Abstract
Positron emission tomography (PET) is a molecular imaging technology that provides quantitative information about function and metabolism in biological processes in vivo for disease diagnosis and therapy assessment. The broad application and rapid advances of PET has led to an increased demand for new radiochemical methods to synthesize highly specific molecules bearing positron-emitting radionuclides. This article provides an overview of commonly-used labeling chemistry in the examples of clinically relevant PET tracers, and highlights most recent development and breakthroughs over the past decade with focus on 11C, 18F, 13N, and 15O.
Keywords: positron emission tomography, carbon-11, fluorine-18, nitrogen-13, oxygen-15
Graphical Abstract

1. Introduction
Positron emission tomography (PET), a non-invasive molecular imaging technique, is capable of probing biological processes in vivo, monitoring the progression of disease states, and evaluating drug treatment efficacy.[1–4] Unlike structural and anatomical imaging techniques, including X-ray and ultrasound, PET offers functional information via the interaction between a targeted molecule bearing a positron-emitting radionuclide (‘radiotracer’) and biological system. Since only trace amounts of radioactive materials (10−6−10−9 grams) are necessary to obey the ‘tracer principle’ in such diagnostic imaging studies, it is feasible to directly monitor the biological process via PET without eliciting pharmacological effects, particularly when radiotracers with high specific activity are employed. PET radiotracers possess desired binding and physiochemical properties to interact with biological targets of interest, including receptors, enzymes and ion channels.[5–7] The growing role of PET imaging in drug discovery efforts has prompted significant investment in novel PET ligand discovery from both industry and academic communities, yielding a steady flow of novel radioligands that showed requisite specificity toward intended biological targets to enable target engagement studies. PET imaging has also been used to examine whether the desired tissue partition of therapeutic agents are achieved and can also serve as a disease-state biomarker by detecting expression level changes in biological target between healthy and disease state, modulation of which by therapeutic agents can be used to monitor treatment efficacy in longitudinal studies.
The advance of PET is often paced by the availability and facile synthesis of targeted radiotracers.[5] To a considerable extent, the key for successful clinical translation depends not only on the biological characteristics of the PET tracer, but also the implementation of an efficient and practical radiolabeling method to enable widespread use. Such radiochemical methods are strongly influenced by the characteristics of the PET nuclides, including chemical reactivity and half-life. Numerous review articles have captured the basic principles of PET[1–6] and overviewed radiotracer development.[7, 8] Herein, we aim to emphasize the importance of commonly-used methods, and highlight recent advances and breakthroughs in the chemistry of carbon-11, fluorine-18, nitrogen-13 and oxygen-15 in the last decade (2008–2018). The proof-of-concept application and synthesis of a diverse range of representative and clinically relevant small molecule PET pharmaceuticals are discussed. We also present unsolved challenges in PET chemistry with these radionuclides and potential area for methodology development as a unified theme to stimulate discovery research in PET chemistry.
2. Labeling methods with carbon-11
Since carbon is the backbone element in the whole spectrum of pharmaceutical molecules, isotopologue labeling using carbon-11 (11C, t½ = 20.4 min) presents a unique opportunity for radiochemistry and PET tracer development since no changes of structural, biological, and pharmacological properties are anticipated after the radiolabel is implemented. The short half-life of 11C requires highly-efficient transformation which usually occurs on the ultimate or penultimate step to maximize the use of rapidly-decaying radioactivity. Complementary to previous 11C reviews,[5, 9–14] we highlight some recent developments and breakthroughs in 11C chemistry with an emphasis on commonly used synthons and novel building blocks (Scheme 1). The nuclear reactions and production of the respective synthons have been reviewed elsewhere.[5, 12]
Scheme 1.
Overview of 11C chemistry
2.1. [11C]CH3X (X = I or OTf) chemistry
[11C]CH3I is by far the most frequently used 11C-labeling agent. Nucleophilic substitution of [11C]CH3X (X = I or OTf) can generate 11C-methyl heteroatomic (N, O, or S) compounds or activated carbonic 11C-methylated compounds under neutral or basic conditions. This simple and direct 11C-methylating method has been well applied in the synthesis of many 11C-labeled tracers, including [11C]PIB, [11C]DASB, [11C]flumazenil, [11C]DTBZ, [11C]MET and [11C]fenoprofen (Scheme 2, A). While [11C]CH3I is satisfactory for most 11C-labeling reactions, the more reactive [11C]CH3OTf (produced from [11C]CH3I with silver triflate at 200 °C) may offer an alternative solution for improved radiochemical yield (RCY) and chemoselectivity, shorter reaction time, lower reaction temperature and less precursor loading. For example, 11C-N-methylation could be selectively achieved with [11C]CH3OTf under neutral conditions in the synthesis of [11C]PIB without the protection of hydroxyl group.[15] Pd-Mediated cross coupling reactions, particularly Stille and Suzuki-type, are often used to synthesize 11C-labeled aryl or alkenyl tracers. Representative examples in each category include (15R)-[11C]TIC, [11C]MNQP and a 11C-labeled mGlu1 ligand from tributylstannes,[16] [11C]celecoxib, [11C]cibbi-772, and [11C]UCB-J from organoborates, respectively (Scheme 2, B1). Complementary to these major approaches, proof-of-concept 11C-labeling methodology studies involving Negishi[17] / Sonogashira-type[18] reactions, and Schwartz’s reagent,[19] were also reported. Recently, a novel 11C-acetylation method was developed using transition metal-mediated cross coupling reactions via [11C]CH3I and a CO source (Scheme 2, B2–3).[20, 21]
Scheme 2.
[11C]CH3X (X=I or OTf) chemistry
2.2. [11C]CO2 chemistry
2.2.1. Formation of 11C-carbonyl labeled carboxylic acids and derivatives via [11C]CO2
[11C]CO2 is the feedstock virtually for all 11C chemistry and can be readily converted to other synthons in high RCYs and high specific activity.[9, 12] Trapping [11C]CO2 with strongly basic organometallic reagents, such as Grignard and organolithium reagents, represents an early and conventional approach to generate 11C-labeled carboxylic acids (Scheme 3, A1). 11C-Labeled fatty acids, such as acetic, propionic, butyric and palmitic acids, have been prepared by this method.[12] The addition of [11C]CO2 to the lithium aldimine intermediate yielded 11C-labeled α-keto acids, such as pyruvic acid.[22] A recent example entailed [11C]CO2 insertion into organolithium (from organostannane precursor), leading to an 11C-labeled of RXR- partial agonist (Scheme 3, A1).[23]
Scheme 3.
Synthesis of 11C-labeled carboxylic acids and derivatives via [11C]CO2
[11C]Carboxylic derivatives including 11C-labeled esters and amides, as well as 11C-labeled amines could also be produced from [11C]carboxymagnesium halides depending on the nature of nucleophiles and proper reducing agents if necessary (Scheme 3, A2).[9, 12, 24] Representative examples include the preparation of [11C]melatonin, and radiopharmaceuticals routinely used in clinical research including (+)-[11C]PHNO and [11C]WAY100635.
In addition, 11C-labeled alkyl halides, for example, [11C]benzyl iodide, can also be prepared by Grignard reaction, followed by reduction and halogenation.[25]
Grignard and organolithium reagents are reactive species which are unstable towards air and moisture, and technical challenges of such radiosyntheses have restricted their widespread use. In 2012, Riss and Pike et al. developed a novel strategy to synthesize 11C-labeled carboxylic acids and derivatives from boronic esters in the presence of a copper catalyst in high RCYs and high specific activity (Scheme 3B).[26] The conversion was tolerant with diverse functional groups, such as halides, nitro and carbonyl groups, demonstrating a significantly broader substrate scope than that of organometallic reagents. In 2014, Vasdev and Liang et al. adopted this method to prepare [11C]bexarotene under modified conditions and translated this ligand for PET-MR imaging studies in nonhuman primates.[27] Recently, Gee and Bongarzone et al. developed a method for synthesizing 11C-labeled amides from primary amines using Grignard reagents (Scheme 3C). This work utilized DBU to trap [11C]CO2, followed by the formation of 11C-oxyphosphonium or [11C]isocyanate intermediates, to afford 11C-labeled amides upon Grignard addition.[28]
2.2.2. Formation of 11C-carbonyl labeled ureas and carbamates using [11C]CO2
The first attempt to prepare 11C-labeled urea from [11C]CO2 was carried out via 11C-carbonylation reaction with amines in the presence of diphenylphosphite and pyridine albeit in low RCYs. Other methods[9, 12] including the use of lithium hexamethyldisilazide (LHMDS), and the formation of reactive intermediate [11C]cyanamides or [11C]isocyanates, offered diverse approaches to synthesize symmetrical 11C-labeled ureas from [11C]CO2.
In 2006, a practical protocol to prepare unsymmetrical 11C-ureas from amines and phosphinimines was reported (Scheme 4A).[29] Phosphinimines derived from azides or primary amines generated 11C-isocyanate intermediate, followed by second amine addition, to provide unsymmetrical 11C-ureas in 8–49% RCYs. In 2009, Hooker and Fowler et al. reported a novel method using DBU as [11C]CO2 trapping base and carried out the synthesis of 11C-labeled carbamate from amines and alkyl halides (Scheme 4B).[30] Several radioligands based on drug scaffolds including [11C]metergoline and [11C]MS-275,[31] were labeled in 25–40% RCYs. In 2011, Wilson and Vasdev et al. discovered a unified method using a phosphazene base, BEMP (as [11C]CO2 trapping base) and POCl3 (as dehydrating or chlorinating reagent) to achieve the formation of unsymmetrical 11C-labeled ureas and carbamates (Scheme 4, C1).[32] This protocol was applied in the synthesis of a broad scope of bioactive molecules,[9, 12] including [11C]CURB, [11C]PF-04457845 and [11C]SL25.1188. Recently, this process was automated by using an ‘in-loop’ synthesis module, and produced [11C]SL25.1188 and [11C]JNJ1661010 by flow chemistry.[33, 34] Another method to obtain unsymmetrical 11C- labeled ureas was developed by Gee et al. in 2013 using Mitsunobu conditions (Scheme 4, C2).[35] Both aliphatic and less reactive aromatic amines proceeded well to offer unsymmetrical 11C-labeled ureas in high RCYs of 69–94%. Most noteworthy is that these [11C]CO2 fixation reactions are simple, one-pot, generally require no heating or cooling, and are easily automated. Consequently, [11C]CURB and [11C]SL2511.88 have been translated for human use, thereby paving the way to numerous PET radiopharmaceuticals synthesized directly from [11C]CO2.[9, 12]
Scheme 4.
Synthesis of 11C-labeled carbamates and ureas via [11C]CO2
Different with 11C-carbonylation, Billard et al. developed a simple method to achieve 11C-methylation for amines from [11C]CO2 under reducing conditions.[36] This direct 11C-labeling strategy was applied to the synthesis of [11C]PIB in 45% RCY and 50 GBq/μmol molar activity.
2.3. [11C]CO chemistry
2.3.1. Aromatic and activated alkyl 11C-carbonylation via [11C]CO
[11C]CO is an established building block for the synthesis of 11C-carbonyl labeled carboxylic acids, esters, amides, ketones and aldehydes via transition-metal mediated carbonylation reactions[10–14] Pd-mediated cross coupling reaction between aryl/benzylic/methyl halides and [11C]CO can provide 11C-labeled carboxylic acids or esters in the presence of hydroxides or alcohols, respectively (Scheme 5, A1).[12, 37–39] For example, [11C]eprosartan was synthesized in 54% RCY by using the corresponding aryl iodide and [11C]CO in the presence of Pd(PPh3)4 and Bu4NOH.[40] Similarly, aryl(mesityl)iodonium saltscould replace aryl halides to react with [11C]CO and generate11C-labeled carboxylic acids.[41] Aryl halides could also be converted into 11C-labeled aryl formyl chlorides in situ in the presence of Bu4NCl, then transformed into 11C-labeled amides, esters, acids, aldehydes, alcohols and ketones.[42, 43] In addition, boronate precursors via Pd-mediated transmetalation could proceed 11C-carbonylation with [11C]CO (Scheme 5, A2). Representative examples include a retinoid compound [11C]Am80[44] and [11C]aspirin[45] obtained from the corresponding boronates.
Scheme 5.
Pd-mediated formation of 11C-labeled carboxylic acid and its derivatives via [11C]CO
11C-Labeled amides could also be obtained from appropriate primary or secondary amines in Pd-mediated cross coupling reactions between aryl halides and [11C]CO.[10–14] Several representative radiotracers are shown in Scheme 5, B1. This is one of three primary methods to access 11C-labeled amides, in addition to [11C]CO2 (via acylation and aminolysis) and [11C]CN (via controlled hydrolysis) methods. Since [11C]CO has limited solubility in organic solvents, efficient radioactive gas trapping in the reaction system is challenging. Several novel strategies were developed to address this problem,[11, 14] including use of a high-pressure reactor, continuous flow microreactor, gas-liquid segmented microfluidics, use of palladium dimer catalyst or NHC ligand, trapping [11C]CO by Pd catalyst, NHC ligand, borane, and copper(I) complex, xenon gas carrier, and improved methods to produce [11C]CO.[46–48] Among these methods, a recent example by Skrydstrup and Antoni et al. highlighted the use of [11C]CO in a simple low-pressure vessel with pre-activated aryl-Pd species as stoichiometric reagents and produce well-functionalized radioligands in high RCYs (Scheme 5, B2).[49] The same authors also applied this strategy in the N-11C-acetylation of peptides with [11C]CO and bisphosphine-ligated Pd complexes (Scheme 5, B3).[50] Several representative N-11C-acetylated bioactive peptides, such as [11C]lacosamide, [11C]acetyl LULUPhol and [11C]acetyl cRGDfK were obtained in high RCYs of 33–46% and high molar activity of 281–404 GBq/μmol.[50]
The synthesis of 11C-carbonyl labeled ketones can be accomplished by [11C]CO-involved three-component Stille-type cross coupling reactions (Scheme 6A).[10–14, 51,52] For example, a potential PET radioligand for histamine subtype 3 receptors, was 11C-labeled by this method using the corresponding arylstannane, aryl iodide and [11C]CO.[52] [11C]Benzaldehyde could be obtained when Et3SiH was employed instead of an organotin reagent.[51] Furthermore, Suzuki-type reactions were also capable of providing 11C-carbonyl labeled ketones (Scheme 6B).[53] The products were obtained in the range of 10–70% RCYs and high molar activity (>150 GBq/μmol).
Scheme 6.
Pd-mediated formation of 11C-ketones and aldehydes via [11C]CO
2.3.2. 11C-Carbonylation of nonactivated aliphatic substrates via [11C]CO
Despite the exciting advances in the Pd-mediated 11C- carbonylation reactions with [11C]CO, the scope is commonly limited to aryl, alkenyl, methyl and benzyl substrates without liability of inclined β-H elimination from ensuing oxidative Pd(II) complex. To expand substrate chemotype, other transition metals or radical-mediated methods were used to activate alkyl halides containing β-hydrogen atoms. In 2016, Rahman et al. developed a Ni-mediated method for the synthesis of 11C-carbonyl labeled alkyl amides starting from non-activated alkyl iodides (Scheme 7A).[54] Cyclic or acyclic alkyl iodides were converted to 11C- carbonyl labeled amides in 33–90% RCYs. Alkyl iodides could also be utilized in free radical-mediated reactions with [11C]CO, to generate 11C-carbonyl labeled carboxylic acids, esters and amides in good RCYs (44–76%) with high molar activity (Scheme 7B).[55–57]
Scheme 7.
11C-Carbonylation of nonactivated aliphatic substrates via [11C]CO
2.3.3. Synthesis of 11C-carbonyl labeled urea and carbamate via [11C]CO
Complementary to [11C]CO2 and [11C]COCl2 chemistry, Rh-mediated 11C-carbonylation reactions convert aryl azides and [11C]CO into the corresponding Rh-coordinated 11C-isocyanates, which subsequently form 11C-carbonyl labeled ureas and carbamates, respectively (Scheme 8).[58] This method was applied in the preparation of a wide range of 11C-compounds, including 11C-labeled VEGFR-2/PDGFR-β dual inhibitors,[59]
Scheme 8.
Formation of 11C-carbonyl labeled ureas and carbamates via [11C]CO
[11C]phenytoin,[60] 11C-labeled sulfonyl ureas and carbamates,[61, 62] and 11C-labeled hydroxyurea derivatives.[63] in addition, Se-mediated 11C-carbonylation of amines and alcohols with [11C]CO offered an alternative method for the preparation of 11C-carbonyl labeled carbamoyl compounds.[64]
2.4. [11C]CN chemistry
2.4.1. Synthesis of 11C-labeled aliphatic nitriles and derivatives
While aromatic nitriles are often found as key scaffolds and pharmacophores in drug molecules, aliphatic nitriles typically exist as intermediates for the preparation of carboxylic acids, amides, amines, and related derivatives. This principle is also applicable for chemistry with [11C]CN. 11C-Labeled alkyl nitriles are commonly prepared by nucleophilic substitution or addition reactions with H[11C]CN as the reagent. The most frequent derivatization is to generate 11C-carboxylic acids or derivatives (Scheme 9A). For example, [11C]lactic acid was produced by [11C]CN addition to acetaldehyde or substitution of aldehyde- bisulfite adduct, followed by hydrolysis. The same protocol could be also applied in the synthesis of [11C]valine (via hydrolysis), [11C]glutamine (via aminolysis) and [11C]dopamine (via reduction). Another method to obtain 11C-labeled amino acids involved the formation of [11C]hydantoin, which was produced via the Bücherer-Bergs reaction from ketones or aldehydes (Scheme 9B). Then [11C]hydantoin was converted to the corresponding 11C- labeled amino acids via hydrolysis in basic conditions. Furthermore, ring-opening of aziridine with [11C]CN also led to alkyl-11CN compounds, which could be converted to 11C-labeled carboxylic acids in 30–40% RCYs (Scheme 9C).[65]
Scheme 9.
Formation of alkyl-11CN and its derivatives
2.4.2. Formation of 11C-labeled aromatic nitriles and derivatives
Pioneered with the nucleophilic substitution of Cr(CO)3 complexes with [11C]CN, aryl-11CN formation was gradually replaced by Pd- or Cu-mediated cross coupling reactions attributed to more extensive substrate scope and higher reaction efficiency (Scheme 10, A1).[12] Representative examples of Pd- mediated reactions include [11C]AZD9272, and compounds derived from aryl-11CN, like [11C]NAD-299. The recent discovery of biaryl phosphine ligands for Pd catalysts, such as t-BuXPhos, further advanced new aromatic 11CN chemistry. In 2015, Hooker and Buchwald et al. developed a Pd-mediated 11C-cyanation of aryl halides/triflates at ambient temperature with rapid reaction rate (<1 min; Scheme 10, A2).[66] These biaryl phosphine ligands, e.g., t-BuXPhos or BrettPhos, were the key to stabilize Pd-aryl complex and accelerate transmetalation and reductive elimination under mild reaction conditions. These authors further extended this concept to the labeling of cysteine-containing peptides via a sequential Pd-mediated C-S and C-11CN bonding formation in one-pot.[67] The formal ‘nucleophile-nucleophile coupling’ provided 11C-labeled peptides in high RCYs (10–50%). Arylboron
Scheme 10.
Formation of aromatic 11CN and its derivatives
Alternative to Pd-mediated 11C-cyanation, Ponchant et al. found that [11C]CN could be trapped by copper to produce Cu[11C]CN in situ, which was then reacted with aryl halides to synthesize 11C-labeled aryl nitriles under high temperature (150250 °C).[68] compounds and arylstannanes can also be used as precursors in the Cu-mediated cyanation. In 2017, Liang and Vasdev et al. developed a 11C-cyanation methodology that takes advantage of aryl boronic acids. This strategy could be realized in aqueous solutions and was compatible with a wide range of aryl boronic precursors, resulting in diverse radiolabeled compounds in 9–70% RCYs (Scheme 10, B2).[69] In 2018, Scott and Sanford et al. further extended labeling precursors to other arylboron compounds and arylstannanes using Cu(II)(OTf)2. This method was compatible with a broad range of substrates. For example, [11C]perampanel was prepared in 10% RCY and 70 GBq/μmol molar activity using an automated radiosynthesis module (Scheme 10, B2).[70]
2.5. Miscellaneous 11C synthons and reagents
Some commonly used / recent developed 11C synthons and reagents prepared by 1–4 step reactions from [11C]CO2 are highlighted in Scheme 11 to demonstrate the diversity and creativity of 11C chemistry.
Scheme 11.
Selected 11C Synthons prepared by 1–4 step reactions
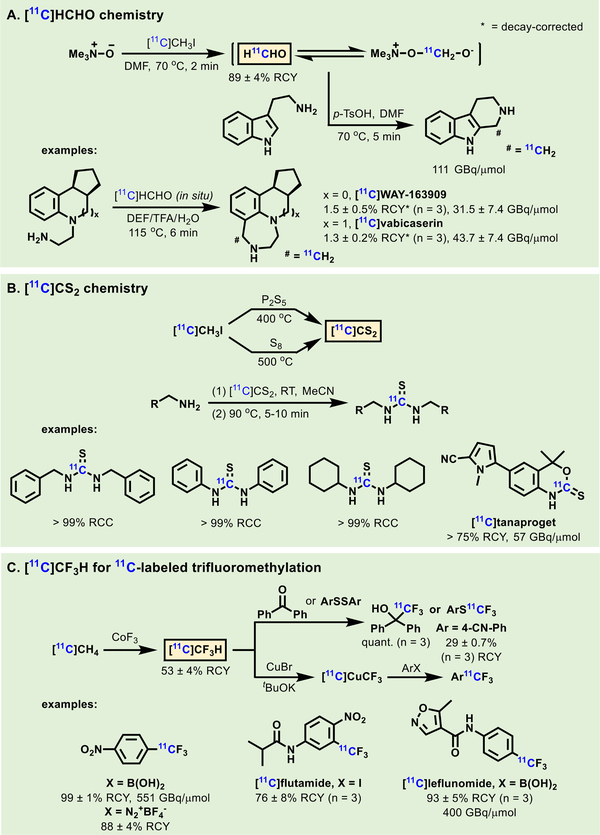
Selected examples of recent 11C-labeled building blocks are described below. [11C]HCHO, produced from [11C]CH3OH oxidation, has been applied in reductive methylations, ringclosure reactions, electrophilic aromatic substitutions and others.[12, 13] In 2008, Hooker et al. developed a new method to synthesize [11C]HCHO from [11C]CH3I with trimethylamine N- oxide (Scheme 12A).[71] Several 11C-labeled tracers including [11C]vabicaserin and [11C]WAY-163909, were efficiently synthesized via the Pictet-Spengler reaction using [11C]HCHO.[72] [11C]CS2 was first prepared from [11C]CO2 and H2S in low RCYs.[73] In 2012, Miller et al. developed a novel method to produce [11C]CS2 by gas phase reaction between [11C]CH3I and a thionating agent P2S5.[74] Then they improved the method by switching the thionating reagent from P2O5 to elemental sulfur (Scheme 12B).[75] [11C]CS2 was found to rapidly react with a broad spectrum of primary amines to give 11C-labeled thiocarbonyl thioureas, including [11C]tanaproget, in high RCYs and high molar activity. In 2017, Pike et al. described a new type 11C-labeled agent [11C]CF3H, based on the fluorination of [11C]CH4 with CoF3 (Scheme 12C).[76] This 11C-labeled reagent was applied to demonstrate a wide range of trifluoromethylations, including nucleophilic additions with ketones, cross-coupling reactions with aryl boronic acids, aryl iodides and aryl diazonium salts via Cu[11C]CF3 generated in situ. 11C-Labeled trifluoromethyl arenes, including [11C]flutamide and [11C]lefunomide, exhibited high RCYs and high molar activity (>200 GBq/μmol). In particular, the molar activity of 11C-labeled trifluoromethyl arenes far exceeded its 18F- counterpart (aryl [18F]CF3), providing a viable access to radiolabel mutlifluoromethylated compounds in high molar activity. Diverse methods to prepare [11C]COCl2 from [11C]CO2 or [11C]CH4 have been developed since the 1970s. Among these methods, an efficient automated [11C]COCl2 synthesis with high RCYs (>80%) was reported in 2010.[77] [11C]COCl2 is an effective reagent to synthesize 11C-labeled ureas and carbamates via [11C]isocyanate or [11C]carbamoyl chloride intermediates. A diverse range of ureas and carbamates, including 11C-labeled 18-β-glycyrrhetinic acid derivative,[78] [11C]MFTC,[79] [11C]SAR127303[80] and [11C]MAGL-0519,[81] were efficiently obtained via [11C]COCl2.
Scheme 12.
Miscellaneous 11C-synthons and reagents
3. Labeling methods with fluorine-18
Fluorine-18 (18F) is the most widely used PET nuclide attributed to the extensive clinical use of 2-deoxy-2-[18F]fluoro-D- glucose ([18F]FDG) in the diagnosis of cancers, cardiovascular and neurological diseases. Fluorine-18 (as [18F]fluoride) is most commonly produced via the 18O(p,n)18F nuclear reaction in high radioquantity and high specific activity, and has a relatively long half-life (109.8 min) compared to 11C, which allows for multi-step synthesis, extended imaging protocols, and off-site use in satellite PET facilities without a cyclotron. These favorable characteristics provide a strong stimulus to develop novel 18F-labeled compounds, and efficient and translational radiofluorination methods. Complementary to previous fluorine-18 reviews,[16, 82–88] we focus on the recent development and breakthroughs in the nucleophilic and electrophilic reactions with 18F (Scheme 13). Indirect 18F-labeling methods, i.e., reactions involved with 18F- building blocks, are discussed elsewhere.[88, 89]
Scheme 13.
Overview of 18F chemistry.
3.1. Aliphatic 18F-fluorination with nucleophilic [18F]fluoride
3.1.1. Aliphatic nucleophilic substitution
Aliphatic nucleophilic substitution with [18F]fluoride is a reliable method that has been commonly used to generate Csp3- 18F bonds in high RCYs and high specific activity. The most common reaction of this type involves the substitution of an adequate leaving group that is susceptible to displacement by [18F]fluoride (Scheme 14A). These leaving groups include -triflate (-OTf), -tosylate (−OTs), -mesylate (−OMs) and halides, with a decreasing order of leaving ability (−OTf > −OTs ~ −OMs > −I > −Br > −Cl). Another type of aliphatic nucleophilic 18F-fluorination entails the addition of [18F]fluoride into strained cyclic substrates, for example, ring opening reactions of epoxides and aziridines (Scheme 14B). The potential regio- and stereo-genicity makes this method attractive for the radiosynthesis of PET tracers, including clinically-relevant [18F]FES and [18F]FLT. Complementary to one-step nucleophilic [18F]fluoride displacement approaches, indirect methods are also widely used,[89–91] which typically involve the utilization of [18F]fluoroalkyl prosthetic groups to conjugate with O, N, or S-nucleophiles, especially when the corresponding precursors are labile during 18F-fluorination (Scheme 14C).
Scheme 14.
Common strategies for synthesis of 18F-alkyl groups
A large number of 18F-labeled PET tracers are prepared through aliphatic nucleophilic 18F-fluorination and can be categorized into four major chemotypes, including unbranched and branched acyclic alkyl-18F, cyclic alkyl-18F, and alkyl-18F with [18F]fluorine atom at activated positions (Scheme 15). The first common form is the unbranched acyclic alkyl-18F. Representative examples include [18F]FMeNER-D2, [18F]FMISO, [18F]FET, [18F]fallypride and [18F]florbetapir (Scheme 15A). The two deuterium (D) atoms in [18F]FMeNER-D2 are introduced to improve metabolic stability and reduce the rate of in vivo defluorination during PET studies.[92] An rigorous overview of 18F- tracer metabolism can be found elsewhere.[93] The second chemotype entails branched acyclic alkyl-18F (Scheme 15B), which includes but is not limited to [18F]iNOS-9, [18F]FTHA, and [18F]FDS. Cyclic alkyl-18F is another common chemotype (Scheme 15C), including the most widely used PET radiopharmaceutical [18F]FDG, and other tracers, like [18F]FES, [18F]FLT, [18F]FACBC, and [18F]FP. In the last category, 18F- fluorination takes advantage of activated positions on the molecule, including the benzylic, allylic or α-position of the carbonyl group, to facilitate 18F-incorporation (Scheme 15D). A few representative examples include [18F]SP203, [18F]AB5186, 18F-labeled allyl, propargyl, and α-carbonyl compounds, such as 4-[18F]fluoroglutamine and [18F]PBR06.
Scheme 15.
Representative chemotypes of aliphatic [18F]fluorides
Compared with nucleophilic 18F-fluorination reactions which are often conducted in polar aprotic solvents, Chi and co-workers used ionic liquids[94] and unusual protic solvents[95] to improve 18F- labeling efficiency (Scheme 16, A1). These conditions could not only dramatically increase 18F reactivity and reaction rate, but also decrease the formation of undesired products, such as alkenes, alcohols or ethers. For instance, [18F]FP-CIT was synthesized in 36% RCY under new conditions containing tBuOH, in contrast to only 3% RCY using CH3CN as a solvent (Scheme 16, A2).[96] These new labeling conditions were also applied in the synthesis of several radioligands, including [18F]FP-CIT, [18F]FLT and [18F]PF05270430 (Scheme 16, A3).[97] The conversion of alcohols to alkyl-18F typically involve a two-step sequence, namely, the formation of alcohol-derived leaving groups, such as -OTs or - OMs, and the subsequent aliphatic 18F-fluorination. In 2015, Doyle et al. reported a ‘one-pot’ direct synthesis of alkyl fluorides from the corresponding alcohols with 2-pyridinesulfonyl fluoride (PyFluor), a new deoxyfluorination reagent (Scheme 16B).[98] As proof of concept, [18F]FDG was achieved by 18F-deoxyfluorination of O-protected carbohydrate in 15% radiochemical conversion (RCC).
Scheme 16.
Unconventional aliphatic 18F-fluorination reactions
An azeotropic [18F]fluoride drying is nearly always employed to ensure high reactivity of 18F under anhydrous conditions. However, this process may lead to decreased RCY/molar activity attributed to prolonged reaction and/or surface adsorption. In 2015, van Dam and Sergeev et al. developed TiO2-catalyzed 18F- fluorination of tosylated precursors in highly aqueous medium without the need of [18F]fluoride drying (Scheme 16C).[99] TiO2 was the key in the 18F-fluorination. First, solvated [18F]fluoride in water was adsorbed at the active surface of TiO2, thus enhancing fluorine-18 reactivity. Synergistically, TiO2 also activated the tosylate precursor via Ti-O coordination with two oxygen atoms of the sulfonyl group.
18F-Fluorination was not merely limited to organic reactions. O’Hagan et al. developed a novel 18F-labeling protocol using enzymatic biosynthesis (Scheme 16D). 18F-Fluorination of (S)- adenosyl-L-methionine (SAM) to 5’-[18F]fluoro-5’-deoxyadenosine (5’-[18F]FDA) was carried out smoothly by fluorinase with up to 95% RCY.[100] Recently, they further extended this enzymatic 18F- fluorination to obtain 5’-[18F]fluorodeoxy-2-ethynyladenosine labeled RGD peptides in 12% RCY and no radiodefluorination was observed in PET imaging studies.[101]
3.1.2. Transition metal-mediated aliphatic 18F-fluorination
Aliphatic nucleophilic 18F-fluorination is usually performed in basic conditions at high temperature; however, these conditions may not be compatible with well-functionalized molecules, resulting in the formation of undesired side products. Transition metal-mediated aliphatic 18F-fluorination may thus provide an alternative to generate alkyl-18F bonds under mild conditions. In 2011, Gouverneur and Brown et al. reported Pd-catalyzed fluorination of highly active allyl p-nitrobenzoates with TBAF at room temperature.[102] Allylic 18F-fluorination was also achieved using [18F]TBAF (Scheme 17, A1). In the same year, Nguyen et al. developed Ir-catalyzed allylic fluorination of trichloroacetimidates.[103] The 18F-labeling reaction was conducted with K[18F]F/K222, and the ensuing allyl [18F]fluoride was achieved in 38% RCY (Scheme 17, A2). In 2013, Gouverneur and Brown et al. reported another Ir-catalyzed fluorination of allyl carbonates,[104] in which a wide range of branched, linear (E)- and (Z)- allyl substrates were labeled with [18F]Et4NF in 8–76% RCYs (Scheme 17, A3).
Scheme 17.
Transition metal-mediated aliphatic 18F-fluorination
Ring-opening of epoxides with [18F]fluoride is a stereogenic method to prepare 18F-tracers. In 2014, Doyle et al. developed an enantioselective 18F-fluorination of epoxides with chiral [18F](salen)CoF (Scheme 17B).[105] The group also developed a dimeric chiral cobalt catalyst to overcome the challenges associated with epoxide substrates bearing Lewis basic nitrogen or α-branching substituents. Several PET tracers were synthesized by this method, including [18F]THK-5105 (85% ee) and [18F]FMISO (90% ee).
Groves and Hooker et al. developed a novel Mn-catalyzed benzylic C-H 18F-fluorination method (Scheme 17C).[106] Several fluorinated drug moieties, including a [18F]celecoxib analog and a 18F-labeled fingolimod, were efficiently labeled. The authors also extended this method to non-activated aliphatic substrates.[107] The use of a more reactive Mn-porphyrin complex Mn(TPFPP)OTs was essential to enable efficient transformation. A diverse range of bioactive molecules was efficiently labeled, including [18F]ACPC (48% RCC) and a [18F]flutamide analog.
Lu and Li et al. recently reported Ag-promoted intramolecular cyclization of unsaturated carbamates using [18F]fluorobenziodoxole.[108] A range of 18F-labeled heterocycles were achieved by using this method but the efficiency of 18F-labeling needs to be further improved (RCYs < 10%). Szabó et al. also reported a metal-free intramolecular cyclization of unsaturated amides with isolated [18F]fluorobenziodoxole.[109] 18F-Fluorocyclization of a wide range of o-styrilamides were performed with high RCCs (54–90%).
3.2. Aromatic 18F-fluorination with nucleophilic [18F]fluoride
3.2.1. Aromatic nucleophilic substitution
Aromatic nucleophilic substitution (SNAr) with [18F]fluoride represents a direct and commonly used method to form CSp2-18F bonds (Scheme 18A). An optimal precursor will generally bear both a leaving group and an activating group (usually electron- withdrawing) in the ortho or para position to facilitate the SNAr reaction by stabilizing the Meisenheimer complex. Representative PET tracers are shown in Scheme 18B. Alternatively, molecules with non-activating substituents could also be synthesized through SNAr conversion of arenes with electron-withdrawing groups, followed by post-SNAr functional group manipulations.[110, 111] Nitrogenous heteroarene, such as pyridine, is electron-deficient than its homoarenes counterpart, which provides a unique opportunity for 18F-labeling via SNAr without the need of activating groups. A wide range of 18F-heteroarenes (particularly 2- [18F]fluoropyridine), were synthesized by this approach (Scheme 18C).
Scheme 18.
Aromatic nucleophilic substitution (SNAr) with [18F]fluoride ion
Efficient 18F-labeling via SNAr requires the presence of an activating group and a leaving group on the arene, which significantly limits the substrate scope. To overcome this challenge, the search for novel precursors carrying new activating and/or leaving groups continues. Recently, research efforts have been made in the development of novel sulfur-based precursors. The first example was demonstrated by Maeda et al. using aryldimethylsulfonium salts in 1987.[112] However, the formation of undesired methyl [18F]fluoride made this method unfavorable for labeling arenes. In 2012, Ametamey et al. developed triarylsulfonium salts as a new class of precursors for 18F-labeling arenes (Scheme 19, A1).[113] 18F-fluorination preferred to occur on the relatively electron-deficient aryl group and the remaining diarylsulfonium group served as the leaving group. In 2015, Arstad et al. extended the substrate scope to electron-rich arenes by using diarylsulfonium bearing para-methoxyphenyl groups as the leaving group (Scheme 19, A2).[114] Besides triarylsulfonium salts, diaryl sulfoxides were also potential precursors for 18F- labeling reactions discovered by Pike et al. in 2016.[115] A higher reaction temperature (150–200 °C) was required probably attributed to low reactivity of diaryl sulfoxides. Electron-deficient diaryl sulfoxides offered products in high RCCs, such as 1-[18F]fluoro-4-nitrobenzene (78%), whereas the labeling of electron-rich diaryl sulfoxides remained a challenge.
Scheme 19.
Unconventional aromatic nucleophilic substitution (SNAr) with [18F]fluoride
Murphy et al. developed N-arylsydnones as novel precursors for 18F-labeling of arenes bearing electron-deficient substituents (Scheme 19B).[116] It was found that the sydnone was not only inductively more electron-withdrawing than the nitro group, but also positioned away from the aryl plane and limited full resonance with the arene. This makes the sydnone a weak anion stabilizer as an excellent activating group for 18F-incorporation.
Phenols are common building blocks in organic synthesis, which makes 18F-deoxyfluorination an attractive strategy to achieve 18F-labeled arenes. Ritter and Hooker et al. developed a novel 18F-fluorination method of phenols based on a concerted nucleophilic aromatic substitution (CSNAr) mechanism (Scheme 20A).[117] This reaction involved the formation of a uronium intermediate, which was distinct from the Meisenheimer complex in the conventional SNAr. Electron-deficient phenols were efficiently converted to aryl-18F in high RCCs (>80%). Recently, Ritter and Hooker et al. further utilized a ruthenium catalyst via η6 π-complex for 18F-deoxyfluorination of electron-rich phenols (Scheme 20B).[118]
Scheme 20.
18F-Deoxyfluorination of phenols via concerted SNAr
Different with 18F-deoxyfluorination, Gouverneur et al. reported a novel method for ipso 18F-substitution of t-butyl group at the para position of phenols.[119] As an alternative SNAr-type pathway, this reaction involved two steps, i.e., de- aromatization/fluorination under oxidative conditions, and re- aromatization under acidic conditions. A series of 4-tert- butylphenols were converted to 4-[18F]fluorophenols using PhI(OAc)2 as the oxidant with 7–20% RCYs.
3.2.2. 18F-Fluorination of hypervalent iodine(III) compounds
Despite significant advances in the 18F-labeling of arenes via the SNAr method, there is an unmet need for efficient 18F- fluorination methods of non-activated arenes. The low efficiency of using Balz-Schiemann and Wallach reactions in the 18F- labeling shifted the research focus initially towards the application of trimethylammonium triflates[120] and more recently major advances have been made with hypervalent iodine(III) based precursors. The first example of diaryliodonium salts as precursors in the synthesis of aryl-18F was reported by Pike et al. (Scheme 21A),[121] in which arenes with electron-deficient or -rich substituents showed high 18F-labeling efficiency. The relatively electron-deficient aryl moiety of the corresponding salt was preferrably labeled by fluorine-18. To achieve high regioselectivity of unsymmetrical diaryliodonium salts, Coenen et al. developed a novel class of aryl(2-thienyl)iodonium salts and demonstrated 18F- labeling in a highly regioselective manner (Scheme 21B).[122] Specifically, 18F-fluorination of aryl(2-thienyl)iodonium salts preferred to occur on the counterpart. An ortho substitution on the aryl group of diaryliodonium salts has substantial effects on the regioselectivity, with the preference of radiofluorination occurs on the ortho-substituted arene (Scheme 21C).[123] Mechanistically, a trigonal bipyramidal iodine intermediate was proposed during 18F- fluorination of diaryliodonium salts (Scheme 21D). While the two aryl groups could rapidly exchange their positions via Berry pseudorotation process, steric bulky ortho-substituted aryl group was situated in an equatorial position to minimize the steric repulsion, leading to a favored reductive elimination pathway to form an Csp2-18F bond on the equatorial arene in both monomeric and oligomeric state. [123, 124]
Scheme 21.
18F-Fluorination of diaryliodonium salts
Recent optimization of reaction conditions and involvement of transition metal catalysts have advanced diaryliodonium salts based radiofluorinations. In 2012, DiMagno and Snyder et al. reported a new method for 18F-fluorination of diaryliodonium salts in nonpolar solvents with high RCYs.[125] In particular, diaryliodonium triflate and [18F]fluoride were first dissolved in a polar aprotic solvent (e.g., MeCN) to facilitate ion exchange between OTf and 18F. After solvent removal, a nonpolar medium (e.g., toluene) was then added for reductive radiofluorination, which was beneficial to inhibit side reactions, including internal electron transfer and disproportionation of diaryliodonium salts. The new method has been used in the synthesis of several 18F- pharmaceuticals,[126] including [18F]FDOPA, [18F]mFBG, [18F]FDA, and [18F]flutemetamol.
In 2014, Scott and Sanford et al. reported Cu-mediated 18F- fluorination of mesityl(aryl)iodonium salts (Scheme 22A).[127] Contrary to the “ortho effect”, 18F-Csp2 reductive elimination occurred on the counterpart of steric bulky mesityl group to allow access to a variety of electron-rich, -neutral and -deficient 18F- arenes. Recently, the same authors improved their method by the formation of electron-rich mesityl(aryl)iodonium salts in situ, which offers a potential solution to synthesize electron-rich aryl-18F bonds without the need of isolation for certain unstable electron- rich diaryliodonium salts.[128]
Scheme 22.
Recent application in the 18F-fluorination of diaryliodonium salts
The traditional method for the preparation of diaryliodonium salts was limited to oxidation of aryl iodides, followed by ligand exchange. In 2016, Liang and Liu et al. developed a new one-pot method to synthesize aryl(isoquinoline)iodonium salts via the mesoionic carbene silver complex (Scheme 22B).[129] Radiofluorination of a board range of aryl(isoquinoline)iodonium salts were achieved with [18F]Et4NF in 25–65% RCYs. As proof of concept, a 18F-labeled natural product, [18F]fluoroaspergillitine, was achieved in 10% RCY with molar activity of 37 GBq/μmol.
In all, attributed to its high efficiency in the 18F-labeling of arenes, particularly for the substrates without proper activating substituents, diaryliodonium salts method has been well applied in the synthesis of a wide range of clinically relevant PET tracers (Scheme 22C).[126]
Among the methods for 18F-labeling of hypervalent iodine(III) species, little attention was paid to other type λ3-iodine compounds until radiofluorination of (diacetoxyiodo)arenes[130] and aryliodonium ylides were developed in recent years. Notably, the latter method has shown excellent progress to achieve unprecedented radiofluorination of non-activated arenes.
In an early report by Ermert et al. in 2014, aryliodonium ylides with Meldrum acid auxiliaries were developed for 18F-labeling of arenes.[131] In the same year, Liang and Vasdev et al. independently developed a novel class of spirocyclic iodonium ylides (SCIDY) and demonstrated regioselective radiofluorination of non-activated arenes (Scheme 23A).[132] SCIDY with cyclopentyl auxiliary gave the highest 85% RCC in the 18F- fluorination of biphenyl iodonium ylides. Using this auxiliary, a wide range of [18F]fluoroarenes were readily achieved via SCIDY in high RCYs. Thermal stability of aryliodonium ylides highly depended on auxiliary substitution, which showed a positive correlation with 18F-incorporation efficiency. A second generation auxiliary, namely SPIAd (spiroadamantyl-1,3-dioxane-4,6-dione), developed by the same authors, outperformed all the other auxiliaries of aryliodonium ylides in stability tests and exhibited highly efficient 18F-labeling capability (Scheme 23B).[133] In addition, Riss et al. reported an improved PPh3-assisted 18F- fluorination of aryliodonium ylides, which showed increased fluorination yield and reaction rate.[134]
Scheme 23.
18F-Fluorination of aryliodonium ylides
18F-Labeling of aryliodonium ylides demonstrated substantially improved regioselectivity over diaryliodonium salts method.[133] The auxiliary group has a preference for the axial position and aryl group takes the equatorial position. The calculation results showed that undesired Csp3-F reductive elimination had ca. 25 kcal/mol higher barrier than favorable Csp2- F pathway, which explained the unique regioselectivity in the 18F- labeling of aryliodonium ylides (Scheme 23C).
As radiofluorination of aryliodonium ylides is an efficient and metal-free method for non-activated arenes, a diverse range of clinically relevant radiopharmaceuticals and 18F-labeled drug scaffolds were obtained by this route (Scheme 24).[81, 132, 133, 135–143] It is noteworthy that [18F]FPEB synthesized via SCIDY technology was fully automated, resulting in a substantial improvement (3–10 fold) in RCYs and molar activity compared with the traditional SNAr method, and has been validated for human use.[144]
Scheme 24.
Representative examples for 18F-fluorination of aryliodonium ylides
3.2.3. Transition metal-mediated aromatic 18F-fluorination
Significant advances have been achieved in the aromatic radiofluorination using transition metals with enhanced reactivity, selectivity and tolerance towards functional groups, which may provide an alternative approach to access [18F]fluoroarenes. In 2011, Ritter and Hooker et al. developed a novel aromatic 18F- fluorination method based on Pd(IV) complex (Scheme 25A).[145] The highly fluorophilic Pd species 1 and [18F]fluoride generated Pd-18F complex 2, which not only served as an electrophilic 18F- fluorination reagent but also oxidized aryl-Pd(II) complex 3 to aryl- Pd(IV)-18F complex, to afford [18F]fluoroarenes after reductive elimination. This method was applied in the synthesis of [18F]fluoroestrone, [18F]paroxetine and a 18F-labeled 5-HT2C agonist (> 1% RCYs).[145, 146] In 2012, Ritter et al. developed another oxidative 18F-fluorination by using aryl-Ni(II) complexes (Scheme 25B).[147] The 18F-Ni(II) complex occurred directly with aqueous [18F]fluoride at room temperature. Both aryl and alkenyl [18F]fluoride could be obtained in high RCYs and representative examples include 3-[18F]fluorobenzamide, [18F]fluorophenylalanine, 5-[18F]fluorouracil and [18F]MDL100907, as well as several 18F-labeled aromatic amino acids, 6-[18F]FDA, 6-[18F]FDOPA, 6-[18F]FMT under an improved ‘low base’ protocol.[148–150]
Scheme 25.
Pd- or Ni-mediated aromatic fluorination with [18F1fluoride
Copper catalysts are widely used in the aromatic fluorination reactions with aryl boronates and arylstannanes. In 2014, Gouverneur et al. developed Cu-mediated 18F-fluorination of aryl boronic esters (e.g., aryl-BPin) with [18F]fluoride (Scheme 26A). The reaction with aryl-BPin precursors was performed in the presence of Cu(OTf)2Py4 and O2, the latter of which was necessary and beneficial for 18F-incorporation.[151, 152] Besides aryl-BPin and copper triflate pyridine system, Sanford and Scott et al. independently developed Cu-mediated 18F-fluorination from aryl boronic acid precursors (Scheme 26B).[153] The reaction was mediated by Cu(OTf)2 in the presence of pyridine with high 18F- labeling efficiency.
Scheme 26.
Cu-mediated aromatic fluorination with [18F]fluoride
Arylstannanes are excellent arylating reagents in transmetalation processes, leading them as potential labeling precursors for Cu-based radiofluorinations. In 2016, Murphy et al. reported copper-mediated oxidative fluorination of arylstannanes with fluoride using TBAT and copper(II) triflate.[154] In the same year, Sanford and Scott et al. independently developed Cu- mediated radiofluorination of arylstannanes (Scheme 26C).[155] The reaction was performed with [18F]fluoride in the presence of pyridine, and compatible with electron-rich and -deficient arylstannanes, as well as vinyl substrates.
These Cu-mediated 18F-labeling methods from boron or tin precursors have been applied in the synthesis of a number of clinical relevant PET ligands under original or improved ‘low base’ conditions.[156] Representative examples from boron esters, boronic acids and arylstannanes are shown in Scheme 27, respectively[151–153, 155, 157]
Scheme 27.
Representative examples for Cu-mediated aromatic 18F- fluorination
3.3. Formation of 18F-labeled multifluoromethyl motifs from nucleophilic [18F]fluoride
3.3.1. 18F-Labeled aryl-CF3/CF2H/OCF3/OCF2H groups via halogen-18F exchange
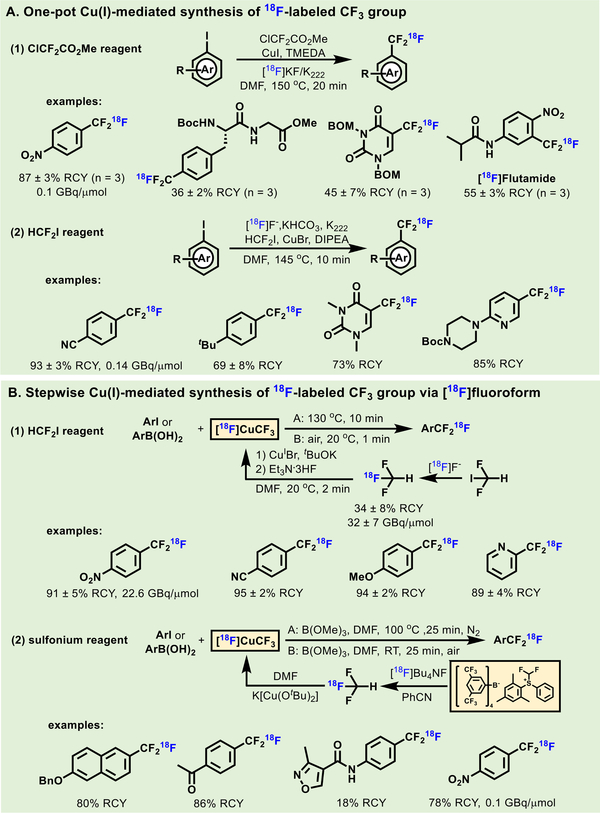
Continuous development of CF3/CF2H-containing pharmaceuticals provides a strong impetus to develop novel radiochemical methods to label these groups that were previously not accessible to drug discovery and PET tracer development. Halogen exchange of CF2X (X = F, Cl, Br) with [18F]fluoride represents a traditional way to access 18F-labeled trifluoromethyl motifs. For example, 18F-labeled aryl-CF3 could be obtained by either 19f/18F isotope exchange, or halogen-18F exchange with aryl-ClF2 or -BrF2 as shown in the synthesis of 18F-celecoxib (Scheme 28).[158] Since the presence of two α-fluorine severely inhibits displacement reactions with [18F]fluoride, harsh conditions including corrosive reagents and high temperature, are usually employed, thus limiting the compatibility with well-functionalized groups.
Scheme 28.
Traditional halogen-18F exchange reactions
In recent years, Ag(I) was found to be an accelerant for halogen-18F exchange under mild reaction conditions. In 2015, Gouverneur et al. found aryl-SCF2Br, -OCF2Br, -OCHFCl precursors could be converted to 18F-labeled aryl-SCF3, -OCF3 and -OCHF2 groups, respectively, in the presence of AgOTf (Scheme 29A).[159] Subsequently, they also found AgOTf could promote the formation of a broad array of 18F-labeled aryl-CF3 and aryl-CF2H from the corresponding aryl-CF2Br and aryl-CHFCl precursors, respectively (Scheme 29A).[160] Another Ag(I) application entailed the synthesis of 18F-labeled Umemoto reagent for trifluoromethylthiolation by Gouverneur et al. (Scheme 29B).[161] The reagent was synthesized from an oxidative ring closure of 18F-labeled [1,1’-biphenyl]-4- yl(trifluoromethyl)-sulfane. 18F-Trifluoromethylthiolation was tolerated with various functional groups and exhibited high chemoselectivity for the radiolabeling of the cysteine residue of unmodified peptides. An αvβ3 integrin-targeted peptide (cRGDfC- [18F]SCF3) was prepared in 19% RCY with 0.15 GBq/μmol molar activity.
Scheme 29.
Facilitated halogen-18F exchange for [18F]CF3/[18F]CF2H formation
Metal-free halogen-18F approaches via activating groups have also been developed in recent years. In 2016, a practical method for 18F-labeled aryl-CF2H compounds was developed by the Ritter, Vasdev and Liang groups (Scheme 29C).[162] The desired product was generated in a highly efficient ‘one-pot’ sequence, including C-H bromination to generate α-Br-α-F- acetophenone in situ, followed by facilitated 18F/Br-exchange at the α position of the carbonyl group and subsequent benzophenone cleavage. A number of well-functionalized 18F- difluoromethylarene analogs including fenofibrate, Claritin and fluoxetine was efficiently synthesized. In the same year, Liang et al. developed another metal-free oxidative C-H activation method for 18F-labeled aryl-CF2H compounds (Scheme 29D).[163] The procedure involved the conversion of benzyl halides into aryl- [18F]CFH2, followed by oxidative C-H activation and fluorination. The method was well tolerated with a diverse range of electron- donating and -neutral functional groups with 10–45% RCCs, and achieved the highest molar activity of 22 GBq/μmol for aryl- [18F]CF2H compounds to date.
3.3.2. 18F-Labeled aryl-CF3 groups via transition metal-mediated cross coupling
The preparation of aryl-CF2X (X = Cl or Br) as labeling precursors is challenging and involves multi-step synthesis in most cases, which limits the substrate scope of halogen-18F exchange method. In 2013, Passchier and Gouverneur et al. introduced an efficient method for aromatic 18F- trifluoromethylation of aryl iodides with Cu(I) catalyst (Scheme 30, A1).[164] [18F]CuCF3 was proposed to generate in situ from CuI,
Scheme 30.
Cu(I)-mediated 18F-labeled CF3 formation via [18F]fluoride and difluorocarbene
ClCF2CO2Me (a difluorocarbene reagent) and [18F]fluoride in the presence of TMEDA. This procedure was operationally simple and tolerated with a variety of functional groups with high RCYs. Another Cu(I)-mediated 18F-trifluoromethylation using CHF2I to generate [18F]CuCF3 was reported by Riss et al. in 2014.[165] Several representative 18F-labeled CF3 molecules, including [18F]flutamide, [18F]trifluorothymine and 18F-labeled Boc-protected piperazine were synthesized by these methods (Scheme 30, A2).
Other than the ‘one-pot’ protocol, a stepwise procedure for [18F]CuCF3 formation with isolated [18F]HCF3 was also viable. In 2014, Vugts et al. employed cross coupling reactions between [18F]CuCF3 and aryl iodides or boronic acids, to generate 18F- labeled CF3 motifs (Scheme 30, B1).[166] Et3N· 3HF was added to stabilize [18F]CuCF3[167] so that this method could offer an increased molar activity of 18F-labeled products to 25 GBq/μmol. The [18F]HCF3 was also used in the reactions with ketones and aldehydes.[168] Using a difluoromethyl sulfonium salt as difluorocarbene regent, Jubault and Labar et al. developed an alternative method to generate [18F]HCF3 (Scheme 30, B2), which was used for the formation of [18F]CuCF3 and subsequent 18F- trifluoromethylation in high RCYs (78–88%).[169]
Other than Cu-mediated cross coupling reactions, Carroll et al. developed site-selective oxidative fluorination of benzylic difluoromethyl groups with Mn(salen)Cl.[170] Based on the mechanism of 18F-transfer with Mn catalyst,[106] 18F-labeled CF3 groups could be obtained in 16–61% RCYs except for a few electron-deficient derivatives.
3.3.3. 18F-Labeled alkyl-CF3 compounds
18F-Labeled trifluoroethyl tosylate can be obtained either by 19F/18F exchange[171] or 18F-addition of 2,2-difluorovinyl tosylate (Scheme 31A-B).[172, 173] Using the protocol in Scheme 31B, 18F- labeled trifluoroethyl tosylate could further react with nucleophiles, including phenols, carboxylic acids and amines, in 77–93% RCYs. As PET imaging application, Scott et al. used this strategy to produce [18F]lansoprazole in 4–14% RCYs with 37 GBq/μmol molar activity.[174]
Scheme 31.
Formation of 18F-labeled alkyl-CF3 compounds
In 2017, Toste and O’Neil et al. reported a novel method for borane-catalyzed 18F-labeled alkyl-CF3 compound formation from Au(III) complex (Scheme 31C).[175] Treatment with [18F]KF and B(C6F5)3, Au(III)-OAc complex was converted to alkyl-[18F]CF3 compound via ligand exchange and reductive elimination. Three representative alkyl-CF3 analogs were labeled in reasonable RCCs, and [18F]BAY 59–3074 was isolated with 6% RCY and 0.3 GBq/μmol molar activity.
3.3.4. 18F-Labeled alkyl- and aryl-SCF3/SeCF3 groups via difluorocarbene
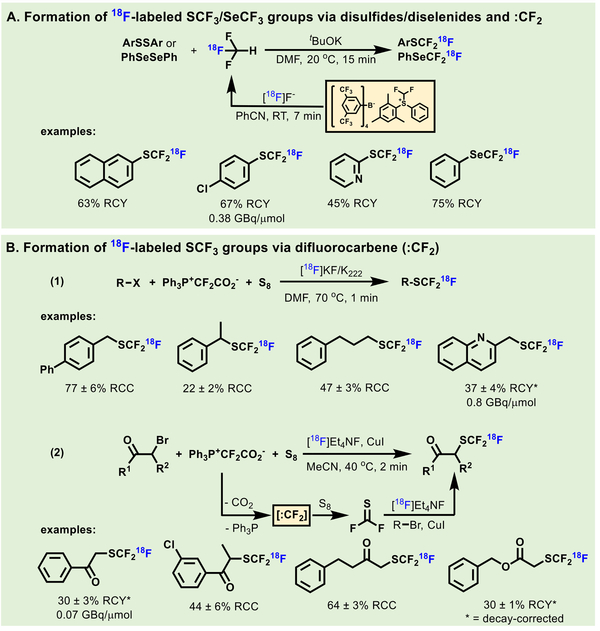
Complementary to Ag(I)-accelerated exchange method of aryl-SCF2X, Jubault and Labar et al. prepared 18F-labeled aryl- SCF3 or -SeCF3 from disulfides or diphenyl diselenides with [18F]fluoroform, respectively (Scheme 32A).[176] Different sulfur- containing derivatives including 2-(phenylthio)isoindoline-1,3- dione and S-phenyl benzenesulfonothioate could also produce the desired product in 84–99% RCYs.
Scheme 32.
18F-Labeled SCF3/SeCF3 formation with [18F]fluoride and :CF2
Despite impressive progress has been achieved in synthesis of 18F-labeled aromatic-SCF3 compounds, 18F- trifluoromethylthiolation on aliphatic carbon was challenging until Liang and Xiao et al. reported an effective method in 2015.[177] 18F- Labeled SCF3 anion generated in situ from [18F]fluoride, S8 and Ph3P+CF2CO2− (PDFA, a novel difluorocarbene reagent[178]), was reported to enable 18F-trifluoromethylthiolation of alkyl electrophiles (Scheme 32, B1). The method was compatible with aliphatic substrates bearing electron-withdrawing or -donating groups in 22–83% RCCs. Subsequently, the authors found α- bromo carbonyls could also be converted to α-[18F]SCF3 carbonyls (Scheme 32, B2).[179] An unconventional reaction mechanism was discovered in the formation of alkyl-SCF3 compounds by the identification of a key intermediate, thiocarbonyl fluoride.
3.4. Non-canonical 18F-labelings via bond formations with B, Si, Al, Ga and S
A diverse array of noncanonical 18F-labelings via bond formations with B, Si, Al, Ga and S have been established in the past decades.[86, 88] In 2005, Perrin et al. reported the first example of 18F-labeling of biomolecules via 18F-B bond formation (Scheme 33A).[180] A board range of 18F-labeled arylfluoroborates, generated from pinacol boronate esters, borimidines and trifluoroborates, were utilized.[180–182] Recently, a new aliphatic trifluoroborates stabilized by adjacent ammonium groups (in a zwitterionic form) developed by Perrin et al. was demonstrted in the synthesis of a number of clinically relevant peptides.[183] Other labeled molecules via B-18F bond formation have been showcased in the synthesis of [18F]BODIPY,[184, 185] NHC-[18F]BF3 adduct[186] and [18F]tetrafluoroborate through halogen-18F exchange.[187] Recently Chen and Liu et al. reported several 18F- labeled boramino acids (replacement of COO− in amino acids with BF3−), synthesized by the 18F-B method, for imaging amino acid transporters.[188]
Scheme 33.
Non-canonical 18F-labeling methods
In 2006, Jurkschat and Schirrmacher et al. reported the synthesis of stable [18F]fluorosilanes via 19f/18F exchange (Scheme 33, B1).[189] Their silicon-based fluoride acceptor (SiFA) methodology took advantage of two bulky teri-butyl groups to shield Si-18F bond and showed excellent in vitro stability. To further reduce high lipophilicity of silicon building blocks, structural modifications including PEGylation and/or zwitterion scaffold were further developed.[190] In parallel, Klar and Ametamey et al. reported the synthesis of [18F]fluorosilanes via H/18F or OH/18F exchange of dialkylsilane precursors (Scheme 33, B2).[191] These 18F-Si bond formation methods were successfully applied in the 18F-labeling peptides, including Tyr3-octreotate and bombesin derivatives, with high efficiency.
In 2009, McBride et al. reported the first method utilizing 18F- Al bond formation to access radiolabeled peptides, where the Al-F complex was stabilized by a hexadentate chelating ligand, NOTA (Scheme 33, C1).[192] The Al-18F chelation strategy has enriched the landscape for biomolecule labeling and showcased in the labeling of many thermostable peptides, including IMP467, dimeric RGDyK and folate receptor-targeting peptides.[88] Recently, new chelators for 18F-Al complex formation were developed by Bormans et al. to tackle with heat-sensitive biologics. The new acyclic polydentate ligands possessed less rigid structure than macrocyclic NOTA, which substantially reduced activation energy for Al-F complexation, and exhibited rapid and efficient labeling with a diverse range of peptides and antibodies (including affibody & nanobody type) at 20–40°C (Scheme 33, C2&C3).[193, 194]
Recently Reid et al. developed a chelating strategy for the preparation of a series of 18F-labeled Ga compounds via halogen- 18F exchange.[195–197] For example, [18F]GaF3(BnMe2-tacn) could be obtained by 19f/18F exchange in high RCYs (81 ± 1%; Scheme 33D). 18F-Labeled sulfur-containing molecules are also important building blocks and imaging agents,[198] such as [18F]FS-PTAD prepared from chlorosulfonyl intermediate for bioconjugation with tyrosine residues,[199] and [18F]SF6, a 18F-labeled gas prepared via isotopic exchange for PET imaging assessment of lung ventilation (Scheme 33E).[200]
3.5. Electrophilic 18F-fluorination with [18F]F2 and [18F]F2-derived reagents
3.5.1. Preparation of electrophilic [18F]F2 reagents
The first and simplest electrophilic 18F-fluorination reagent is [18F]F2, which can be produced via 20Ne(d,α)18F or 18O(p,n)18F nuclear reaction using F2 as carrier (Scheme 34). Molar activity of [18F]F2 is usually in the range of 0.04–0.4 GBq/μmol. An improved preparation of [18F]F2 was reported using [18F]CH3F and a low amount of F2, to (produce high molar activity up to 55 GBq/μmol).[201] Attributed to high chemical reactivity of [18F]F2, poor chemo- and regio-selectivity was often observed, resulting in low RCYs and technical challenges in purification. These limitations have stimulated the recent development of novel electrophilic 18F-fluorination reagents aimed for milder reactivity and higher selectivity. A range of electrophilic 18F-reagents were prepared from [18F]F2 (Scheme 34), including previously reviewed[5] [18F]XeF2, [18F]CF3OF, [18F]CH3COOF, [18F]FClO3, N- [18F]fluoropyridinium triflate, 1-[18F]fluoro-2-pyridone, and most recent [18F]NFSI[202] and [18F]Selectfluor.[203, 204]
Scheme 34.
Preparation of electrophilic 18F-fluorinating reagents
3.5.2. Electrophilic [18F]fluoroalkyl group formation
The original synthesis of [18F]FDG was conducted via electrophilic 18F-fluorination, albeit in low RCY and accompanied with undesired regioisomers (Scheme 35, A1).[205] The analogous reaction was also applied in the electrophilic 18F-fluorination of electron-deficient fluorinated alkenes, such as [18F]EF5 (Scheme 35, A2).[206] The recent development of [18F]F2-derived reagents, such as [18F]NFSI, provided a new opportunity for enantio- and diastereo-selective 18F-fluorination. In 2015, Gouverneur et al. developed an organocatalyst-mediated enantioselective 18F- fluorination of aldehydes with [18F]NFSI (Scheme 35B).[207] The ‘one-pot’ reaction was commenced with a chiral imidazolidinone and [18F]NFSI, followed by oxidation of CHO to COOH and deprotection, to provide 18F-labeled amino acids. For instance, (2S,4S)-4-[18F]fluoroglutamic acid was synthesized in 62% RCC with high enantioselectivity (>99% ee) and high diastereoselectivity (19:1 d.r). In 2017, Britton, Schaffer, Bénard and Martin et al. reported a selective photocatalytic 18F- fluorination of nonactivated C-H bonds with [18F]NFSI (Scheme 35C).[208] The reaction was initiated by abstracting H from the aliphatic chain using photoactivated decatungstate catalyst, followed by [18F]fluorine atom transfer from [18F]NFSI. As proof of concept, branched aliphatic amino acids 4-[18F]fluoroleucine (4- [18F]FL) and 5-[18F]fluorohomoleucine (5-[18F]FHL) were synthesized in 23% and 28% RCYs, respectively.
Scheme 35.
Electrophilic 18F-fluorination for 18F-alkyl formation
3.5.3. Electrophilic [18F]fluoroarene formation
The most representative electrophilic aromatic 18F- fluorination entails the synthesis of 6-[18F]FDOPA. It was first synthesized from electrophilic aromatic substitution of 3,4- dihydroxy-phenyl-L-alanine with [18F]F2 in low RCYs and low regioselectivity. Electrophilic 18F-fluorination of aryl organometallic reagents was regioselective compared with direct electrophilic 18F-fluorination. As a result, the synthesis was improved to 25% RCY by a regioselective radiofluorodestannylation (Scheme 36A).[209] Another direction to improve regioselectivity of electrophilic 18F-fluorination involved the use of milder electrophilic 18F-fluorinating reagents, such as [18F]NFSI[207]206] and more recently, [18F]Selectfluor in the Ag- mediated 18F-fluorination of arylstannanes or arylboronic esters precursors for [18F]FDOPA synthesis reported by Gouverneur et al. (Scheme 36B-C).[203, 204]
Scheme 36.
Electrophilic 18F-fluorination for arenes
4. Labeling methods with nitrogen-13 and oxygen-15
Considering the extremely short half-lives of 15O (t1/2 = 2.04 min) and 13N (t1/2 = 9.97 min), it is challenging to perform multiple- step radiochemical synthesis of complex molecules compared to 18F. Automated inline production of 15O and 13N agents is often used to improve the synthesis efficiency and quality, so that repetitive PET studies can be conducted on the same subject within a short study period. Together with previous 13N and 15O reviews,[210, 211] we highlight the recent development of major synthons and transformations.
4.1. 13N chemistry
Nitrogen-13 is routinely produced by nuclear reactions 16O(p,α)13N, 12C(p,n)13N or 13C(p,n)13N in the cyclotron (Scheme 37A).[212] [13N]NH3, widely used in clinical PET imaging for cardiovascular diseases[213] and glutamate metabolism,[214] was produced by proton irradiation of water.[212] [13N]NH3 could also serve as a building block for 13N transformations including enzymatic reaction, Hofmann rearrangement, amide or imine reduction, amination of organoboranes, substitution or amidation. These reactions were used to prepare 13N-labeled amino acids, primary amines, amides, ureas, carbamates and metal complexes (Scheme 37B).[212, 215–217]
Scheme 37.
13N chemistry
[13N]NO2− is another commonly used synthon for 13N chemistry, which can be prepared by proton irradiation of water, oxidation of [13N]NH3 using gallium or cobalt oxides, or reduction of [13N]NO3− through metals or eukaryotic nitrate reductase (Scheme 37A).[212, 218, 219] 13N-Nitrosation of ureas, secondary amines and thiols could be realized via [13N]NO2−.[218, 220] For example, GSNO, a most widely studied S-nitrosothiol, was synthesized in 24% RCY.[218] [13N]NO2− is also used to prepare 13N-labeled diazo[221, 222] and azido[223] compounds via diazonium intermediate. The ensuing 13N-labeled azido compounds can be further used in the cyclization reactions to form [13N]triazoles,[224] [13N]tetrazoles,[225] and a furaxan analog [13N]PRG150 (Scheme 37C).[226] Other 13N-labeling agents, such as [13N]N2 and [13N]N2O, were reported as well. [212, 227]
4.2. 15O chemistry
The short half-life of 15O necessitates rapid and single-step- preferred radiosynthetic process. [15O]O2 gas, produced from 14N(d,n)15O nuclear reaction, is the feedstock virtually for all 15O- labeled tracers. The synthetic routes and PET imaging applications are depicted in Scheme 38. The [15O]O2 was converted to other 15O gases including [15O]CO2, [15O]CO and [15O]N2O under appropriate reductive or oxidative conditions at high temperature.[228, 229] Other essential tracers, for example, [15O]H2O2, [15O]butanol and 6-[15O]-2-deoxy-D-glucose, were also efficiently prepared by the one-step reaction.[230–232]
Scheme 38.
15O chemistry
The most important 15O-labeled PET tracer is [15O]H2O, commonly used for the study of regional cerebral blood flow and metabolism.[233] Several methods for the production of [15O]H2O have been reported,[5] including direct bombardment of water using 16O(p,pn)15O reaction, reaction of [15O]O2 with H2 over Pt or Pd at high temperature, and in vivo 15O exchange of [15O]CO2 by carbonic anhydrase.
5. Summary and Outlook
Small molecule PET ligand discovery has long been hampered by the stringent constraints imparted by challenges in working with the short-lived radionuclides, 11C, 18F, 13N and 15O. Over the last decade, we have observed a rapid growth in PET radiochemistry, partially driven by the growing importance of PET imaging in pharmaceutical research, yielding a plethora of exciting new methodologies that equipped researchers with not only greater synthetic options, but also a broader scope of functional groups. These advances provide greater flexibility when designing new PET ligands, with optimized pharmacological, physicochemical and ADME properties. Among several new 18F-fluorination methods for labeling non-activated arenes, the most advanced for human use at this time are the hypervalent iodine(III) methods (diaryl iodonium salts and iodonium ylides). Copper-mediated radiofluorination using boron/tin precursors is also promising for clinical translation. In 11C chemistry, carbonylation via [11C]CO2 fixation has advanced to human use and has been useful for 11C-labeled carbamates, unsymmetrical ureas, carboxylic acids, amides and oxazolidinones. More advanced methods and board substrate scope for 11C-labeled amides and nitriles have been seen in the transition metal mediated cross coupling reactions with [11C]CO.
It is important, however, to realize that many emerging methodologies were established primarily using simple substrates with minimal functionalizations. Broad functional group toleration and robustness of a given methodology need to be rigorously assessed to gain a realistic understanding on its suitability in PET labeling of fully elaborated drug-like molecules. Other promising but underdeveloped areas may also include electrophilic fluorination and multifluoromethylation in high specific activity, electrochemical or light-induced reactions, radiolabeling of unmodified biologics under physiological conditions. Although it may be unrealistic to think of 13N and 15O as substitutes for 11C or 18F, these radionuclides are rarely considered by PET chemists and have potential for generating an arsenal of new radiotracers. Furthermore, with any new labeling methodology, amenability to simple purification, automation, quality control, and most importantly GMP practice needs to be considered upfront to ensure a clear pathway into the clinic. Finally, efforts toward expansion of functional groups that are amenable to radiolabeling with PET nuclides will increase the probability of successfully identifying PET ligands to guide the development of life-saving therapeutics.
Acknowledgement
Financial support from the NIH grants (DA038000, DA043507, MH117125 to S.L., and AG054473 to N.V.), NSFC (81701751) and the Fundamental Research Funds for the Central Universities (21617311) to L.W. is gratefully acknowledged.
Biography
 Steven H. Liang obtained his B.S. at Tianjin University, followed by his Ph.D. in Chemistry with Professor Marco Ciufolini in the University of British Columbia. Then he started as a NSERC fellow with Professor EJ Corey at Harvard University. Dr. Liang is currently the Director of Radiochemistry and Biomarker Development Program, Nuclear Medicine and Molecular Imaging at Massachusetts General Hospital and Assistant Professor of Radiology at Harvard Medical School.
Steven H. Liang obtained his B.S. at Tianjin University, followed by his Ph.D. in Chemistry with Professor Marco Ciufolini in the University of British Columbia. Then he started as a NSERC fellow with Professor EJ Corey at Harvard University. Dr. Liang is currently the Director of Radiochemistry and Biomarker Development Program, Nuclear Medicine and Molecular Imaging at Massachusetts General Hospital and Assistant Professor of Radiology at Harvard Medical School.
References
- [1].Fowler JS, Wolf AP, Acc. Chem. Res 1997, 30, 181. [Google Scholar]
- [2].Phelps ME, Proc. Natl. Acad. Sci 2000, 97, 9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS, Nat. Rev. Drug Discov 2008, 7, 591. [DOI] [PubMed] [Google Scholar]
- [4].Ametamey SM, Honer M, Schubiger PA, Chem. Rev 2008, 108, 1501. [DOI] [PubMed] [Google Scholar]
- [5].Miller PW, Long NJ, Vilar R, Gee AD, Angew. Chem. Int. Ed 2008, 47, 8998. [DOI] [PubMed] [Google Scholar]
- [6].Liang SH, Vasdev N, Aust. J. Chem 2015, 68, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pike VW, Curr. Med. Chem 2016, 23, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Piel M, Vernaleken I, Rösch F, J. Med. Chem 2014, 57, 9232. [DOI] [PubMed] [Google Scholar]
- [9].Rotstein BH, Liang SH, Holland JP, Collier TL, Hooker JM, Wilson AA, Vasdev N, Chem. Commun 2013, 49, 5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kealey S, Gee A, Miller PW, J. Labelled Compd. Radiopharm. 2014, 57, 195. [DOI] [PubMed] [Google Scholar]
- [11].Rahman O, J. Labelled Compd. Radiopharm. 2015, 58, 86. [DOI] [PubMed] [Google Scholar]
- [12].Rotstein BH, Liang SH, Placzek MS, Hooker JM, Gee AD, Dolle F, Wilson AA, Vasdev N, Chem. Soc. Rev 2016, 45, 4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dahl K, Halldin C, Schou M, Clin. Transl. Imaging 2017, 5, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Taddei C, Gee AD, J. Labelled Compd. Radiopharm. 2018, 61, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilson AA, Garcia A, Chestakova A, Kung H, Houle S, J. Labelled Compd. Radiopharm. 2004, 47, 679. [Google Scholar]
- [16].Fujinaga M, Yamasaki T, Maeda J, Yui J, Xie L, Nagai Y, Nengaki N, Hatori A, Kumata K, Kawamura K, Zhang M-R, J. Med. Chem 2012, 55, 11042. [DOI] [PubMed] [Google Scholar]
- [17].Kealey S, Passchier J, Huiban M, Chem. Commun 2013, 49, 11326. [DOI] [PubMed] [Google Scholar]
- [18].Wüst F, Zessin J, Johannsen B, J. Labelled Compd. Radiopharm. 2003, 46, 333. [Google Scholar]
- [19].Wuest FR, Berndt M, J. Labelled Compd. Radiopharm. 2006, 49, 91. [Google Scholar]
- [20].Dahl K, Schou M, Halldin C, Eur. J. Org. Chem 2016, 2775. [Google Scholar]
- [21].Dahl K, Nordeman P, Eur. J. Org. Chem 2017, 5785. [Google Scholar]
- [22].Kilbourn MR, Welch MJ, Int. J. Appl. Radiat. Isot 1982, 33, 359. [DOI] [PubMed] [Google Scholar]
- [23].Shibahara O, Watanabe M, Yamada S, Akehi M, Sasaki T, Akahoshi A, Hanada T, Hirano H, Nakatani S, Nishioka H, Takeuchi Y, Kakuta H, J. Med. Chem 2017, 60, 7139. [DOI] [PubMed] [Google Scholar]
- [24].Mossine AV, Brooks AF, Jackson IM, Quesada CA, Sherman P, Cole EL, Donnelly DJ, Scott PJH, Shao X, Bioconjugate Chem 2016, 27, 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pekosak A, Filp U, Rotteveel L, Poot AJ, Windhorst AD, J. Labelled Compd. Radiopharm. 2015, 58, 342. [DOI] [PubMed] [Google Scholar]
- [26].Riss PJ, Lu S, Telu S, Aigbirhio FI, Pike VW, Angew. Chem. Int. Ed 2012, 51, 2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rotstein BH, Hooker JM, Woo J, Collier TL, Brady TJ, Liang SH, Vasdev N, ACS Med. Chem. Lett 2014, 5, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bongarzone S, Runser A, Taddei C, Dheere AKH, Gee A, Chem. Commun 2017, 53, 5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van Tilburg EW, Windhorst AD, van der Mey M, Herscheid JD, J. Labelled Compd. Radiopharm. 2006, 49, 321. [Google Scholar]
- [30].Hooker JM, Reibel AT, Hill SM, Schueller MJ, Fowler JS, Angew. Chem. Int. Ed 2009, 48, 3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hooker JM, Kim SW, Alexoff D, Xu Y, Shea C, Reid A, Volkow N, Fowler JS, ACS Chem. Neurosci 2009, 1, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilson AA, Garcia A, Houle S, Sadovski O, Vasdev N, Chem. Eur. J 2011, 17, 259. [DOI] [PubMed] [Google Scholar]
- [33].Dahl K, Collier TL, Chang R, Zhang X, Sadovski O, Liang SH, Vasdev N, J. Labelled Compd. Radiopharm. 2018, 61, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Downey J, Bongarzone S, Hader S, Gee AD, J. Labelled Compd. Radiopharm. 2018, 61, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dheere AKH, Yusuf N, Gee A, Chem. Commun 2013, 49, 8193. [DOI] [PubMed] [Google Scholar]
- [36].Liger F, Eijsbouts T, Cadarossanesaib F, Tourvieille C, Le Bars D, Billard T, Eur. J. Org. Chem 2015, 6434. [Google Scholar]
- [37].Lu S, Hong J, Itoh T, Fujita M, Inoue O, Innis RB, Pike VW, J. Labelled Compd. Radiopharm. 2010, 53, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kealey S, Plisson C, Collier TL, Long NJ, Husbands SM, Martarello L, Gee AD, Org. Biomol. Chem 2011, 9, 3313. [DOI] [PubMed] [Google Scholar]
- [39].Cornilleau T, Audrain H, Guillemet A, Hermange P, Fouquet E, Org. Lett 2015, 17, 354. [DOI] [PubMed] [Google Scholar]
- [40].Aberg O, Lindhe Ö, Hall H, Hellman P, Kihlberg T, Längström B, J. Labelled Compd. Radiopharm. 2009, 52, 295. [Google Scholar]
- [41].Altomonte S, Telu S, Lu S, Pike VW, J. Org. Chem 2017, 82, 11925. [DOI] [PubMed] [Google Scholar]
- [42].Dahl K, Nordeman P, Eur. J. Org. Chem 2017, 2648. [Google Scholar]
- [43].Roslin S, Dahl K, Nordeman P, J. Labelled Compd. Radiopharm. 2018, 61, 447. [DOI] [PubMed] [Google Scholar]
- [44].Takashima-Hirano M, Ishii H, Suzuki M, ACS Med. Chem. Lett 2012, 3, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ishii H, Minegishi K, Nagatsu K, Zhang M-R, Tetrahedron 2015, 71, 1588. [Google Scholar]
- [46].Taddei C, Bongarzone S, Dheere AKH, Gee AD, Chem. Commun 2015, 51, 11795. [DOI] [PubMed] [Google Scholar]
- [47].Nordeman P, Friis SD, Andersen TL, Audrain H, Larhed M, Skrydstrup T, Antoni G, Chem. Eur. J 2015, 21, 17601. [DOI] [PubMed] [Google Scholar]
- [48].Anders DA, Bongarzone S, Fortt R, Gee AD, Long NJ, Chem. Commun 2017, 53, 2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Andersen TL, Friis SD, Audrain H, Nordeman P, Antoni G, Skrydstrup T, J. Am. Chem. Soc 2015, 137, 1548. [DOI] [PubMed] [Google Scholar]
- [50].Andersen TL, Nordeman P, Christoffersen HF, Audrain H, Antoni G, Skrydstrup T, Angew. Chem. Int. Ed 2017, 56, 4549. [DOI] [PubMed] [Google Scholar]
- [51].Dahl K, Schou M, Amini N, Halldin C, Eur. J. Org. Chem 2013, 1228. [Google Scholar]
- [52].Siméon FG, Culligan WJ, Lu S, Pike VW, Molecules 2017, 22, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rahman O, Kihlberg T, Längström B, Eur. J. Org. Chem 2004, 474. [DOI] [PubMed] [Google Scholar]
- [54].Rahman O, Längström B, Halldin C, ChemistrySelect 2016, 1, 2498. [Google Scholar]
- [55].Itsenko O, Kihlberg T, Längström B, J. Org. Chem 2004, 69, 4356. [DOI] [PubMed] [Google Scholar]
- [56].Itsenko O, Kihlberg T, Längström B, Eur. J. Org. Chem 2005, 3830. [DOI] [PubMed] [Google Scholar]
- [57].Itsenko O, Längström B, J. Org. Chem 2005, 70, 2244. [DOI] [PubMed] [Google Scholar]
- [58].Doi H, Barletta J, Suzuki M, Noyori R, Watanabe Y, Längström B, Org. Biomol. Chem 2004, 2, 3063. [DOI] [PubMed] [Google Scholar]
- [59].Ilovich O, Aberg O, Längström B, Mishani E, J. Labelled Compd. Radiopharm. 2009, 52, 151. [Google Scholar]
- [60].Verbeek J, Eriksson J, Syvänen S, Labots M, de Lange ECM, Voskuyl RA, Mooijer MPJ, Rongen M, Lammertsma AA, Windhorst AD, EJNMMI research 2012, 2, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aberg O, Längström B, J. Labelled Compd. Radiopharm. 2011, 54, 38. [Google Scholar]
- [62].Stevens MY, Chow SY, Estrada S, Eriksson J, Asplund V, Orlova A, Mitran B, Antoni G, Larhed M, Aberg O, Odell LR, ChemistryOpen 2016, 5, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Barletta J, Karimi F, Längström B, J. Labelled Compd. Radiopharm. 2006, 49, 429. [Google Scholar]
- [64].Kihlberg T, Karimi F, Längström B, J. Org. Chem 2002, 67, 3687. [DOI] [PubMed] [Google Scholar]
- [65].Gillings NM, Gee A, J. Labelled Compd. Radiopharm. 2001,44, 909. [Google Scholar]
- [66].Lee HG, Milner PJ, Placzek MS, Buchwald SL, Hooker JM, J. Am. Chem. Soc 2015, 137, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhao W, Lee HG, Buchwald SL, Hooker JM, J. Am. Chem. Soc 2017, 139, 7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ponchant M, Hinnen F, Demphel S, Crouzel C, Appl. Radiat. Isot 1997, 48, 755. [Google Scholar]
- [69].Ma L, Placzek MS, Hooker JM, Vasdev N, Liang SH, Chem. Commun 2017, 53, 6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Makaravage KJ, Shao X, Brooks AF, Yang L, Sanford MS, Scott PJH, Org. Lett 2018, 20, 1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hooker JM, Schönberger M, Schieferstein H, Fowler JS, Angew. Chem. Int. Ed 2008, 47, 5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Neelamegam R, Hellenbrand T, Schroeder FA, Wang C, Hooker JM, J. Med. Chem 2014, 57, 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Niisawa K, Ogawa K, Saito J, Taki K, Karasawa T, Nozaki T, Int. J. Appl. Radiat. Isot 1984, 35, 29. [Google Scholar]
- [74].Miller PW, Bender D, Chem. Eur. J 2012, 18, 433. [DOI] [PubMed] [Google Scholar]
- [75].Haywood T, Kealey S, Sánchez-Cabezas S, Hall JJ, Allott L, Smith G, Plisson C, Miller PW, Chem. Eur. J 2015, 21, 9034. [DOI] [PubMed] [Google Scholar]
- [76].Haskali MB, Pike VW, Chem. Eur. J 2017, 23, 8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ogawa M, Takada Y, Suzuki H, Nemoto K, Fukumura T, Nucl. Med. Biol 2010, 37, 73. [DOI] [PubMed] [Google Scholar]
- [78].Asakawa C, Ogawa M, Fujinaga M, Kumata K, Xie L, Yamasaki T, Yui J, Fukumura T, Zhang M-R, Bioorg. Med. Chem. Lett 2012, 22, 3594. [DOI] [PubMed] [Google Scholar]
- [79].Kumata K, Yui J, Hatori A, Maeda J, Xie L, Ogawa M, Yamasaki T, Nagai Y, Shimoda Y, Fujinaga M, Kawamura K, Zhang M-R, ACS Chem. Neurosci 2014, 6, 339. [DOI] [PubMed] [Google Scholar]
- [80].Wang L, Mori W, Cheng R, Yui J, Hatori A, Ma L, Zhang Y, Rotstein BH, Fujinaga M, Shimoda Y, Yamasaki T, Xie L, Nagai Y, Minamimoto T, Higuchi M, Vasdev N, Zhang M-R, Liang SH, Theranostics 2016, 6, 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cheng R, Mori W, Ma L, Alhouayek M, Hatori A, Zhang Y, Ogasawara D, Yuan G, Chen Z, Zhang X, Shi H, Yamasaki T, Xie L, Kumata K, Fujinaga M, Nagai Y, Minamimoto T, Svensson M, Wang L, Du Y, Ondrechen MJ, Vasdev N, Cravatt BF, Fowler C, Zhang M-R, Liang SH, J. Med. Chem 2018, 61, 2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cai L, Lu S, Pike VW, Eur. J. Org. Chem 2008, 2853. [Google Scholar]
- [83].Tredwell M, Gouverneur V, Angew. Chem. Int. Ed 2012, 51, 11426. [DOI] [PubMed] [Google Scholar]
- [84].Liang SH, Vasdev N, Angew. Chem. Int. Ed 2014, 53, 11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJ, Chem. Sci 2014, 5, 4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chansaenpak K, Vabre B, Gabbai FP, Chem. Soc. Rev 2016, 45, 954. [DOI] [PubMed] [Google Scholar]
- [87].Preshlock S, Tredwell M, Gouverneur V, Chem. Rev 2016, 116, 719. [DOI] [PubMed] [Google Scholar]
- [88].Krishnan HS, Ma L, Vasdev N, Liang SH, Chem. Eur. J 2017, 23, 15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].van der Born D, Pees A, Poot AJ, Orru RVA, Windhorst AD, Vugts DJ, Chem. Soc. Rev 2017, 46, 4709. [DOI] [PubMed] [Google Scholar]
- [90].Zhang M-R, Suzuki K, Curr. Top. Med. Chem 2007, 7, 1817. [DOI] [PubMed] [Google Scholar]
- [91].Kniess T, Laube M, Brust P, Steinbach J, MedChemComm 2015, 6, 1714. [Google Scholar]
- [92].Schou M, Halldin C, Sovago J, Pike VW, Hall H, Gulyas B, Mozley PD, Dobson D, Shchukin E, Innis RB, Farde L, Synapse 2004, 53, 57. [DOI] [PubMed] [Google Scholar]
- [93].Kuchar M, Mamat C, Molecules 2015, 20, 16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kim DW, Choe YS, Chi DY, Nucl. Med. Biol 2003, 30, 345. [DOI] [PubMed] [Google Scholar]
- [95].Kim DW, Ahn D-S, Oh Y-H, Lee S, Kil HS, Oh SJ, Lee SJ, Kim JS, Ryu JS, Moon DH, Chi DY, J. Am. Chem. Soc 2006, 128, 16394. [DOI] [PubMed] [Google Scholar]
- [96].Chaly T, Dhawan V, Kazumata K, Antonini A, Margouleff C, Dahl JR, Belakhlef A, Margouleff D, Yee A, Wang S, Tamgnan G, Neumeyer JL, Eidelberg D, Nucl. Med. Biol 1996, 23, 999. [DOI] [PubMed] [Google Scholar]
- [97].Zhang L, Villalobos A, Beck EM, Bocan T, Chappie TA, Chen L, Grimwood S, Heck SD, Helal CJ, Hou X, Humphrey JM, Lu J, Skaddan MB, McCarthy TJ, Verhoest PR, Wager TT, Zasadny K, J. Med. Chem 2013, 56, 4568. [DOI] [PubMed] [Google Scholar]
- [98].Nielsen MK, Ugaz CR, Li W, Doyle AG, J. Am. Chem. Soc. 2015, 137, 9571. [DOI] [PubMed] [Google Scholar]
- [99].Sergeev ME, Morgia F, Lazari M, Wang C Jr., van Dam RM, J. Am. Chem. Soc 2015, 137, 5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Deng H, Cobb SL, Gee AD, Lockhart A, Martarello L, McGlinchey RP, O’Hagan D, Onega M, Chem. Commun 2006, 42, 652. [DOI] [PubMed] [Google Scholar]
- [101].Thompson S, Zhang Q, Onega M, McMahon S, Fleming I, Ashworth S, Naismith JH, Passchier J, O’Hagan D, Angew. Chem. Int. Ed 2014, 53, 8913. [DOI] [PubMed] [Google Scholar]
- [102].Hollingworth C, Hazari A, Hopkinson MN, Tredwell M, Benedetto E, Huiban M, Gee AD, Brown JM, Gouverneur V, Angew. Chem. Int. Ed 2011,50, 2613. [DOI] [PubMed] [Google Scholar]
- [103].Topczewski JJ, Tewson TJ, Nguyen HM, J. Am. Chem. Soc 2011, 133, 19318. [DOI] [PubMed] [Google Scholar]
- [104].Benedetto E, Tredwell M, Hollingworth C, Khotavivattana T, Brown JM, Gouverneur V, Chem. Sci 2013, 4, 89. [Google Scholar]
- [105].Graham TJA, Lambert RF, Ploessl K, Kung HF, Doyle AG, J. Am. Chem. Soc 2014, 136, 5291. [DOI] [PubMed] [Google Scholar]
- [106].Huang X, Liu W, Ren H, Neelamegam R, Hooker JM, Groves JT, J. Am. Chem. Soc 2014, 136, 6842. [DOI] [PubMed] [Google Scholar]
- [107].Liu W, Huang X, Placzek MS, Krska SW, McQuade P, Hooker JM, Groves JT, Chem. Sci 2018, 9, 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yang B, Chansaenpak K, Wu H, Zhu L, Wang M, Li Z, Lu H, Chem. Commun 2017, 53, 3497. [DOI] [PubMed] [Google Scholar]
- [109].Cortes Gonzalez MA, Nordeman P, Bermejo Gomez A, Meyer DN, Antoni G, Schou M, Szabo KJ, Chem. Commun 2018, 54, 4286. [DOI] [PubMed] [Google Scholar]
- [110].Lemaire C, Gillet S, Guillouet S, Plenevaux A, Aerts J, Luxen A, Eur. J. Org. Chem 2004, 2004, 2899. [Google Scholar]
- [111].Wagner FM, Ermert J, Coenen HH, J. Nucl. Med 2009, 50, 1724. [DOI] [PubMed] [Google Scholar]
- [112].Maeda M, Fukumura T, Kojima M, Appl. Radiat. Isot 1987, 38, 307. [Google Scholar]
- [113].Mu L, Fischer CR, Holland JP, Becaud J, Schubiger PA, Schibli R, Ametamey SM, Graham K, Stellfeld T, Dinkelborg LM, Lehmann L, Eur. J. Org. Chem 2012, 889. [Google Scholar]
- [114].Sander K, Gendron T, Yiannaki E, Cybulska K, Kalber TL, Lythgoe MF, Arstad E, Sci. Rep 2015, 5, 9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chun J-H, Morse CL, Chin FT, Pike VW, Chem. Commun 2013, 49, 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Narayanam MK, Ma G, Champagne PA, Houk KN, Murphy JM, Angew. Chem. Int. Ed 2017, 56, 13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Neumann CN, Hooker JM, Ritter T, Nature 2016, 534, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Beyzavi MH, Mandal D, Strebl MG, Neumann CN, D’Amato EM, Chen J, Hooker JM, Ritter T, ACS Cent. Sci 2017, 3, 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gao Z, Lim YH, Tredwell M, Li L, Verhoog S, Hopkinson M, Kaluza W, Collier TL, Passchier J, Huiban M, Gouverneur V, Angew. Chem. Int. Ed 2012, 51, 6733. [DOI] [PubMed] [Google Scholar]
- [120].Haka MS, Kilbourn MR, Watkins GL, Toorongian SA, J. Labelled Compd. Radiopharm. 1989, 27, 823. [Google Scholar]
- [121].Pike VW, Aigbirhio FI, J. Chem. Soc., Chem. Commun 1995, 2215. [Google Scholar]
- [122].Ross TL, Ermert J, Hocke C, Coenen HH, J. Am. Chem. Soc 2007, 129, 8018. [DOI] [PubMed] [Google Scholar]
- [123].Chun J-H, Lu S, Lee Y-S, Pike VW, J. Org. Chem 2010, 75, 3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lee Y-S, Chun J-H, Hodoscek M, Pike VW, Chem. Eur. J. 2017, 23, 4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Neumann K, Qin L, Vavere A, Snyder S, DiMagno S, J. Nucl. Med 2012, 53, no. supplement 1 71. [Google Scholar]
- [126].Pike VW, J. Labelled Compd. Radiopharm. 2018, 61, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH, Org. Lett 2014, 16, 3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].McCammant MS, Thompson S, Brooks AF, Krska SW, Scott PJH, Sanford MS, Org. Lett 2017, 19, 3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yuan Z, Cheng R, Chen P, Liu G, Liang SH, Angew. Chem. Int. Ed 2016, 55, 11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Haskali MB, Telu S, Lee Y-S, Morse CL, Lu S, Pike VW, J. Org. Chem 2016, 81, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cardinale J, Ermert J, Humpert S, Coenen HH, RSC Adv. 2014, 4, 17293. [Google Scholar]
- [132].Rotstein BH, Stephenson NA, Vasdev N, Liang SH, Nat. Commun 2014, 5, 4365. [DOI] [PubMed] [Google Scholar]
- [133].Rotstein BH, Wang L, Liu RY, Patteson J, Kwan EE, Vasdev N, Liang SH, Chem. Sci 2016, 7, 4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Jakobsson JE, Gronnevik G, Riss PJ, Chem. Commun 2017, 53, 12906. [DOI] [PubMed] [Google Scholar]
- [135].Petersen IN, Kristensen JL, Herth MM, Eur. J. Org. Chem 2017, 2017, 453. [Google Scholar]
- [136].Kugler F, Ermert J, Kaufholz P, Coenen HH, Molecules 2015, 20, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang L, Jacobson O, Avdic D, Rotstein BH, Weiss ID, Collier L, Chen X, Vasdev N, Liang SH, Angew. Chem. Int. Ed 2015, 54, 12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yuan G, Jones GB, Vasdev N, Liang SH, Bioorg. Med. Chem. Lett 2016, 26, 4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Drerup C, Ermert J, Coenen HH, Molecules 2016, 21, 1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Collier TL, Normandin MD, Stephenson NA, Livni E, Liang SH, Wooten DW, Esfahani SA, Stabin MG, Mahmood U, Chen J, Wang W, Maresca K, Waterhouse RN, El Fakhri G, Richardson P, Vasdev N, Nat. Commun 2017, 8, 15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang L, Cheng R, Fujinaga M, Yang J, Zhang Y, Hatori A, Kumata K, Yang J, Vasdev N, Du Y, Ran C, Zhang MR, Liang SH, J. Med. Chem 2017, 60, 5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Li S, Cai Z, Zheng M-Q, Holden D, Naganawa M, Lin S-F, Ropchan J, Labaree D, Kapinos M, Lara-Jaime T, Navarro A, Huang Y, J. Nucl. Med 2018, 59, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stephenson NA, Holland JP, Kassenbrock A, Yokell DL, Livni E, Liang SH, Vasdev N, J. Nucl. Med 2015, 56, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Stephenson NA, Holland JP, Kassenbrock A, Yokell DL, Livni E, Liang SH, Vasdev N, J. Nucl. Med 2015, 56, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, Choi DC, Hooker JM, Ritter T, Science 2011,334, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kamlet AS, Neumann CN, Lee E, Carlin SM, Moseley CK, Stephenson N, Hooker JM, Ritter T, PLoS One 2013, 8, e59187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lee E, Hooker JM, Ritter T, J. Am. Chem. Soc 2012, 134, 17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hoover AJ, Lazari M, Ren H, Narayanam MK, Murphy JM, van Dam RM, Hooker JM, Ritter T, Organometallics 2016, 35, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ren H, Wey H-Y, Strebl M, Neelamegam R, Ritter T, Hooker JM, ACS Chem. Neurosci 2014, 5, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zlatopolskiy BD, Zischler J, Urusova EA, Endepols H, Kordys E, Frauendorf H, Mottaghy FM, Neumaier B, ChemistryOpen 2015, 4, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, Mercier J, Genicot C, Gouverneur V, Angew. Chem. Int. Ed 2014, 53, 7751. [DOI] [PubMed] [Google Scholar]
- [152].Taylor NJ, Emer E, Preshlock S, Schedler M, Tredwell M, Verhoog S, Mercier J, Genicot C, Gouverneur V, J. Am. Chem. Soc 2017, 139, 8267. [DOI] [PubMed] [Google Scholar]
- [153].Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, Scott PJH, Org. Lett 2015, 17, 5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gamache RF, Waldmann C, Murphy JM, Org. Lett 2016, 18, 4522. [DOI] [PubMed] [Google Scholar]
- [155].Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH, Org. Lett 2016, 18, 5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E, Endepols H, Neumaier B, Chem. Eur. J 2015, 21, 5972. [DOI] [PubMed] [Google Scholar]
- [157].Preshlock S, Calderwood S, Verhoog S, Tredwell M, Huiban M, Hienzsch A, Gruber S, Wilson TC, Taylor NJ, Cailly T, Schedler M, Collier TL, Passchier J, Smits R, Mollitor J, Hoepping A, Mueller M, Genicot C, Mercier J, Gouverneur V, Chem. Commun 2016, 52, 8361. [DOI] [PubMed] [Google Scholar]
- [158].Prabhakaran J, Underwood MD, Parsey RV, Arango V, Majo VJ, Simpson NR, Van Heertum R, Mann JJ, Kumar JSD, Bioorg. Med. Chem 2007, 15, 1802. [DOI] [PubMed] [Google Scholar]
- [159].Khotavivattana T, Verhoog S, Tredwell M, Pfeifer L, Calderwood S, Wheelhouse K, Collier TL, Gouverneur V, Angew. Chem. Int. Ed 2015, 54, 9991. [DOI] [PubMed] [Google Scholar]
- [160].Verhoog S, Pfeifer L, Khotavivattana T, Calderwood S, Collier TL, Wheelhouse K, Tredwell M, Gouverneur V, Synlett 2016, 27, 25. [DOI] [PubMed] [Google Scholar]
- [161].Verhoog S, Kee CW, Wang Y, Khotavivattana T, Wilson TC, Kersemans V, Smart S, Tredwell M, Davis BG, Gouverneur V, J. Am. Chem. Soc 2018, 140, 1572. [DOI] [PubMed] [Google Scholar]
- [162].Shi H, Braun A, Wang L, Liang SH, Vasdev N, Ritter T, Angew. Chem. Int. Ed 2016, 55, 10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yuan G, Wang F, Stephenson NA, Wang L, Rotstein BH, Vasdev N, Tang P, Liang SH, Chem. Commun 2017, 53, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Huiban M, Tredwell M, Mizuta S, Wan Z, Zhang X, Collier TL, Gouverneur V, Passchier J, Nat. Chem 2013, 5, 941. [DOI] [PubMed] [Google Scholar]
- [165].Rühl T, Rafique W, Lien VT, Riss PJ, Chem. Commun 2014, 50, 6056. [DOI] [PubMed] [Google Scholar]
- [166].van der Born D, Sewing C, Herscheid JDM, Windhorst AD, Orru RVA, Vugts DJ, Angew. Chem. Int. Ed 2014, 53, 11046. [DOI] [PubMed] [Google Scholar]
- [167].Zanardi A, Novikov MA, Martin E, Benet-Buchholz J, Grushin VV, J. Am. Chem. Soc 2011, 133, 20901. [DOI] [PubMed] [Google Scholar]
- [168].van der Born D, Herscheid JDM, Orru RVA, Vugts DJ, Chem. Commun 2013, 49, 4018. [DOI] [PubMed] [Google Scholar]
- [169].Ivashkin P, Lemonnier G, Cousin J, Grégoire V, Labar D, Jubault P, Pannecoucke X, Chem. Eur. J 2014, 20, 9514. [DOI] [PubMed] [Google Scholar]
- [170].Carroll L, Evans HL, Spivey AC, Aboagye EO, Chem. Commun 2015, 51, 8439. [DOI] [PubMed] [Google Scholar]
- [171].Suehiro M, Yang G, Torchon G, Ackerstaff E, Humm J, Koutcher J, Ouerfelli O, Bioorg. Med. Chem 2011, 19, 2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Riss PJ, Aigbirhio FI, Chem. Commun 2011,47, 11873. [DOI] [PubMed] [Google Scholar]
- [173].Riss PJ, Ferrari V, Brichard L, Burke P, Smith R, Aigbirhio FI, Org. Biomol. Chem 2012, 10, 6980. [DOI] [PubMed] [Google Scholar]
- [174].Fawaz MV, Brooks AF, Rodnick ME, Carpenter GM, Shao X, Desmond TJ, Sherman P, Quesada CA, Hockley BG, Kilbourn MR, Albin RL, Frey KA, Scott PJH, ACS Chem. Neurosci 2014, 5, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Levin MD, Chen TQ, Neubig ME, Hong CM, Theulier CA, Kobylianskii IJ, Janabi M, O’Neil JP, Toste FD, Science 2017, 356, 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Carbonnel E, Besset T, Poisson T, Labar D, Pannecoucke X, Jubault P, Chem. Commun 2017, 53, 5706. [DOI] [PubMed] [Google Scholar]
- [177].Zheng J, Wang L, Lin J-H, Xiao J-C, Liang SH, Angew. Chem. Int. Ed 2015, 54, 13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zheng J, Cai J, Lin J-H, Guo Y, Xiao J-C, Chem. Commun 2013, 49, 7513. [DOI] [PubMed] [Google Scholar]
- [179].Zheng J, Cheng R, Lin J-H, Yu DH, Ma L, Jia L, Zhang L, Wang L, Xiao J-C, Liang SH, Angew. Chem. Int. Ed 2017, 56, 3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Ting R, Adam MJ, Ruth TJ, Perrin DM, J. Am. Chem. Soc 2005, 127, 13094. [DOI] [PubMed] [Google Scholar]
- [181].Liu Z, Li Y, Lozada J, Schaffer P, Adam MJ, Ruth TJ, Perrin DM, Angew. Chem. Int. Ed 2013, 52, 2303. [DOI] [PubMed] [Google Scholar]
- [182].Liu Z, Li Y, Lozada J, Wong MQ, Greene J, Lin K-S, Yapp D, Perrin DM, Nucl. Med. Biol 2013, 40, 841. [DOI] [PubMed] [Google Scholar]
- [183].Liu Z, Pourghiasian M, Radtke MA, Lau J, Pan J, Dias GM, Yapp D, Lin K-S, Benard F, Perrin DM, Angew. Chem. Int. Ed 2014, 53, 11876. [DOI] [PubMed] [Google Scholar]
- [184].Hendricks JA, Keliher EJ, Wan D, Hilderbrand SA, Weissleder R, Mazitschek R, Angew. Chem. Int. Ed 2012, 51, 4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Liu S, Li D, Zhang Z, Prakash GS, Conti PS, Li Z, Chem. Commun 2014, 50, 7371. [DOI] [PubMed] [Google Scholar]
- [186].Chansaenpak K, Wang M, Wu Z, Zaman R, Li Z, GabbaY FP, Chem. Commun 2015, 51, 12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Jiang H, Bansal A, Pandey MK, Peng K-W, Suksanpaisan L, Russell SJ, DeGrado TR, J. Nucl. Med 2016, 57, 1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Liu Z, Chen H, Chen K, Shao Y, Kiesewetter DO, Niu G, Chen X, Sci. Adv 2015, 1, 1:e1500694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Schirrmacher R, Bradtmoller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wangler B, Niemeyer CM, Jurkschat K, Angew. Chem. Int. Ed 2006, 45, 6047. [DOI] [PubMed] [Google Scholar]
- [190].Niedermoser S, Chin J, Wangler C, Kostikov A, Bernard-Gauthier V, Vogler N, Soucy J-P, McEwan AJ, Schirrmacher R, Wangler B, J. Nucl. Med 2015, 56, 1100. [DOI] [PubMed] [Google Scholar]
- [191].Mu L, Hohne A, Schubiger PA, Ametamey SM, Graham K, Cyr JE, Dinkelborg L, Stellfeld T, Srinivasan A, Voigtmann U, Klar U, Angew. Chem. Int. Ed 2008, 47, 4922. [DOI] [PubMed] [Google Scholar]
- [192].McBride WJ, Sharkey RM, Karacay H, D’Souza CA, Rossi EA, Laverman P, Chang C-H, Boerman OC, Goldenberg DM, J. Nucl. Med 2009, 50, 991. [DOI] [PubMed] [Google Scholar]
- [193].Cleeren F, Lecina J, Billaud EM, Ahamed M, Verbruggen A, Bormans GM, Bioconjugate Chem 2016, 27, 790. [DOI] [PubMed] [Google Scholar]
- [194].Cleeren F, Lecina J, Ahamed M, Raes G, Devoogdt N, Caveliers V, McQuade P, Rubins DJ, Li W, Verbruggen A, Xavier C, Bormans G, Theranostics 2017, 7, 2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bhalla R, Darby C, Levason W, Luthra SK, McRobbie G, Reid G, Sanderson G, Zhang W, Chem. Sci 2014, 5, 381. [Google Scholar]
- [196].Bhalla R, Levason W, Luthra SK, McRobbie G, Sanderson G, Reid G, Chem. Eur. J 2015, 21, 4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Monzittu FM, Khan I, Levason W, Luthra S, McRobbie G, Reid G, Angew. Chem. Int. Ed 2018, 57, 6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Pascali G, Matesic L, Zhang B, King AT, Robinson AJ, Ung AT, Fraser BH, EJNMMI Radiopharm. Chem 2017, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Al-Momani E, Israel I, Buck AK, Samnick S, Appl. Radiat. Isot 2015, 104, 136. [DOI] [PubMed] [Google Scholar]
- [200].Gómez-Vallejo V, Lekuona A, Baz Z, Szczupak B, Cossío U, Llop J, Chem. Commun 2016, 52, 11931. [DOI] [PubMed] [Google Scholar]
- [201].Bergman J, Solin O, Nucl. Med. Biol 1997, 24, 677. [DOI] [PubMed] [Google Scholar]
- [202].Teare H, Robins EG, Arstad E, Luthra SK, Gouverneur V, Chem. Commun 2007, 43, 2330. [DOI] [PubMed] [Google Scholar]
- [203].Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V, Angew. Chem. Int. Ed 2010, 49, 6821. [DOI] [PubMed] [Google Scholar]
- [204].Stenhagen ISR, Kirjavainen AK, Forsback SJ, Jorgensen CG, Robins EG, Luthra SK, Solin O, Gouverneur V, Chem. Commun 2013, 49, 1386. [DOI] [PubMed] [Google Scholar]
- [205].Ido T, Wan C-N, Casella V, Fowler JS, P. WA, J. Labelled Compd. Radiopharm 1978, 14, 175. [Google Scholar]
- [206].Dolbier WR Jr., Li A-R, Koch CJ, Shiue C-Y, Kachur AV, Appl. Radiat. Isot 2001,54, 73. [DOI] [PubMed] [Google Scholar]
- [207].Buckingham F, Kirjavainen AK, Forsback S, Krzyczmonik A, Keller T, Newington IM, Glaser M, Luthra SK, Solin O, Gouverneur V, Angew. Chem. Int. Ed 2015, 54, 13366. [DOI] [PubMed] [Google Scholar]
- [208].Nodwell MB, Yang H, Colovic M, Yuan Z, Merkens H, Martin RE, Benard F, Schaffer P, Britton R, J. Am. Chem. Soc 2017, 139, 3595. [DOI] [PubMed] [Google Scholar]
- [209].Namavari M, Bishop A, Satyamurthy N, Bida G, Barrio JR, Int. J. Radiat. Appl. Instrum. Part A. Appl. Radiat. Isot 1992, 43, 989. [DOI] [PubMed] [Google Scholar]
- [210].Miller PW, Kato K, Langstrom B, in The Chemistry of Molecular Imaging, Vol. 1 (Eds.: Long N, Wong W-T), John Wiley & Sons, Inc, Hoboken, New Jersey, 2014, pp. 79. [Google Scholar]
- [211].Ermert J, J. Labelled Compd. Radiopharm. 2018, 61, 179. [DOI] [PubMed] [Google Scholar]
- [212].Gomez-Vallejo V, Gaja V, Gona KB, Llop J, J. Labelled Compd. Radiopharm. 2014, 57, 244. [DOI] [PubMed] [Google Scholar]
- [213].Statuto M, Galli E, Bertagna F, Migliorati E, Zanella I, Di Lorenzo D, De Agostini A, Rodella C, Apostoli P, Caimi L, Giubbini R, Biasiotto G, Nucl. Med. Commun 2016, 37, 412. [DOI] [PubMed] [Google Scholar]
- [214].Cooper AJL, Neurochem. Int 2011,59, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].da Silva ES, Gomez-Vallejo V, Baz Z, Llop J, Lopez-Gallego F, Chem. Eur. J 2016, 22, 13619. [DOI] [PubMed] [Google Scholar]
- [216].da Silva ES, Gómez-Vallejo V, Llop J, López-Gallego F, Process Biochem 2017, 57, 80. [Google Scholar]
- [217].Blower JE, Cousin SF, Gee AD, EINMMI Radiopharm Chem 2017, 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Llop J, Gomez-Vallejo V, Bosque M, Quincoces G, Penuelas I, Appl. Radiat. Iso 2009, 67, 95. [DOI] [PubMed] [Google Scholar]
- [219].da Silva ES, Gómez-Vallejo V, Llop J, López-Gallego F, Catal. Sci. Technol 2015, 5, 2705. [Google Scholar]
- [220].Gaja V, Gómez-Vallejo V, Cuadrado-Tejedor M, Borrell JI, Llop J, J. Labelled Compd. Radiopharm. 2012, 55, 332. [Google Scholar]
- [221].Gómez-Vallejo V, Borrell JI, Llop J, Eur. J. Med. Chem 2010, 45, 5318. [DOI] [PubMed] [Google Scholar]
- [222].Gaja V, Gomez-Vallejo V, Puigivila M, Perez-Campana C, Martin A, Garcia-Osta A, Calvo-Fernandez T, Cuadrado-Tejedor M, Franco R, Llop J, Mol. Imaging Biol 2014, 16, 538. [DOI] [PubMed] [Google Scholar]
- [223].Joshi SM, de Cozar A, Gomez-Vallejo V, Koziorowski J, Llop J, Cossio FP, Chem. Commun 2015, 51, 8954. [DOI] [PubMed] [Google Scholar]
- [224].Joshi SM, Gómez-Vallejo V, Salinas V, Llop J, RSC Adv 2016, 6, 109633. [Google Scholar]
- [225].Joshi SM, Mane RB, Pulagam KR, Gomez-Vallejo V, Llop J, Rode C, New J Chem. 2017, 41, 8084. [Google Scholar]
- [226].Pippin AB, Arshad ZHM, Voll RJ, Nye JA, Ghassabian S, Williams CM, Mancini A, Liotta DC, Smith MT, Goodman MM, ACS Med. Chem. Lett 2016, 7, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Nickles RJ, Gatley SJ, Hichwa RD, Simpkin DJ, Martin JL, Int. J. Appl. Radiat. Isot 1978, 29, 225. [Google Scholar]
- [228].Iwata R, Ido T, Radiochim. Acta 1993, 60, 75. [Google Scholar]
- [229].Diksic M, Yamamoto YL, Feindel W, J. Nucl. Med 1983, 24, 603. [PubMed] [Google Scholar]
- [230].Takahashi K, Murakami M, Hagami E, Sasaki H, Kondo Y, Mizusawa S, Nakamichi H, Iida H, Miura S, Kanno I, Uemura K, J. Labelled Compd. Radiopharm. 1989, 27, 1167. [Google Scholar]
- [231].Berridge MS, Cassidy EH, Terris AH, J. Nucl. Med 1990, 31, 1727. [PubMed] [Google Scholar]
- [232].Yorimitsu H, Murakami Y, Takamatsu H, Nishimura S, Nakamura E, Angew. Chem. Int. Ed 2005, 44, 2708. [DOI] [PubMed] [Google Scholar]
- [233].Fan AP, Jahanian H, Holdsworth SJ, Zaharchuk G, J. Cereb. Blood FlowMetab. 2016, 36, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]