Abstract
STUDY QUESTION
Which different pathways and functions are altered in rhesus monkey oocytes that fail to mature after an ovulatory stimulus?
SUMMARY ANSWER
Failed to mature (FTM) oocytes complete a large portion of the transition in transcriptome composition associated with normal maturation, but also manifest numerous differences that indicate incomplete transcriptional repression and cytoplasmic maturation affecting multiple processes.
WHAT IS KNOWN ALREADY
Oocyte maturation defects contribute to unexplained female infertility. Failure of some oocytes to undergo germinal vesicle breakdown or progress to second meiotic metaphase in response to an ovulatory stimulus can limit the number of high quality oocytes available for ART.
STUDY DESIGN, SIZE, DURATION
The transcriptome of rhesus monkey oocytes that failed to mature (FTM; n = 11, 5 donors) in response to an ovulatory stimulus in vivo was compared to those of normal germinal vesicle stage (GV, n = 7, 2 donors) and metaphase II stage (MII, n = 7, 5 donors) oocytes by RNA-sequencing (RNAseq).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Female rhesus monkeys of normal breeding age (6–12 years old) and with regular menstrual cycles were used. Animals underwent a controlled ovarian stimulation protocol for the collection of oocytes by ultrasound-guided needle aspiration of follicles.
MAIN RESULTS AND THE ROLE OF CHANCE
We obtained a high quality RNAseq dataset consisting of n = 7, n = 7, and n = 11 libraries for normal GV, normal MII and FTM oocytes, respectively. Total reads acquired were an average of 34 million for each GV sample, 41 million for each FTM sample and 59 million for each MII oocyte sample. Approximately 44% of the total reads were exonic reads that successfully aligned to the rhesus monkey genome as unique non-rRNA gene transcript sequences, providing high depth of coverage. Approximately 44% of the mRNAs that undergo changes in abundance during normal maturation display partial modulations to intermediate abundances, and 9.2% fail to diverge significantly from GV stage oocytes. Additionally, a small group of mRNAs are grossly mis-regulated in the FTM oocyte. Differential expression was seen for mRNAs associated with mitochondrial functions, fatty acid beta oxidation, lipid accumulation, meiosis, zona pellucida formation, Hippo pathway signaling, and maternal mRNA regulation. A deficiency DNA methyltransferase one mRNA expression indicates a potential defect in transcriptional silencing.
LARGE SCALE DATA
All RNAseq data are published in the Gene Expression Omnibus Database (GSE112536).
LIMITATIONS, REASONS FOR CAUTION
These results do not establish cause of maturation failure but reveal novel correlates of incompetence to mature. Transcriptome studies likely do not capture all post-transcriptional or post-translational events that inhibit maturation, but do reveal mRNA expression changes that lie downstream of such events or that are related to effects on upstream regulators. The use of an animal model allows the study of oocyte maturation failure independent of covariates and confounders, such as pre-existing conditions of the female, which is a significant concern in human studies. Depending on the legislation, it may not be possible to collect and study oocytes from healthy women; and using surplus oocytes from patients undergoing ART may introduce confounders that vary from case to case. FTM oocytes were at various stages of meiotic progression, so correlates of specific times of arrest are not revealed. All the FTM oocytes failed to respond appropriately to an ovulatory stimulus in vivo. Therefore, this analysis informs us about common transcriptome features associated with meiotic incompetence.
WIDER IMPLICATIONS OF THE FINDINGS
These results reveal that some diagnostic markers of oocyte quality may not reflect developmental competence because even meiotically incompetent oocytes display many normal gene expression features. The results also reveal potential mechanisms by which maternal and environmental factors may impact transcriptional repression and cytoplasmic maturation, and prevent oocyte maturation.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grants from the National Institutes of Health Office of Research Infrastructure Programs Division of Comparative Medicine Grants R24 [OD012221 to K.E.L., OD011107/RR00169 (California National Primate Research Center), and OD010967/RR025880 to C.A.V.]; the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under the award number T32HD087166; MSU AgBioResearch, Michigan State University. Authors have nothing to disclose.
Keywords: infertility, meiosis, RNAseq, oocyte maturation, gene expression, cytoplasmic maturation, developmental competence, rhesus monkey
Introduction
The United States Centers for Disease Control estimates that more than 12% of women aged 15–44 years have impaired fecundity, and that more than half of these are infertile (Chandra et al., 2013). The incidence of unexplained infertility ranges from 15% to 30% of infertile couples (Gelbaya et al., 2014). Male factors are the sole cause in ~30% of these cases, indicating that female factors are common in unexplained infertility (Quaas and Dokras, 2008). The most commonly cited female factors include ovulatory defects (e.g. polycystic ovary syndrome, diminished ovarian reserve and primary ovarian insufficiency), fallopian tube obstruction and uterine defects.
Recently, oocyte maturation defects have emerged as an additional cause of unexplained female infertility (Avrech et al., 1997; Levran et al., 2002). Oocytes can arrest aberrantly with germinal vesicles (GV) intact or at the first meiotic division (metaphase I: MI), or may reach metaphase II (MII) but fail to activate upon insemination (Levran et al., 2002); the former two classes will also fail to activate when inseminated, as they are incompetent to respond, and if artificially activated following insemination without completing meiosis, will give rise to aneuploid embryos. GV intact immature oocytes arise somewhat rarely. Small numbers may be observed overall (4.4% of oocytes and ~24% of patients undergoing ART) (Avrech et al., 1997), with arrest at MI and MII being more prevalent among oocytes retrieved (Levran et al., 2002). Complete failure of oocyte maturation is rare, but does arise in some patients (Levran et al., 2002; Chen et al., 2010). Complete maturation failure can be accompanied by a fully normal karyotype (Chen et al., 2010), but genetic abnormalities, such as Turner mosaicism, can also contribute to a high rate of failed oocyte maturation (Moore et al., 2008). Accordingly, for some patients, failure in oocyte maturation creates severe infertility, while for most other patients failed maturation limits the numbers of oocytes available for ART. Thus, maturation defects contribute to the overall occurrence of unexplained infertility for patients with severe compromise, but may also reduce fecundity and assisted reproduction efficiencies in some other cases.
A better understanding of the molecular mechanisms and genetic factors that underlie normal and defective oocyte maturation could enhance early diagnosis and thereby prevent unproductive attempts to use routine ART methods to achieve pregnancy. Additionally, knowledge of the physiological or endocrine factors that predispose a fraction of the oocytes to be retrieved without successful maturation could indicate procedural modifications to reduce occurrence during ART. Multiple mechanisms can be postulated to inhibit oocyte maturation. For example, deficiencies in oocyte-intrinsic factors could be responsible. Alternatively, a deficiency in communication with supporting cumulus cells could compromise maturation ability. Finally, maternal health or physiology, or environmental factors could interfere with essential processes. And while specific factors essential for oocyte maturation have been identified, those factors may not be pertinent to oocyte maturation failure following an ovulatory stimulus. Moreover, it has not been determined to what degree failed-to-mature (FTM) oocytes progress down the pathway to maturation: essential information for devising appropriate experimental hypotheses for testing.
Oocytes that fail to undergo GV breakdown and/or meiotic progression following an ovulatory stimulus could acquire some of the gene expression features of oocytes that successfully mature, could remain like normal GV stage oocytes, could take on unusual characteristics unlike either stage, or could display a combination of these characteristics. A detailed transcriptome analysis may discriminate between these possibilities, and reveal specific target molecules and processes for mechanistic studies, enabling discovery of the causal mechanisms leading to oocyte maturation failure. Additionally, transcriptome analysis of FTM oocytes could uncover novel genetic markers useful for diagnosing potential genetic barriers to normal oocyte maturation that could guide patient care. Finally, use of an experimentally tractable animal model that closely resembles humans in such analyses would allow such mechanistic studies to be undertaken that cannot be pursued in humans. The rhesus monkey (Macaca mulatta) is ideal for this because it demonstrates highly similar reproductive physiology (length of menstrual cycle, ovulation time, monovulatory, corpus luteum formation) and other characteristics similar to humans (Schramm and Bavister, 1999; Chaffin and Vandevoort, 2013). Experimental procedures in the monkey closely resemble clinical procedures in humans. There is also a high degree of genome similarity, and the rhesus monkey genome is well characterized.
We undertook a detailed transcriptome analysis of rhesus monkey FTM oocytes retrieved following ovulatory stimulation for standard IVF cycles. This is the largest study of its kind yet performed in this species. The results revealed numerous abnormalities in the FTM oocyte transcriptome. In addition, we show that although FTM oocytes undergo much of the normal maturation process, they manifest inadequate transcriptional regulation of mitochondrial functions, among other defects in biological processes and signaling pathways. The significance of these results to understanding and possibly preventing oocyte maturation failure, as well as improving the development of biomarkers for oocyte quality, is discussed.
Materials and Methods
Ovarian stimulation, oocyte retrieval and oocyte lysis
Female adult rhesus macaques were housed at the California National Primate Center, as described (VandeVoort et al., 2015). All experimental procedures for collecting oocytes and the handling of animals were conducted in accordance with the ethics guidelines established and approved by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California-Davis, CA, USA. To ensure that the study captured differences between normal and abnormal oocyte maturation response following an ovulatory stimulus with an otherwise healthy endocrine profile (avoiding potential confounding endocrine effects) and eliminating age of females as a confounding variable, we used optimal breeding age (6–12 years old) females with normal menstrual cycles and a history of successful pregnancy.
We used a controlled ovarian stimulation protocol for the collection of oocytes. Rhesus macaques were observed daily for signs of menses. Within Day 1–4 of menses, all animals (n = 12) were administered an i.m. injection of recombinant human FSH (rhFSH; La Jolla Discount Pharmacy, La Jolla, CA, USA), 37.5 IU twice daily for a total of 7 days. On Day 8, a subset of animals (n = 2) was used for collection of GV oocytes and another subset of animals (n = 10) was administrated an i.m. injection of 1000 IU hCG (La Jolla Discount Pharmacy, La Jolla, CA, USA). On the day following the last injection, we used ultrasound-guided needle aspiration of follicles for the collection of oocytes according to established procedures (VandeVoort et al., 2015). Oocytes and any attached cumulus cells were placed into drops of hamster embryo culture medium 9 (HECM-9) under oil (VandeVoort et al., 2011) and incubated at 5% CO2 for 4–6 h prior to observation for maturation status for both normal GV and MII, and FTM oocytes. FTM oocytes retrieved after hCG either had an expanded cumulus and may or may not have undergone GV break down (GVBD) but no polar body was evident, or they displayed a tight ring of cumulus cells around them at recovery and had undergone GVBD. Although FTM oocytes were at various stages of meiotic progression, they all were meiotically incompetent, having failed to respond appropriately to an ovulatory stimulus in vivo. Analysis of these oocytes will thus inform about common transcriptome features associated with meiotic incompetence. Any oocytes from animals treated with hCG that had tight cumulus and intact GV were not used because they might have been inadvertently aspirated from secondary or small antral follicles while collecting the cohort from large follicles. Regardless of stage of maturation, all cumulus cells were removed by repeatedly pipetting oocytes with a narrow-gauge pipette that was just slightly larger than the oocytes, while observing oocytes with a stereomicroscope. In total, we collected seven samples (one oocyte each) of GV stage oocytes from two females, 11 samples (two to three oocytes each) of FTM oocytes from five females, and seven samples (two oocytes each) of MII stage oocytes from five females for RNA-sequencing (RNAseq) analyses. Between one and three oocytes were lysed for each sample in 20 μl of Pico Pure lysis buffer and heat treated at 42°C for 30 min prior to storage in −80°C.
Preparation and sequencing of libraries for RNAseq
All samples were processed for RNA extraction and RNAseq analysis. Using the PicoPureTM RNA Extraction kit (Life Technologies, Carlsbad, CA, USA), total RNA was isolated from each sample following the manufacturer protocol, including a DNAse digestion (RNase-Free DNase Set; Qiagen, Hilden, Germany) to remove any contaminating DNA. The production of cDNA libraries for sequencing began with processing 100 ng of each RNA sample with the Ovation RNA-Seq System v2 using Ribo-SPIATM Technology (NuGen, San Carlos, CA, USA). After this initial processing, a Covaris-2 sonicator was used to mechanically fragment the cDNA to an average of 300 bp. This was then followed by a brief S1 nuclease digestion, as previously described (Head et al., 2011). After bead purification, the cDNA was processed through the Ovation Ultralow DR Multiplex Systems 1-16 (NuGen) for end repair, adapter ligation and final library amplification.
Using an Illumina HiSeq 4000 (Illumina, San Diego, CA, USA), barcoded libraries were pooled and sequenced in rapid run mode to generate 50 nucleotide unpaired end reads. Samples were loaded at 65% of optimal loading concentration, to enhance the effectiveness of the Illumina cluster identification algorithm, along with 10% of optimal loading concentration of PhiX DNA Control library (adapter-ligated library obtained from randomly sheared PhiX DNA; Illumina, San Diego, CA, USA) to increase initial read sequence complexity. The total numbers of PF (passed-filter) reads ranged from 18 M to 78 M (Supplementary Table S1). The fraction of Q30 bases ranged from 91.6% to 96.4% and average Q from 35.8 to 39.2 (Supplementary Table S1). All sequencing data will be made available at Gene Expression Omnibus (GSE112536: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE112536).
RNAseq data analysis and ingenuity pathway analysis
Reads were aligned using Hisat2 (Kim et al., 2015) to the Ensembl rhesus monkey genome (Mmul build v8.1.0, genome annotation relase-91) (Zimin et al., 2014; Aken et al., 2017). Mapped exonic read counts were generated utilizing featureCounts (Liao et al., 2014). Reads aligning to ribosomal RNA (rRNA) or rRNA like genes were excluded. Successfully aligned non-rRNA exonic reads ranged from 6.8 M to 28.8 M. Differential expression calculations were conducted within the DESeq2 software package (Love et al., 2014). All possible pairwise differential comparisons between the three groups were conducted: FTM/GV, FTM/MII and MII/GV. Genes with a q-value (P-value adjusted for multiple comparisons with a Benjamini-Hochberg correction) below false discovery rate of 0.05 were considered significant and classified to be differentially expressed genes (DEGs). FTM maturation groups (Fig. 1) and the included genes were defined by filtering and comparing the main differential comparisons. Using DESeq2, gene expression levels were converted from raw read counts to normalized FPKM (fragment counts normalized per kilobase of feature length per million mapped fragments) (Trapnell et al., 2010).
Figure 1.
Schematic figure showing transitions of mRNA expression of the failed to mature oocytes in comparison to normal maturation in the rhesus monkey (Categories A–G). Differentially expressed genes (DEGs) identified by Cuffdiff analysis and defined as those with a q-value (false discovery rate) <0.05 are shown. The normal maturation (NM) category represents the mRNAs that are differentially expressed between germinal vesicle (GV) and metaphase II (MII) oocytes. Category A represents mRNAs that changed during normal maturation, being differentially expressed between GV and MII oocytes (i.e. in NM group) and between GV and failed to mature (FTM), but not between MII and FTM oocytes. Category B represents mRNAs that changed partially in FTM oocytes, reaching an abundance between and significantly different from GV and MII oocytes. Category C represents mRNAs that failed to be modulated, being differentially expressed between MII and GV oocytes, but not acquiring a statistical difference in FTM oocytes compared to GV oocytes. Category D represents mRNAs that displayed excessive modulation in FTM oocytes beyond the change seen in NM. Category E represents mRNAs that are oppositely regulated in FTM compared to MII relative to initial GV level of expression. Categories F (increased expression) and G (decreased expression) represent mRNAs displaying significant differences between FTM and both GV and MII, but not in NM.
For each comparison, QIAGEN Ingenuity Pathway Analysis® (IPA; Hilden, Germany) evaluated the resultant differential expression data with the analysis tools including: Canonical Pathway (CP), Disease and Functions (DF) and Upstream Regulator (UR) analyses. For each of the analyses, IPA outputs a P-value detailing the level of enrichment significance between the uploaded DEG list as compared to the number of known molecules in the database for a given analysis entry (CP/UR/DF). Those entries with a P < 0.05 were considered statistically significant. Additionally, a directionality in regulation (activation/increase or inhibition/decrease) is calculated and presented as a z-score, where |z|>1.96 is considered significant. Within DF analysis we created a truncated list of biological functions (BFs) by deselecting all entries containing disease notations from the IPA analysis for our comparisons.
Results
The goal of this study was to better understand oocyte maturation failure by identifying affected biological pathways and processes in primate oocytes that fail to mature following an ovulatory stimulus. Our approach was to use whole transcriptome RNAseq analysis to compare normal oocyte maturation to failed maturation, and thereby determine the extent to which FTM oocytes either completed or failed to complete changes associated with normal oocyte maturation, or deviated from that path (Fig. 1). We obtained a high quality RNAseq dataset consisting of n = 7, n = 7 and n = 11 libraries for normal GV, normal MII and FTM oocytes, respectively. Total reads acquired were an average of 34 million for each GV samples, 41 million for each FTM sample and 59 million for each MII oocyte sample. Approximately 44% of the total reads were exonic reads that successfully aligned to the rhesus monkey genome as unique non-rRNA gene transcript sequences, providing very high depth of coverage (Supplementary Table S1).
Genes, functions and pathways comprising the normal maturational progression in mRNA expression during rhesus monkey oocyte maturation following an ovulatory stimulus
To assess the degree to which FTM oocytes progress down the normal path of oocyte maturation it is necessary first to have a thorough understanding of the transcriptome changes that accompany normal maturation (NM). Comparison of GV and MII stage oocyte transcriptomes revealed that ~12% of the transcriptome (1503 of 12 222 total mRNAs quantified) was significantly modulated during maturation, with 553 mRNAs displaying apparent increases in abundance (likely the result of either mRNA polyadenylation or greater stability relative to overall mRNA population stability) and 950 displaying decreases in abundance, indicative of degradation (category NM, Fig. 1; Supplementary Table S2). To elucidate the major biological pathways and processes affected by this transition, we performed IPA analysis (Supplementary Tables S3-S5). The top activated BF categories with z-scores >1.96 were related to cell death and necrosis, and the top inhibited BFs (z ≤ −1.96) were related to cell cycle progression, cycling of centrosome, cell proliferation, and duplication of centrioles. A high z-score near the threshold of significance (z = 1.70) was seen for meiosis (Table I). Additional BFs related to DNA repair, ubiquitination, apoptosis and cell movement and differentiation were significantly affected (P < 0.05) but lacked significant z-scores. Top affected CPs were related to protein ubiquitination, oxidative phosphorylation, multiple signaling pathways, nucleotide excision repair and mitochondrial function (Table I and Supplementary Table S4).
Table I.
Canonical pathways, biological functions and upstream regulators that are altered in rhesus monkey oocytes that failed to mature after an ovulatory stimulus compared to normal maturation.a
| −log(P-value)b | CPs | Z-scorec |
|---|---|---|
| Top Z-Scores | ||
| 1.41 | Cardiac Hypertrophy Signaling | −2.18 |
| 2.97 | Glioblastoma Multiforme Signaling | −2.00 |
| 1.38 | Gα12/13 Signaling | −1.90 |
| 1.87 | Ovarian Cancer Signaling | −1.67 |
| 1.48 | CCR3 Signaling in Eosinophils | −1.67 |
| 5.58 | EIF2 Signaling | −1.60 |
| 1.78 | Rac Signaling | −1.51 |
| 1.40 | P2Y Purigenic Receptor Signaling Pathway | −1.51 |
| 1.91 | Endothelin-1 Signaling | −1.50 |
| Selected CPs | ||
| 7.38 | Protein Ubiquitination Pathway | |
| 6.41 | Oxidative Phosphorylation | |
| 4.56 | Regulation of eIF4 and p70S6K Signaling | −0.82 |
| 4.51 | Mitochondrial Dysfunction | |
| 3.88 | Nucleotide Excision Repair Pathway | |
| 3.87 | GADD45 Signaling | |
| 3.67 | Insulin Receptor Signaling | −1.00 |
| 3.45 | Sirtuin Signaling Pathway | 1.40 |
| 3.44 | Adipogenesis pathway | |
| 3.02 | Integrin Signaling | −0.89 |
| 2.39 | JAK/Stat Signaling | −1.27 |
| 1.77 | IL-8 Signaling | −1.29 |
| 1.75 | VEGF Family Ligand-Receptor Interactions | −1.41 |
| −log(P-value) | BFs | Z-score |
| Top Z-Scores | ||
| 3.59 | Cycling of centrosome | −2.55 |
| 2.26 | Development of muscle cell lines | 2.45 |
| 4.54 | Cell proliferation of tumor cell lines | −2.42 |
| 4.17 | Duplication of centriole | −2.42 |
| 4.46 | Cell death of cervical cancer cell lines | 2.26 |
| 3.75 | Cell proliferation of breast cancer cell lines | −2.11 |
| 4.30 | Cell cycle progression of tumor cell lines | −2.02 |
| 3.16 | Contraction of uterus | −1.95 |
| 3.12 | Apoptosis of cervical cancer cell lines | 1.94 |
| 2.90 | Cell viability of cervical cancer cell lines | −1.87 |
| 3.02 | Cell proliferation of kidney cell lines | −1.77 |
| 3.06 | Meiosis of oocytes | 1.70 |
| 5.08 | Cell cycle progression | −1.68 |
| 2.58 | Cell death of fibroblast cell lines | 1.64 |
| 2.36 | Dilation of heart ventricle | 1.63 |
| 2.32 | Quantity of ovarian follicle | −1.62 |
| 3.14 | Phosphorylation of phosphatidic acid | −1.56 |
| 2.85 | Cell movement of cervical cancer cell lines | 1.53 |
| 2.24 | Repair of DNA | −1.50 |
| Selected BFs | ||
| 8.44 | Cell death | 0.31 |
| 6.82 | Apoptosis | −0.10 |
| 4.34 | Transactivation of RNA | 0.55 |
| 3.67 | Meiosis of germ cells | 0.37 |
| 2.86 | Organization of cytoplasm | 0.73 |
| 2.75 | Organization of cytoskeleton | 0.73 |
| 2.66 | Leukocyte migration | 0.52 |
| 2.56 | Microtubule dynamics | 0.76 |
| 2.52 | Mitosis | −0.76 |
| 2.28 | Formation of cytoskeleton | 0.22 |
| −log(P-value) | URsd | Z-score |
| Top Z-Scores | ||
| 7.49 | RICTOR | 4.56 |
| 1.48 | INSR | −3.05 |
| 2.08 | KDM5A | 3.05 |
| 2.97 | PPARGC1A | −2.88 |
| 1.75 | CD3 | 2.73 |
| 4.11 | Guanidinopropionic acid | −2.51 |
| 2.97 | GnRH analog | 2.41 |
| 1.50 | CAT | −2.41 |
| 4.71 | TRAP1 | 2.35 |
| 2.63 | CD247 | −2.33 |
| 5.71 | Beta-estradiol | −2.24 |
| 1.69 | mir-30 | −2.22 |
| 2.11 | PLA2R1 | −2.22 |
| 3.05 | miR-30c-5p (and other miRNAs w/seed GUAAACA) | 2.14 |
| 1.85 | NFE2L1 | −2.00 |
| 1.82 | FUT8 | 2.00 |
| 1.55 | HEXIM1 | 1.98 |
| 1.50 | AMH | 1.98 |
| 2.31 | PPP3CA | 1.96 |
| Selected URs | ||
| 10.84 | HNF4A | 1.06 |
| 6.25 | MYC | −1.87 |
| 4.86 | NUPR1 | 0.89 |
| 3.78 | TP53 | 0.75 |
| 3.56 | E2F1 | 0.99 |
| 3.33 | ESR1 | −1.72 |
| 3.33 | ERBB2 | −0.19 |
| 2.57 | ↓CDK4 | |
| 2.40 | Progesterone | 1.93 |
| 2.37 | CREB1 | 0.05 |
| 2.36 | NR3C1 | −1.09 |
| 1.99 | ↓CTNNB1 | −0.14 |
| 1.79 | HTT | |
| 1.71 | APP | −0.13 |
| 1.54 | IL4 | 0.76 |
| 1.46 | IL6 | 1.52 |
| 1.36 | TGFB1 | −0.21 |
| 1.32 | TNF | 0.27 |
aCanonical pathways (CP), biological functions (BF) and upstream regulators (UR) included here were selected for having a z-score and/or a number of differentially expressed genes (DEG) affected within each entry. Results are identified by Ingenuity Pathway Analysis® (IPA) from the metaphase II/germinal vesicle (MII/GV) comparison.
b−log(P-value): The P-value, statistical overlap of DEG list and gene set for CP, BF and UR with applied ‘-log10’ transform. Only values -log10(P)≥1.30 (equivalent to P ≤ 0.05) are shown in the table.
cZ-score: z > 1.96 to be significantly activated or increased, and those with z< −1.96 to be significantly inhibited.
dUR as defined from IPA. ↑ or ↓ preceding UR indicates that UR itself is significantly different between GV and MII.
Upstream regulator analysis revealed massive changes in cellular regulation consistent with the large number of affected mRNAs, with >700 URs showing significant effects (P < 0.05). This included 18 URs with downstream pathways predicted to have increased activity and 15 URs predicted to have decreased activity (z-scores ± 1.96 or more). Prominent affected URs included rapamycin-insensitive companion of MTOR (RICTOR), lysine demethylase 5 A (KDM5A), insulin receptor (INSR) and peroxisome proliferator-activated receptor, gamma, coactivator 1 alpha (PPARGC1A). Twenty-five URs related to cell cycle, transcription, mitochondrial function, endoplasmic reticulum stress and antioxidant capacity were themselves differentially expressed at the mRNA level and showed significant changes in activity (P < 0.05) but not significant z-scores (Supplementary Table S5).
Overall comparison of FTM oocytes to normal GV and MII stage oocytes
FTM oocytes displayed 1413 mRNAs significantly different from normal GV oocytes and 293 mRNAs differing from normal MII oocytes (Supplementary Tables S6–S7). The lesser divergence from the MII pattern indicates that the transcriptome profile of FTM oocytes resembled the MII profile more than the GV profile. Principal component analyses (Fig. 2A) and frequency distribution curves of DEGs (Fig. 2B–D) confirmed this, and further revealed that the overall variance in mRNA expression pattern was no greater among FTM oocytes than among GV or MII stage oocytes.
Figure 2.
Principle component analyses and frequency distribution graphs were used to visualize the differences between FTM, GV and MII oocytes. A. Principle component (PC) analysis plots show sample relationships between GV, FTM and MII samples. There is a clear delineation between GV and MII oocytes with FTM falling in between the two. B–D. Frequency distributions of the log two-fold changes between comparisons are indicated. Note that the overall variance in mRNA expression patterns was no greater among FTM oocytes than GV or MII stage oocytes. B = GV to FTM; C = FTM to MII; D = GV to MII. All graphs were created in R package ggplot2.
To determine what aspects of NM were correctly executed, partially executed, or lacking in FTM oocytes, we classified the 1503 GV-MII DEGs into categories A–E based on their expression in FTM oocytes compared to GV and MII oocytes (Figs 1, 3). We also determined what mRNAs underwent aberrant modulation in FTM oocytes (categories F & G). IPA analysis of these categories was then used to identify biological pathways and processes associated with these different categories in FTM oocytes.
Figure 3.
Distribution of mRNA expression values for FTM oocytes amongst categories A–E in comparison to NM. For each category, the total number of genes and percentage of the NM gene set are shown. Category NM includes mRNAs that are differentially expressed between GV and MII oocytes. Category A includes mRNAs that are differentially expressed between GV and MII and between GV and FTM, but not between MII and FTM. Category B includes NM mRNAs that are modulated in abundance during NM but only partially modulated in FTM oocytes (FTM different from both GV and MII stage oocytes). Category C includes mRNAs that failed to be modulated in abundance in FTM oocytes. Categories D and E include mRNAs that displayed excess and opposite regulation in abundance, respectively, compared to NM.
Genes, functions and pathways with normal progression in FTM oocytes
We observed 704 mRNAs that underwent normal maturation-associated changes in abundance in the FTM oocytes (Category A, Fig. 1; Supplementary Table S8). IPA analysis of these genes revealed BFs and CPs that were modulated correctly in FTM oocytes, including two signaling pathways with significant activation (ataxia-telangiectasia mutated (ATM), Cell Cycle: G2/M DNA Damage Checkpoint Regulation Signaling) and one with significant inhibition (Cyclins and Cell Cycle Regulation), and known to play important roles in oocyte maturation (|z|-score > 1.96). Additional signaling pathways for category A mRNAs were related to inflammation, metabolism and multiple signaling pathways with known roles in oocytes (Table II; P < 0.05). BFs associated with category A mRNAs included cell movement, cell death, cycling of centrosome, cell cycle progression, repair of DNA, cell trafficking and microtubule dynamics. Overall, 55 of the 107 CPs and 132 of 330 BFs associated with NM were modulated correctly in FTM oocytes (Table I, Supplementary Tables S9, S10). Similarly, 348 of the 731 URs significantly associated with NM (48%) were associated with category A genes (Table I; Supplementary Table S11). Eight of 26 URs with significant z-scores returned by analysis of the category A genes also had significant z-scores in the same direction as seen for NM. Collectively, the IPA analysis of BFs, CPs and URs for category A genes indicates considerable overlap with results from the NM set.
Table II.
Selected IPA® CPs, BFs and URs affected in category Aa
| −log(P-value)b | CPs | Z-scorec |
|---|---|---|
| Top Z-scores | ||
| 2.21 | ATM Signaling | 2.45 |
| 1.63 | Cell Cycle: G2/M DNA Damage Checkpoint Regulation | 2.00 |
| 1.49 | Cyclins and Cell Cycle Regulation | −2.00 |
| 1.53 | Production of Nitric Oxide and Reactive Oxygen Species in Macrophages | 1.67 |
| 2.99 | Glioblastoma Multiforme Signaling | −1.51 |
| Selected CPs | ||
| 5.49 | Insulin Receptor Signaling | −0.28 |
| 5.01 | EIF2 Signaling | −0.71 |
| 4.93 | mTOR Signaling | 0.38 |
| 4.29 | Regulation of eIF4 and p70S6K Signaling | |
| 2.78 | Protein Kinase A Signaling | 0.00 |
| 2.75 | ERK/MAPK Signaling | 0.58 |
| 2.42 | Integrin Signaling | −0.30 |
| 2.15 | PPARα/RXRα Activation | 0.45 |
| 1.99 | Adipogenesis pathway | |
| 1.89 | Actin Cytoskeleton Signaling | 0.63 |
| 1.75 | p38 MAPK Signaling | −0.82 |
| 1.70 | Sumoylation Pathway | −0.82 |
| −log(P-value) | BFs | Z-score |
| Top Z-scores | ||
| 1.81 | Proliferation of hematopoietic cells | −2.88 |
| 2.05 | Development of bone marrow | 2.62 |
| 2.14 | Mitosis | −2.55 |
| 3.19 | Development of muscle cell lines | 2.24 |
| 2.14 | Microtubule dynamics | 2.20 |
| 2.36 | Cell death of connective tissue cells | −2.19 |
| 3.12 | Transcription | −2.14 |
| 2.08 | Trafficking of cells | 2.08 |
| 2.20 | Relaxation of muscle | −2.00 |
| 1.86 | Cell viability of hepatoma cell lines | 1.99 |
| 1.81 | Checkpoint control | −1.98 |
| 1.66 | Quantity of nucleus | 1.98 |
| 2.23 | Binding of bone marrow cell lines | 1.97 |
| 2.80 | Transcription of RNA | −1.96 |
| 2.16 | Expression of RNA | −1.85 |
| 2.75 | Organization of cytoplasm | 1.85 |
| 2.57 | Organization of cytoskeleton | 1.85 |
| 2.85 | Cell movement of leukocytes | 1.77 |
| 6.13 | Migration of cells | 1.51 |
| 2.47 | Cell cycle progression | −1.48 |
| Selected BFs | ||
| 6.44 | Cell movement | 1.21 |
| 6.30 | Cell death | −0.86 |
| 5.75 | Apoptosis | −1.00 |
| 4.37 | Ejection of first polar body | |
| 3.63 | Meiosis of oocytes | |
| 3.39 | Cell viability | 1.31 |
| 2.82 | Arrest in mitosis | |
| 2.79 | Quantity of cells | −0.02 |
| 2.79 | Morphology of reproductive system | |
| 2.46 | Concentration of lipid | −0.55 |
| 1.88 | Arrest in proliferation of cells | |
| −log(P-value) | URsd | Z-score |
| Top Z-scores | ||
| 1.76 | mir-21 | 2.71 |
| 2.33 | Hdac | −2.65 |
| 2.71 | TBX2 | −2.59 |
| 1.95 | hydrogen peroxide | 2.26 |
| 1.68 | miR-199a-5p (and other miRNAs w/seed CCAGUGU) | −2.20 |
| 1.53 | MTOR | −2.16 |
| 1.91 | CAV1 | 2.13 |
| 2.10 | GATA6 | 2.02 |
| 1.82 | KMT2A | −2.00 |
| 1.37 | HMGB1 | 2.00 |
| 2.61 | RBL2 | 1.98 |
| 1.77 | MAX | −1.98 |
| 2.11 | mir-30 | −1.98 |
| 1.39 | ELK1 | 1.97 |
| 1.37 | SMAD4 | 1.88 |
| 1.65 | BMP6 | 1.81 |
| 1.62 | FOXM1 | −1.79 |
| 2.20 | AMPK | 1.73 |
| 1.31 | HIF1A | 1.73 |
| 5.25 | ESR1 | −1.68 |
| 2.23 | FOXO1 | 1.64 |
| 1.82 | SMAD2 | 1.58 |
| 6.62 | HNF4A | 1.48 |
| Selected URs | ||
| 4.71 | MYC | −0.49 |
| 3.92 | CCND1 | −1.13 |
| 3.46 | KDM5B | 0.95 |
| 3.23 | ↑CTBP1 | |
| 2.69 | HDAC5 | −0.56 |
| 2.52 | ERBB2 | −1.05 |
| 2.43 | NUPR1 | 0.43 |
| 1.95 | ↓CTNNB1 | 0.76 |
| 1.89 | Histone h3 | |
| 1.69 | HRAS | −0.56 |
| 1.66 | TGFB1 | 0.20 |
| 1.62 | PPARG | −0.42 |
aCategory A is defined as those genes differentially expressed between GV and MII and between GV and GVII, but having no statistically significant difference between FTM versus MII. Results are identified from the FTM/GV comparison.
b−log(P-value): The P-value, statistical overlap of DEG list and gene set for CPs, BFs and URs with applied ‘-log10’ transform. A -log(P-value) of 1.3 or higher is consider statistically significant at a P < 0.05.
c Z-score: z > 1.96 to be significantly activated or increased, and those with z <−1.96 to be significantly inhibited or decreased.
dURs as defined from IPA. ↑ or ↓ preceding UR indicates that UR itself is significantly different between FTM and GV.
Genes, functions and pathways displaying partial progression in FTM oocytes
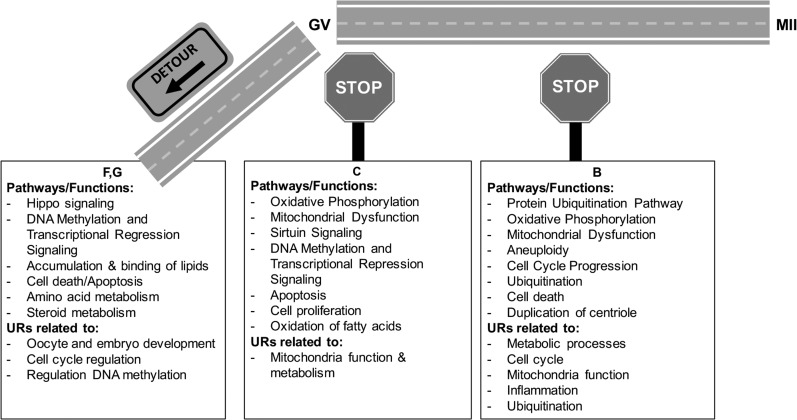
There were 658 category B mRNAs displaying partial modulations in abundance in FTM oocytes (Fig. 1, Table III, Supplementary Table S12), representing 42% of the mRNAs undergoing modulation during NM. IPA analyses identified 229 BFs associated with these partially modulated genes (Supplementary Table S13), including aneuploidy and cell death of fibroblast cell lines, duplication of centriole, ubiquitination, cell cycle progression and DNA fragmentation (Table IV). Eighty-five of category B significant BFs were shared with the list of 264 category NM gene BFs (Supplementary Table S3). These effects on BFs were accompanied by significant associations with CPs important for regulation of oocyte maturation and cell proliferation (Table IV; Supplementary Table S14). Other affected CPs associated with category B mRNAs included protein ubiquitination, nuclear factor, erythroid 2 like 2 (NRF2)-mediated oxidative stress response, oxidative phosphorylation, mitochondrial dysfunction, pentose phosphate pathway and inflammatory related signaling, which were also associated with NM (Supplementary Table S4). Upstream regulator analysis revealed over 350 affected URs associated with category B genes (Supplementary Table S15, P < 0.05). These URs were related to metabolic processes, mitochondria function, inflammation, cell proliferation, cell cycle and ubiquitination, consistent with some of the effects we identified in both CP and BF analyses (Table IV; Fig. 4). All the URs related to the above processes except cyclin dependent kinase inhibitor 2 A (CDKN2A) were associated with NM (Supplementary Table S5). These IPA results highlight aspects of NM changes that are likely compromised in FTM oocytes, including mitochondrial function and oxidative phosphorylation, protein metabolism (ubiquitination), pentose phosphate metabolism, DNA methylation and cell cycle (Fig. 4).
Table III.
Selected DEGs in Category Ba
| FPKMb | FTM/GV | FTM/MII | MII/GV | |||
|---|---|---|---|---|---|---|
| Gene Symbol | GV | FTM | MII | L2FCc | L2FCd | L2FCe |
| PTGS2 | 0.00 | 0.34 | 2.95 | 7.18 | −3.12 | 10.30 |
| RHOU | 0.08 | 0.50 | 3.82 | 2.64 | −2.92 | 5.57 |
| HIST1H2AJ | 3.98 | 1.66 | 0.32 | −1.30 | 2.36 | −3.66 |
| CYP19A1 | 80.78 | 28.82 | 5.69 | −1.49 | 2.34 | −3.83 |
| TIMP1 | 2.50 | 14.46 | 70.58 | 2.53 | −2.29 | 4.81 |
| HIST3H2BB | 111.42 | 45.45 | 13.01 | −1.29 | 1.81 | −3.10 |
| IGFBP5 | 5.57 | 12.74 | 38.15 | 1.19 | −1.58 | 2.77 |
| AASDH | 5.72 | 1.70 | 0.62 | −1.75 | 1.46 | −3.21 |
| CYC1 | 3.58 | 2.06 | 0.75 | −0.80 | 1.45 | −2.26 |
| GSTM3 | 273.40 | 126.77 | 46.48 | −1.11 | 1.45 | −2.56 |
| PAM16 | 13.43 | 6.40 | 2.78 | −1.07 | 1.20 | −2.27 |
| UFD1 | 5.02 | 2.40 | 1.04 | −1.07 | 1.20 | −2.27 |
| LRP5 | 1.10 | 3.05 | 6.98 | 1.47 | −1.20 | 2.67 |
| FOXM1 | 0.14 | 0.83 | 1.72 | 2.52 | −1.06 | 3.58 |
| NDUFA4 | 150.97 | 80.76 | 43.51 | −0.90 | 0.89 | −1.80 |
| COX7 C | 9.09 | 3.91 | 2.09 | −1.25 | 0.89 | −2.14 |
| CENPW | 28.32 | 16.84 | 9.55 | −0.75 | 0.82 | −1.57 |
| DSN1 | 67.64 | 41.51 | 23.88 | −0.70 | 0.80 | −1.50 |
| ZAR1L | 81.85 | 58.80 | 35.81 | −0.48 | 0.72 | −1.19 |
| ABCF2 | 29.67 | 16.27 | 9.99 | −0.87 | 0.70 | −1.57 |
| COX5B | 52.50 | 29.77 | 18.83 | −0.82 | 0.66 | −1.48 |
| PDCD5 | 1011.54 | 732.38 | 473.38 | −0.47 | 0.63 | −1.10 |
| SOD1 | 938.89 | 700.25 | 464.63 | −0.42 | 0.59 | −1.01 |
| BMP3 | 0.08 | 0.68 | 0.95 | 3.15 | −0.48 | 3.63 |
| CCAR2 | 0.94 | 2.48 | 3.46 | 1.41 | −0.48 | 1.89 |
| NSUN2 | 7.20 | 9.38 | 12.98 | 0.38 | −0.47 | 0.85 |
| ZFX | 7.74 | 1.20 | 1.65 | 1.37 | −0.46 | 1.83 |
aCategory B is defined as those gene that are differentially expressed between GV vs MII, and the expression of FTM is in between GV and MII.
bFPKM: Fragments Per Kilobase of transcript per Million mapped reads.
cL2FC: Log fold change of base 2. Positive values indicate an increase in FTM versu. GV with a FDR q-value of <0.05.
dL2FC: Log fold change of base 2. Positive values indicate an increase in FTM versu. MII with a FDR q-value of <0.05.
eL2FC: Log fold change of base 2. Positive values indicate an increase in MII versus GV with a FDR q-value of <0.05.
Table IV.
Selected IPA® CPs, BFs and URs Affected in Category Ba
| −log(P-value)b | Selected CPs |
|---|---|
| 7.92 | Protein Ubiquitination Pathway |
| 4.00 | Hypoxia Signaling in the Cardiovascular System |
| 3.56 | Oxidative Phosphorylation |
| 2.72 | Mitochondrial Dysfunction |
| 2.23 | p53 Signaling |
| 2.08 | Leukocyte Extravasation Signaling |
| 2.01 | Role of NANOG in Mammalian Embryonic Stem Cell Pluripotency |
| 1.88 | NRF2-mediated Oxidative Stress Response |
| 1.86 | GM-CSF Signaling |
| 1.80 | Antiproliferative Role of Somatostatin Receptor 2 |
| 1.77 | Non-Small Cell Lung Cancer Signaling |
| 1.77 | Aldosterone Signaling in Epithelial Cells |
| 1.76 | Mouse Embryonic Stem Cell Pluripotency |
| 1.66 | Melanoma Signaling |
| 1.65 | JAK/Stat Signaling |
| 1.56 | Integrin Signaling |
| 1.55 | VEGF Family Ligand-Receptor Interactions |
| 1.49 | Endothelin-1 Signaling |
| 1.48 | Actin Cytoskeleton Signaling |
| 1.47 | CNTF Signaling |
| 1.41 | IL-8 Signaling |
| −log(P-value) | Selected BFs |
| 4.26 | Cell cycle progression of tumor cell lines |
| 3.92 | Cell cycle progression |
| 3.84 | Organismal death |
| 3.84 | Ubiquitination |
| 3.57 | Duplication of centriole |
| 3.44 | Migration of cervical cancer cell lines |
| 3.44 | Metabolism of DNA |
| 3.43 | Ubiquitination of protein |
| 3.27 | Cell death of connective tissue cells |
| 2.90 | Fragmentation of DNA |
| 2.68 | Cell proliferation of breast cancer cell lines |
| 2.55 | Arrest in interphase |
| 2.51 | Development of reproductive system |
| 2.44 | Cell death |
| 2.41 | Tubulogenesis |
| 2.14 | Degradation of extracellular matrix |
| 2.02 | Aneuploidy |
| 1.97 | Development of genitourinary system |
| 1.94 | Invasion of colorectal cancer cell lines |
| 1.77 | Ploidy of cells |
| 1.77 | Survival of organism |
| 1.77 | Cell death of fibroblast cell lines |
| −log(P-value) | Selected URsc |
| 4.04 | TP53 |
| 4.01 | RICTOR |
| 3.50 | AGT |
| 3.29 | Progesterone |
| 3.27 | HNF4A |
| 3.13 | FUT8 |
| 2.82 | PGR |
| 2.31 | MYC |
| 2.17 | EGFR |
| 2.14 | EGF |
| 2.09 | ↓SOD1 |
| 1.98 | CD3 |
| 1.91 | beta-estradiol |
| 1.80 | miR-145-5p (and other miRNAs w/seed UCCAGUU) |
| 1.79 | CDKN2A |
| 1.70 | AKT1 |
| 1.65 | PRL |
| 1.60 | LIF |
| 1.59 | RB1 |
| 1.58 | Ins1 |
| 1.49 | EGR1 |
| 1.49 | INS |
| 1.42 | MYD88 |
| 1.40 | APC |
| 1.35 | Esrra |
| 1.32 | KDM5A |
aCategory B is defined as those gene that are differentially expressed between GV and MII, and the expression of FTM is between GV and MII.
b−log(P-value): The P-value, statistical overlap of DEG list and gene set for canonical pathway, biological functions and UR with applied ‘−log10’ transform. A -log(P-value) of 1.3 or higher is considered statistically significant at a P < 0.05.
cUR as defined from IPA. ↑ or ↓ preceding UR indicates it is differentially expressed between FTM and GV, and itself is a member of category B.
Figure 4.
Summary of the top biological functions, pathways and upstream regulators aberrantly regulated in FTM, displaying (B) partial modulation, (C) failure to be modulated, and (F + G) altered expression in FTM oocytes but not during NM. All data came from IPA® canonical pathways, biological functions and upstream regulators (UR) analyses for these different gene categories (B, C and F + G).
Genes, functions and pathways failing to change in FTM oocytes
Category C contains 138 mRNAs that failed to change significantly in abundance in response to an ovulatory stimulus (Fig. 1, Supplementary Table S16). These mRNAs were expressed at abundances not significantly different from GV oocytes, indicating they were not mRNAs that failed to accumulate, but rather ones that failed to initiate the change in abundance that accompanies NM. A large majority of these mRNAs are involved in oxidative phosphorylation, mitochondrial function, DNA methylation and transcription (Table V). Some mRNAs (e.g. growth differentiation factor 9 (GDF9), RNA polymerase II subunit A (POLR2A), zygote arrest 1 (ZAR1) and a few mitochondrial related genes) appeared to be expressed outside of the normal range of GV or MII expression (Supplementary Table S16) but expression values were not significantly different from GV stage oocytes. IPA analysis revealed CPs for category C mRNAs that are important for oocyte function, including sirtuin signaling, oxidative phosphorylation, mitochondrial function, DNA repair, DNA methylation and transcription repression (Table VI, Supplementary Table S17). Some of the DEGs contained in these signaling pathways were among the most highly expressed within category C genes, with roles in mitochondrial function and metabolism (Table V). Affected BFs for category C mRNAs were related to apoptosis, cell proliferation, and fatty acid oxidation, were also associated with NM (Table VI, Supplementary Table S18, and Fig. 4). IPA analysis revealed 148 URs associated with category C genes, 31 of which were also associated with NM (Supplementary Table S19). These URs were also associated with downstream effectors related to mitochondrial function and metabolism (Table VI, Supplementary Table S19 column H). Thus, the failure to regulate category C mRNAs reinforces many of the deficiencies seen with category B mRNAs, such as effects on mitochondrial function, oxidative metabolism and DNA methylation.
Table V.
Selected DEGs in Category Ca
| FPKMb | FTM/MII | |||
|---|---|---|---|---|
| Gene Symbol | GV | FTM | MII | L2FCc |
| TMEM208 | 1.13 | 1.47 | 0.16 | 3.21 |
| SLC2A1 | 3.61 | 1.95 | 0.27 | 2.83 |
| NDUFA5 | 8.61 | 11.84 | 1.71 | 2.79 |
| C11H12orf57 | 4.53 | 3.31 | 0.57 | 2.52 |
| SIRPB2 | 3.36 | 3.24 | 0.57 | 2.52 |
| YJEFN3 | 1.91 | 2.09 | 0.36 | 2.52 |
| PRDX5 | 3.85 | 5.12 | 0.97 | 2.40 |
| NDUFA1 | 34.03 | 25.48 | 5.04 | 2.34 |
| COPS9 | 64.32 | 55.74 | 11.36 | 2.30 |
| PTRHD1 | 14.98 | 9.06 | 1.86 | 2.28 |
| BTNL8 | 9.82 | 5.70 | 1.24 | 2.20 |
| NDUFV1 | 2.76 | 3.01 | 0.67 | 2.17 |
| UQCRH | 8.44 | 5.27 | 1.22 | 2.11 |
| ACAA1 | 3.79 | 3.60 | 0.91 | 1.98 |
| NDUFS6 | 7.11 | 7.88 | 2.04 | 1.95 |
| COX6 C | 384.73 | 252.41 | 71.13 | 1.83 |
| ACTL8 | 335.56 | 295.46 | 1038.09 | −1.81 |
| NPM3 | 10.85 | 12.78 | 38.75 | −1.60 |
| SNRPF | 10.27 | 8.44 | 23.87 | −1.50 |
| SRI | 15.74 | 11.74 | 29.51 | −1.33 |
| BSCL2 | 19.94 | 17.41 | 7.12 | 1.29 |
| SAP30 | 13.85 | 14.52 | 33.57 | −1.21 |
| DNMT3B | 33.39 | 34.63 | 50.40 | −0.54 |
aCategory C is defined as those genes differentially expressed between MII/GV and FTM/MII, while there is no statistically significant difference between FTM/GV.
bFPKM: Fragments Per Kilobase of transcript per Million mapped reads.
cL2FC: Log fold change of base 2. Positive values indicate an increase in FTM versus MII with a FDR q-value of <0.05.
Table VI.
Selected IPA® CPs, BFs and URs affected in Category Ca
| −log(P-value)b | CPs |
|---|---|
| 11.30 | Oxidative Phosphorylation |
| 10.80 | Mitochondrial Dysfunction |
| 5.11 | Sirtuin Signaling Pathway |
| 3.98 | Adipogenesis pathway |
| 3.61 | Nucleotide Excision Repair Pathway |
| 3.15 | Assembly of RNA Polymerase II Complex |
| 2.21 | DNA Methylation and Transcriptional Repression Signaling |
| 2.00 | Estrogen Receptor Signaling |
| 1.92 | Androgen Signaling |
| 1.54 | RAR Activation |
| −log(P-value) | BFs |
|---|---|
| 2.53 | Apoptosis of stem cells |
| 2.15 | Cell proliferation of kidney cell lines |
| 1.73 | Oxidation of fatty acid |
| −log(P-value) | URs |
|---|---|
| 7.40 | RICTOR |
| 6.03 | guanidinopropionic acid |
| 3.95 | KDM5A |
| 3.46 | MAPT |
| 3.15 | AIFM1 |
| 2.94 | SURF1 |
| 2.63 | DOT1L |
| 2.58 | HNF4A |
| 2.57 | PSEN1 |
| 2.39 | HTT |
| 2.16 | 1,2-dithiol-3-thione |
| 2.09 | SNAI1 |
| 1.95 | ERK |
| 1.94 | PPARGC1A |
| 1.88 | RB1 |
| 1.84 | APP |
| 1.55 | CD3 |
| 1.54 | palmitic acid |
| 1.44 | CD28 |
aCategory C is defined as those genes differentially expressed between MII/GV and FTM/MII, while there is no statistically significant difference between GV and FTM (i.e. change during normal maturation but not in FTM).
b−log(P-value): The P-value, statistical overlap of DEG list and gene set for canonical pathway, biological functions and upstream regulators with applied ‘−log10’ transform. A -log(P-value) of 1.3 or higher is consider statistically significant at a P < 0.05.
Comparison of mitochondrial function gene expression between NM and FTM oocytes
Changes in expression of genes related to mitochondrial function for category B and C genes was a prominent feature of FTM oocytes. To explore this in more detail, we assessed effects on such genes as a group (Table VII, genes extracted from categories A to C). This group includes genes that are involved in multiple mitochondrial functions including oxidative phosphorylation, fatty acid oxidation and sirtuin signaling. Many of the mRNAs encoding mitochondrial proteins showed changes in expression similar to NM (category A), and consisted primarily of ones related to ATP binding, transport, and synthesis, and mitochondrial ribosomal proteins. However, many other mitochondrial mRNAs were abnormally elevated in FTM oocytes (categories B and C), encoding components of mitochondrial complexes I, II and III, such as cytochrome C oxidase subunits and nicotinamide adenine dinucleotide (NADH) ubiquinone oxidase subunits. Superoxide dismutase 1 (SOD1) mRNA was also elevated in FTM oocytes compared to MII oocytes.
Table VII.
Mitochondrial function related DEGs identified in the FTM oocytes from gene categories A, B and C in rhesus monkeys.
| FPKMa | FTM/GV | FTM/MII | |||||
|---|---|---|---|---|---|---|---|
| Gene expression category | Symbol | Gene name | GV | FTM | MII | L2FCb | L2FCc |
| A | ATP6V1A | ATPase H+ Transporting V1 Subunit A | 0.78 | 5.47 | 3.42 | 2.83 | 0.677 |
| A | TIMM10B | Translocase of Inner Mitochondrial Membrane 10B | 0.15 | 1.04 | 0.87 | 2.76 | 0.249 |
| A | MUL1 | Mitochondrial E3 Ubiquitin Protein Ligase 1 | 0.46 | 3.04 | 2.27 | 2.71 | 0.420 |
| A | RUVBL1 | RuvB Like AAA ATPase 1 | 0.65 | 3.04 | 2.90 | 2.21 | 0.068 |
| A | DARS2 | Aspartyl-tRNA Synthetase 2, Mitochondrial | 3.44 | 0.74 | 0.87 | −2.21 | −0.228 |
| A | MRPS18B | Mitochondrial Ribosomal Protein S18B | 18.27 | 5.69 | 5.10 | −1.67 | 0.161 |
| A | ND4 | NADH Dehydrogenase Subunit 4 | 16 565 | 19 123 | 18 027 | 1.63 | 0.085 |
| A | ATP5I | ATP Synthase, H+ Transporting, Mitochondrial Fo Complex Subunit E | 4.76 | 1.70 | 0.67 | −1.48 | 1.339 |
| A | ABCC10 | ATP Binding Cassette Subfamily C Member 10 | 4.03 | 10.74 | 10.49 | 1.41 | 0.035 |
| A | ATP6 | ATP Synthase F0 Subunit 6 | 7423 | 7961 | 7583 | 1.28 | 0.070 |
| A | COX3 | Cytochrome C Oxidase Subunit III | 12 715 | 11 173 | 9801 | 1.24 | 0.189 |
| A | ND2 | NADH Dehydrogenase Subunit 2 | 12 001 | 13 671 | 13 327 | 1.24 | 0.037 |
| A | COX7C | Cytochrome C Oxidase Subunit VIIc | 154.41 | 66.75 | 43.08 | −1.21 | 0.633 |
| A | MRPS22 | Mitochondrial Ribosomal Protein S22 | 66.69 | 32.94 | 32.47 | −1.02 | 0.021 |
| A | COA1 | Cytochrome C Oxidase Assembly Factor 1 Homolog (S. Cerevisiae) | 139.20 | 72.98 | 53.60 | −0.93 | 0.445 |
| A | ATP6V0A1 | ATPase H+ Transporting V0 Subunit A1 | 10.19 | 18.55 | 19.99 | 0.86 | −0.107 |
| A | WWOX | WW Domain Containing Oxidoreductase | 21.62 | 11.96 | 8.12 | −0.85 | 0.558 |
| A | ABCB1 | ATP Binding Cassette Subfamily B Member 1 | 24.35 | 13.58 | 10.62 | −0.84 | 0.354 |
| A | MRPS31 | Mitochondrial Ribosomal Protein S31 | 374 | 261 | 248 | −0.52 | 0.074 |
| B | LOC717879 | Cytochrome C Oxidase Subunit 7A2, Mitochondrial | 26.62 | 11.32 | 4.88 | −1.23 | 1.220 |
| B | LOC100423866 | Cytochrome C Oxidase Subunit 7C, Mitochondrial-like | 9.09 | 3.91 | 2.09 | −1.25 | 0.891 |
| B | NDUFA4 | NDUFA4, Mitochondrial Complex Associated | 150.97 | 80.76 | 43.51 | −0.90 | 0.893 |
| B | ABCF2 | ATP Binding Cassette Subfamily F Member 2 | 29.67 | 16.27 | 9.99 | −0.87 | 0.704 |
| B | LOC701661 | Cytochrome C Oxidase Subunit 5B, Mitochondrial | 52.50 | 29.77 | 18.83 | −0.82 | 0.662 |
| B | MTERF3 | Mitochondrial Transcription Termination Factor 3 | 31.42 | 22.70 | 19.92 | −0.47 | 0.189 |
| B | SOD1 | Superoxide Dismutase 1 | 939 | 700 | 465 | −0.42 | 0.592 |
| C | NDUFA5 | NADH:ubiquinone Oxidoreductase Subunit A5 | 8.61 | 11.84 | 1.71 | 0.46 | 2.791 |
| C | LOC710937 | NADH Dehydrogenase [ubiquinone] 1 Alpha Subcomplex Subunit 3 Pseudogene | 8.55 | 4.17 | 0.83 | −1.03 | 2.354 |
| C | NDUFA1 | NADH:ubiquinone Oxidoreductase Subunit A1 | 34.03 | 25.48 | 5.04 | −0.42 | 2.340 |
| C | NDUFV1 | NADH:ubiquinone Oxidoreductase Core Subunit V1 | 2.76 | 3.01 | 0.67 | −0.42 | 2.170 |
| C | UQCRH | Ubiquinol-cytochrome C Reductase Hinge Protein | 8.44 | 5.27 | 1.22 | −0.66 | 2.112 |
| C | NDUFS6 | NADH:ubiquinone Oxidoreductase Subunit S6 | 7.11 | 7.88 | 2.04 | 0.15 | 1.948 |
| C | LOC703685 | Cytochrome C Oxidase Subunit 6C | 385 | 252 | 71 | −0.61 | 1.828 |
| C | NDUFB2 | NADH:ubiquinone Oxidoreductase Subunit B2 | 146.92 | 82.95 | 26.26 | −0.82 | 1.660 |
| C | NDUFB6 | NADH:ubiquinone Oxidoreductase Subunit B6 | 23.99 | 27.09 | 9.29 | 0.18 | 1.545 |
| C | ATP5G1 | ATP Synthase, H+ Transporting, Mitochondrial Fo Complex Subunit C1 (subunit 9) | 56.95 | 40.76 | 18.49 | −0.48 | 1.141 |
| C | MRPL33 | Mitochondrial Ribosomal Protein L33 | 98.73 | 98.72 | 52.07 | −0.003 | 0.922 |
| C | COX4I1 | Cytochrome C Oxidase Subunit IV Isoform 1 | 40.54 | 31.61 | 19.23 | −0.36 | 0.718 |
aFPKM: Fragments Per Kilobase of transcript per Million mapped reads.
bL2FC: Log fold change of base 2. Positive values indicate an increase in FTM relative to GV.
cL2FC: Log fold change of base 2. Positive values indicate an increase in FTM relative to MII.
Aberrant changes in mRNA expression in FTM oocytes and associated functions and pathways (D, E, F, G)
Failure to mature was also accompanied by a small number of highly abnormal changes in mRNA expression. Category D mRNAs showed excessive change in the same direction as MII compared to GV oocytes, and category E genes were regulated opposite to their regulation during NM. Categories F and G are not normally modulated during maturation but are altered in FTM oocytes compared to GV oocytes. A total of just two annotated protein coding mRNAs (and one uncharacterized hypothetical protein coding mRNA) comprised categories D and E (Fig. 1, Supplementary Table S20). Forty-nine DEGs comprised categories F and G (Fig. 1, Supplementary Table S21). Of these 49 mRNAs, 12 showed increased abundances and 37 showed decreased abundances in FTMs relative to normal GV oocytes. Of the mRNAs identified within these aberrantly regulated gene category, notable mRNAs with increased expression in FTM oocytes relative to MII oocytes included large tumor suppressor kinase 1 (LATS1), desmoglein 2 (DSG2), and very low density lipoprotein receptor (VLDLR), and notable mRNAs with decreased expression in FTM oocytes included hydroxysteriod 11-beta dehydrogenase 2 (HSD11B2), transmembrane protein 138 (TMEM138), marker of proliferation Ki-67 (MKI67), DNA methyltransferase 1 (DNMT1), b-cell lymphoma 2 like 10 (BCL2L10), and retinoblastoma-binding protein 7 (RBBP7) (Table VIII). These genes are related to histone deacetylation, DNA methylation, genomic integrity and spindle formation, metabolism, and cell polarity, all of which could impact oocyte maturation.
Table VIII.
Selected DEGs in category D, E, F and Ga,b.
| FPKMc | FTM/GV | FTM/MII | |||
|---|---|---|---|---|---|
| Gene symbol | GV | FTM | MII | L2FCd | L2FCe |
| EPHA2 | 0.01 | 2.81 | 0.19 | 9.14 | 3.91 |
| MKI67 | 19.48 | 43.01 | 11.29 | 1.14 | 1.93 |
| NIPAL3 | 11.26 | 21.38 | 11.54 | 0.93 | 0.89 |
| RPF1 | 74.52 | 113.82 | 68.63 | 0.61 | 0.73 |
| MRPL39 | 0.61 | 2.03 | 0.57 | 1.71 | 1.82 |
| LATS1 | 0.68 | 2.80 | 0.68 | 2.04 | 2.05 |
| DERL1 | 29.21 | 26.26 | 17.53 | 0.52 | 0.58 |
| SLC41A2 | 5.35 | 10.14 | 5.70 | 0.92 | 0.83 |
| MKI67 | 26.41 | 57.98 | 16.24 | 1.13 | 1.84 |
| VLDLR | 46.78 | 84.99 | 52.56 | 0.86 | 0.69 |
| ZNF263 | 0.25 | 1.04 | 0.25 | 2.07 | 2.07 |
| DSG2 | 17.86 | 27.86 | 18.09 | 0.64 | 0.62 |
| HSD11B2 | 9.22 | 1.03 | 8.18 | −3.17 | −2.99 |
| KIAA1210 | 1.75 | 0.21 | 1.26 | −3.05 | −2.58 |
| TSEN15 | 6.12 | 1.74 | 4.33 | −1.81 | −1.31 |
| PKD1L3 | 12.43 | 5.97 | 14.24 | −1.06 | −1.25 |
| SLC36A1 | 17.09 | 6.45 | 14.72 | −1.41 | −1.19 |
| LMNB1 | 29.67 | 15.58 | 33.78 | −0.93 | −1.12 |
| TMEM138 | 27.13 | 14.82 | 31.42 | −0.88 | −1.09 |
| DNMT1 | 1488.86 | 919.26 | 1828.09 | −0.70 | −0.99 |
| BCL2L10 | 711.64 | 458.07 | 896.20 | −0.64 | −0.97 |
| RBBP7 | 490.58 | 345.48 | 512.97 | −0.51 | −0.57 |
| SMAD2 | 226.71 | 160.43 | 232.96 | −0.50 | −0.54 |
| PARD3 | 20.93 | 14.63 | 21.13 | −0.52 | −0.53 |
| ING3 | 51.52 | 34.43 | 47.39 | −0.58 | −0.46 |
| BOD1 | 588.04 | 442.14 | 694.66 | −0.41 | −0.65 |
| NEIL3 | 65.66 | 38.84 | 62.65 | −0.76 | −0.69 |
aCategory D&E. Category D&E are defined as those genes differentially expressed between MII/GV, FTM/GV, FTM/MII.
bCategory F + G. Category F + G is defined as those genes where there is statistically significant difference FTM and both GV and MII, while there is no difference between MII/GV.
cFPKM: Fragments Per Kilobase of transcript per Million mapped reads.
dL2FC: Log fold change of base 2. Positive values indicate an increase in FTM vs. GV with a FDR q-value of <0.05.
fL2FC: Log fold change of base 2. Positive values indicate an increase in MII versus FTM with a FDR q-value of <0.05.
IPA analysis was performed on the combined mRNAs from sets F and G. Affected CPs included HIPPO signaling, dolichol and dolichyl phosphate biosynthesis (regulation of glycosylation), granzyme B signaling (initiates apoptosis), and valine degradation I (amino acid metabolism) (Table IX, Supplementary Table S22). Decreased activity of the CPs DNA methylation and transcriptional repression was associated with the F + G set. Analysis of affected biological functions for F + G similarly yielded results for dolichol metabolism, amino acid metabolism, DNA methylation, cell death, accumulation and transport of lipids and steroid metabolism, which besides cell death were not associated with NM (Table IX, Supplementary Table S23, P < 0.05). Nine URs were shared between F + G and C. Interestingly there were 13 URs shared between NM and F + G gene, suggesting the potential importance of these URs to NM (Table IX, Supplementary Table S24). Notably, DNMT1 was a significantly downregulated mRNA among the F + G set, echoing the effects on DNA methylation seen for canonical pathway and biological function analyses for B, C and F + G.
Table IX.
Selected IPA® CPs, BFs and URs affected in category F + Ga
| −log(P-value)b | Selected CPs |
|---|---|
| 3.60 | HIPPO signaling |
| 2.97 | DNA Methylation and Transcriptional Repression Signaling |
| −log(P-value) | Selected BFs |
|---|---|
| 2.89 | Apoptosis |
| 1.91 | Necrosis |
| 2.51 | Apoptosis of tumor cell lines |
| 1.72 | Quantity of cells |
| 1.58 | Cell survival |
| 2.44 | Growth of epithelial tissue |
| 1.58 | Cell death of connective tissue cells |
| 2.61 | Accumulation of lipid |
| 1.39 | Quantity of leukocytes |
| 2.22 | Interphase of tumor cell lines |
| 1.96 | Growth of neurites |
| 1.69 | Development of neurons |
| 2.86 | Senescence of fibroblast cell lines |
| 2.69 | Steroid metabolism |
| 2.67 | Proliferation of dermal cells |
| 2.09 | Metabolism of membrane lipid derivative |
| 1.82 | Stimulation of cells |
| 1.58 | Morphology of neurons |
| 1.47 | Outgrowth of neurites |
| 1.37 | Neuritogenesis |
| 2.86 | Abnormal morphology of Nucleus |
| 2.75 | Neurogenesis of embryo |
| 2.49 | Pluripotency of cells |
| 2.42 | Catabolism of lipid |
| 2.15 | Binding of lipid |
| 2.15 | Metabolism of glycosphingolipid |
| 1.85 | Apoptosis of gonadal cells |
| 1.71 | DNA recombination |
| 1.65 | Differentiation of adipocytes |
| 1.41 | Transport of lipid |
| −log(P-value) | Selected URsc |
|---|---|
| 1.72 | MYC |
| 1.66 | ERBB2 |
| 2.32 | LMNA |
| 2.14 | YY1 |
| 1.83 | Cg |
| 1.72 | EGFR |
| 1.69 | CEBPB |
| 1.64 | IL15 |
| 1.46 | KRAS |
| 1.45 | NUPR1 |
| 2.26 | ↓DNMT1 |
| 2.15 | AREG |
| 1.88 | FOXM1 |
| 1.85 | FGF1 |
| 1.68 | RORC |
| 1.58 | MAP2K1 |
| 1.51 | miR-124-3p (and other miRNAs w/seed AAGGCAC) |
| 1.50 | RORA |
| 1.42 | RAF1 |
| 1.42 | CDKN1A |
| 1.38 | E2F4 |
| 1.32 | LDL |
aCategory F + G is defined as those genes where there is a statistical difference FTM and both GV and MII, while there is no difference between MII/GV.
b-log(P-value): The P-value, statistical overlap of DEG list and gene set for CPs, BFs and URs with applied ‘-log10’ transform. A -log(P-value) of 1.3 or higher is considered statistically significant at a P < 0.05.
cUR as defined from IPA. ↑ or ↓ preceding UR indicates it is differentially expressed between FTM/GV.
Discussion
A failure in oocyte maturation could be attributable to a complete lack of response to an ovulatory stimulus, a defect in competence to undergo GVBD and resume meiotic progression, or specific deficiencies in cytoplasmic maturation or meiosis that block nuclear maturation and progression to second meiotic metaphase. Using whole transcriptome and pathway analysis in a nonhuman primate species that closely models human oogenesis, our results demonstrate that FTM oocytes do indeed fulfill a large portion (47%) of the normal maturation-associated changes in mRNA expression. However, FTM oocytes also manifest a large number of deficiencies in the regulation of many other mRNAs. Approximately 44% of the mRNAs that undergo changes in abundance during NM display partial modulations to intermediate abundances, and 9.2% fail to diverge significantly from GV stage oocytes. Additionally, a small group of mRNAs are grossly mis-regulated.
These results exclude the scenario in which FTM oocytes simply fail to respond to an ovulatory stimulus. The results also indicate that failure to mature is associated with a failure to correctly regulate mRNAs that support a diverse array of pathways and processes, and that deficiencies are evident in transcriptional control as well as mRNA turnover. These deficiencies may be markers of maturation incompetence, or may be primary deficiencies that inhibit correct cytoplasmic maturation, which in turn could be responsible for the block to nuclear maturation and/or successful re-entry into meiosis.
Several striking features of FTM oocytes emerged in this analysis. First, the expression of DNMT1 mRNA is reduced and therefore DNA methylation activity is likewise projected to be diminished. Second, the expression of genes encoding mitochondrial proteins is broadly elevated, and there are projected disturbances in oxidative phosphorylation, and cellular redox state, associated with mitochondrial dysfunction. Further gene expression defects support the idea that mitochondria are not regulated correctly in FTM oocytes. This includes elevated expression of steroid receptor RNA activator (SRA) stem-loop interacting RNA binding protein (SLIRP), which in addition to inhibiting nuclear steroid receptor signaling also promotes intra-mitochondrial mRNA translation (Lagouge et al., 2015). Additionally, we observed increased expression of mitochondrial complex I, cytochrome C oxidase and other electron transport genes in FTM oocytes, contrasting with reductions of these genes in NM, and indicating a widespread failure to correctly regulate mitochondrial function in FTM oocytes. Third, FTM oocytes manifest disturbances in fatty acid beta oxidation and lipid metabolism and lipid droplet clustering. Related to those lipid metabolic functions, increased expression of BSCL2, seipin lipid droplet biogenesis associated (BSCL2; lipid droplet formation) and acetyl-coa acyltransferase 1 (ACAA1; fatty acid beta oxidation) are observed.
A mechanistic link between DNMT1, mitochondrial function, lipid metabolism and oocyte quality in oocytes was suggested recently in a study reporting that melatonin treatment of porcine oocytes led to enhanced DNMT1 gene expression, reduced mitochondrial gene expression, decreased reactive oxygen species and increased lipid accumulation (He et al., 2018). Another study from that same group reported that upregulation of DNMT1 expression led to mitochondrial DNA hypermethylation and downregulation of mitochondrial DNA content (Jia et al., 2016). In other cell types, DNMT1 deficiency is associated with deficient fatty acid beta oxidation and abnormal citrate metabolism (Himes et al., 2015). Deficiencies in mitochondrial replication can occur in oocytes and other cells through suppression of nuclear encoded DNA polymerase gamma A (POLG) and other enzymes that promote mitochondrial DNA replication and repair (Lee et al., 2015; El-Hattab et al., 2017). Because these were not among affected genes showing reduced expression in FTM oocytes, defects in mitochondrial DNA repair would not be expected to contribute to the FTM phenotype. However, the effects observed here on DNMT1 expression, mitochondrial gene regulation, oxidative phosphorylation, lipid accumulation and beta oxidation may be mechanistically linked in a common pathway that can be disrupted in a fraction of oocytes, and could inhibit successful nuclear maturation and/or meiotic progression.
Other affected mRNAs impact diverse pathways including, but not limited to, cell cycle functions. Among partially modulated mRNAs, prominent affected pathways included cell cycle progression as well as aneuploidy, cell death, ubiquitination, metabolism and stress response pathways. Meiosis-related affected functions and pathways also include oocyte activation, arrest in oogenesis, and organization of mitotic spindle (protein kinase, DNA-activated, catalytic polypeptide (PRKDC), siah E3 ubiquitin protein ligase 1 (SIAH1)). One gene, RBBP7, was under-expressed in FTM oocytes, and this protein plays a key role in meiotic spindle formation (Balboula et al., 2014). Additional severely affected mRNAs, which remained at the GV abundances and therefore failed entirely to be modulated normally, contribute to oocyte-specific functions such as zona pellucida formation (zona pellucida glycoprotein 3 (ZP3) mRNA elevated in FTM oocytes); alterations in zona pellucida protein secretion could also result from defects in dolichol phosphorylation, which was also highlighted among the F + G defects seen for genes with grossly abnormal regulation. Additionally, ZP3 is required for GVBD and may affect serine/threonine kinase (AKT), lamin and microfilament functions in oocytes (Gao et al., 2017). Sustained high expression of ZP3 in FTM oocytes may indicate that not only is ZP3 required for GVBD, but that correct downregulation may also be important.
The Hippo signaling pathway also emerged as an affected pathway in analysis of the F + G highly aberrantly regulated gene group. This pathway plays a crucial role in regulating follicle growth, and decreasing Hippo signaling in conjunction with AKT activation can increase follicle growth (Kawamura et al., 2016). Hippo signaling via mammalian ste20-like kinases 1/2 (MST1/2) ultimately leads to LATS1 phosphorylation, decreased YAP nuclear localization and inhibition of growth promoting (i.e. pro-folliculogenesis) genes. The LATS1 mRNA is aberrantly elevated in FTM oocytes compared to GV and MII stage oocytes; and LATS1 mRNA is a member of the category F genes. Thus, aberrant activation of the Hippo pathway may inhibit complete development of follicles giving rise to the FTM oocytes following an ovulatory stimulus.
Our data provide additional insight into the potential downstream functions of DNA methylation in primate oogenesis. DNMT1 mRNA is reduced in FTM oocytes compared to both GV and MII stage oocytes. The deficiency of DNMT1 expression throughout oogenesis could lead to a failure to maintain suppression of a wide variety of genes. Indeed, many of the aberrantly over-expressed nuclear genes encoding mitochondrial proteins possess CpG islands near their promoters that can be regulated by DNA methylation. DNMT1 is widely regarded as a DNA maintenance methylase, but was recently reported to direct methylation of hemimethylated loci following de novo methylation by other DNA methylases (Shirane et al., 2013). This provides an opportunity for DNMT1 deficiency to contribute to aberrant gene transcription in oocytes. The apparent link between DNMT1 expression, decreased mitochondrial activity and oxidative phosphorylation, modulation of other oocyte characteristics (He et al., 2018), and the correct regulation of fatty acid beta oxidation (Dunning et al., 2014) provides heightened interest in understanding DNMT1 functions during oogenesis.
In contrast to DNMT1, the DNA methyltransferease 3 beta (DNMT3B) mRNA shows a modest 53% increase in relative abundance between GV and MII stages, but does not display this increase in FTM oocytes. The modest increase in apparent abundance most likely reflects some degree of DNMT3B mRNA stabilization during NM, but which is not occurring in FTM oocytes. DNMT3B is one of a much larger collection of 245 maternal mRNAs that are not correctly stabilized in FTM oocytes (553 from NM group shared with groups C or B). Further study of the mechanisms that underlie the failure to stabilize these mRNAs in FTM oocytes would provide new understanding of the critical process of correct and timely maternal mRNA regulation in mammalian oocytes.
Accordingly, this study of FTM oocytes has revealed key aspects of cytoplasmic maturation that contribute to the successful production of high quality MII stage oocytes, and provides a valuable model for the further study of determinants of oocyte quality. These results give new insight into the acquisition of oocyte developmental competence. This is relevant to considering possible diagnostics markers of oocyte quality, as successful modulation of many mRNAs (e.g. group A genes) and their associated protein expression values need not signify fully normal cytoplasmic maturation and high oocyte quality. Even if GVBD occurs, these markers may not be fully indicative of high oocyte quality. Other features of oocyte cytoplasmic maturation revealed in our dataset may also need to be considered. For example, correct regulation of genes in groups B and C would be strong markers of more complete cytoplasmic maturation and their incorrect regulation would be indicative of poor quality. Additionally, aberrant regulation of group D, E, F and G genes may reflect compromised oocyte quality even if other genes display more normal expression values. These same principles could apply to the expression of corresponding proteins or associated metabolites. The large dataset of rhesus monkey FTM oocytes presented here provides a new and powerful reference dataset for assisting in the development of novel oocyte quality diagnostic tools.
Supplementary Material
Acknowledgements
The authors thank Dana Dagget for her technical assistance.
Authors’ roles
M.L.R. analyzed and interpreted data, prepared figures and tables, and wrote manuscript; P.Z.S processed, analyzed and interpreted data; U.M. processed, analyzed and interpreted data; K.A.V. and B.G. processed samples and edited manuscript; C.A.V. produced samples, provided funding, and edited manuscript; K.E.L. conceived study, provided funding, interpreted data and wrote manuscript.
Funding
This work was supported by grants from the National Institutes of Health Office of Research Infrastructure Programs Division of Comparative Medicine Grants R24 [OD012221 to K.E.L., OD011107/RR00169 (California National Primate Research Center), and OD010967/RR025880 to C.A.V.]; the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under the award number T32HD087166; MSU AgBioResearch, Michigan State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Aken BL, Achuthan P, Akanni W, Amode MR, Bernsdorff F, Bhai J, Billis K, Carvalho-Silva D, Cummins C, Clapham P et al. Ensembl 2017. Nucleic Acids Res 2017;45:D635–D642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrech OM, Goldman GA, Rufas O, Stein A, Amit S, Yoles I, Pinkas H, Fisch B. Treatment variables in relation to oocyte maturation: lessons from a clinical micromanipulation-assisted in vitro fertilization program. J Assist Reprod Genet 1997;14:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboula AZ, Stein P, Schultz RM, Schindler K. Knockdown of RBBP7 unveils a requirement of histone deacetylation for CPC function in mouse oocytes. Cell Cycle 2014;13:600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Vandevoort CA. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp Biol Med (Maywood) 2013;238:539–548. [DOI] [PubMed] [Google Scholar]
- Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report 2013;67:1–18. [PubMed] [Google Scholar]
- Chen ZQ, Ming TX, Nielsen HI. Maturation arrest of human oocytes at germinal vesicle stage. J Hum Reprod Sci 2010;3:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction 2014;148:R15–R27. [DOI] [PubMed] [Google Scholar]
- El-Hattab AW, Craigen WJ, Scaglia F. Mitochondrial DNA maintenance defects. Biochim Biophys Acta 2017;1863:1539–1555. [DOI] [PubMed] [Google Scholar]
- Gao LL, Zhou CX, Zhange XL, Liu P, Jin GY, Ma Y, Li J, Yang ZX, Zhang D. ZP3 is required for germinal vesicle breakdown in mouse oocyte meiosis. Sci Rep 2017;7:41272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv 2014;69:109–115. [DOI] [PubMed] [Google Scholar]
- He B, Yin C, Gong Y, Liu J, Guo H, Zhao R. Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J Cell Physiol 2018;233:302–312. [DOI] [PubMed] [Google Scholar]
- Head SR, Komori GT, Hart J, Shimashita L, Schaffer DR, Salomon PT, Ordoukhanian PT. Method for improved Illumina Sequencing Library Preparation using NuGEN Ovation RNA-Seq system. Biotechniques 2011;50:177–181. [DOI] [PubMed] [Google Scholar]
- Himes KP, Young A, Koppes E, Stolz D, Barak Y, Sadovsky Y, Chaillet JR. Loss of inherited genomic imprints in mice leads to severe disruption in placental lipid metabolism. Placenta 2015;36:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Li J, He B, Jia Y, Niu Y, Wang C, Zhao R. Abnormally activated one-carbon metabolic pathway is associated with mtDNA hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Sci Rep 2016;6:19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol 2016;28:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015;12:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Mourier A, Lee HJ, Spahr H, Wai T, Kukat C, Silva Ramos E, Motori E, Busch JD, Siira S et al. SLIRP regulates the rate of mitochondrial protein synthesis and protects lRPPRC from degradation. PLoS Genet 2015;11:e1005423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Zhao MH, Zheng Z, Kwon JW, Liang S, Kim SH, Kim NH, Cui XS. Polymerase subunit gamma 2 affects porcine oocyte maturation and subsequent embryonic development. Theriogenology 2015;83:121–130. [DOI] [PubMed] [Google Scholar]
- Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod 2002;17:1604–1609. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014;30:923–930. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AK, Lynch K, Arny MJ, Grow DR. Turner mosaicism (45,X/46,XX) diagnosed in a young woman subsequent to low oocyte maturity and failed ICSI. Fertil Steril 2008;90:e2013–e2015. [DOI] [PubMed] [Google Scholar]
- Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol 2008;1:69–76. [PMC free article] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod 1999;14:2544–2555. [DOI] [PubMed] [Google Scholar]
- Shirane K, Toh H, Kobayashi H, Miura F, Chiba H, Ito T, Kono T, Sasaki H. Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases. PLoS Genet 2013;9:e1003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010;28:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVoort CA, Mtango NR, Latham KE, Stewart DR. Primate preimplantation embryo is a target for relazin during early pregnancy. Fertil Steril 2011;96:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVoort CA, Mtango NR, Midic U, Latham KE. Disruptions in follicle cell functions in the ovaries of rhesus monkeys during summer. Physiol Genomics 2015;47:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin AV, Cornish AS, Maudhoo MD, Gibbs RM, Zhang X, Pandey S, Meehan DT, Wipfler K, Bosinger SE, Johnson ZP et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol Direct 2014;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.