Abstract
Mycobacterium tuberculosis monoarthritis is a rare form of TB, occurring in 1-2% of cases in the United States. Delays in definitive diagnosis and subsequent treatment are common. While case reports of tuberculous arthritis have been presented in international literature, there is a relative paucity of literature from within the United States. Given the difficulty in diagnosis and adverse outcomes of delayed diagnosis, we present the case of an 11-year-old otherwise healthy male with isolated monoarticular TB septic arthritis of the right knee. A discussion, including review of current literature, regarding presentation, diagnosis, and treatment of tuberculous monoarthritis follows. The emerging role of arthroscopy as a diagnostic and treatment modality for tuberculous monoarthritis of the knee is discussed.
Level of Evidence: VI
Introduction
Tuberculosis (TB) is a systemic disease that is endemic in many countries outside of the United States.1 The annual incidence of TB in the United States is reported to be 9,287 cases, compared to 10.4 million cases worldwide.2 Within the United States, there is a significant difference the incidence of TB between pediatric (0.5 to 1.2 per 100,000) and adult populations (3.0 per 100,000).3
Myocbacterium tuberculosis, the causative organism of TB, can manifest in a variety of ways. A remote history of fever is the most common clinical manifestation of primary tuberculosis, occurring in 70% of symptomatic cases.4 Pulmonary symptoms, including cough, pleuritic chest pain, and shortness of breath are present in 60-80% of pediatric TB infection.4-9 Overall, over 90% of older children and adults exposed to TB do not develop overt clinical symptoms.9 In contrast, children under the age of two have an increased risk (60-80%) of developing clinical TB.9
Tuberculosis monoarthritis is a rare form of TB, occurring in 1-2% of cases of cases in the United States.5,10-13 Most cases of monoarthritis TB are a result of reactivation of latent disease, marked by a latency period of 1-3 years.9 Hip and knee monoarticular arthritis accounts for 30% of all cases of skeletal tuberculosis in children.14,15 Interestingly, systemic symptoms are present in only one-third of patients with skeletal tuberculosis.9 Septic arthritis secondary to TB is often indolent and may be indistinguishable from much more common etiologies of knee pain and inflammation in children, such as juvenile idiopathic arthritis (JIA).7,10,13 Given the lack of systemic symptoms and indolent disease course, delays in definitive diagnosis and subsequent treatment of monoarthritis secondary to TB are common.5,6,10,11,16 Delayed diagnosis of monoarticular TB arthritis can lead to synovial erosion, formation of draining sinuses, osteomyelitis, and pathologic fracture of the bone.6,7,13 Additionally, articular cartilage erosion and joint space narrowing may occur in late-stage disease.7,17
While case reports of tuberculous arthritis have been presented in international literature,10,18-20 there is a relative paucity of literature from within the United States. Given the difficulty in diagnosis and adverse outcomes of delayed diagnosis, we present the case of an 11-year-old otherwise healthy male with isolated monoarticular TB septic arthritis of the right knee.
Case Report
KF is an 11-year-old healthy Chinese-American male who initially presented to orthopaedic clinic with a several-month history of intermittent right knee pain, swelling and difficulty bearing weight. He denied any recent fevers, chills, cough, weight loss, or pulmonary symptoms. The patient had recently travelled to China on multiple occasions, and noted that he had received previous treatment for his knee pain in China, including intra-articular steroid injections, knee aspirations, and hyaluronic acid injections. On physical examination, patient had a moderate effusion of the right knee joint without noticeable erythema. He was able to bear weight on the affected extremity in clinic and was afebrile. He also had limited range of motion, with active knee range of motion from 15° of flexion to 100° of flexion. The remainder or the physical examination was unremarkable.
Plain radiographs of his right knee revealed a large knee effusion but were unremarkable for any bony erosion or joint space narrowing (Figure 1). An MRI of the right knee with and without contrast was obtained and demonstrated a large effusion of the right knee joint with the presence of diffuse synovitis throughout the knee joint (Figure 2). Additionally, multiple intra-articular loose bodies were identified. Laboratory workup included an elevated erythrocyte sedimentation rate (27 mm/ Hr [reference range 0-15 mm/Hr]), C-reactive protein (1.0 mg/dL [reference range ≤0.5 mg/dL]), and platelet count (502 x 103/mm3 [reference range 150-400 x 103/ mm3]). His complete blood count was normal without evidence of leukocytosis.
Figure 1.
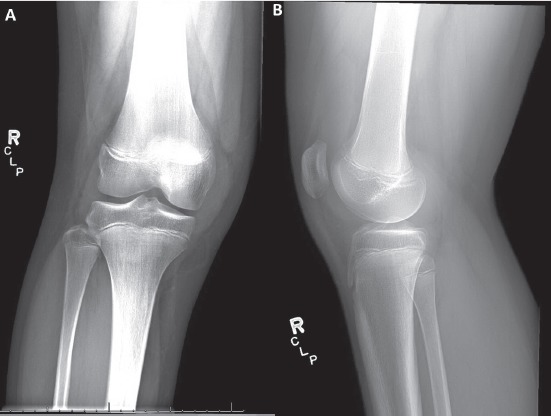
(a,b) – AP (a) and lateral (b) radiographs of the right knee. Overall alignment is anatomic, with the presence of a knee effusion. No osseus pathology observed.
Figure 2.
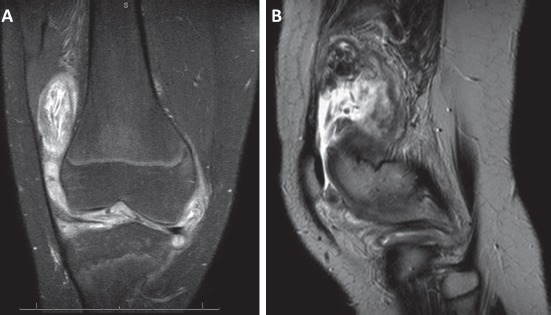
(a,b) – Coronal section of T2-weighted MRI (a) and sagittal section of T2-FS weighted MRI of the right knee demonstrating diffuse synovitis and free intra-articular bodies.
Given his elevated inflammatory labs and knee effusion, a knee aspirate was performed in clinic. Synovial fluid aspirated from the joint was turbid and red, but contained only 7568 total nucleated cells/mm3, with 2043 total polymononuclear cells/mm3. His initial intra-articular gram stain, aerobic cultures, anaerobic cultures, and acid-fast bacilli culture were negative. We elected to obtain a comprehensive infection workup, including a Manotoux tuberculin skin test (PPD), QuantiFERON TB-gold serum test, and HIV test. Although his antibody assays for HIV-1 and HIV-2 were negative, his PPD test was remarkable for a site of skin reaction 20 mm in diameter. His follow-up QuantiFERON TB-gold serum testing was read as indeterminate.
In the setting of a positive PPD test and inconclusive knee aspiration assay, the decision was made to perform a diagnostic knee arthroscopy, tissue biopsy, and repeat right knee joint aspiration to improve our diagnostic yield. We elected to hold intraoperative antibiotics until cultures were performed. Prior to our diagnostic arthroscopy, we performed a repeat intra-articular knee aspirate. This aspirate yielded 15 ml of brown-tinged synovial fluid without evidence of gross purulence. During our diagnostic arthroscopy, the patient was noted to have diffuse nodular synovitis as well as numerous circular rice bodies (Figure 3). Multiple soft tissue samples were obtained for microbial culture as well as histologic examination. Tissue samples taken intraoperatively were positive for acid-fast bacilli. Histologic examination of soft tissue biopsy demonstrated granulomatous synovitis with focal necrosis (Figure 4). Synovial fluid cultures from the knee aspirate were positive for Mycobacterium tuberculosis. Repeat QuantiFERON TB-gold testing was positive for TB.
Figure 3.
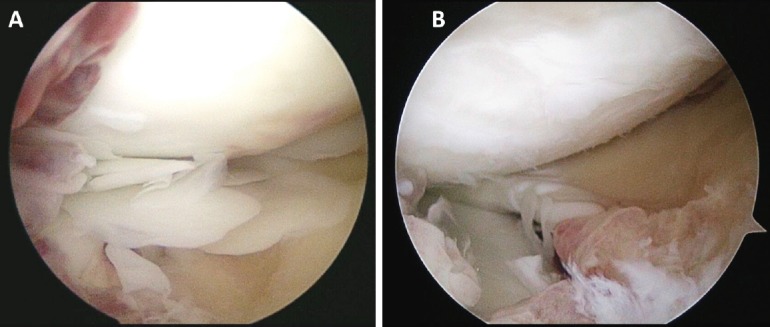
(a, b) – Intra-operative photographs demonstrating synovitis (a,b) and free-floating articular bodies (a).
Figure 4.
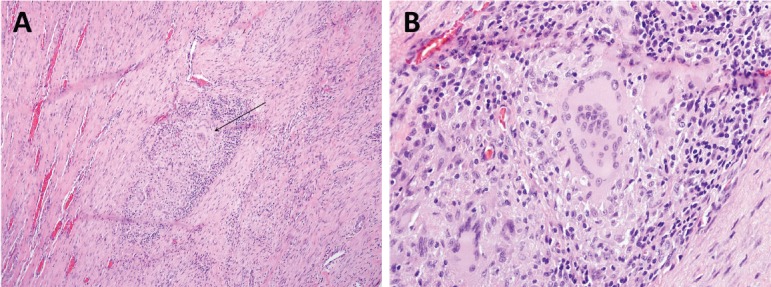
(a,b) – a. Histopathology slide (100x power) of resected synovial tissue demonstrating granulomatous synovitis with central focal necrosis (black arrow). Histopathology slide (400x power) re-demonstrating granuloma with focal necrosis.
The patient was diagnosed with monoarticular TB infection, and he was initiated on a multi-drug treatment for TB by the infectious disease team. The treatment regimen included rifampin, isoniazid, pyrazinamide, and ethambutol. Speciation and sensitivity results of cultures demonstrated the bacterium to be resistant to isoniazid, with susceptibility of fluoroquinolones. The patient was then initiated on levofloxacin after discontinuation of the isoniazid. At the patient’s four month follow up appointment, he was doing well, with marked improvements in knee stiffness and swelling. Physical examination demonstrated a 5° flexion contracture in the affected knee, but was otherwise unremarkable. He is currently scheduled for a 9 month course of antibiotic treatment.
Discussion
The clinical case above illustrates the history and physical exam findings in an adolescent male with tuberculous arthritis of the knee. Previously undiagnosed disseminated tuberculosis that presents as isolated monoarticular arthritis is rare, with an estimated incidence of 1-2% within the United States.5,10-13,16 Risk factors for developing TB in the United States include being foreign born, having a foreign born parent, and living outside of the United States for greater than two months.21 A history of close contact with infected persons or travel to endemic areas may be difficult to establish, or may be absent entirely in up to 16% of patients.10,13 Our subject had a recent travel to China and was born in China (as were both of his parents); both of these are identifiable risk factors for developing septic TB of the knee. According to the World Health Organization (WHO) annual report in 2016, China had 804,163 cases of TB with an annual incidence of 67 cases per 100,000 and a mortality rate of 2.6 cases per 100,000.1 China’s annual expenditure for TB prevention and treatment was $372 million USD per year.1 Another well-described cohort at an increased risk of developing symptomatic TB are children with juvenile rheumatoid arthritis patients on immunomodulating agents, such as tumor necrosis factor-alpha.22,23 Often times patients with presumed juvenile rheumatoid arthritis are treated with intra-articular steroids.10 The patient in our case report did not have a prior history of juvenile rheumatoid arthritis or immunomodulating agents.
Our case of monoarticular tuberculosis presented with knee pain, swelling, stiffness, and decreased range of motion 6 months after foreign travel. In a recent case review, Rosenberg et al. found that only approximately 15% of children present with erythema and joint effusion that would be suggestive of a septic arthritis.9 Bone pain proximal or distal to the joint and mechanical locking of the knee have also been reported.10,13 These indolent findings are in stark contrast to the clinical presentation of a child with a bacterial septic arthritis secondary to a more common pathogen (i.e. Staphylococcus aureus). Common pathogenic organisms for bacterial septic arthritis in children include Staphylococcus aureus, Kingella kingae, Neisseria gonorrhoeae, and Haemophilus influenza type B.24 Fever, pain, and swelling are common clinical symptoms of these organisms.24. Transient synovitis may also present in a similar manner to septic arthritis with pain and swelling of the affected joint.
Plain radiographs are often the first imaging modality employed to evaluate children with knee pain, swelling, or decreased range of motion. These radiographs may be unremarkable in the acute phase of TB.6,25 In later stages, joint effusion, osteopenia, widening of the intercondylar notch, osseous erosion and cortical defects, bone cysts and peri-articular lytic lesions represent common findings of monoarticular TB arthritis. A unique finding of monoarticular TB is its ability to cross the epiphysis into the joint space. This trans-epiphysial spread of TB osteomyelitis is not characteristic of other causes of septic arthritis.26 In young children, the epiphysis may not be ossified and joint destruction is often underestimated. The epiphysis can also appear inappropriately mature for the patient’s age secondary to hyperemia as a result of the infection.19 Involvement of the epiphysis, often as a result of the spread of infection from a metaphyseal nidus, may manifest as epiphyseal widening.6,19,26 Periosteal reaction may be present or absent.27 In chronic cases, radiographic joint space narrowing can occur.11,12,15 Phemister’s triad, first described by D.B. Phemister in 1924, encompasses the classical radiographic findings of mycobacterial septic arthritis: periarticular osteoporosis, erosion of subchondral bone, and joint space narrowing indicative of destruction of articular cartilage.28 Kerri and Martini developed a classification system that placed patients into one of four stages based on radiographic findings (Table 1).25 Numerous studies have demonstrated that radiographic stage at the time of presentation is predictive of overall functional outcome of the affected knee.7,8,25 Our patient would fall into stage I based on the Kerri and Martini classification, and therefore would be predicted to make near to full recovery in terms of knee motion and function.7,8,25 Ultrasound may be helpful in identifying the presence of a joint effusion.26 Advanced imaging, including CT and MRI, may be useful for identifying the extent of bone destruction as well as synovial and soft tissue involvement.26 MRI may also be useful for identifying free-floating bodies within the joint, including rice bodies, which may be seen in tuberculous arthritis, as was the case in this patient.29 Although imaging of the affected joint may reveal the presence of a pathologic process within the joint, findings are often not specific enough for a concrete diagnosis, and further laboratory workup is often necessary.
Table 1.
Kerri and Martini Classification
| Stage | Radiographic Findings |
|---|---|
| I | Localized osteopenia, no bone lesions, +/- soft tissue swelling |
| II | One or more areas of osseus erosion, without narrowing of the joint space |
| III | Narrowing of the joint space without gross anatomical disorganization |
| IV | Narrowing of the joint space with gross anatomical disorganization |
Pediatric patients presenting with knee pain, swelling, and erythema often undergo numerous laboratory assays including complete blood counts with differential, ESR, and CRP. If clinical suspicion is high, more specific tests for tuberculosis (PPD skin testing, QuantiFERON TB-gold serum testing) may be warranted. Several case series have shown than ESR is often elevated above 20 mm/ Hr in 80-96% of children with TB monoarthritis.11,23 CRP and platelet count were not frequently reported in the reviewed studies, but were elevated in the case of KF. Mantoux skin testing is frequently positive (≥10mm) in immunocompetent patients with TB, ranging from 87%-97% in recent case series.7,8,13,30 Examination with QuantiFERON TB-gold serum was not widely reported, but was indeterminate in this case. Cell count from synovial fluid in tuberculous arthritis is often less than more traditional cases of septic arthritis, ranging from 5,000-20,000 total cells with a predomination of polymorphonuclear leukocytes (PMNs).1012,13
The gold standard for diagnosing TB arthritis is isolating the causative bacterium on tissue culture or histological study.6,7,10 Needle biopsy of synovium and bone has been established as an effective method of obtaining material for culture and histopathologic examination as described above, with the advantage of being much less invasive than an open synovial or bone biopsy.7,12,31 However, the synovial aspirate may yield a negative result, especially in the earlier stages of the disease potentially due to a lack of an early immune response.6,7,12 Culturenegative intraarticular tissue samples during arthroscopy may undergo histologic evaluation, and more often yields a definitive diagnosis.8,10,20,24,25 Characteristic histologic findings from open biopsies include caseating granulomas and the presence of giant cells.6-8,10
Recently, arthroscopy has been proposed as an alternative to needle biopsy for the diagnosis of tuberculous arthritis in older children.13,24,25 Arthroscopy of affected joints remains minimally invasive relative to open biopsy, and synovial tissue from multiple sites within the joint may be collected for culture and histopathology. Guo et al. reported a recent series of 41 patients with tuberculous arthritis of the knee, in which the diagnostic yield of arthroscopy was >90% of its cohort.32 Additionally, direct visualization of the affected synovium has diagnostic value, and is not accomplished with needle biopsy. Synovial projections described as “tongue-like” or “nodular” may be observed in TB arthritis.32 Additionally, free-floating rice bodies described previously may be observed, retrieved, and sent for histopathologic examination.29 Arthroscopy has the added benefit of being therapeutic in addition to diagnostic, the ability to debride and resect inflamed synovium and free-floating bodies within the joint.29,31,32 Multiple studies have reported rapid and sustained improvement in knee range of motion following arthroscopy.31,32 Arthroscopic arthrolysis has been welldescribed as an effective long-term treatment of arthrofibrosis, as may be seen following ACL reconstruction and total knee arthroplasty.33,34 We believe that arthroscopy may play a similar role in the treatment of tuberculous arthritis, especially in cases of delayed presentation with prolonged periods of inflammation and decreased knee range of motion. Restoration of range of motion early in the disease course and post-operative physical therapy have also been proposed as adjunct treatments aimed at preserving range of motion. 31,32
Chemotherapeutic regimens often involve three to four drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) for an extended period of time (e.g. ≥12 months).6,8,35,36 In a recent sudy, the Treatment Action Group demonstrated that worldwide TB research funding has increased from $358 million USD in 2005 to $676 million USD in 2013, with 38% of the total expenditure related to drug therapies.35 The use of this three to four drug regimen has increased substantially in the United States from 40.3% of TB patients in 1993 to 84.7% in 2015.3 Additionally, treatment monitoring is advocated by the CDC, with 92.1% of patients undergoing treatment monitoring in 2013.3 Although the rate of multi-drug resistant TB has remained stable in the United States at 1.1% (37 persons) in 2015, foreign-born individuals represent over 86% of these cases. Additionally, the World Health Organization (WHO) 2016 report shows a significantly higher rate of multi-drug resistant TB in China (5.1 cases per 100,000) when compared to the United States.1
Timing of intervention directly influences patient outcomes in TB arthritis. Early initiation of antibacterial therapy has been shown to yield improved ROM and function in children with monoarticular TB.6-8 Recurrence of infection does occur in patients treated with medical and surgical management, with rates estimated to be as high as 29% in adult population in endemic countries.37 Recurrence is often due to drug-resistant strains of tuberculosis, and usually occurs within the first 6 months following completion of the initial course of therapy. End stage arthritis secondary to late-stage monoarticular TB can be treated with a total knee arthroplasty after the completion of antituberculosis medication.38,39 Antituberculosis medication is often continued after surgery. Unfortunately, re-infection rates following total knee arthroplasty secondary to monoarticular TB infection ranges from 14-31%.38,39 Arthrodesis remains an option for patients that fail treatment with arthroplasty, offering pain relief and joint stability.39
Conclusion
Tuberculosis monoarthritis of the knee in children, while rare in the United States, remains a difficult condition to diagnose and treat. A high clinical index of suspicion coupled with a comprehensive history, physical examination, and diagnostic workup is required to make a timely and accurate diagnosis. Consistent follow-up during a protracted treatment course with both orthopedic and infectious disease physicians is necessary to ensure treatment efficacy, and to identify treatment failures or complications in their early stages. Knee arthroscopy has a developing and increasing role in both the diagnosis and treatment of tuberculous monoarthritis.
References
- 1. World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016.
- 2.Schmit KM, Wansaula Z, Pratt R, Price SF, Langer AJ. Tuberculosis - United States, 2016. MMWR. Morbidity and mortality weekly report. 2017;66:289–294. doi: 10.15585/mmwr.mm6611a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2017.
- 4.Poulsen A. Some clinical features of tuberculosis. Acta tuberculosea Scandinavica. 1957;33:37–92;. concl. [PubMed] [Google Scholar]
- 5.Garrido G, Gomez-Reino JJ, Fernandez-Dapica P, Palenque E, Prieto S. A review of peripheral tuberculous arthritis. Seminars in Arthritis and Rheumatism. 1988;18:142–149. doi: 10.1016/0049-0172(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Watts HG, Lifeso RM. Tuberculosis of bones and joints. The Journal of Bone and Joint Surgery. American Volume. 1996;78:288–298. doi: 10.2106/00004623-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman EB, Allin J, Campbell JA, Leisegang FM. Tuberculosis of the knee. Clinical Orthopaedics and Related Research. 2002:100–106. doi: 10.1097/00003086-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Lee AS, Campbell JA, Hoffman EB. Tuberculosis of the knee in children. The Journal of Bone and Joint Surgery. British volume. 1995;77:313–318. [PubMed] [Google Scholar]
- 9.Cruz AT, Starke JR. Clinical manifestations of tuberculosis in children. Paediatric Respiratory Reviews. 2007;8:107–117. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Al-Matar MJ, Cabral DA, Petty RE. Isolated tuberculous monoarthritis mimicking oligoarticular juvenile rheumatoid arthritis. The Journal of Rheumatology. 2001;28:204–206. [PubMed] [Google Scholar]
- 11.Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American Family Physician. 2005;72:1761–1768. [PubMed] [Google Scholar]
- 12.Jacobs JC, Li SC, Ruzal-Shapiro C, Kiernan H, Parisien M, Shapiro A. Tuberculous arthritis in children. Diagnosis by needle biopsy of the synovium. Clinical Pediatrics. 1994;33:344–348. doi: 10.1177/000992289403300606. [DOI] [PubMed] [Google Scholar]
- 13.Rajakumar D, Rosenberg AM. Mycobacterium tuberculosis monoarthritis in a child. Pediatric Rheumatology Online Journal. 2008;6(15) doi: 10.1186/1546-0096-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris BS, Varma R, Garg A, Awasthi M, Maheshwari M. Multifocal musculoskeletal tuberculosis in children: appearances on computed tomography. Skeletal Radiology. 2002;31:1–8. doi: 10.1007/s00256-001-0439-y. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson SP, Ormerod LP. Ten-year experience of bone and joint tuberculosis in Blackburn 1978- 1987. Journal of the Royal College of Surgeons of Edinburgh. 1990;35:259–262. [PubMed] [Google Scholar]
- 16.Fernandes S, Vieira-Sousa E, Furtado C, Costa A, Barros R, Fonseca JE. A diagnosis of disseminated tuberculosis based on knee arthroscopic guided synovial biopsy in the context of monoarthritis. Acta Reumatologica Portuguesa. 2016;41:256–259. [PubMed] [Google Scholar]
- 17.Wilkinson MC. Tuberculosis of the hip and knee treated by chemotherapy, synovectomy, and debridement. A follow-up study. The Journal of Bone and Joint Surgery. American Volume. 1969;51:1343–1359. doi: 10.2106/00004623-196951070-00013. [DOI] [PubMed] [Google Scholar]
- 18.Shahin MA, Sultan MI, Alam MJ, Saeed A, Azad AK, Choudhury MR. Tuberculosis is a Mimicker of JIA: A Rare Case Report. Mymensingh Medica Journal : MMJ. 2016;25:575–579. [PubMed] [Google Scholar]
- 19.Guillou-Debuisson C, Salanne S, Marechal C, Laporte E, Claudet I, Grouteau E. [Osteoarticular tuberculosis: a differential diagnosis of idiopathic juvenile arthritis] Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 2010;17:1553–1558. doi: 10.1016/j.arcped.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Ciobanu LD, Pesut DP. Tuberculous synovitis of the knee in a 65-year-old man. Vojnosanitetski Pregled. 2009;66:1019–1022. doi: 10.2298/vsp0912019c. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention (CDC). Tuberculosis: Slide Set - Epidemiology of Pediatric Tuberculosis in the United States, 1993- 2012. 2015.
- 22.Hsin YC, Zhuang LZ, Yeh KW, Chang CW, Horng JT, Huang JL. Risk of Tuberculosis in Children with Juvenile Idiopathic Arthritis: A Nationwide Population-Based Study in Taiwan. PloS one. 2015;10 doi: 10.1371/journal.pone.0128768. e0128768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunelli JB, Bonfiglioli KR, Silva CA, et al. Latent tuberculosis infection screening in juvenile idiopathic arthritis patients preceding anti-TNF therapy in a tuberculosis high-risk country. Revista Brasileira de Reumatologia. 2016 doi: 10.1016/j.rbre.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Arthritis P K. Septic . In: Cherry JD HG, Kaplan SL, ed. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Philadelphia:: Elsevier Saunders;; 2014. pp. 727–734. [Google Scholar]
- 25.Kerri O, Martini M. Tuberculosis of the knee. International Orthopaedics. 1985;9:153–157. doi: 10.1007/BF00268165. [DOI] [PubMed] [Google Scholar]
- 26.Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatric Radiology. 2004;34:853–860. doi: 10.1007/s00247-004-1223-7. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Gupta N, Mishra M, Agrawal N, Kumar D. Primary epiphyseal and metaepiphyseal tubercular osteomyelitis in children A series of 8 case. Acta Orthopaedica Belgica. 2016;82:797–805. [PubMed] [Google Scholar]
- 28.Phemister DB. THE EFFECT OF PRESSURE ON ARTICULAR SURFACES IN PYOGENIC AND TUBERCULOUS ARTHRITIDES AND ITS BEARING ON TREATMENT. Annals of Surgery. 1924;80:481–500. doi: 10.1097/00000658-192410000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karaoglu S, Karaaslan F, Mermerkaya MU. A mechanically locked knee joint due to free-floating flake-shaped rice bodies: a case report. Acta Orthopaedica et Traumatologica Turcica. 2015;49:565–567. doi: 10.3944/AOTT.2015.14.0124. [DOI] [PubMed] [Google Scholar]
- 30.Martini M, Ouahes M. Bone and joint tuberculosis: a review of 652 cases. Orthopedics. 1988;11:861–866. doi: 10.3928/0147-7447-19880601-04. [DOI] [PubMed] [Google Scholar]
- 31.Masood S. Diagnosis of tuberculosis of bone and soft tissue by fine-needle aspiration biopsy. Diagnostic Cytopathology. 1992;8:451–455. doi: 10.1002/dc.2840080505. [DOI] [PubMed] [Google Scholar]
- 32.Guo L, Yang L, Duan XJ, Chen GX, Zhang Y, Dai G. Arthroscopically assisted treatment of adolescent knee joint tuberculosis. Orthopaedic Surgery. 2010;2:58–63. doi: 10.1111/j.1757-7861.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayr HO, Brandt CM, Weig T, et al. Long-term Results of Arthroscopic Arthrolysis for Arthrofibrosis After Anterior Cruciate Ligament Reconstruction. Arthroscopy : the Journal of Arthroscopic & Related Surgery : Official Oublication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2017;33:408–414. doi: 10.1016/j.arthro.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Hegazy AM, Elsoufy MA. Arthroscopic arthrolysis for arthrofibrosis of the knee after total knee replacement. HSS journal : the Musculoskeletal Journal of Hospital for Special Surgery. 2011;7:130–133. doi: 10.1007/s11420-011-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frick M. 2014 Report on Tuberculosis Research Funding Trends, 2005-2013. 2nd Edition. New York, NY:: Treatment Action Group;; 2015. pp. 1–56. [Google Scholar]
- 36.Campos LR, Sztajnbok FC, Galvao S, Lessa MD, Aymore IL, Sztajnbok F. [Presence of riziform bodies in a patient with juvenile idiopathic arthritis: case report and literature review.] Revista brasileira de reumatologia. 2014. doi: 10.1016/j.rbr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Huang TY, Wu TS, Yang CC, Chiang PC, Yu KH, Lee MH. Tuberculous arthritis--a fourteen-year experience at a tertiary teaching hospital in Taiwan. Journal of Microbiology, Immunology, and Infection (Wei mian yu gan ran za zhi) 2007;40:493–499. [PubMed] [Google Scholar]
- 38.Kim YH. Total knee arthroplasty for tuberculous arthritis. The Journal of Bone and Joint Surgery. American Volume. 1988;70:1322–1330. [PubMed] [Google Scholar]
- 39.Su JY, Huang TL, Lin SY. Total knee arthroplasty in tuberculous arthritis. Clinical Orthopaedics and Related Research. 1996:181–187. doi: 10.1097/00003086-199602000-00024. [DOI] [PubMed] [Google Scholar]


