Evaluation of candidates for mechanical circulatory support devices
In advanced heart failure (HF), the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) proposed seven clinical profiles (and modifiers) for a convenient, easy classification of disease status, risk of implantation of mechanical circulatory support devices (MCSDs) and adequate time for intervention (Chart 1).1
Chart 1.
Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles
| Profile | Description | Hemodynamic status | Time frame for definitive intervention |
|---|---|---|---|
| 1 | Critical cardiogenic shock | Persistent hypotension despite the use of inotropes and intra-aortic balloon pumps, associated with organic dysfunction | Hours |
| 2 | Progressive decline, but inotrope dependent | Deterioration of renal and hepatic function, nutritional status and lactate levels, despite use of inotropes in optimized doses | Days |
| 3 | Stable but inotrope dependent | Clinical stability on continuous inotropic therapy, and history of failure to wean from it | Weeks - months |
| 4 | Frequent hospitalization | Signs of water retention, symptoms at rest and frequent admissions to emergency departments | Weeks - months |
| 5 | At home, exertion intolerant | Intolerant to activity, comfortable at rest despite water retention | Intervention emergency depends on nutritional status and organic dysfunction severity |
| 6 | Exertion limited | Moderate limitation to activity; absence of signs of hypervolemia | Intervention emergency depends on nutritional status and organic dysfunction severity |
| 7 | NYHA III | Hemodynamic stability and absence of hypervolemia | Intervention is not indicated |
NYHA: New York Heart Association.
One of the main determinant factors for a successful MCSD implantation is patient eligibility. Correct selection of patients involves – (1) patients with advanced HF to which the risk of MCSD implantation surpasses mortality risk for current disease (making it a beneficial intervention); (2) patients with moderately advanced HF, i.e., implantation of MCSD would not increase patient’s morbidity and mortality due to increased complication rate; (3) no contraindications for MCSD implantation.2,3
Perioperative renal failure, pre-existing right HF, liver dysfunction, mechanical ventilation in the pre-operative period, low weight or overweight and reoperation have been related to worse clinical outcomes after MCSD implantation.3-5
The main scores for risk prediction in MCSD implantation are described in Chart 2.
Chart 2.
Risk predictors for mechanical circulatory support device implantation
| Risk score for destination therapy6 | Risk score for bridge/destination therapy (HMII score)7 | Pre-operative risk score8 | Pre-operative risk score9 |
|---|---|---|---|
| Risk of 90-day in-hospital mortality (pulsatile flow) |
Ninety-day mortality (continuous flow) |
Mortality risk after MCSD implantation (mean of 84 days) |
Mortality risk after MCSD implantation (mean of 100 days) |
| Platelets < 148.000/µL OR: 7.7 |
Age (for 10 years) OR: 1.32 |
Urine flow < 30 mL/hour RR: 3.9 |
Respiratory failure /sepsis OR: 11,2 |
| Albumin < 3.3 mg/dL OR: 5.7 |
Albumin OR: 0.49 |
CVP > 16 mmHg RR: 3.1 |
Right heart failure OR: 3.2 |
| INR > 1,1 OR: 5.4 |
Creatinine OR: 2.1 |
Mechanical ventilation RR: 3 |
Age > 65 years OR: 3.01 |
| Use of vasodilator OR: 5.2 |
INR OR: 3.11 |
Prothrombin time > 16 seconds RR: 2.4 |
Postcardiotomy acute ventricular failure OR: 1.8 |
| Pulmonary artery medium pressure < 25
mmHg OR: 4.1 |
Center volume < 15 implants OR: 2.24 |
Reoperation RR: 1.8 |
Acute myocardial infarction OR: 1.7 |
| ALT > 45 U/mL OR: 2.6 |
Leucocytes > 15.000 RR: 1.1 |
||
| Hematocrit < 34% OR: 3,0 |
Temperature > 101.5 F RR: 0 |
||
| BUN > 51 U/dL OR: 2.9 |
|||
| Intravenous inotropic support OR: 2.9 |
HMII: HeartmateII; OR: odds ratio; RR: relative risk; CVP: central venous pressure; INR: international normalized ratio; ALT: alanine transaminase; BUN: Blood Urea Nitrogen. MCSD: mechanical circulatory support device
Echocardiography
Evaluation of patients candidates for MCDS should include a transthoracic echocardiogram (TEE) complemented by a transesophageal echocardiography (TEE).
The effects of MCDS on right ventricular function depend on the balance between the benefits of decompression of the left chambers (reduction of the left ventricular afterload) and greater volumetric load to the right atrium (RA; increase of the right ventricular preload). Decompression of left chambers also cause changes in the geometry of the right chambers, such as leftward shift of interatrial (IAS) and interventricular septum (IVS), structural changes of tricuspid annulus, which can aggravate a pre-existing tricuspid insufficiency (TI) and right ventricular overload.10
Considering that right ventricular cardiac output determines left ventricular preload, a significant reduction in right ventricular function results in decreased output by the MCSD. It is estimated that approximately 30% of patients with left ventricular assist device develop limiting right ventricular dysfunction. For these reasons, a careful evaluation of right ventricular function is mandatory before MCDS implantation. In the presence of moderate-to-severe dysfunction, the requirement of a permanent biventricular support cannot be ruled out.11
In the assessment of right ventricular function before MCSD implantation, it is recommended the measurement of the right ventricle, as well as a semiquantitative assessment of right ventricular longitudinal and radial contractility combined with quantitative parameters, including fractional area change (FAC; FAC < 20% are associated with increased risk of right ventricular dysfunction after MCSD implantation),12 tricuspid annular plane systolic excursion (TAPSE) determined by M mode, peak systolic velocity of lateral tricuspid ring, measured by tissue Doppler (s’), and right ventricular performance index.13,14
Predictors of right ventricular dysfunction before mechanical circulatory support device implantation
Right ventricular dysfunction is multifactorial and includes an increase in preload, ventricular ischemia and mechanical interdependence of ventricular geometry. It is one of the most severe complications of left ventricular assist device, observed in up to 30% of cases and associated with a six-fold increase in morbidity and mortality (increased risk in up to 67%).11,15
Risk factors and the main risk score for right ventricular dysfunction after MCSD implantation are described in Charts 3 and 4.
Chart 3.
Risk factors for right ventricular dysfunction after mechanical circulatory support device implantation (MCSD)16
| Indication of MCDS | Destination therapy |
|---|---|
| Sex | Female |
| Pre-implantation support | Intra-aortic balloon pump and vasopressor requirement |
| Organic dysfunctions | Respiratory: invasive ventilatory support |
| Hepatic: ALT ≥ 80 UI/L. bilirubin > 2.0 mg/dL | |
| Renal: serum creatinine ≥ 2.3 g/dLHistory of kidney replacement therapy | |
| Nutritional: albumin ≤ 3.0 g/dL | |
| Coagulation: platelets < 120,000 | |
| Others: increased BNP. PCR. Procalcitonin | |
| Right ventricular dysfunction | Right ventricular diastolic diameter > 35 mm. FAC < 30%. Right atrium > 50 mm |
| Hemodynamic measures | CVP ≥ 15 mmHg or CVP/PCP ≥ 0.63. right ventricular work index ≤ 300 mmHg mL/m2; low pulmonary artery pressures, low cardiac index or increased pulmonary vascular resistance |
| Others | Non-ischemic cardiomyopathy, reoperation, important TI, history of PTE |
ALT: alanine transaminase; BNP: brain natriuretic peptide; CRP: C-reactive protein; FAC: fractional area change; CVP: central venous pressure; PCP pulmonary capillary pressure; TI: tricuspid insufficiency; PTE: pulmonary thromboembolism
Chart 4.
Main risk scores for right ventricular failure after left ventricular mechanical circulatory support device implantation
| Score | Variables | Prediction |
|---|---|---|
| University of Michigan, RV Failure Risk Score, Matthews et al.17 | Vasopressor requirement: 4 points TGP ≥ 80 IU/L: 2 points Bilirubin ≥ 2.0 mg/dL: 2.5 points Creatinine ≥ 2.3 mg/dL or hemodialysis: 3 points |
Likelihood of right ventricular
failure • ≥ 5.5 points: 7.6 • 4.0-5.0 points: 2.8 • ≤ 3.0 points: 0.49 |
| Kormos et al.18 | Pre-operative predictors for early left
ventricular dysfunction: CVP/PCP > 0.63 Ventilatory support BUN > 39 mg/dL |
One-year survival: • Absent right ventricular dysfunction: 78% • Early right ventricular dysfunction: 59% (p< 0.001) |
| University of Pennsylvania, RV Failure Risk Score, Fitzpatrick et al.19 | Cardiac index ≤ 2.2 L/min/m2:
18 points SVRI ≤ 0.25 mmHg-L/m2: 18 points Important right ventricular dysfunction: 17 points Serum creatinine ≥ 1.9 mg/dL: 17 points Previous cardiac surgery: 16 points Systolic arterial pressure ≤ 96 mmHg: 13 points |
< 30: 96%, isolated left ventricular assist
device ≥ 65 points: 11%, isolated left ventricular assist device |
| CRITT score20 | CVP > 15 mmHg: 1 point Severe right ventricular dysfunction: 1 point Pre-operative mechanical ventilation: 1 point Important tricuspid insufficiency: 1 point Tachycardia (> 100 bpm) = 1 point |
1-2 points: low risk for right ventricular
dysfunction 2-3 points: moderate risk for right ventricular dysfunction 4-5 points: high risk for right ventricular dysfunction |
ALT: alanine transaminase; CVP: central venous pressure; PCP pulmonary capillary pressure; BUN: Blood Urea Nitrogen; SVRI: systemic vascular resistance index
Implantation of a MCSD in the left ventricle should be performed with caution in patients with important right ventricular dilation, moderate-to-severe tricuspid insufficiency, tricuspid valve annulus > 45 mm and CVP > 15 mmHg. By this means, hemodynamic variables directly reflect a preload or afterload increase and right ventricular contractility reductions, whereas venous congestion and organ hypoperfusion, consequence of right ventricular dysfunction, indicate hepatic and renal dysfunctions15,21
Positive hemodynamic indicators of adequate right ventricular function that might reduce the risk of post-MCSD implantation dysfunction are: CVP ≤ 8 mmHg; PCP ≤ 18 mmHg; CVP/PCP ≤ 0,66; pulmonary vascular resistance (PVR) < 2 wood units and right ventricular work index ≥ 400 mL/m2.
Temporary devices
Selection of strategies for temporary mechanical circulatory support devices
Temporary MCSD can be used for hemodynamic and clinical stability restoration, aiming at improvement of cardiac function and transplantation. Three strategies (which may be overlapped) can be defined:
Bridge to decision: should be considered in severely ill patients, who requires immediate hemodynamic support due to high risk of cardiac failure. It may occur in different situations – lack of neurological recovery, multiple organ failure, hemodynamic stabilization and requirement of other devices – in which the final strategy of therapy cannot be established during device implantation (e.g. after cardiorespiratory arrest).22
Bridge to recovery: situation in which support device is removed for ventricular function recovery, such as ventricular dysfunction following acute myocardial infarction, Takotsubo cardiomyopathy and myocarditis.23
Bridge to transplantation: situations in which the patient is in progressive severity and heart transplantation cannot be performed in a short term. Support devices may provide hemodynamic support and clinical stability until transplantation is performed.
Types of temporary mechanical circulatory support devices
Main characteristics of temporary MCSDs available in Brazil are described in Chart 5.24
Chart 5.
Temporary mechanical circulatory support devices available in Brazil
| Characteristics | Intra-aortic balloon | ECMO | TandemHeart™ | Impella 2.5® Impella CP® Impella 5.0® |
CentriMag® | EXCOR® |
|---|---|---|---|---|---|---|
| Mechanism | Pneumatic | Centrifugal | Centrifugal | Axial | Centrifugal | Pulsatile |
| Access | Percutaneous | Percutaneous / thoracotomy | Percutaneous | Percutaneous Percutaneous Dissection |
Thoracotomy | Thoracotomy |
| Cannulation | 7-9 F | 18-21 F Inflow 15-22 F Outflow |
21 F Inflow 15-17 F Outflow |
12 F 14 F 21 F |
24-34 F | 27-48 F Inflow 36-48 F Outflow |
| Insertion technique | Descending aorta via femoral artery | Percutaneous: - Inflow: right atrium via femoral or jugular vein - Outflow: descending aorta via femoral artery Thoracotomy: - Inflow: right atrium - Outflow: pulmonary artery (left mechanical circulatory assist device) or ascending aorta (biventricular assist device) |
Inflow: left atrium via femoral vein and
transseptal puncture Outflow: femoral artery |
Insertion into left ventricle via femoral artery | ACM-E: - Inflow: left ventricle (via left atrium or apex of left ventricle) - Outflow: ascending aorta ACM-D:- Inflow: right atrium - Outflow: pulmonary artery |
ACM-E: - Inflow: left ventricle (apex of left ventricle) - Outflow: ascending aorta ACM-D: - Inflow: right atrium - Outflow: pulmonary artery |
| Hemodynamic support | 0.5 L/min | > 4.5 L/min | 4 L/min | 2.5 L/min 3.7 L/min 5.0 L/min |
Up to 8-10 L/min | Up to 8 L/min |
ECMO: Extracorporeal membrane oxygenation
Indications and contraindications
Although temporary MCSDs are primarily indicated for patients INTERMACS levels 1 and 2, INTERMACS level 3 patients, dependent of high doses of inotropes or at high risk of hemodynamic instability may also be considered eligible.
Contraindications for temporary MCDS include limiting clinical situations that require individualized approach and involvement of other professionals (e.g. oncologist for establishment of cancer prognosis).
Intra-aortic balloon pump (IABP)
The mechanism of action of the IABP is aortic counterpulsation, which increases diastolic pressure at aortic root, promoting an increase in coronary perfusion, afterload reduction, and consequently an increment in cardiac output by 15%.
Although IABP is still used in the clinical practice especially in younger patients with less severe cardiogenic shock, the efficacy of the method should be carefully evaluated based on improvement of objective parameters of tissue microperfusion. Lack of improvement of these variables in a short time period (hours) justifies the selection of more invasive devices.
Recommendations for intra-aortic balloon pump implantation
| Recommendation | Class | Evidence level |
|---|---|---|
| Post-AMI cardiogenic shock | IIa | B |
| Post-AMI mechanical complication with cardiogenic shock | IIa | C |
| Refractory angina after standard therapy for acute coronary syndrome | IIa | C |
| Cardiogenic shock in ischemic / non-ischemic chronic cardiomyopathy | IIa | C |
| Intervention support for patients at high cardiac risk | IIb | C |
AMI: acute myocardial infarction
Percutaneous circulatory devices
Definition and benefits
Percutaneous circulatory devices enable active support without requiring a synchronism with the cardiac cycle. The main benefits are maintenance of tissue perfusion, improvement of coronary perfusion, and reduction of myocardial oxygen consumption, filling pressures and ventricular wall stress, providing a circulatory support in cardiogenic shock.25,26
Recommendations for percutaneous circulatory support device implantation
| Recommendation | Class | Evidence level |
|---|---|---|
| Post-AMI cardiogenic shock | IIa | C |
| Support for interventions in patients at high cardiac risk | IIb | C |
AMI: acute myocardial infarction
Types of percutaneous circulatory devices
Impella®
Impella device is composed of a continuous axial flow pump, that aspirates blood directly from the left ventricle and directs it to the aorta (works in series with left ventricle). It allows the flow of 2.5 L/min (Impella® 2.5), 4.0 L/min (Impella® CP) or 5.0 L/min (Impella® 5.0). The model currently available in Brazil is Impella® CP.24,27
TandemHeart™
TandemHeart™ system is composed of a centrifugal extracorporeal pump, a femoral cannula, a transseptal cannula and a control console. It pumps blood from the left atrium through a transseptal cannula to the ileo-femoral arterial system. Both TandemHeart™ and the left ventricle work in parallel and contribute to aortic blood flow.24,27
Extracorporeal membrane oxygenation
Definition, types and benefits
Extracorporeal membrane oxygenation (ECMO) is an invasive temporary mechanical support that provides partial or total cardiopulmonary support for patients with cardiogenic shock and/or acute respiratory insufficiency. There are two types of ECMO – venoarterial and venovenous. With quick installation technology, ECMO promotes rapid reversal of circulatory failure and/or anoxia.
Recommendations for extracorporeal membrane oxygenation implantation
| Recommendation | Class | Level of evidence |
|---|---|---|
| Bridge to decision or recovery | I | C |
| Bridge to transplantation | IIa | C |
Paracorporeal circulatory support
Definition, types and benefits
Paracorporeal circulatory support devices are surgically implanted pumps that promote hemodynamic support in individuals with refractory cardiogenic shock with high mortality risk.
A CentriMag® is a continuous flow, magnetically levitated centrifugal blood pump. It provides up to 10 L/minute of blood flow and low shear stress, promoting low thrombogenicity, moderate anticoagulation levels and minimum hemolysis during support.24
Berlin Heart EXCOR® is a pulsatile-flow pump that provides up to 8 L/min of blood flow, with batteries connected to a transport system, allowing an up to ten hours of patient’s mobility.
Recommendations for implantation of paracorporeal circulatory pumps
| Recommendation | Class | Level of evidence |
|---|---|---|
| Bridge to decision or recovery | IIa | C |
| Bridge to transplantation | IIa | C |
Other conventional centrifugal pumps may be used with the same purpose.
Long term devices
Types of long-term mechanical circulatory support devices
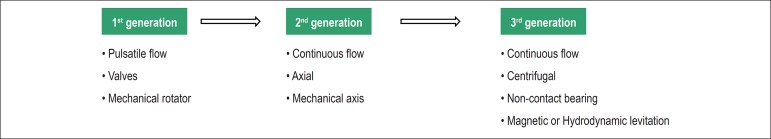
Due to technological progress, advances in long-term MCSD models have occurred during the last years, regarding pumping system and flow type, enabling its reduction in size, greater efficiency and lower complication rates (Figure 1).
Figure 1.
Progress of long-term mechanical circulatory support devices.
The long-term MCSDs available in Brazil are described in Chart 6.
Chart 6.
Long-term mechanical circulatory support devices available in Brazil
| Name | Company | Type of pump | Type of support | Presence of bearing | Anvisa Approval |
|---|---|---|---|---|---|
| HeartMate II® | Thoratec | Axial flow | Left | Yes | Yes |
| INCOR® | Berlin Heart | Axial flow | Left | No (electromagnetic levitation) | Yes |
| HeartWare® | HeartWare | Centrifugal flow | Left | No (electromagnetic levitation) | Yes |
Anvisa: Agência Nacional de Vigilância Sanitária (The Brazilian Health Regulatory Agency); NA: not applicable
Indications and contraindications
In making decision process for long-term MCSDs, some important factors should be considered. In case of bridge to transplantation, transplant waiting time should be taken into account; for waiting time shorter than 30 days, there would be a low benefit-cost ratio. Also, the use of these devices in INTERMACS level 2 patients may have unfavorable results.
Recommendations for long-term mechanical circulatory support devices as bridge to transplant
| Recommendation | Class | Level of evidence |
|---|---|---|
| Systolic heart failure - INTERMACS levels 2 and 3 | Class IIa | C |
| Systolic heart failure - INTERMACS level 4 | Class IIb | C |
| Systolic heart failure -INTERMACS levels 1, 5, 6 and 7 | Class III | C |
Recommendations for long-term mechanical circulatory support devices as destination therapy
| Recommendation | Class | Level of evidence |
|---|---|---|
| Systolic heart failure - INTERMACS 3 | Class IIa | B |
| Systolic heart failure - INTERMACS 2 | C | |
| Systolic heart failure - INTERMACS 4 | Class IIb | C |
| Systolic heart failure - INTERMACS 1, 5, 6 e 7 | Class III | C |
Recommendations for long-term mechanical circulatory support devices as bridge to decision
| Recommendation | Class | Level of evidence |
|---|---|---|
| Systolic heart failure - INTERMACS 2 and 3 | Class IIa | C |
| Systolic heart failure - INTERMACS 4 | Class IIb | C |
| Systolic heart failure - INTERMACS 1, 5, 6 and 7 | Class III | C |
Patients eligible for MCSD should be evaluated for the presence of factors that may contraindicate or negatively influence patients’ survival after transplant. Main contraindications are listed in Chart 7.
Chart 7.
Contraindications for long-term mechanical circulatory support devices
| Absolute | Coumarin intolerance |
| Absence of trained caregivers | |
| Severe psychiatric disorders or nonadherence to the staff instructions | |
| Severe motor deficit or cognitive deficit related after stroke | |
| Neoplastic disease with unfavorable prognosis | |
| Vascular malformation of the small bowel that predisposes to bleeding | |
| Severe pulmonary obstructive disease | |
| Severe hepatic dysfunction | |
| Active infection | |
| Hematologic changes (platelets < 50,000 mm3 and thrombophilia) | |
| Relative | Moderate-to-severe right ventricular dysfunction |
| Dialytic therapy for renal failure | |
| Difficult-to-control diabetes | |
| Partial motor deficit after stroke | |
| Severe malnutrition | |
| Significative peripheral artery disease |
Strategy for selection of long-term MCSDs
Bridge to decision: long-term MCSDs may be indicated for patients with clinical conditions that contraindicate heart transplantation, but if modified, patients may become eligible for transplant (for example: pulmonary hypertension and curable cancers).
Bridge to transplant: Situations in which MCSDs may provide hemodynamic support and clinical stability until heart transplant, in patients with progressive severity and when a short-term transplant is not possible.
Destination therapy: Situations in which MCSDs may provide hemodynamic support and clinical stability in patients with refractory heart failure with contraindication for cardiac transplant, promoting higher survival and better quality of life as compared with clinical treatment with drugs.
Optimization and management of right ventricular function
Right ventricular failure is still one of the main factors that affect patients’ survival after MCSD implantation.28 Its diagnostic criteria are – signs and symptoms for persistent right ventricular dysfunction; CVP > 18 mmHg with cardiac index < 2,0 L/min.m2 in the absence of ventricular arrhythmias or pneumothorax; requirement of ventricular support devices; or requirement for inhaled nitric oxide or inotropic therapy for more than one week after device implantation.29
Implantation of a MCSD increases cardiac output and consequently causes an increment in venous return to the right ventricle. To counteract this preload increase, right ventricular compliance should improve with reduction of its afterload (decrease in left ventricular filling pressure and pulmonary arterial pressure). However, leftward shift of IVS may occur in case of excessive left ventricular emptying.29
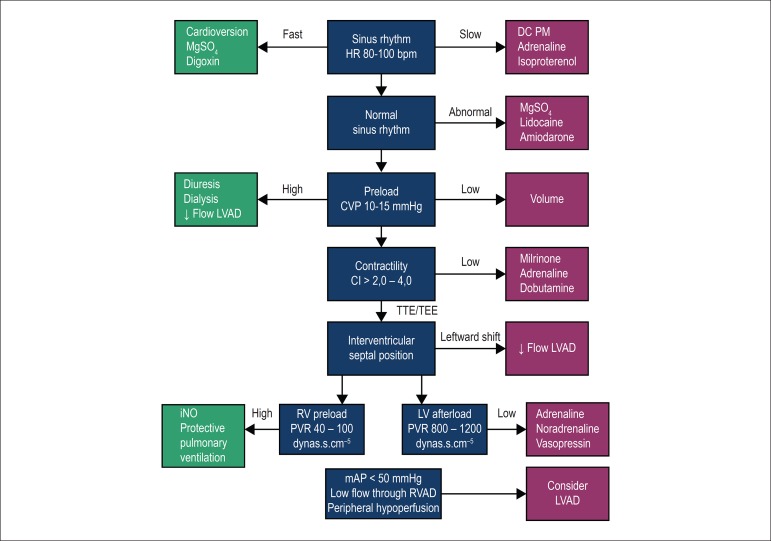
In addition to its contractility, optimization of right ventricular preload and afterload is crucial to prevent right ventricular failure in the perioperative period. CVP and systolic pulmonary pressure should be maintained lower than 16 mmHg and 65 mmHg, respectively. For maintenance of coronary perfusion, use of inotropes that cause pulmonary vasodilation (milrinone or dobutamine) and maintain adequate systemic pressure (adrenaline) is recommended. In addition, the use of specific pulmonary vasodilators, such as nitric oxide should be considered (Figure 2).30
Figure 2.
Optimization and management of right ventricular function. MgSO4: magnesium sulfate; HR: heart rate; DC PM: dual-chamber pacemaker with right atrial and ventricular stimulation and sensitivity; LVAD: Left ventricular assist device; CVP: central venous pressure; CI: cardiac index; TTE: transthoracic echocardiogram; TEE: transesophageal echocardiography; RV: right ventricular; PVR: pulmonary vascular resistance; LV: left ventricular; SVR: systemic vascular resistance; RVAD: right ventricular assist device; mAP: mean arterial pressure.
Complications after long-term MCSD implantation
The main complications related to long-term MCSD implantation are described in Chart 8.
Chart 8.
Complications of long-term mechanical circulatory support devices (MCSDs)
| Bleeding | Pericardial effusion | Respiratory insufficiency |
|---|---|---|
| Right ventricular dysfunction | Hypertension | Non-neurological arterial thromboembolism |
| Neurological events | Arrhythmias | Venous thromboembolism |
| Infections | Myocardial infarction | Surgical wound dehiscence |
| MCSD malfunction | Hepatic dysfunction | Psychiatric / behavioral change |
| Hemolysis | Renal dysfunction |
Proposal of prioritization criteria for cardiac transplant in patients with MCSD
With increasing number of MCDSs, this document proposes a change in the prioritization criteria for patients in the cardiac transplant waiting list. These new criteria are described in Chart 9.
Chart 9.
Proposal of prioritization criteria for cardiac transplant
| Priority | Criterium |
|---|---|
| 1 | Cardiogenic shock in patients using
short/medium-term paracorporeal MCDS (including intra-aortic
balloon) Long-term MCDS with complications and substitution of device is not possible |
| 2 | Cardiogenic shock in patients using inotropes or vasopressors |
| 3 | Stable long-term MCDS without complications |
| 4 | Outpatient management of advanced heart failure |
MCDS: mechanical circulatory device support
References
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30(2):115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz AJ, Rose EA, Gelijns AC. The cost of long-term LVAD implantation. Ann Thorac Surg. 2001;71(3 Suppl):S195–S198. doi: 10.1016/s0003-4975(00)02621-7. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure Study Group Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Reedy JE, Swartz MT, Termuhlen DF, Pennington DG, McBride LR, Miller LW, et al. Bridge to heart transplantation: importance of patient selection. J Heart Transplant. 1990;9(5):473–480. [PubMed] [Google Scholar]
- 5.Lietz K, Miller LW. Patient selection for left-ventricular assist devices. Curr Opin Cardiol. 2009;24(3):246–251. doi: 10.1097/HCO.0b013e32832a0743. [DOI] [PubMed] [Google Scholar]
- 6.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116(5):497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 7.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61(3):313–321. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Oz MC, Goldstein DJ, Pepino P, Weinberg AD, Thompson SM, Catanese KA, et al. Screening scale predicts patients successfully receiving long-term implantable left ventricular assist devices. Circulation. 1995;92(9 Suppl):II169–II173. doi: 10.1161/01.cir.92.9.169. [DOI] [PubMed] [Google Scholar]
- 9.Deng MC, Loebe M, El-Banayosy A, Gronda E, Jansen PG, Vigano M, et al. Mechanical circulatory support for advanced heart failure: effect of patient selection on outcome. Circulation. 2001;103(2):231–237. doi: 10.1161/01.cir.103.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Santamore WP, Gray LA., Jr. Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61(1):350–356. doi: 10.1016/0003-4975(95)01056-4. [DOI] [PubMed] [Google Scholar]
- 11.Loforte A, Stepanenko A, Potapov EV, Musumeci F, Dranishnikov N, Schweiger M, et al. Temporary right ventricular mechanical support in high-risk left ventricular assist device recipients versus permanent biventricular or total artificial heart support. Artif Organs. 2013;37(6):523–530. doi: 10.1111/aor.12038. [DOI] [PubMed] [Google Scholar]
- 12.Scalia GM, McCarthy PM, Savage RM, Smedira NG, Thomas JD. Clinical utility of echocardiography in the management of implantable ventricular assist devices. J Am Soc Echocardiogr. 2000;13(8):754–763. doi: 10.1067/mje.2000.105009. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 14.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. International Society for Heart and Lung Transplantation The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014 Mar;6(Suppl 1):S52–S59. doi: 10.3978/j.issn.2072-1439.2013.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51(22):2163–2172. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et al. HeartMate II Clinical Investigators Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139(5):1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick 3rd JR, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27(12):1286–1292. doi: 10.1016/j.healun.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013;96(3):857–863. doi: 10.1016/j.athoracsur.2013.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman WL, Acharya D, Siric F, Loyaga-Rendon RY. Assessment and management of right ventricular failure in left ventricular assist device patients. Circ J. 2015;79(3):478–486. doi: 10.1253/circj.CJ-15-0093. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein D, Neragi-Miandoab S. Mechanical bridge to decision: what are the options for the management of acute refractory cardiogenic shock? Curr Heart Fail Rep. 2011;8(1):51–58. doi: 10.1007/s11897-010-0041-5. [DOI] [PubMed] [Google Scholar]
- 23.Kar B, Basra SS, Shah NR, Loyalka P. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation. 2012;125(14):1809–1817. doi: 10.1161/CIRCULATIONAHA.111.040220. [DOI] [PubMed] [Google Scholar]
- 24.Gilotra NA, Stevens GR. Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol. 2014;8(Suppl 1):75–85. doi: 10.4137/CMC.S15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104(24):2917–2922. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 26.Raess DH, Weber DM. Impella 2.5. J Cardiovasc Transl Res. 2009;2(2):168–172. doi: 10.1007/s12265-009-9099-4. [DOI] [PubMed] [Google Scholar]
- 27.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, et al. Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Patlolla B, Beygui R, Haddad F. Right-ventricular failure following left ventricle assist device implantation. Curr Opin Cardiol. 2013;28(2):223–233. doi: 10.1097/HCO.0b013e32835dd12c. [DOI] [PubMed] [Google Scholar]
- 30.Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res Clin Anaesthesiol. 2012;26(2):217–229. doi: 10.1016/j.bpa.2012.03.006. [DOI] [PubMed] [Google Scholar]