| Yes | 0.559 | (0.559–0.391) | 0.002 |
| Unknown | 1.051 | (0.142–7.797) | 0.961 |
| Radiotherapy | |||
| No/unknown | Reference | ||
| Yes | 1.082 | (0.777–1.505) | 0.641 |
| Chemotherapy | |||
| No/unknown | Reference | ||
| Yes | 0.747 | (0.540–1.035) | 0.080 |
HR hazard ratio, CI confidence interval, TNBC triple negative breast cancer, HER2 human epidermal growth factor receptor 2
†Cox multivariate regression analysis
Fig. 3.
Kaplan–Meier survival curves according to molecular subtype in patients with brain metastasis from breast cancer without visceral (lung and liver) metastasis. a Covariate-unadjusted and b Covariate-adjusted Kaplan–Meier survival graphs stratified by molecular subtype after adjusting for age, surgery, chemotherapy, radiotherapy, and bone metastasis. HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer. *Log-rank test, **Cox multivariate regression analysis
Bone metastasis was not a significant prognostic factor even in the patients without visceral metastasis (p = 0.357). Surgery improved patient OS, radiotherapy did not increase OS, and chemotherapy may improve OS, but more studies are needed (p = 0.002, p = 0.080, and p = 0.641, respectively).
Discussion
In the cohort of breast cancer patients from 2010 to 2014 (N = 206,913), HER2 subtype breast cancer showed the highest incidence of brain metastasis (1.0%, 104/9942) and HER2 subtype patients younger than 40 years of age had a particularly high incidence of brain metastasis (1.7%). TNBC cancer patients showed a sporadic distribution of brain metastasis irrespective of T and N stages. When patients had bone, lung, and liver metastases together, the probability of brain metastasis was 19.6, 13.2, 28.0%, and 30.8% in the luminal A, B, HER2, and TNBC subtypes, respectively (p = 0.001). For the patients with brain metastasis, high rates of visceral metastases were also observed in the HER2 and TNBC subtypes. This was true particularly for the HER2 subtype, in which patients who were diagnosed with brain metastasis had a 30.8% probability of having metastases in both the liver and lung, as well. The median survival time for HER2 subtype patients with brain and bone metastases, but without visceral metastasis was 17 months, and survival for those with visceral metastasis was 6 months. Unlike the Breast Cancer Specific Graded Prognostic Assessment (BS-GPA) for patients with brain metastasis from breast cancer, the prognostic ranking of the HER2 subtype among molecular subtypes was reversed with the luminal A subtype, when the presence of visceral metastasis was considered. In patients without visceral metastasis, the HER2 subtype showed a favorable prognosis compared to the luminal A subtype. Therefore, molecular subtype and visceral metastasis are correlated with incidence and prognosis for patients with brain metastasis.
The cut-off point for brain evaluation may be determined under clinical considerations. For example, if there is a risk of brain metastasis more than 30% by a nomogram and the patient has a suspicious symptom, brain magnetic resonance imaging (MRI) might be recommended. In our nomogram, the cut-off point of predictive probability of brain metastasis that is related with the accuracy of the nomogram with the highest sensitivity and specificity was close to zero (0.2–0.3%) (Supplementary Table 1). However, the positive predictive value (PPV) increased as the probability of brain metastasis increased, and negative predictive value (NPV) was favorable regardless of the predictive probability. Since the PPV and NPV reflect the usefulness of a nomogram, this nomogram may be more useful as the probability of a patient’s brain metastasis is higher.
One study provided a nomogram of the probability of subsequent brain metastasis in patients with stage IV breast cancer (Graesslin et al. 2010). Unlike this nomogram, our study generated a nomogram of the probability of accompanying brain metastasis at the time of breast cancer diagnosis among all breast cancer patients. Because of the nature of a cross-sectional study, our nomogram might be more appropriate to determine whether to add a brain evaluation test, such as brain MRI, when multiple extracranial metastases have been found in a patient.
Considering the high incidence of brain metastasis in patients with the HER2 subtype and multiple visceral metastases (Hicks et al. 2006), and the sporadic incidence of brain metastasis in patients with the TNBC subtype regardless of disease stage, individuals in these two subgroups may benefit from close surveillance for brain metastasis even in early stages of disease. Intracranial metastases associated with oligometastases of small disease burden may be effectively controlled, therefore improving quality of life (Niwińska et al. 2010) and survival of affected patients (Kondziolka et al. 2011).
The impact of molecular subtype on prognosis may be integrated into existing information regarding the intrinsic nature of each subtype, availability of effective targeted therapies for the specific subtype, and other subtype-associated variables (Hennigs et al. 2016; Mendes et al. 2015; Savci-Heijink et al. 2015). Circulating tumor cells (CTC) may provide a key molecular mechanism of metastasis in breast cancer, and the number of CTCs before treatment predicts the prognosis in patients with metastatic breast cancer (Cristofanilli et al. 2004). Stem cell-like tumor cell and epithelial-to-mesenchymal transition (EMT) appear to be associated with the CTC, and relevant markers (CD44, CD24 and ALDH1 for stem cell and Twist1, Akt2, and PI3Kα for EMT) are overexpressed in CTCs of metastatic breast cancer patients (Aktas et al. 2009). Stem cell markers are observed more frequently in TNBC (ALDH1, CD44, CD24) and HER2 (ALDH1) which could increase metastatic propensities and poor clinical outcomes of these subtypes. EMT is also likely to confer resistant to targeted therapy and chemotherapy in HER2-positive breast cancer patients (De Mattos-Arruda et al. 2015).
In our study, the TNBC subtype showed the worst prognosis irrespective of brain metastasis, and is known to be an aggressive form of cancer without effective therapeutic options (Lin et al. 2008). The median survival time for patients with TNBC who had bone metastasis only was 13 months. For the HER2 subtype patients, however, a significant prognostic difference was observed among stage IV patients in which patients with only bone metastasis showed a median survival time of more than 58 months, whereas patients with brain and bone metastases, without visceral metastasis, had a median survival time of 17 months. Median survival time for patients with brain and visceral metastases, without bone metastasis, was only 6 months.
In this study, the prognosis of HER2 subtype brain metastasis was poor with a median survival time of 10 months even with the high rate of chemotherapy in this group (77.9%). Given the majority HER2 subtype patients had visceral organ metastases (68.3%) as well, we speculated that this metastasis contributed to poor outcome. Even after multivariate analysis, the high rate of visceral metastasis remained a likely candidate for contributing to the poor prognosis in the HER2 subtype group.
The BS-GPA did not take visceral metastasis into account, but the KPS in the BS-GPA reflected the burden of visceral metastasis and brain metastasis (Sperduto et al. 2012). However, survival outcomes for patients with brain and visceral metastases may not be the same as those brain metastasis patients with similar KPS and identical molecular subtype, but who do not have combined visceral metastases. Similar to the GPA for brain metastasis from non-small cell and small cell lung cancers, which includes both KPS and extracranial metastasis as prognostic factors (Sperduto et al. 2011), our study implied that visceral metastasis and brain metastasis from breast cancer might be independent prognostic factors that should be considered together with KPS and molecular subtype.
In agreement with our results, another SEER data (2010–2013) based study (Wang et al. 2017) demonstrated that molecular subtype and multiple metastases are significantly prognostic in stage IV breast cancer. However, this study calculated the percentage of brain metastasis within the stage IV breast cancer patients not in the whole breast cancer cohort, resulting in that luminal A (HR+/HER2−) showed the highest incidence of brain metastasis. It seems more reasonable that the incidence of brain metastasis among molecular subtypes should be analyzed in all breast cancers: our study showed high susceptibilities of brain metastasis in the HER2 and TNBC subtypes, and these results are identical to those reported in the literature (Heitz et al. 2009; Hicks et al. 2006; Lim et al. 2017). In addition, Wang et al. demonstrated that HER2 subtype brain metastasis showed the most favorable cancer-specific survival (CSS) rate among subtypes. However, many large studies of brain metastasis from breast cancer consistently reported luminal B subtype (HR+/HER2+) as the most favorable subtype (Nam et al. 2008; Niwinska et al. 2017; Sperduto et al. 2012). With one-year updated SEER database from 2010 to 2014 in our study, luminal B subtype showed the most favorable OS rates as well as CSS rates (data not shown). Lastly, Wang et al. insisted that multiple organ metastases eliminated the prognostic impact of the molecular subtype. In our study, however, the molecular subtype maintained the significant prognostic value even with multiple visceral metastases.
To improve treatment outcomes of brain metastases from breast cancer, subtype-specific clinical trials are ongoing. The Radiation Therapy Oncology Group (RTOG) 1119 is a phase II randomized study of whole brain radiotherapy/stereotactic radiosurgery in combination with concurrent lapatinib therapy (a small-molecule tyrosine kinase inhibitor) in patients with brain metastasis from HER2 subtype breast cancer (White et al. 2017). Intra-arterial cerebral infusion of trastuzumab is being investigated (NCT02571530), and phosphoinositide 3-kinase (NCT02000882) and cyclin-dependent kinase 4/6 inhibitors (NCT02774681, NCT02308020) are also being tested.
There were some limitations in this study that included the following: the SEER database did not provide information about patients’ performance status, the number or size of brain metastases, or use of hormone therapy. Information was unavailable regarding whether HER2-targeted therapy was administered with chemotherapy, and detailed information about radiotherapy (site, extent, and technique—stereotactic radiosurgery or whole brain radiotherapy) was also not available. The cut-off values of HER2 tests (IHC, FISH, or CISH) were not provided. In this study, molecular subtypes were classified using information on combinations of ER, PR, and HER2 results provided in SEER database.
Taken together, the molecular subtype of the breast cancer and the presence or absence of visceral metastasis should be taken into consideration for prediction of prognosis for patients with brain metastasis from breast cancer. In addition, patients with HER2 and TNBC cancer subtypes having visceral metastasis, close surveillance for brain metastasis should be considered. This policy could contribute to early detection of brain metastasis and may putatively lead to improved quality of life and survival of breast cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This work was supported by the Grant (No. 0820010) for Cancer Control Program from Korean Ministry of Health & Welfare and SNUBH Research Fund (No. 18-2018-001 & No. 13-2018-003) to In Ah Kim.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required because of the retrospective nature of the study.
References
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11:R46. 10.1186/bcr2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartmann C et al (2017) Factors influencing the development of visceral metastasis of breast cancer: a retrospective multi-center study. The Breast 31:66–75 [DOI] [PubMed] [Google Scholar]
- Cho E et al (2015) The use of stereotactic radiosurgery for brain metastases from breast cancer: who benefits most? Breast Cancer Res Treat 149:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer New England. J Med 351:781–791. 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2009) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattos-Arruda L et al (2015) MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast. Cancer Patients Oncotarget 6:37269–37280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- Finn RS et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35 [DOI] [PubMed] [Google Scholar]
- Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini A, Fasola G, Puglisi F (2015) Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 32:125–133 [DOI] [PubMed] [Google Scholar]
- Gondi V et al (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graesslin O et al (2010) Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28:2032–2037 [DOI] [PubMed] [Google Scholar]
- Heitz F et al (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases European. J Cancer 45:2792–2798 [DOI] [PubMed] [Google Scholar]
- Hennigs A et al (2016) Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer 16:734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks DG et al (2006) Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR The American. J Surg Pathol 30:1097–1104 [DOI] [PubMed] [Google Scholar]
- Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK (2015) Emerging strategies for treating brain metastases from breast cancer. Cancer Cell 27:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D et al (2011) Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg 114:792–800 [DOI] [PubMed] [Google Scholar]
- Lim YJ et al (2017) Failure patterns according to molecular subtype in patients with invasive breast cancer following postoperative adjuvant radiotherapy: long-term outcomes in contemporary clinical practice Breast. Cancer Res Treat 163:555–563. 10.1007/s10549-017-4206-8 [DOI] [PubMed] [Google Scholar]
- Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative. Breast Cancer 113:2638–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes D et al (2015) The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer–a systematic review. Breast Cancer Res 17:140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam B-H, Han H-S, Ro J, Lee KS, Kim SY, Kim TH, Kwon Y (2008) Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 10:R20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwinska A, Pogoda K, Rudnicka H, Jagiello-Gruszfeld AI, Rybski S, Nowecki Z (2017) Outcomes from 735 patients with breast cancer brain metastases (BM) according to biological subtype, number of BMs, and systemic treatment after local therapy. J Clin Oncol 35(15 suppl):2078 [Google Scholar]
- Niwińska A, Tacikowska M, Murawska M (2010) The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death International. Int J Radiat Oncol Biol Phys 77:1134–1139 [DOI] [PubMed] [Google Scholar]
- Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J, van de Vijver MJ (2015) Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 150:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016 CA: a cancer. J Clin 66:7–30 [DOI] [PubMed] [Google Scholar]
- Solomayer E-F, Diel I, Meyberg G, Gollan C, Bastert G (2000) Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat 59:271–278 [DOI] [PubMed] [Google Scholar]
- Sperduto PW et al (2011) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduto PW et al (2012) Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 82:2111–2117. 10.1016/j.ijrobp.2011.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer New England. J Med 372:724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang C, Zhang J, Kong L, Zhu H, Yu J (2017) The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER. Based study Oncotarget 8:26368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Moughan J, Kim I, Peereboom D, De Los Santos J, Sperduto P, Mehta M (2017) Abstract OT1-04-02: NRG oncology/RTOG 1119: Phase II randomized study of whole brain radiotherapy with concurrent lapatinib in patients with brain met from HER2-positive breast cancer—A collaborative study of RTOG & KROG (NCT01622868). AACR
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.