Abstract
Cystic fibrosis (CF), one of the most common human genetic diseases worldwide, is caused by a defect in the CF transmembrane conductance regulator (CFTR). Patients with CF are highly susceptible to infections caused by opportunistic pathogens including Burkholderia cenocepacia, which induce excessive lung inflammation and the eventual loss of pulmonary function. Abundant neutrophil recruitment into the lung is a key characteristic of bacterial infections in CF patients. In response to infection, inflammatory neutrophils release reactive oxygen species (ROS) and toxic proteins, leading to aggravated lung-tissue damage in patients with CF. The present study shows a defect in ROS production by mouse Cftr−/− and human F508del-CFTR, CF neutrophils, and consequently, reduced antimicrobial activity against B. cenocepacia. Furthermore, dysregulated Ca2+ homeostasis led to increased intracellular concentrations of Ca2+ that correlated with significantly diminished NADPH oxidase response and impaired secretion of neutrophil extracellular traps (NETs) in human CF neutrophils. Functionally deficient human CF neutrophils recovered their antimicrobial killing capacity following treatment with pharmacological inhibitors of Ca2+ channels and CFTR channel potentiators. Our findings suggest that regulation of neutrophil Ca2+ homeostasis, via CFTR potentiation or by the regulation of Ca2+ channels, can be used as a new therapeutic approach for reestablishing immune function in patients with CF.
Keywords: Cystic fibrosis, Neutrophils, Burkholderia cenocepacia, NETs, Calcium homeostasis
Introduction
Cystic fibrosis (CF) affects approximately 30,000 individuals in the US alone (1). CF is caused by a functional defect in the cystic fibrosis transmembrane conductance regulator (CFTR), which functions as a cyclic AMP-activated, ATP-gated chloride (Cl−) and thiocyanate (SCN−) channel (2, 3). Since the CFTR gene was discovered in 1989, more than 1800 mutations of this single gene have been identified, with about 242 mutations confirmed to cause cystic fibrosis (1). F508del-CFTR is the most common mutation in the CF population; 86.4 percent of patients have at least one copy of this mutation. The F508del-CFTR mutation results in a misfolded protein that cannot escape the endoplasmic reticulum for further processing (1, 4).
In the lung, CF mutations have long been associated with viscid mucus, resulting in an ineffective mucociliary clearance, leading to colonization of the airways with opportunistic pathogens, and consequently, respiratory infections that are the main cause of mortality in CF patients (5, 6). Burkholderia cepacia complex (Bcc) is a group of opportunistic bacterial pathogens that infect CF patients causing heightened lung inflammation and the devastating, sepsis-like Cepacia syndrome (7, 8). B. cenocepacia infections have been associated with high morbidity and mortality in CF patients (9).
Although CF has long been recognized as an epithelial disease, cumulative evidence suggests that deficient CFTR function in immune cells leads to aberrant inflammation and deficient pathogen clearance (10). CFTR is expressed in phagocytic cells including macrophages (11, 12) and neutrophils (2, 13). Neutrophils, first line of defense against microbial infections, possess multiple antimicrobial mechanisms to clear microorganisms including phagocytosis, neutrophil granule release, production of reactive oxygen species (ROS), and generation of neutrophil extracellular traps (NETs) (14, 15). ROS are critical to ensuring an effective killing by phagocytosis and to inducing NETs (15). Infected CF airways harbor abundant neutrophils, which can contribute to further pulmonary damage (16). Upon infecting CF neutrophils, B. cenocepacia can induce necrosis (17), and release of neutrophil granule contents, such as myeloperoxidase, enzymes and DAMPs. The materials released contribute to inflammation, and eventually, may cause lung failure.
The changes in the tissue microenvironment of patients with CF modify cell functions of phagocytes. For instance, CF neutrophils show reduced phagocytic capacity (18), and consequently, defective killing against Pseudomonas aeruginosa (19) due to a reduction of phagolysosome chlorination (2). Despite B. cenocepacia mechanisms to scavenge ROS of host cells, these bacteria succumb to ROS-dependent killing mechanisms upon efficient neutrophil responses in healthy individuals. However patients with chronic granulomatous disease (CGD), which is characterized by defective NADPH oxidase, are highly susceptible to develop infections by B. cenocepacia (20). Similar susceptibility of CF patients to B. cenocepacia infections has led investigators to search for abnormalities in the oxidative responses of the CF neutrophil. Although some reports indicate defective NADPH oxidase activity and reduced ROS production in human CF neutrophils (21, 22), others have found no differences in respiratory burst activity between CF patients and healthy individuals (23–25). Thereby, additional experimental evidence is required from mouse and human neutrophils to help resolving this controversial topic.
In the past decade, Ca2+ mobilization has been shown to have an important role in the regulation of ROS by phagocytic cells (26). In addition, extracellular Ca2+ entry is required for NADPH oxidase activation (26). Abnormal regulation of Ca2+ has been reported in CF airway epithelia (27). Increased intracellular concentration of calcium appears to enhance the inflammatory profile of CF airway cells, which may contribute to the development of chronic and aggravated inflammatory responses (28). However, the role of Ca2+ signaling in regulating CF neutrophil dysfunction has not been investigated.
Here we report a diminished capacity of CF neutrophils to kill B. cenocepacia, resulting from reduced production of ROS metabolites in CF neutrophils, and consequently, a reduced production of NETs. We propose that dysregulated Ca2+ homeostasis, which directly modifies the function of NADPH oxidase and further reduces ROS-dependent antimicrobial mechanisms in CF neutrophils, causes the changes in the CF neutrophils’ responses. Therefore, the regulation of Ca2+ channel activities, may constitute a new therapeutic approach to improve the immune response of patients with CF.
Materials and Methods
Ethics statement
The Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children’s Hospital IACUC (AR13-00020) approved animal experiments to ensure the humane care and use of animals. All mice studies were performed in strict accordance with the National Institutes of Health standards as set forth in "Guide for the Care and Use of Laboratory Animals" DHSS Publication No. (NIH) 85–23.
Mouse strains
Wild-type (WT) C57BL/6 were bred and maintained in Nationwide Children’s Hospital vivarium. Cftrtm1Unc Tg(FABPCFTR)1Jaw/J, (Cftr−/−) (29) and B6N.129S2-Ncf1tm1Shl/J (p47phox−/−) (30) were purchased from Jackson Laboratory (Bar Harbor, ME).
Bacterial culture
Burkholderia cenocepacia strain K56-2, Pseudomonas aeruginosa 01 wild-type (Pa01) and Staphylococcus aureus SH1000 wild-type were cultured in Luria-Bertani (LB) broth (Difco, MD), Nontypeable Haemophilus Influenzae 86-028NP (NTHI) (clinically isolated) was cultured in brain heart infusion (BHI) broth at 37 °C overnight with shaking. Bacterial concentration was adjusted before each experiment based on absorbance at 600 nm or 490 nm for NTHI.
Bacterial infection
C57BL/6 or Cftr−/− mice were infected intratracheally (i.t.) with 5×106 colony forming units (CFU) of B. cenocepacia, and the survival curve was followed up to 8 days post infection (dpi). Some C57BL/6 mice were pretreated with 250 µg of anti-Ly6G clone 1A8 (Bioxcell, West Lebanon, NH) or 250 µg of Gr-1 clone RB6-8C5 (Bioxcell, West Lebanon, NH) antibody for 24 hours before bacterial infection. Bacterial inoculum was adjusted and administrated i.t., and the survival curve was followed for up to 8 dpi.
Neutrophils migration in vivo
C57BL/6 mice were i.t. infected with 5×106 CFU of B. cenocepacia. Animals were euthanized 24 or 48 hpi, and bronchoalveolar lavage (BAL) was collected with 1mL of PBS plus 10−3 M of EDTA. Lung tissue was collected and treated with collagenase IV plus DNase I for 30 minutes at 37 °C, then mashed, washed and stained with anti CD45 brilliant violet 510 (Biolegend, San Diego CA), CD11b Alexa Fluor 700 (Biolegend, San Diego CA), Ly6G PerCP-Cy5.5 (Biolegend, San Diego CA) and Ly6C PE-Cy7 (Biolegend, San Diego CA) antibodies plus live/dead blue discriminator (Invitrogen, Eugene OR). Cells were acquired with a LSR II flow cytometer (BD, Franklin Lakes, NJ). Some lungs were fixed and embedded in paraffin, slides were stained with Hematoxylin-Eosin (H&E).
Isolation of bone marrow neutrophils
Mouse bone marrow cells were isolated from femurs and tibiae. Polymorphonuclear cells (PMN) were purified by positive selection using biotinylated anti-Ly-6G (Biolegend, San Diego CA) and MACS streptavidin-microbeads (Miltenyi Biotec, Auburn CA), following the manufacturer instructions. PMN purity was ≥95% as assessed by flow cytometry.
Isolation of human blood PMN cells
Human donors gave written informed consent for blood donation at the Nationwide Children’s Hospital as approved by the Institutional Review Board of Nationwide Children’s Hospital. Written consent from legal guardians of minors was obtained as well as written assent from minors aged 9 to 17 years. Inclusion criteria for patients in the study was bearing the F508del mutation. Exclusion criteria included history of B. cepacia complex culture positivity, chronic immunosuppression, CFTR modulator use, and history of transplantation. Chronic azithromycin was the only immunomodulatory medicine taken by some subjects during the study period. Blood was collected (20 mL per subject) in heparinized tubes (BD, Franklin Lakes NJ). Human peripheral blood neutrophils were purified by negative selection (Stemcell Technologies, Vancouver, BC, Canada) according to the manufacturer directions.
Measurement of reactive oxygen species
105 neutrophils were stained with highly reactive oxygen species (hROS) detection reagent, aminophenyl fluorescein (APF), to measure hypochlorite (ClO−) hydroxyradical (•OH), and peroxynitrite (ONOO−) production. According to the manufacturer (Enzo life sciences, Farmingdale NY, USA), this fluorescent reagent has little reactivity towards other ROS such as singlet oxygen, superoxide, hydrogen peroxide, nitric oxide, and alkyl peroxide. Neutrophil staining was followed by addition of 10−5 M of Diphenyleneiodonium chloride (DPI) (Tocris Bioscience, Bristol, UK) or 10−5 M of a voltage-independent CFTR inhibitor PPQ-102 (Tocris Bioscience, Bristol, UK), then cells were stimulated with 10−7 M of PMA, 1µg/ml of LPS, 2.5×10−4 M of Pyocianin, S. aureus or NTHI, the kinetics were measured at 488/515nm using Synergy H1 multi-mode plate reader (Biotek, Winooski VT). Some cells were treated with 10−5 M of PPQ-102 for 20 minutes and incubated with 10−4 M of luminol, followed by addition of 5×10−8 M of PMA, B. cenocepacia and/or 10−5 M of DPI, the kinetics were acquired by luminescence plate reader.
Quantification of bacterial colony forming units
Human or mouse neutrophils (5×105) were infected with Burkholderia cenocepacia (Bc), Pseudomonas aeruginosa (Pa01), Staphylococcus aureus (Sa) or Nontypeable Haemophilus influenzae (NTHI) using a multiplicity of infection of 10 (MOI=10) in RPMI medium with 2% FBS for 45 minutes. Cells were centrifuged at 1200 rpm for 7 minutes, the supernatant was removed, and the pellet was resuspended and seeded in 24 well plates, then some neutrophils were treated with 3×10−6 M of EGTA (Sigma Aldrich, St. Louis, MO) or 10−5 M of 2-APB (Sigma Aldrich, St. Louis, USA) and incubated for 3 hours at 37 °C. Some samples were pretreated with 10−5 M DPI for 10 minutes or with or with 5×10−6 M of VX-770 (Ivacaftor, Selleckchem.com) for 60 minutes at 37 °C and maintained with the potentiator during the course of infection. To quantify intracellular bacteria, neutrophils were lysed with 0.1% Triton X-100 (Sigma Aldrich, St. Louis, MO), and free bacteria were quantified by serial dilution on LB and chocolate agar plates. Relative percent of antimicrobial killing was calculated by dividing the inverse CFU of CF with non-CF neutrophils and multiplied by 100.
NETs visualization and quantitation
105 PMN were attached in coverslips for 30 minutes at 37 °C. Cells were then stimulated with 1µg/mL LPS, 5×10−8 M PMA, or infected with B. cenocepacia or B. cenocepacia-RFP (MOI=10) and incubated for 3 hours at 37 °C. To visualize NETs, we used a method previously described (31). Briefly, for neutrophil elastase (NE) detection; rabbit anti-human/mouse NE (Abcam, Cambridge MA, USA) and chicken anti-rabbit Alexa Fluor 568 or chicken anti-rabbit Alexa Fluor 488 (Invitrogen, Eugene OR, USA) were used. Samples were mounted using fluoroshield mounting media with blue emitting DAPI (Abcam, Cambridge, MA, USA). The slides were acquired using the Nikon Eclipse Ti (Nikon Instrument Inc. Americas) and analyzed using ImageJ software (NIH). To quantify NETs, 105 neutrophils were treated with 10−5 M of DPI or 10−5 M of PPQ-102, then stimulated with 1µg/ml of LPS, 10−7 M of PMA, B. cenocepacia, S. aureus or NTHI using a MOI of 10, some cells were lysed with 1% triton X-100 (to determine total DNA content), after 2 h of incubation, some cells were treated with DNase I for 45 minutes at 37 °C, then extracellular DNA was stained with 5×10−6 M of SyTOX green (Invitrogen, Eugene OR) for 15 minutes, finally, the samples fluorescence was measured at 504/523 nm with a Synergy H1 Multi-mode plate reader. To quantify the amount of extracellular DNA we followed the method previously described (32).
Scanning Electron Microscopy
105 PMN were seeded on coverslips for 30 minutes at 37 °C, followed by stimulation with 5×10−8 M of PMA or infected with B. cenocepacia (MOI=10) for 3 hours at 37 °C. Samples were fixed for 30 minutes with 2.5% glutaraldehyde in PBS buffer, followed by secondary fixation for 30 minutes with 1% osmium tetroxide in PBS. The fixed samples were then triple washed in deionized water (dH2O) and dehydrated with graded ethanol (50%, 70%, 80%, 90%, 95%, and 100%). The dehydrated samples were incubated 1 hour in 50% hexamethyldisilazane (HMDS):ethanol, followed by two 1-hour incubations in 100% HMDS, and allowed to air dry. The cover slips were coated with 2.5 nM gold:palladium in an Emitech K550X sputter coater (Emitech, Ashford, England) and examined on a Hitachi S-4800 field-emission scanning electron microscope (Hitachi High Technologies, Schaumburg, IL). Images were captured at an accelerating voltage of 3–5 kV using the upper secondary electron detector, unless noted.
Expression of CFTR in human neutrophils
105 human neutrophils were seeded in coverslips coated with poly-L-lysine (Sigma Aldrich, St. Louis, MO). Cells were fixed, permeabilized and stained with mouse anti-human CFTR [CF3] (Abcam, Cambridge MA), wheat germ agglutinin Alexa Fluor 594 (Abcam, Cambridge MA), rabbit anti-human LAMP1 (Abcam, Cambridge MA), goat anti-mouse Alexa Fluor 488 (Abcam, Cambridge MA), goat anti-rabbit Alexa Fluor 594 (Abcam, Cambridge MA) and Hoechst 33342 (ThermoFischer scientific). Slides were imaged using apotome fluorescent microscope (Zeiss) and analyzed with ImageJ software (NIH).
Measurement of cytosolic Ca2+ in neutrophils
Human or mouse bone marrow neutrophils were stained with 10−6 M of Flou-4 AM (Invitrogen, Eugene OR) in the presence of 4×10−3 M of probenecid (Invitrogen, Eugene OR) for 30 minutes at 37 °C. Neutrophils (5×105) were aliquoted per tube in Hank's Balanced Salt Solution (HBSS) and maintained on ice and in the dark. Some samples were pretreated with 3×10−3 M EGTA, 10−5 M DPI, 10−5 M of PPQ-102 for 20 minutes or with 5×10−6 M of VX-770 for 30 minutes at 37 °C. The accumulation of intracellular free calcium was assessed by FACS in triplicate with a LSR II cytometer, using the blue laser (488nm) for excitation and 505 LP 530/30 BP filter channel for acquiring fluorescence emission. Neutrophils were collected before stimulation for 30 seconds at low acquisition rate to determine baseline calcium levels, then cells were stimulated with 10−7 M of IL-8 (R&D Systems), 10−7 M of platelet activating factor (PAF) (Sigma Aldrich), 10−7 M of C5a (R&D Systems) or (10−8 - 10−7 M) fMLP (Sigma Aldrich). The fluorescence emission of Fluo-4 was measured for 250–350 seconds. Data was analyzed using the kinetics platform in FlowJo software (Ashland, OR), each second of the kinetics represents the MFI of around 800 events.
Statistical analysis
Statistical evaluation for bar graphs was determined by unpaired Student’s t test or by one-way ANOVA/Tukey. The analysis were performed by using GraphPad Prism software (San Diego CA). A value of p<0.05 was considered statistically significant.
Results
Increased susceptibilty to B. cenocepacia infection in Cftr−/− mice
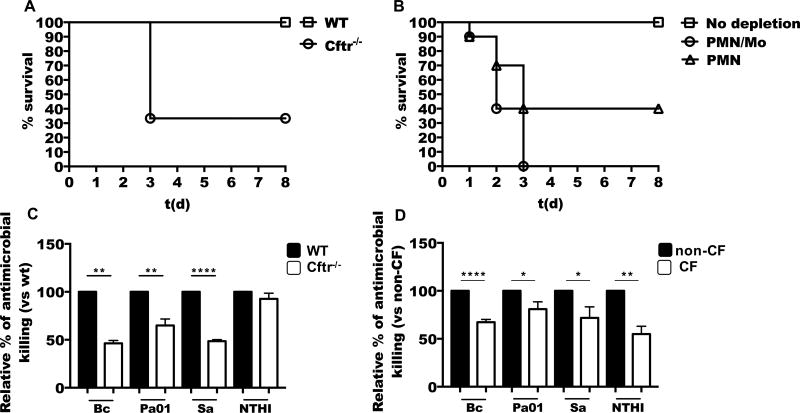
CF patients are prone to develop lung infections by B. cenocepacia (1), however, the establishment of animal models is critical to study the host-microbe interaction. Although CFTR-deficient mice can clear some of the pathogens that affect CF patients, these mice show an impaired ability to control lung infection with Pseudomonas aeruginosa and showed excessive proinflammatory signaling (33–35). In an attempt to mimic the human infection, we intratracheally (i.t.) infected wild type (WT) and Cftr−/− mice with B. cenocepacia (Figure 1A), and studied the course of lung infection. We observed that while all WT mice survived up to 8 days after infection, only 30 percent of Cftr−/− mice survived one week after infection. Infection with 5×106 colony forming units (CFU) of B. cenocepacia killed up to 70 percent of mice in the Cftr−/− group, as early as 72 hours post infection (hpi), but none of the mice in the WT group, suggesting that CFTR-deficient mice are defective in specific antimicrobial responses against this bacteria.
Figure 1. Neutrophils are essential to control B. cenocepacia infection, but CF neutrophils show defective bacterial killing.
(A) C57BL/6 (WT) or Cftr−/− mice were i.t. infected with 5×106 CFU of B. cenocepacia, the infection was followed up to 8 days post-infection, and graph shows survival of mice in each group (n=6 per group). (B) WT mice were pretreated intravenously with 250 µg of anti-Ly6G (PMN) or anti-Gr1 (PMN/Mo) purified antibody 24 h before bacterial infection. Animals were infected with B. cenocepacia and survival was monitored for 8 days. (n=10 mice per group). (C) WT or Cftr−/− bone marrow neutrophils were infected with B. cenocepacia (Bc), P. aeruginosa (Pa01), Staphylococcus aureus (Sa) or nontypeable Haemophilus influenzae (NTHI) (MOI of 10), and CFU were enumerated at 3 h (n≥4 mice). (D) Purified neutrophils from non-CF or CF patients were infected with Bc, Pa01, Sa or NTHI (MOI=10), then CFU were enumerated at 3 h (Bc n=11, Pa01 n=7, Sa n=8, NTHI n=6). Asterisks indicate p value for statistical significance (**p<0.01, ***p<0.001, ****p<0.0001).
Neutrophils are essential to clear B. cenocepacia infections
Phagocytes have an important role in maintaining lung homeostasis by preventing bacterial infections and removing dead cells (15). Patients with CF are prone to develop lung infections due to B. cenocepacia. However, it is not clear whether neutrophils play a protector or pathologic role. We first investigated the contribution of neutrophils to the inflammation induced by B. cenocepacia in murine airways. We i.t. infected WT mice with B. cenocepacia and investigated the migration of neutrophils into the lungs. We found that B. cenocepacia induced a very robust recruitment of neutrophils by 24 and 48 hpi, as evaluated in the bronchoalveolar lavage (BAL) fluid and lung parenchymal tissue (Supplemental Figure 1). This response suggests that neutrophils may have a major role in the immunity against B. cenocepacia. To study the functional role of neutrophils during B. cenocepacia infection, we depleted neutrophils in WT mice by intravenous (i.v.) injection of anti-Ly6G specific antibody, or depleted neutrophils and monocytes with anti-Gr1 antibody (36). Depleted and non-depleted WT mice were i.t. infected with B. cenocepacia (5×106 CFU) and survival was monitored over the next eight days after infection. While WT mice efficiently resolved the bacterial infection, with neutrophils depleted, greater than 50% of the mice succumbed to infection during the first 72 hpi (Figure 1B), furthermore, none of the mice depleted of monocytes and neutrophils survived beyond 72 hpi (Figure 1B). These results demonstrated that both types of phagocytes, monocytes and neutrophils, are essential to clear B. cenocepacia infection in the lungs.
CFTR channel dysfunction reduces antimicrobial capacity in neutrophils
To assess the relationship between CFTR channel expression and antimicrobial functions in neutrophils, we first determined protein expression and cellular localization of CFTR in human neutrophils. The CFTR channel is highly expressed in barrier tissues, such as lung epithelial cells and gut epithelial cells (37). It has been reported that leukocytes also express CFTR, including T cells (38) and macrophages (11), yet studies on neutrophils offered mixed results (2, 11, 18, 39). Using fluorescent anti human CFTR, we detected CFTR expression in human neutrophils (Supplemental Figure 2). Neutrophils expressed CFTR in the plasma membrane, and in intracellular vesicles related to phagolysosomal compartments and the endocytic pathway (Supplemental Figure 2). Next, to evaluate the potential impact of CFTR channel dysfunction on the antimicrobial activities of neutrophils, we infected WT and Cftr−/− mouse neutrophils (Figure 1C) with CF pathogens; B. cenocepacia (Bc), P. aeruginosa (Pa01), S. aureus (Sa) and nontypeable Haemophilus influenza (NTHI). Cftr−/− neutrophils showed significantly reduced antimicrobial killing against B. cenocepacia, P. aeruginosa and S. aureus, however the antimicrobial killing against NTHI was not significantly reduced in mouse Cftr−/− neutrophils. We next evaluated the antimicrobial capacity of CF (delF508-CFTR) and non-CF donors against the same CF related pathogens as tested with mouse neutrophils (Figure 1D). We observed a reduced antimicrobial capacity in CF neutrophils against all the pathogens tested. The results suggest an intrinsic defect in the antimicrobial response of CF neutrophils, which could vary according to the host condition and the pathogen.
Oxidative burst dependent antimicrobial mechanisms are impaired in CF neutrophils
The generation of reactive oxygen species (ROS) is an essential antimicrobial mechanism in neutrophils. Although ROS intermediates are produced by the NADPH oxidase complex in healthy individuals, results regarding the function of NADPH oxidase and the generation of ROS pathway in CF neutrophils are matter of controversy (21–25). Since we observed a defect in antimicrobial mechanism in CF neutrophils, we sought to analyze how the CFTR mutation or inhibition of CFTR function affects ROS production in neutrophils.
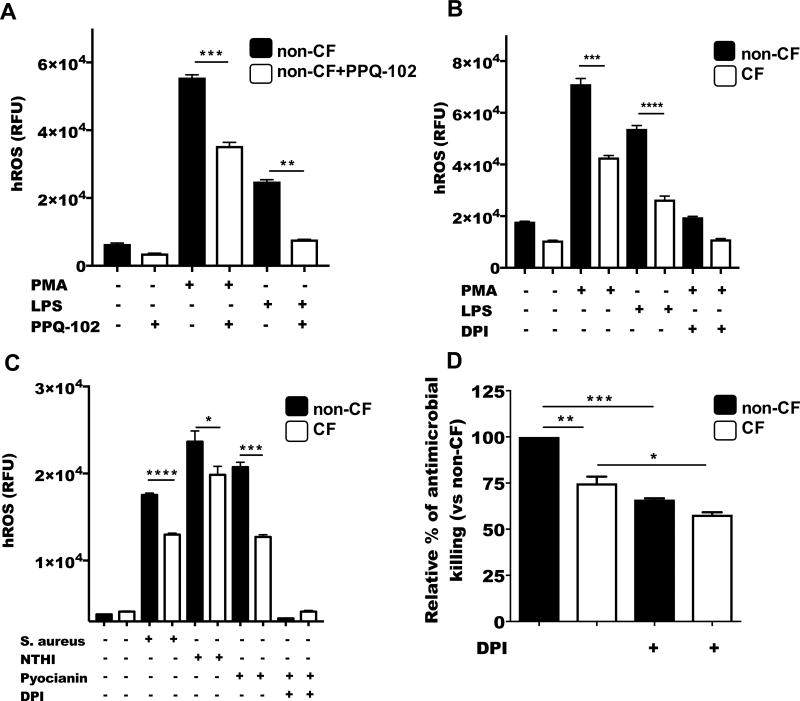
Because activation of Cl− channels causes Cl− efflux and plasma membrane depolarization (40), it is possible that NADPH oxidase activity, which is regulated by membrane depolarization (41) may be affected by deficient Cl− fluxes. To test whether blocking the CFTR channel in non-CF neutrophils would directly impact the oxidative response in neutrophils, we used the cell-permeable pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor (PPQ-102) and the cell permeable dye, aminophenyl fluorescein (APF), to detect the production of highly reactive oxygen species (hROS) in neutrophils, including hypochlorite (ClO−), hydroxyradical (•OH), and peroxynitrite (ONOO−). The inhibition of CFTR was enough to reduce hROS production in non-CF neutrophils when stimulated with PMA or LPS (Figure 2A). Reduced Cl− efflux by CFTR inhibition also affected the production of superoxide products (Supplementary Figure 3A).
Figure 2. ROS-dependent antimicrobial mechanisms are impaired in CF neutrophils.
(A) Neutrophils purified from healthy donors were stained with APF, treated with 10−5 M of PPQ-102 for 20 minutes at 37 °C and stimulated with 5×10−8 M PMA or 1µg/ml LPS. hROS products including hypochlorite (HClO−), hydroxyradical (•OH), and peroxynitrite (ONOO−) were measured with a fluorescence plate reader (488/515 nm). (B) hROS was measured in CF or non-CF neutrophils stimulated with PMA or LPS or by using (C) 2.5×10−4 M of pyocianin, S. aureus or NTHI (MOI=10) as indicated. (D) CF and non-CF neutrophils were pretreated with 10−5 M DPI for 10 minutes followed by the infection with B. cenocepacia (MOI=10). CFU were quantified by serial dilutions and plating. Graphs are representative of 3 independent experiments. Asterisks indicate p value for statistical significance (*=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001).
To test whether CFTR deficiency in neutrophils might impact on Cl− mobilization, and therefore contribute to the production of hROS, we measured hROS. CF neutrophils showed a reduced production of hROS when stimulated with PMA or LPS (Figure 2B), also when CF neutrophils where stimulated with pyocianin, S. aureus, NTHI (Figure 2C) or with B. cenocepacia (Supplementary Figure 3B), showed a reduced concentration of ROS, suggesting that a deficiency in CFTR function could impact in the production of final oxidative products.
To confirm the importance of the ROS response against B. cenocepacia infection, we pretreated neutrophils with the flavoprotein inhibitor, diphenyleneiodonium chloride (DPI) (42), then infected with B. cenocepacia. Both CF neutrophils and non-CF neutrophils showed reduced antimicrobial killing activity when NADPH oxidase was blocked (Figure 2D). These results reinforced the premise that production of ROS by neutrophils is critical to control B. cenocepacia infection.
CF neutrophils are defective in NETosis
NETosis was described in 2004 as the ultimate antimicrobial mechanism of neutrophils to trap and kill extracellular bacteria (43). CF patients that develop chronic inflammation have a profuse neutrophilia in the lungs, correlating with one of the clinical features of these patients, i.e., the highly dense sputum, which some reports relate with high concentration of DNA (44). The clinical manifestations in the lung suggest that the neutrophil extracellular traps’ (NETs) secretion could have a major role in the pathology of CF patients (45). The ROS pathway has been extensively related to NETs production in neutrophils. Thus, our results showing deficient secretions of ROS in CF neutrophils prompted us to analyze whether the ROS-NETs pathway was affected in neutrophils from CF patients.
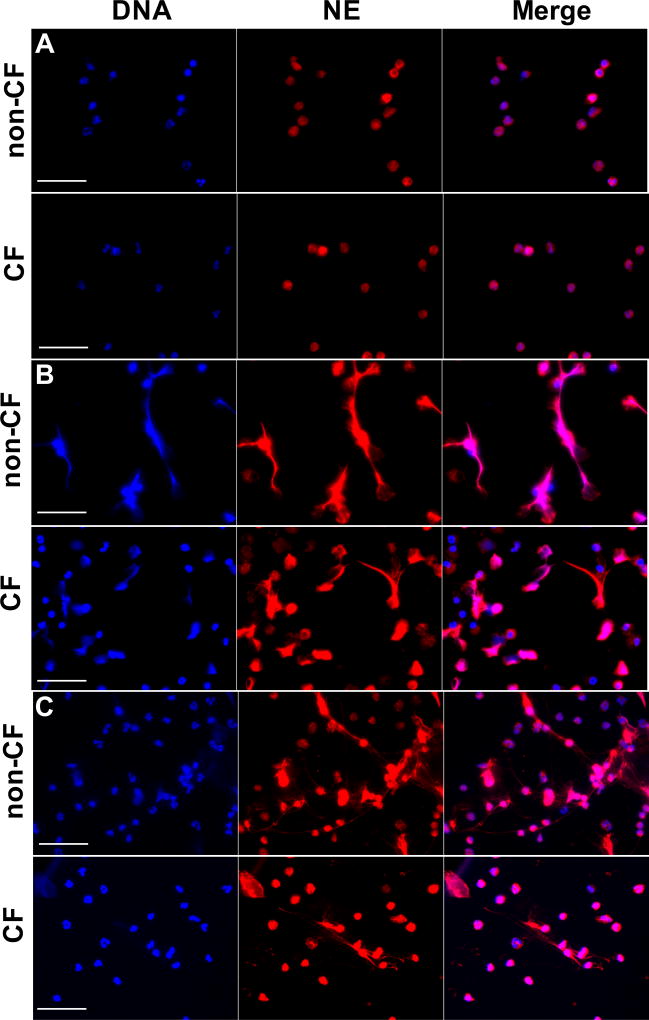
When we stimulated CF and non-CF neutrophils with PMA, CF neutrophils produced less extended “web-like” NETs structures compared to non-CF, which could indicate a defect in the NETs pathway (Figure 3A–B).
Figure 3. NETosis is partially impaired in neutrophils from CF patients.
Non-CF or CF neutrophils were stimulated with (A) 5×10−8 M PMA or (B) 1µg/mL LPS for 3 h. Slides were fixed and stained with rabbit anti-NE plus goat anti-rabbit IgG Alexa Fluor 568 and DAPI. The images were acquired with a fluorescence microscope at 40× (scale bars represent 50 µm). Images are representative of five independent experiments.
When CFTR function was inhibited in non-CF neutrophils, NETosis was partially reduced after stimulation with PMA (Supplemental Figure 3C). We obtained similar results stimulating the cells with LPS (Figure 3C). The NETosis induced by PMA was dependent on the NADPH oxidase pathway (Supplemental Figure 4A), as previously suggested in patients suffering chronic granulomatous disease (CGD)(46), a syndrome characterized by mutations in the NADPH/PHOX complex (47).
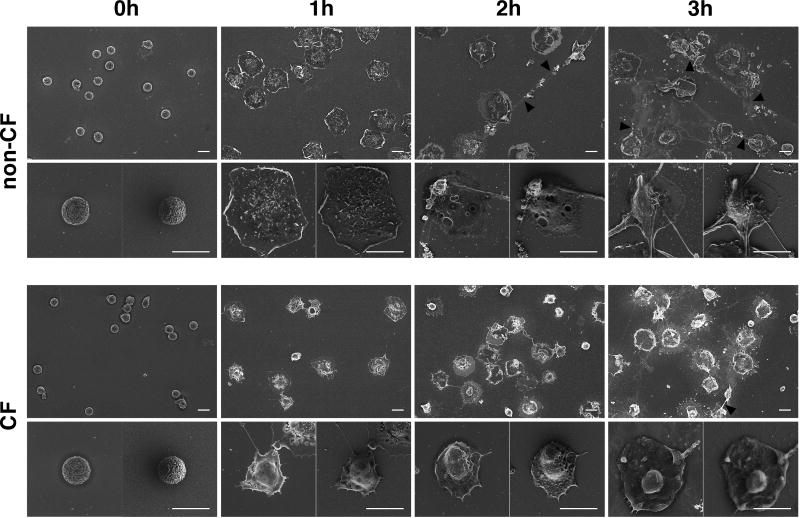
Furthermore, we confirmed the inability of CF neutrophils to produce NETs by comparing the kinetics of NET formation in CF and non-CF neutrophils. We employed scanning electron microscopy (SEM) to visualize the stimulation of neutrophils with PMA, and followed morphological change over 3 hours post-stimulation. PMA stimulation induced cells to flatten and chromatin to decondense, followed by nuclear disintegration (Figure 4, 1hour panels), and ultimately, ejection of DNA filaments into the extracellular space (Figure 4, 2 hour panels). In contrast, PMA failed to induce such dramatic morphological changes in CF neutrophils, even after 3 hours post-stimulation (Figure 4, 3 hour panels).
Figure 4. CF neutrophils show distinct pattern of activation and inability to form NETs.
Non-CF or CF neutrophils were stimulated with 5×10−8 M PMA for 1–3h, then processed for scanning electron microscopy (SEM). The scale bars represents 10 µm. Images are representative of 3 independent experiments.
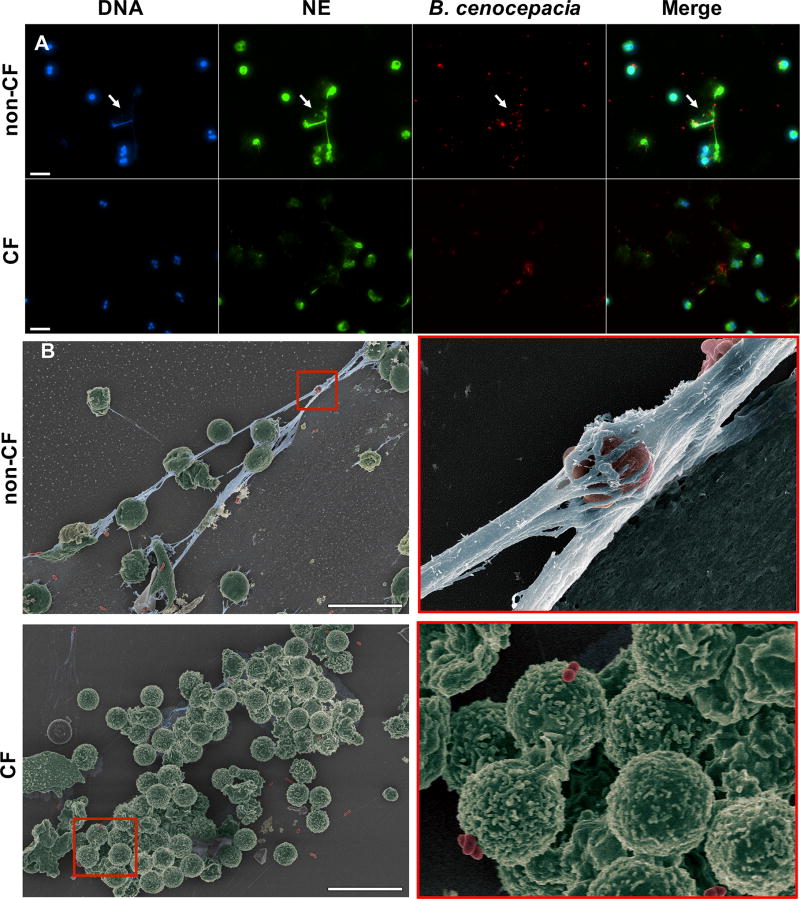
Next, we tested whether B. cenocepacia infection could directly induce NETs formation. CF and non-CF neutrophils were isolated and assessed for NETs using different methodologies. We found that CF neutrophils infected with B. cenocepacia did not produce NETs (Figure 5A), however, non-CF neutrophils released NETs colocalizing with bacteria (Figure 5A, arrows indicate bacteria colocalization with NETs). Further analysis using SEM confirmed that CF neutrophils were unable to releasing NETs in the presence of B. cenocepacia, even when the neutrophils were in contact with bacteria (Figure 5B lower panel, red boxed-inset). In contrast, non-CF neutrophils released NETs abundantly in the presence of B. cenocepacia, which trapped extracellular free bacteria (Figure 5B upper panel, red boxed-inset). Next, we quantified NETs by staining extracellular DNA released (eDNA) with SyTOX green. CF neutrophils showed a reduced production of eDNA or NETs when stimulated with PMA, LPS, B. cenocepacia or S. aureus compared with non-CF neutrophils. Intriguinly, neither CF or non CF neutrophils produced significant amounts of NETs when stimulated with NTHI (Suplemmental figure 4A–B).
Figure 5. CF neutrophils fail to release NETs when infected with B. cenocepacia.
Non-CF or CF neutrophils were infected with B. cenocepacia expressing red fluorescence protein (RFP) (MOI=10) for 3 h, (A) The slides were fixed and stained with rabbit anti-NE plus chicken anti-rabbit IgG Alexa Fluor 488 and DAPI. White arrows indicate bacteria that co-localize with NETs (scale bars represent 20 µm). (B) Neutrophils were infected with B. cenocepacia for 3 h, then fixed and processed for visualization by SEM. Bacteria are pseudo colored red; neutrophils are pseudo colored green; and DNA is pseudo colored blue. Images were taken from one out of three representative experiments. Scale bars indicate 20 µm.
Dysregulated Ca2+ homeostasis in CF neutrophils
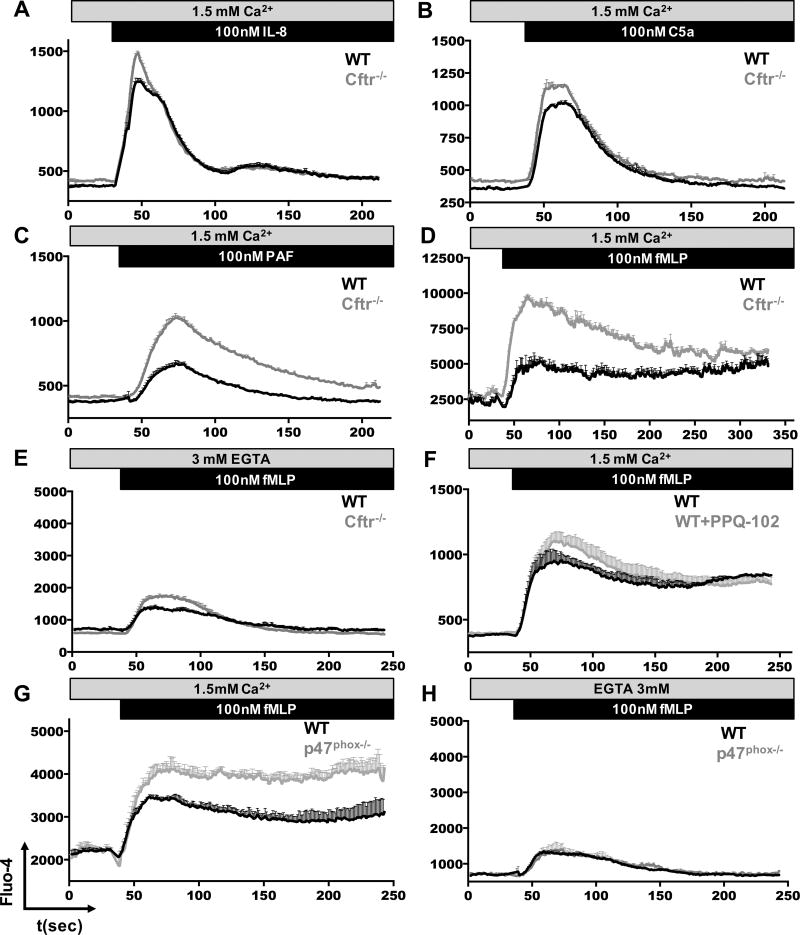
Published studies have demonstrated that the hyper-activation of CF airway epithelia depends on Ca2+ overloading (48, 49). In CF patients, activation of airway epithelia contributes to persistent recruitment of inflammatory neutrophils, which are related to exacerbated lung inflammation and lung damage (6). The role of CFTR dysfunction in modification of Ca2+ homeostasis in CF neutrophils has not yet been investigated. To address this point, we used mouse neutrophils and found slightly elevated Ca2+ responses in Cftr−/− neutrophils, when stimulated with IL-8 (Figure 6A) or C5a (Figure 6B). In contrast, when Cftr−/− neutrophils were stimulated with PAF, we measured significantly greater levels of [Ca2+]i, as compared to responses in their WT counterpart (Figure 6C). Similar trend was observed in Cftr−/− neutrophils when stimulated with fMLP (Figure 6D). Seeking to identify the source of Ca2+ response in Cftr−/− neutrophils, we chelated extracellular Ca2+ using EGTA (Figure 6E). Following depletion of external Ca2+, Cftr−/− neutrophils showed slightly elevated [Ca2+]i when compared to WT neutrophils, suggesting that loss of the CFTR channel may affect the regulation of intracellular concentration of Ca2+ following neutrophil stimulation. We wondered whether CFTR-mediated Cl− currents could directly regulate such intracellular Ca2+ responses in neutrophils. To test this, we treated WT neutrophils with PPQ-102 and analyzed the [Ca2+]i kinetic following fMLP stimulation. Interestingly, inhibition of the CFTR channel was enough to increase the Ca2+ response in neutrophils (Figure 6F).
Figure 6. Calcium homeostasis is dysregulated in Cftr/− neutrophils.
WT, Cftr−/− or p47phox−/− mouse bone marrow neutrophils were loaded with Fluo-4, cells were aliquoted in HBSS buffer containing Ca2+, (A) WT or Cftr−/− neutrophils were stimulated with 10−7 M of IL-8, (B) 10−7 M of C5a, (C) 10−7 M of PAF or (D) 10−7 M of fMLP. (E) Neutrophils were stimulated with fMLP in free Ca2+ media. (F) Cells were pretreated with 10−5 M of PPQ-102 for 20 minutes, then stimulated with fMLP. WT or p47phox−/− neutrophils were stimulated with 10−7 M of fMLP (G) with Ca2+ or (H) without Ca2+, the intracellular free Ca2+ levels were measured by FACS for 250 seconds, each second of the kinetics represents around 103 events. The graphs show MFI±SEM of 3 mice each one, data representative of 3 independent experiments.
To correlate the reduced oxidative response in CF neutrophils with the altered Ca2+ response, we used p47phox−/− neutrophils, which are deficient in NADPH oxidase activity (30), and compared their response against WT neutrophils. Oxidative burst deficient p47phox−/− neutrophils had greater [Ca2+]i levels in response to fMLP stimulation, which did not decline with time, as compared to that of WT neutrophils (Figure 6G). There were no differences in [Ca2+]i levels when the cells were stimulated with fMLP without extracellular Ca2+ (Figure 6H). These data suggest that reduced NADPH oxidase activity altered intracellular Ca2+ regulation upon neutrophil stimulation, and that this dysregulation is likely due to alteration of Ca2+ entry through plasma membrane Ca2+ channels.
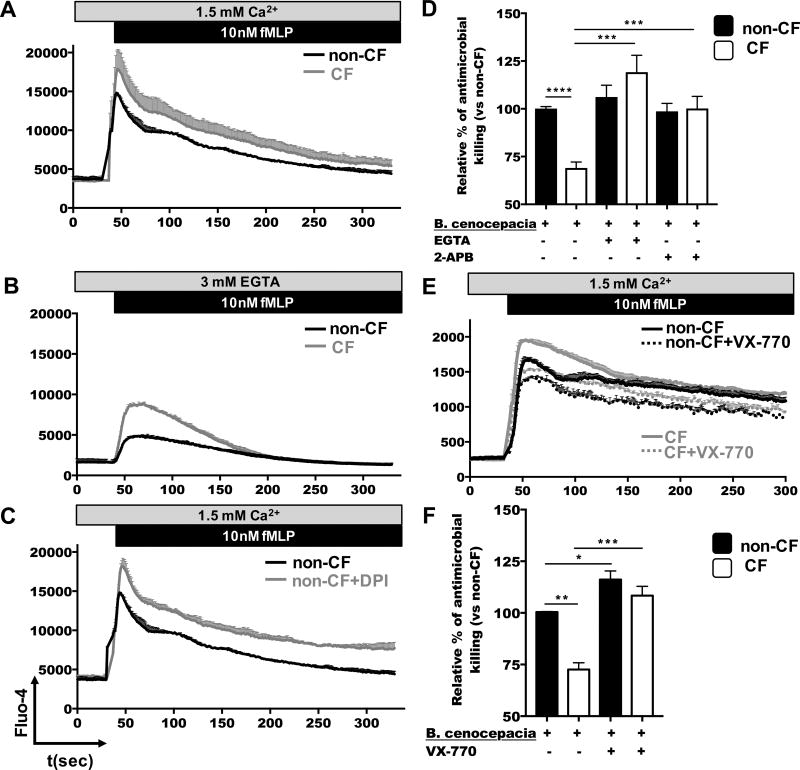
To investigate whether CF neutrophils exhibited similar Ca2+ responses as Cftr−/− mouse neutrophils, we measured [Ca2+]i in human neutrophils after stimulation. We found that CF neutrophils exhibited an elevated intracellular Ca2+ response, as compared to non-CF neutrophils, when stimulated with fMLP (Figure 7A). Similar to Cftr−/− neutrophils in the absence of extracellular Ca2+, following addition of EGTA (Figure 6E), human CF neutrophils developed greater intracellular Ca2+ levels than non-CF neutrophils (Figure 7B). To test whether CF neutrophils responded similar to p47phox−/− neutrophils, with regards to [Ca2+]i upon stimulation, we first added DPI to human non-CF neutrophils, and then stimulated them with fMLP. Interestingly, Ca2+ responses raised in DPI-treated neutrophils, similar to those observed in CF neutrophils (Figure 7C), which indicated that regulation of Ca2+ responses is dependent of NADPH oxidase activity in neutrophils. Altogether, these data suggest a direct correlation between CFTR channel function and the regulation of both NADPH oxidase activity and the Ca2+ responses in neutrophils.
Figure 7. Potentiation of CFTR channel reduces intracellular Ca2+ levels, which results in improved antimicrobial killing of CF neutrophils.
Human CF and non-CF neutrophils (A-C) were loaded with fluorescent Ca2+-detecting dye Fluo-4, in Ca2+ and Mg2+ containing media, and then stimulated with 10−8M fMLP, in the presence of EGTA (B) or DPI (C). The accumulation of intracellular free Ca2+ was assessed by flow cytometry for 340 seconds by measuring the kinetic of fluorescence emission of Fluo-4. The graphs shown are representative of 3 independent experiments. (D) Non-CF and CF neutrophils were infected with B. cenocepacia (MOI=10) for 45 minutes and seeded in 24-well plates. Infected neutrophils were treated with 3×10−6M EGTA or 10−4M 2-APB for 3 hours at 37 °C. Cells were lysed and CFU were calculated (n=5 donors). (E) Non-CF and CF neutrophils were pretreated with VX-770 before loading with Fluo-4 in HBSS media containing Ca2+ and Mg2+. Cells were stimulated with fMLP and the kinetic of Ca2+ measurement recorded by flow cytometry. Graphs are representative of 3 independent experiments. (F) Non-CF and CF neutrophils were treated with VX-770 for 60 minutes, following by the infection with B. cenocepacia (MOI=10) for 45 minutes, then infected cells were incubated 3 hours and CFU enumerated (n=3 donors). Asterisks indicate statistically significant differences between groups (*=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001).
Downregulation of Ca2+ overloading restores antimicrobial response in CF neutrophils
Our hypothesis is that excessive Ca2+ response is responsible for the reduced efficacy of antimicrobial pathways in CF neutrophils. To evaluate whether extracellular Ca2+ entry was contributing to the overloading of Ca2+, and consequently, was responsible for the reduced antimicrobial mechanism in CF neutrophils, we chelated extracellular Ca2+ with EGTA, or inhibited transient receptor potential (TRP) Ca2+ channels with 2-aminoethoxydiphenylborane (2-APB), and then infected the cells with B. cenocepacia (Figure 7D). Both CF and non-CF neutrophils exhibited increased antimicrobial killing when extracellular Ca2+ was depleted. Interestingly, when we inhibited TRP type channels, and therefore blocked the extracellular Ca2+ entry associated to neutrophil activation, CF neutrophils improved antimicrobial killing against B. cenocepacia.
Potentiation of CFTR regulates Ca2+ levels and restores antimicrobial function in CF neutrophils
To understand how CFTR channel activity can impact the regulation of [Ca2+]i levels in neutrophils, we pretreated non-CF and CF neutrophils with VX-770, a potentiator of CFTR channel activity and recorded [Ca2+]i levels. Interestingly, both CF and non-CF neutrophils reduced their intracellular calcium levels under stimulation with fMLP when cells were pretreated with VX-770 (Figure 7E), suggesting a direct functional correlation between CFTR channel activity and Ca2+ regulation in neutrophils. To analyze whether the potentiation of CFTR channel can improbe the antimicrobial activity in neutrophils, we pretreated CF and non-CF neutrophils with VX-770 and intracellular antimicrobial activity was measured as described before. CF neutrophils increased the antimicrobial response against B. cenocepacia when cells were treated with VX-770 (Figure 7F), similarly, non-CF neutrophils treated with VX-770 increased their antimicrobial activity.
Our results suggest that increased intracellular Ca2+ levels in CF neutrophils may result as a CFTR malfunction and directly correlate with reduced antimicrobial capacity in CF neutrophils. Thus, the potentiation of CFTR channels is contributing to the reduction of [Ca2+]i in CF neutrophils, and consequently, is improving antimicrobial responses during infection.
Discussion
Despite the development of new treatments with CFTR modulators, respiratory failure from serious, chronic, lung infections remains the main cause of death in patients with CF (1). While B. cenocepacia occurs less frequently in the CF population than other pathogens such as Pseudomonas aeruginosa, B. cenocepacia is associated with poor outcomes and post-transplant survival leading to exclusion from life-saving lung transplant eligibility (50). Understanding the defects in the immune system that allow pathogens such as B. cenocepacia to persist in CF is key to developing new and more effective therapies.
In this study, we show that neutrophil functions are essential in the host to properly control the bacterial infection induced by B. cenocepacia. Neutrophils use ROS to kill bacteria by activating antimicrobial pathways (15). The fact that B. cenocepacia predominantly infects chronic granulomatous disease (CGD), CF and other immunocompromised patients (20), suggests that ROS may be critical to prevent B. cenocepacia infection. Despite some reports showing contradictory conclusions about a possible defective ROS pathway in CF neutrophils (21, 23), our data show that the ROS pathway is deficient in neutrophils from CF patients. CF neutrophils exhibited reduced oxidative burst when PKC and TLR4 pathways were activated. The reduction in oxidative burst was observed in the production of hROS including ClO−, a critical oxidant involved in bacterial killing that requires chloride anions. Previously, Painter et al., provided evidence of defective intraphagolysosomal production of ClO− in human CF neutrophils (2). Our current data support the idea that the functions of CFTR in neutrophils may directly contribute to supply chloride, which is required for the formation of chlorinated metabolites during oxidative burst. Therefore, CFTR dysfunction may consequently affect neutrophil chlorination and killing of phagocytosed bacteria. Although NADPH oxidase activity is the central microbial killing mechanis, it is conceivable that phagocytes can use NADPH oxidase-independent antimicrobial mechanisms to kill B. cenocepacia, including degranulation and autophagy. Thus, alternative bacterial killing pathways may explain why in our results, neutrophils treated with DPI and then infected with B. cenocepacia preserved significant bacterial killing capacity.
Several molecules derived from neutrophils are markers for prognosis in patients with CF. Such markers include neutrophil elastase, myeloperoxidase, and extracellular DNA or antimicrobial peptides, which may link lung damage with NETosis (16, 44, 51, 52). Indeed, published work suggest that neutrophils undergoing NETosis may contribute to lung’s inflammation and pathology in CF patients (45, 53). However, the work by Young et al., acknowledges that clinical isolates of P. aeruginosa obtained from CF patients early and later in the course of infection demonstrated an acquired capacity to withstand NET-mediated killing (53). Moreover, Gray et al, recently reported that neutrophils from CF patients produced NETs similarly to non-CF neutrophils, furthermore these authors argued that NET production is increased in aged neutrophils from CF patients (45). The method used by Gray et al, was based exclusively in one DNA release assay, and thereby, DNA release due to neutrophil cell death distinct to NETosis may account for increased SYTOX green positive staining in neutrophils from those CF patients. Our results in contrast, showed decreased production of NETs by CF neutrophils and the production of NETs was assessed using three methods, including the visualization of web-like structures by fluorescence and electron microscopy, costaining of neutrophil elastase (NE) and chromatin DNA by immunofluorescence. Finally, unlike the methods used by Gray et al., we substracted non-NETosis release of DNA by treating in each experimental group samples with DNase I at 2 hours post stimulation according to the NETosis quantitative method previously described (32). Thus, by means of three methods we concluded that neutrophils from CF patients exhibited reduced capacity to produce NETs, and this defect was likely a consequence of the reduced activity of NADPH oxidase and lowered production of ROS.
It is also possible that complex pharmacological treatments of CF patients might affect the antimicrobial or immune functions of neutrophils and account, at least in part, for the variability of results from distinct groups of investigators. However, the patients included in this study were only treated with the antibiotic azithromycin as needed, but the use of any CFTR corrector regimen was an exclusion criteria. Other studies are in good agreement with our findings and have shown for instance, that neutrophils with reduced ROS production, such as those from patients with chronic granulomatous disease or myeloperoxidase (MPO) deficiency, produce fewer NETs in response to inflammatory stimuli (54). Further evidence also suggested ClO− as the key ROS involved in human NETosis establishing a potential link between deficient ClO− production and reduced NET formation in CF neutrophils (55). Our scanning electron microscopy results unequivocally show the inability of CF neutrophils to develop NETosis in the presence of bacteria, despite intrinsic morphological changes in unstimulated CF neutrophils that may correlate with the over activated, but inefficient, cell status of circulating neutrophils in CF patients (reviewed in (56)). Indeed, our findings suggest that CF neutrophils are unable to efficiently kill B. cenocepacia, and thereby, in CF patients, bacteria can infect neutrophils and other local cells and disseminate to the lung. In addition to defective production of oxidants and NETs, neutrophils from CF patients have been reported to be impaired in other antimicrobial mechanisms including phagocytosis (57) and degranulation (58). Interestingly, neutrophil chemotaxis and inflammatory recruitment are not affected in clinically stable CF patients (16, 59). In response to infection and because of bacterial spreading, neutrophils accumulate and die likely via pyropoptosis in the lung, as suggested for CF macrophages (60), releasing toxic compounds from their granules into the lung microenvironment, and ultimately promoting tissue destruction. We do not disregard however, the possibility that CF neutrophils in the lung, as opposed to blood neutrophils, could develop distinct patterns of activation. In contrast to the peripheral blood neutrophils tested here, isolated neutrophils from the lung of a CF patient may be continuously exposed to inflammatory stimuli caused by persistent inflammation or induced by recurrent bacterial infections. Nevertheless, our data using blood CF neutrophils is in good agreement with the findings reported in sputum neutrophils from CF patients, which also display a reduced respiratory burst (21).
Although CFTR is not a Ca2+ channel, regulated homeostasis of Ca2+, in both non hematopoietic and hematopoietic cells, seems to be critical for CFTR function. For example, some reports correlated increased [Ca2+]i in CF airway epithelia with hyper-inflammation in the lungs (27, 61), however the sources of such elevated [Ca2+]i were not determined. A recent study showed that calmodulin, considered the major Ca2+ signaling molecule, directly interacts with the R region of CFTR channel, and the presence of the F508del mutation, increases levels of [Ca2+]i in epithelial cells (62). Another study suggested the TRPC6 plasma membrane channel to be responsible for elevated Ca2+ levels in CF airway epithelia (48). In addition, Cl− efflux during activation directly affected Ca2+ signaling in neutrophils (63), however, whether a CFTR channel defect can directly impact the Ca2+ homeostasis in neutrophils it is not known.
We acknowledge the need to identify potential Ca2+ channels that contribute to dysregulation of Ca2+ homeostasis in CF neutrophils. Our initial studies toward this end have focused on determining the expression levels of Na+, Cl− and Ca2+ channels in CF or non-CF neutrophils, following stimulation with B. cenocepacia. Whereas our preliminary genomics analysis of bacterial activated CF neutrophils showed increased expression of several ion channel genes upon stimulation (unpublished results), the strongest upregulation occurred with Ca2+-activated Cl− channels (CaCCs), suggesting dysregulated Ca2+ homeostasis may occur to compensate the lack of CFTR Cl− channels activity in CF cells. Further studies are needed to clarify the origin of ion imbalances in CF neutrophils.
In the present work, we analyzed whether ion imbalances, including Ca2+ mobilization may account for neutrophil dysfunction in CF patients. We showed these cells exhibited major dysregulation of Ca2+ homeostasis, which was stimulus-dependent. In contrast to non-CF neutrophils, CF neutrophils not only responded to stimulation with increased levels of Ca2+ release, but also with long and sustained extracellular entry of Ca2+. These results suggest that CF neutrophils may have increased concentrations of subcellular stored Ca2+ that is rapidly released upon stimulation. Increased Ca2+ levels may consequently activate Ca2+ uptake via opening of plasma membrane Ca2+ channels, expanding the overall mobilization of Ca2+, and resulting in the increased levels of intracellular Ca2+ observed in CF neutrophils. The relationship of Ca2+ signaling and NADPH oxidase has been extensively studied, Ca2+ influx is known to be a crucial element of NADPH oxidase regulation (as reviewed in (26)), however how Ca2+ signaling controls NADPH oxidase activity in neutrophils is not well understood. Our results demonstrate that by blocking NADPH oxidase activity, non-CF neutrophils acquired a phenotype similar to CF neutrophils, characterized by high levels of intracellular Ca2+ and deficient bacterial killing. Thus, impaired NADPH oxidase activity suggests a direct regulatory pathway between [Ca2+]i and NADPH oxidase function. In addition, NET formation is largely dependent on NADPH oxidase activity (64), and therefore, our current working model suggests increased Ca2+ responses of CF neutrophils in response to bacterial or endogenous inflammatory stimuli, which will favor neutrophil inflammation but diminished NADPH oxidase activity, NETosis, and consequently, the reduced efficacy in antimicrobial functions of neutrophils from CF patients.
Although additional studies are needed to define the specific Ca2+ channels involved in the Ca2+ dysregulation in CF neutrophils. We propose that intracellular Ca2+ release mechanisms and members of the TRP family of Ca2+ channels expressed in neutrophils (65, 66), may likely be affected by CFTR malfunction.
Regardless of the channels regulating entry of Ca2+ in CF neutrophils, our data demonstrate that by temporary blocking excessive extracellular Ca2+ entry; either via Ca2+ chelation or by broad inhibition of TRP type plasma membrane channels, deficient CF neutrophils improved their antimicrobial killing capacity against B. cenocepacia. Although the molecular identity of the channel(s) responsible for Ca2+ overloading in CF neutrophils has yet to be defined, our results suggest that regulation of Ca2+ homeostasis could be a new therapeutic target, aiming to restore efficacious immune responses in CF patients. Interestinlgy, the inhibition of TRP type Ca2+ channels is becoming an amenable target for ameliorating pain, the reduction of cell migration or cancer metastasis, and several other physiopathological disorders (67–69). To our knowledge, the specific modulation of neutrophil function in disease, by inhibition of Ca2+ channels has not been attempted and this can become the translational aspect of our research.
We and others have already proposed that TRPM2 and TRPM7 channels may regulate Ca2+ overloading in phagocytic cells (67, 69–74), we speculate that systemic pharmacological inhibition of these channels may help restoring Ca2+ homeostasis and recovering the antimicrobial capacity in phagocytic cells. Indeed the pharmacological use in human of the antifungal drug clotrimazole, which also blocks TRPM2 channels (66, 72), results in dramatic reduction in the recruitment of inflammatory neutrophils (68), indicating downregulation of Ca2+ overloading in neutrophils, may be a feasible therapeutic approach applicable to CF patients. Furthermore, because of Ca2+ dysregulation may result from defective function of CFTR chloride channel, potentiation of CFTR channel by using the drug VX-770 (Ivacaftor) becomes an alternative and feasible strategy to recover impaired CF neutrophil functions. Our results showed that Ivacaftor treated CF neutrophils were able to reduce [Ca2+]i levels, confirming a direct correlation between CFTR function and Ca2+ regulation in these cells, most importantly, Ivacaftor treatment rendered remarkable improvement in the antimicrobial functions of non-CF and CF neutrophils. Altogether, our data provide experimental evidence and highlight the potential clinical relevance for the use of potentiators of CFTR channel, which in combination with pharmacological inhibitors of Ca2+ channels may restore the homeostasis of neutrophils in CF patients.
Supplementary Material
Acknowledgments
We acknowledge Dr. Melody Davis for critically reading and editing the manuscript. We also thank Dr. Miguel Valvano for providing the B. cenocepacia strain.
This work was supported by NIH/NIAID R21 AI120013 grant to SP-S. FR-A was supported by a Post-Doctoral fellowship from CONACyT 208229 (Mexico).
Abbreviations used in this article
- 2-APB
2-Aminoethoxydiphenylborane
- CFTR
Cystic fibrosis conductance regulator
- CFUs
colony-forming units
- DPI
Diphenyleneiodonium chloride
- fMLP
N-Formyl-L-methionyl-L-leucyl-L-phenylalanine
- MOI
multiplicity of infection
- NETs
neutrophil extracellular traps
- NTHI
Nontypeable Haemophilus influenzae
- PMA
phorbol 12-myristate 13-acetate
- PPQ-102
pyrimido-pyrrolo-quinoxalinedione
- ROS
reactive oxygen species.
References
- 1.CFF. Patient registry annual data report 2015 [Google Scholar]
- 2.Painter RG, Valentine VG, Lanson NA, Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childers M, Eckel G, Himmel A, Caldwell J. A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates. Med Hypotheses. 2007;68:101–112. doi: 10.1016/j.mehy.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho CP, Dos Santos SC, Madeira A, Mira NP, Moreira AS, Sa-Correia I. Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: epidemiology, clonal variation, and genome-wide expression alterations. Front Cell Infect Microbiol. 2011;1:12. doi: 10.3389/fcimb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nature medicine. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AM, Dodd ME, Govan JR, Barcus V, Doherty CJ, Morris J, Webb AK. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59:948–951. doi: 10.1136/thx.2003.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahenthiralingam E, Vandamme P. Taxonomy and pathogenesis of the Burkholderia cepacia complex. Chronic respiratory disease. 2005;2:209–217. doi: 10.1191/1479972305cd053ra. [DOI] [PubMed] [Google Scholar]
- 9.De Soyza A, Meachery G, Hester KL, Nicholson A, Parry G, Tocewicz K, Pillay T, Clark S, Lordan JL, Schueler S, Fisher AJ, Dark JH, Gould FK, Corris PA. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:1395–1404. doi: 10.1016/j.healun.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Dekkers JF, van der Ent CK, Kalkhoven E, Beekman JM. PPARgamma as a therapeutic target in cystic fibrosis. Trends Mol Med. 2012;18:283–291. doi: 10.1016/j.molmed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nature cell biology. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 12.Van de Weert-van Leeuwen PB, Van Meegen MA, Speirs JJ, Pals DJ, Rooijakkers SH, Van der Ent CK, Terheggen-Lagro SW, Arets HG, Beekman JM. Optimal complement-mediated phagocytosis of Pseudomonas aeruginosa by monocytes is cystic fibrosis transmembrane conductance regulator-dependent. Am J Respir Cell Mol Biol. 2013;49:463–470. doi: 10.1165/rcmb.2012-0502OC. [DOI] [PubMed] [Google Scholar]
- 13.Ng HP, Valentine VG, Wang G. CFTR targeting during activation of human neutrophils. J Leukoc Biol. 2016;100:1413–1424. doi: 10.1189/jlb.4A0316-130RR. [DOI] [PubMed] [Google Scholar]
- 14.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. The Journal of experimental medicine. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauseef WM, Borregaard N. Neutrophils at work. Nature immunology. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 16.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 17.Bylund J, Campsall PA, Ma RC, Conway BA, Speert DP. Burkholderia cenocepacia induces neutrophil necrosis in chronic granulomatous disease. Journal of immunology. 2005;174:3562–3569. doi: 10.4049/jimmunol.174.6.3562. [DOI] [PubMed] [Google Scholar]
- 18.Morris MR, Doull IJ, Dewitt S, Hallett MB. Reduced iC3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clin Exp Immunol. 2005;142:68–75. doi: 10.1111/j.1365-2249.2005.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter LA, Goldberg JB. Influence of neutrophil defects on Burkholderia cepacia complex pathogenesis. Frontiers in cellular and infection microbiology. 2011;1:9. doi: 10.3389/fcimb.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston N, Stewart N, Smith DS, Bell SC, Champion AC, Reid DW. Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013;12:352–362. doi: 10.1016/j.jcf.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Fruhwirth M, Ruedl C, Ellemunter H, Bock G, Wolf H. Flow-cytometric evaluation of oxidative burst in phagocytic cells of children with cystic fibrosis. Int Arch Allergy Immunol. 1998;117:270–275. doi: 10.1159/000024022. [DOI] [PubMed] [Google Scholar]
- 23.McKeon DJ, Cadwallader KA, Idris S, Cowburn AS, Pasteur MC, Barker H, Haworth CS, Bilton D, Chilvers ER, Condliffe AM. Cystic fibrosis neutrophils have normal intrinsic reactive oxygen species generation. The European respiratory journal. 2010;35:1264–1272. doi: 10.1183/09031936.00089709. [DOI] [PubMed] [Google Scholar]
- 24.Witko-Sarsat V, Allen RC, Paulais M, Nguyen AT, Bessou G, Lenoir G, Descamps-Latscha B. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol. 1996;157:2728–2735. [PubMed] [Google Scholar]
- 25.Kolpen M, Hansen CR, Bjarnsholt T, Moser C, Christensen LD, van Gennip M, Ciofu O, Mandsberg L, Kharazmi A, Doring G, Givskov M, Hoiby N, Jensen PO. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65:57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- 26.Brechard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. Journal of leukocyte biology. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. The Journal of biological chemistry. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 28.Antigny F, Jousset H, Konig S, Frieden M. Thapsigargin activates Ca(2)+ entry both by store-dependent, STIM1/Orai1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium. 2011;49:115–127. doi: 10.1016/j.ceca.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 30.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. The Journal of experimental medicine. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vong L, Sherman PM, Glogauer M. Quantification and visualization of neutrophil extracellular traps (NETs) from murine bone marrow-derived neutrophils. Methods in molecular biology. 2013;1031:41–50. doi: 10.1007/978-1-62703-481-4_5. [DOI] [PubMed] [Google Scholar]
- 33.Davidson DJ, Dorin JR, McLachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous DJ. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat Genet. 1995;9:351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- 34.Gosselin D, Stevenson MM, Cowley EA, Griesenbach U, Eidelman DH, Boule M, Tam MF, Kent G, Skamene E, Tsui LC, Radzioch D. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:1253–1262. doi: 10.1164/ajrccm.157.4.9702081. [DOI] [PubMed] [Google Scholar]
- 35.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 37.Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushwah R, Gagnon S, Sweezey NB. Intrinsic predisposition of naive cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir Res. 2013;14:138. doi: 10.1186/1465-9921-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsunoda Y, Matsumiya H. Calcium-activated membrane depolarization via modulation of chloride efflux from parietal cells during gastrin stimulation. FEBS Lett. 1987;222:149–153. doi: 10.1016/0014-5793(87)80209-0. [DOI] [PubMed] [Google Scholar]
- 41.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 42.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 44.Dwyer M, Shan Q, D'Ortona S, Maurer R, Mitchell R, Olesen H, Thiel S, Huebner J, Gadjeva M. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. Journal of innate immunity. 2014;6:765–779. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, Mackellar A, Felton JM, Paemka L, McCullagh BN, Lucas CD, Dorward DA, McKone EF, Cooke G, Donnelly SC, Singh PK, Stoltz DA, Haslett C, McCray PB, Whyte MKB, Rossi AG, Davidson DJ. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–144. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiriaco M, Salfa I, Di Matteo G, Rossi P, Finocchi A. Chronic granulomatous disease: Clinical, molecular, and therapeutic aspects. Pediatr Allergy Immunol. 2016;27:242–253. doi: 10.1111/pai.12527. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antigny F, Norez C, Becq F, Vandebrouck C. CFTR and Ca Signaling in Cystic Fibrosis. Frontiers in pharmacology. 2011;2:67. doi: 10.3389/fphar.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antigny F, Norez C, Dannhoffer L, Bertrand J, Raveau D, Corbi P, Jayle C, Becq F, Vandebrouck C. Transient receptor potential canonical channel 6 links Ca2+ mishandling to cystic fibrosis transmembrane conductance regulator channel dysfunction in cystic fibrosis. Am J Respir Cell Mol Biol. 2011;44:83–90. doi: 10.1165/rcmb.2009-0347OC. [DOI] [PubMed] [Google Scholar]
- 50.Assani K, Shrestha CL, Robledo-Avila F, Rajaram MV, Partida-Sanchez S, Schlesinger LS, Kopp BT. Human Cystic Fibrosis Macrophages Have Defective Calcium-Dependent Protein Kinase C Activation of the NADPH Oxidase, an Effect Augmented by Burkholderia cenocepacia. J Immunol. 2017;198:1985–1994. doi: 10.4049/jimmunol.1502609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. The European respiratory journal. 2008;32:783–795. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- 52.Kelly E, Greene CM, McElvaney NG. Targeting neutrophil elastase in cystic fibrosis. Expert opinion on therapeutic targets. 2008;12:145–157. doi: 10.1517/14728222.12.2.145. [DOI] [PubMed] [Google Scholar]
- 53.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akong-Moore K, Chow OA, von Kockritz-Blickwede M, Nizet V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One. 2012;7:e42984. doi: 10.1371/journal.pone.0042984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laval J, Ralhan A, Hartl D. Neutrophils in cystic fibrosis. Biol Chem. 2016;397:485–496. doi: 10.1515/hsz-2015-0271. [DOI] [PubMed] [Google Scholar]
- 57.Alexis NE, Muhlebach MS, Peden DB, Noah TL. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J Cyst Fibros. 2006;5:17–25. doi: 10.1016/j.jcf.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, Jundi B, Bergin DA, McCarthy C, McElvaney OJ, White MM, Clynes M, Reeves EP, McElvaney NG. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sener B, Hascelik G, Ozcelik U, Gunalp A, Gocmen A. Neutrophil chemotaxis in acutely infected and clinically stable cystic fibrosis patients. Pediatr Int. 1999;41:514–518. doi: 10.1046/j.1442-200x.1999.01116.x. [DOI] [PubMed] [Google Scholar]
- 60.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123:1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. The Journal of biological chemistry. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 62.Bozoky Z, Ahmadi S, Milman T, Kim TH, Du K, Di Paola M, Pasyk S, Pekhletski R, Keller JP, Bear CE, Forman-Kay JD. Synergy of cAMP and calcium signaling pathways in CFTR regulation. Proc Natl Acad Sci U S A. 2017;114:E2086–E2095. doi: 10.1073/pnas.1613546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busetto S, Trevisan E, Decleva E, Dri P, Menegazzi R. Chloride movements in human neutrophils during phagocytosis: characterization and relationship to granule release. J Immunol. 2007;179:4110–4124. doi: 10.4049/jimmunol.179.6.4110. [DOI] [PubMed] [Google Scholar]
- 64.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heiner I, Eisfeld J, Luckhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell calcium. 2003;33:533–540. doi: 10.1016/s0143-4160(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 66.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Seminars in cell & developmental biology. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, Herson PS. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab. 2011;31:2160–2168. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson D, Hebecker B, Moyes DL, Miramon P, Jablonowski N, Wisgott S, Allert S, Naglik JR, Hube B. Clotrimazole dampens vaginal inflammation and neutrophil infiltration in response to Candida albicans infection. Antimicrob Agents Chemother. 2013;57:5178–5180. doi: 10.1128/AAC.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang J, Furuya H, Faouzi M, Zhang Z, Monteilh-Zoller M, Kawabata KG, Horgen FD, Kawamori T, Penner R, Fleig A. Inhibition of TRPM7 suppresses cell proliferation of colon adenocarcinoma in vitro and induces hypomagnesemia in vivo without affecting azoxymethane-induced early colon cancer in mice. Cell Commun Signal. 2017;15:30. doi: 10.1186/s12964-017-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beceiro S, Radin JN, Chatuvedi R, Piazuelo MB, Horvarth DJ, Cortado H, Gu Y, Dixon B, Gu C, Lange I, Koomoa DL, Wilson KT, Algood HM, Partida-Sanchez S. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017;10:493–507. doi: 10.1038/mi.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heiner I, Radukina N, Eisfeld J, Kuhn F, Luckhoff A. Regulation of TRPM2 channels in neutrophil granulocytes by ADP-ribose: a promising pharmacological target. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:325–333. doi: 10.1007/s00210-005-1033-y. [DOI] [PubMed] [Google Scholar]
- 72.Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- 73.Schilling T, Miralles F, Eder C. TRPM7 regulates proliferation and polarisation of macrophages. J Cell Sci. 2014;127:4561–4566. doi: 10.1242/jcs.151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HS, Hong C, Kim BJ, So I. The Pathophysiologic Roles of TRPM7 Channel. Korean J Physiol Pharmacol. 2014;18:15–23. doi: 10.4196/kjpp.2014.18.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.